Abstract
Objective
To update and summarize evidence of risk factors for breast cancer.
Summary Background Data
Women who are at high risk for breast cancer have a variety of options available to them, including watchful waiting, prophylactic surgery, and chemoprevention. It is increasingly important to accurately assess a patient’s risk profile to ensure that the cost/benefit ratio of the selected treatment is favorable.
Methods
Estimates of relative risk for documented risk factors were obtained from seminal papers identified in previous reviews. These estimates were updated where appropriate with data from more recent reports using large sample sizes or presenting meta-analyses of previous studies. These reports were identified from a review of the Medline database from 1992 to 2002.
Results
Risk factors that have received a great deal of publicity (hormone use, alcohol consumption, obesity, nulliparity) present a relatively modest relative risk for breast cancer (<2). Factors associated with a prior history of neoplastic disease or atypical hyperplasia and factors associated with a genetic predisposition significantly affect the risk of breast cancer, with relative risks ranging from 3 (for some cases of positive family history) to 200 (for premenopausal women positive for a BRCA mutation).
Conclusions
More precise tools, based on techniques of molecular biology such as microarray analysis, will be needed to assess individual risk for breast cancer.
It is estimated that women who survive to the age of 85 will have a 1 in 9 lifetime chance of developing breast cancer. This degree of risk is not homogeneously spread across the population, however. While some individuals will never get breast cancer, others are at increased risk. For these high-risk women, aggressive surveillance and/or treatment may be recommended. Rather than undergo extended periods of watchful waiting, some women may choose prophylactic oophorectomy or mastectomy to reduce their level of risk. More recently, with the findings from the NSABP P-01 trial that tamoxifen can reduce the incidence of breast cancer by almost 50% in high-risk patients, 1 some women are opting for long-term tamoxifen treatment as a chemopreventive strategy. However, tamoxifen treatment is not without side effects and risks of its own. In addition to exacerbated menopausal symptoms, tamoxifen treatment has been linked to an increased incidence of pulmonary emboli and endometrial cancer. Other drugs on the horizon as potential chemopreventive agents will doubtlessly have their own spectra of side effects. Thus, it has become increasingly important to be able to accurately assess a patient’s risk profile to ensure that the cost/benefit ratio of the selected treatment is favorable.
This review will discuss factors that have been determined to increase the risk of invasive breast cancer and will also consider various models currently being used to estimate the additive effects of multiple risk factors. Future possibilities for risk analysis based on new techniques for sampling breast tissue and for measuring abnormalities at the molecular level will also be discussed.
DOCUMENTED RISK FACTORS FOR BREAST CANCER
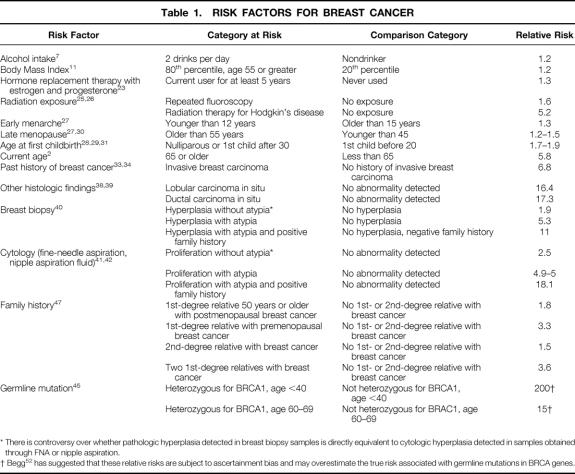
Documented risk factors for breast cancer are shown in Table 1, arranged roughly in order of relative risk. Relative risk denotes the risk for an individual who is positive for a factor versus the risk for an individual who is negative for a factor. A relative risk of 1 indicates that there is no increased risk, whereas a relative risk of 10 indicates that there is a 10-fold increase in risk.
Table 1. RISK FACTORS FOR BREAST CANCER
* There is controversy over whether pathologic hyperplasia detected in breast biopsy samples is directly equivalent to cytologic hyperplasia detected in samples obtained through FNA or nipple aspiration.
† Begg 52 has suggested that these relative risks are subject to ascertainment bias and may overestimate the true risk associated with germline mutations in BRCA genes.
Age
One of the best-documented risk factors for breast cancer (and for many other cancers) is age. As seen in Figure 1, the incidence of breast cancer is extremely low before age 30 (incidence <25 cases per 100,000), after which it increases linearly until the age of 80, reaching a plateau of slightly less than 500 cases per 100,000. 2 If all women less than 65 years of age are compared with women aged 65 or older, the relative risk of breast cancer associated with increased age is 5.8.
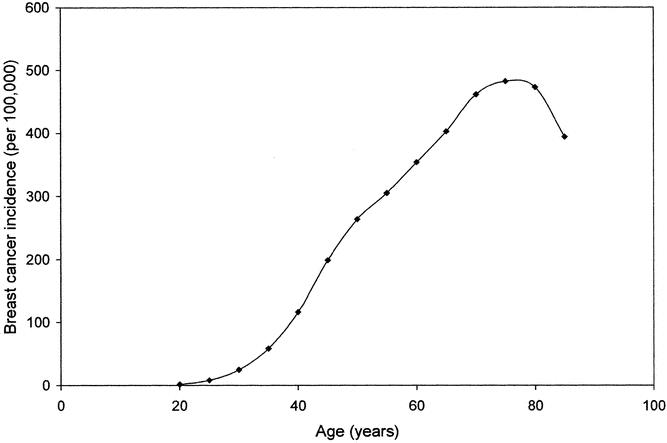
Figure 1. Breast cancer incidence (per 100,000) as a function of age. (Data derived from SEER Cancer Statistics Review, 1973–1997. 2)
Lifestyle and Environmental Factors
There is a keen interest on the part of the general public in risk factors that may lie under the direct control of the individual, as is the case with cigarette smoking and lung cancer. Researchers are investigating the exciting potential for preventing cancer through behavioral modifications or by the avoidance of carcinogenic agents. Unlike the case of cigarette smoking and lung cancer, however, no factors of this kind have yet been identified that have a major effect on the risk of breast cancer. There are, however, several factors that have a more limited effect.
Alcohol Consumption
A number of mechanisms have been proposed whereby the consumption of alcohol might increase the risk of breast cancer. The proposed mechanisms have ranged from the relatively specific (stimulating the metabolism of carcinogens such as acetaldehyde) to those that are more global (decreasing DNA repair efficiency or reducing intake of protective nutrients). 3 That being said, the documented effect of alcohol consumption on the incidence of breast cancer appears to be modest. Numerous studies have reported that consumption of one drink per day or less (approximately 12 g alcohol) does not significantly affect the risk of breast cancer. 4–6 This was confirmed in a meta-analysis by Ellison et al. of relevant studies published between 1966 and 1999. 7 In the 42 reports that met the study criteria, the meta-analysis showed a relative risk of 1.1 for individuals having one drink per day, 1.21 for individuals having two drinks per day, and 1.4 for individuals having three drinks per day, when compared to individuals who did not drink alcohol. There are few data from these 42 studies about the effect of heavier doses of alcohol, but the indications are that the relative risk does not exceed 2, even at very high doses (seven or eight drinks per day). There was no difference in relative risk based on the type of alcohol consumed (wine vs. beer vs. distilled spirits).
There is significant variation among the studies reviewed in the Ellison studies, and two recent reports have suggested that this may be due to the results of confounding factors. Vachon et al. examined the interaction between alcohol consumption and family history in 426 multigenerational breast cancer families. 8 They found that the relative risk associated with daily alcohol consumption was 2.45 in first-degree relatives of breast cancer patients and 1.27 in second-degree relatives, but only 0.99 in unrelated women who had married into the families. These results have not been confirmed in the more recent study by Ursin et al. 9 Royo-Bordonado et al. reported that the relative risk associated with alcohol consumption was increased in individuals with a Body Mass Index (BMI) larger than the median. 10
Body Mass Index
In the largest study to date looking at the relationship between BMI and breast cancer index, 570,000 Norwegian women aged 30 to 69 were weighed and measured and then followed for 6 to 18 years with respect to the incidence and outcomes of breast cancer. 11 BMI was estimated from height and weight measurements as weight divided by height squared (Quetelet’s index). Subjects were divided into quintiles based on BMI, and comparisons were made between the highest and lowest quintile in each 5-year age group (30–34, 35–39, etc.). In premenopausal women, BMI was not a risk factor for breast cancer incidence and may even have played a minor protective role. In postmenopausal women, however, the relative risk of breast cancer incidence in the highest versus the lowest BMI quintile was 1.1 in women aged 55 to 59, 1.18 in women aged 60 to 64, and 1.22 in women aged 65 to 69.
It is not surprising that high BMI should be at least a minor risk factor for breast cancer in this age group, as adipose tissue is an important extragonadal source of bioavailable estrogens in postmenopausal women. 12,13 Exposure to these estrogens postmenopausally increases the time frame in which they may affect both the initiation and promotion of breast cancer. 12 In addition, several studies suggest that high BMI is associated with increased levels of insulin and insulinlike growth factors, which have been associated with increased risk of breast cancer. 14–16 This is of special importance in peri- and postmenopausal women because accumulation of body fat in this age group is usually abdominal, and abdominal obesity is strongly associated with hyperinsulinemia, a risk factor for breast cancer. 17
Hormone Replacement Therapy
As of 1995, nearly 40% of postmenopausal women in the United States used hormone replacement therapy (HRT) for the control of menopausal symptoms and the prevention of osteoporosis. 18 Although HRT was first recommended for menopausal symptoms in the early 1970s, 19 it was nearly 20 years later that troublesome reports started to appear linking HRT with an increased incidence of breast cancer. 20,21 Before the release of data from the Women’s Health Initiative (WHI), the largest study investigating this issue was a meta-analysis of 51 epidemiologic studies by the Collaborative Group on Hormonal Factors in Breast Cancer published in 1997. 22 This analysis considered data from 52,705 women with breast cancer and 108,411 women without breast cancer. For current HRT users or for those who had ceased use 1 to 4 years previously, the risk of developing breast cancer increased for each year of use, showing a relative risk of 1.35 after 5 or more years of use. This study did not find any difference in relative risk based on the type of hormone therapy used (estrogen vs. estrogen and progesterone). While these data were compelling, they were based on epidemiologic evidence. Many felt that the definitive answer to the question of how HRT affects the risk of breast cancer would come when the WHI results were made public.
The WHI is a large multi-institutional study that has enrolled more than 16,000 postmenopausal women aged 50 to 79 to prospectively assess the risks and benefits of HRT using the most commonly prescribed form of estrogen-plus-progesterone or of estrogen alone. In July 2002, the National Institutes of Health suddenly halted the estrogen-plus-progesterone arm of the study because interim analysis of the data indicated that the risks of continuing HRT outweighed the benefits. In addition to the expected increase in risk of stroke, women in this study arm also showed an unexpected increase in the risk of coronary disease. Of relevance here, this large-scale prospective study demonstrated a 26% increase in the risk of breast cancer over a 5-year period. 23 This is fully consistent with the results of the earlier meta-analysis of epidemiologic studies.
Importantly, both studies indicate that the increased risk of breast cancer is only in current or recent users of HRT. Among users who stopped HRT more than 5 years previously, the risk is no greater than in someone who never used HRT. Also of interest, a recent study by Ursin et al. 9 found that the risk associated with HRT was not increased as a function of BMI, alcohol use, parity, history of benign breast disease, or family history of breast cancer.
Current statistics about the effects of HRT on the risk of breast cancer apply to estrogen-plus-progesterone regimens only. While epidemiologic studies indicate that estrogen alone may also increase the risk of breast cancer, this has not been apparent in the WHI study, and the estrogen-only arm of that study is currently being allowed to continue.
Radiation Exposure
Although much of our early knowledge about the carcinogenic effects of radiation exposure in a human population was derived from studies of atomic bomb survivors, 24 therapeutic radiation exposure to monitor or treat disease is now the most significant cause of radiation-induced carcinogenesis. Studies by Boice et al. involving tuberculosis patients have documented that multiple fluoroscopies are a significant risk factor for breast cancer. 24,25 In a 1991 update, breast cancer incidence was tabulated for 2,573 women who were examined by x-ray fluoroscopy an average of 88 times during therapy for tuberculosis and who were followed for an average of 30 years. 25 Extrapolating from the data collected in this population, the relative risk for 1 Gy of radiation exposure at a latency period of 10 years was estimated to be 1.61. They found that younger women were at higher risk than older women, and that the increased risk for breast cancer, beginning 10 to 15 years after the initial exposure, remained high for the duration of the woman’s lifetime.
The risk of breast cancer is also increased in women receiving radiation therapy to the chest area for the treatment of Hodgkin’s lymphoma, especially in women treated from the time of puberty to the age of 30. 26 As with fluoroscopic exposure, there is again a long latency period (approximately 15 years), but the resulting cancers still often occur in women who have not yet begun regular mammographic screening. Clemons et al. 26 reviewed 17 published studies examining the incidence of breast cancer in Hodgkin’s disease survivors. They found a median relative risk of 5.2 (range 1.4–33, plus an outlier value of 75.3), at an average latency period of 14 years (range 5–15.1).
Reproductive Factors
Brinton et al. examined the relationship between reproductive factors and the risk of breast cancer in a series of studies using subjects from the Breast Cancer Detection Demonstration Project. 27–29 This was a multicenter breast cancer screening program involving over 280,000 women at 29 centers. Information about reproductive variables (timing of menarche and menopause, number and timing of children) was obtained from home interviews conducted by trained nurse interviewers. Case-control data were ultimately obtained from 2,908 breast cancer patients and 3,180 controls matched for ethnicity and age.
These data indicated that women who began menstruating before the age of 12 had a relative risk for invasive breast cancer of 1.3 compared to those who began after the age of 15. 27 At the other end of the reproductive period, those who did not reach menopause until age 55 or after showed a relative risk of 1.22 compared with those who experienced menopause before the age of 45. 27 This is somewhat lower than the relative risk of 1.5 reported in an earlier study by Trichopoulos et al. 30 Based on such data, Vogel has suggested that the risk of breast cancer stemming from these gynecologic variables is a simple function of the number of ovulatory menstrual cycles that a woman undergoes during her lifetime. 3 As support for this idea, it has been observed that women who have both ovaries removed before the age of 40 show a 45% reduction in risk compared with women who undergo a natural menopause at the age of 50 to 54. 27
The Brinton studies also demonstrated that the risk of breast cancer increased if a woman was nulliparous or experienced her first live birth at or after the age of 30. Compared to a woman with a first live birth at an age less than 20, the relative risk for the nulliparous woman was 1.67, and the risk for the woman giving birth at or after the age of 30 was 2.23. 28,29 This is consistent with the later work of White, who estimated a relative risk of 1.9 for both nulliparous women and for those giving birth at or after age 30. 31 The Brinton study further showed that there was no protective effect from an early pregnancy if it was not carried to full term. 28,29 This may be because of cell differentiation that occurs in the breast during the last part of a woman’s first full-term pregnancy and subsequent lactation. Sharpe has suggested that epithelial stem cells may be less susceptible to carcinogenesis after this final epithelial differentiation. 32
Prior History of Neoplastic Disease or Hyperplasia in the Breast
Individuals who have a prior history of invasive carcinoma, carcinoma in situ, or atypical hyperplasia in the breast can have a significantly increased risk for the future development of invasive breast carcinoma. Most physicians prefer to manage such women conservatively with close surveillance, although a few women at very high risk may opt for prophylactic mastectomy.
Invasive Ductal Carcinoma
Hankey et al. 33 recorded the incidence of second cancers in 27,275 patients with primary breast cancer and found an average annual incidence of 0.7% for second cancers. This is in agreement with a study by Rosen et al., 34 who found an average annual incidence of 0.8% per year for second cancers. This contrasts with an average breast cancer incidence of approximately 0.11% per year in the general population. 2
Lobular Carcinoma In Situ
Lobular carcinoma in situ (LCIS) has always posed a management dilemma for physicians. Although it is a recognized risk factor for the development of invasive carcinoma, it is not itself a preinvasive condition. 35 The increased risk extends to all breast tissue, not just to the region of the original lesion or to the ipsilateral breast. Furthermore, the subsequent invasive lesion is more likely to be ductal in origin rather than lobular. 36,37 Thus, limited surgery to excise the lesion has no therapeutic value, nor is there a benefit in having the patient undergo more extensive biopsies to detect additional LCIS lesions. In a review of four studies published by Stybo and Wood, 38 the incidence of invasive carcinoma (ipsilateral and contralateral) reported in patients with a previous diagnosis of LCIS ranged from 7% to 36% (median 33%) over a follow-up period ranging from 17 to 24 years (median 18.5 years). This translates to an approximate annual incidence rate of 1.8% and a relative risk of 16.4 compared with individuals having no prior history of LCIS.
Ductal Carcinoma In Situ
Unlike LCIS, ductal carcinoma in situ (DCIS) is a preinvasive lesion, so it is not surprising that a diagnosis of DCIS is associated with an increased risk of invasive carcinoma. The seminal study by Page et al. 39 followed women with DCIS treated by biopsy only and reported a 28% incidence of invasive carcinoma at an average follow-up time of 15 years. This translates to an annual incidence of 1.9% and a relative risk of 17.3.
Hyperplasia
Epithelial hyperplasia (usual or typical) is marked by an increase in the number of normal epithelial cells in normal arrangement relative to the basement membrane. Atypical hyperplasia is characterized by cells that have taken on some of the neoplastic characteristics of DCIS. Both usual hyperplasia and atypical hyperplasia, as ascertained through histologic analysis of biopsy specimens, are associated with an increased relative risk of invasive breast cancer, as documented in a 1985 study by Dupont et al. 40 This study re-evaluated breast biopsies from 1,925 patients with proliferative disease (hyperplasia) and 1,378 patients with nonproliferative benign breast disease who had been followed for a median duration of 17 years. In comparison to patients without hyperplasia, they found that the relative risk for invasive breast cancer was 1.9 in patients with early hyperplasia, 5.3 in patients with atypical hyperplasia, and 11 in patients with atypical hyperplasia with a positive family history (mother, sister, or daughter) with breast cancer.
An association between the presence of atypical cells and breast cancer risk has also been demonstrated in cytologic studies. Fabian et al. 41 performed random periareolar fine-needle aspiration cytology on 480 women who were designated as high risk based on a family history of breast cancer, prior precancerous biopsy, or prior invasive cancer. Estimated risk of future cancer development was calculated using the Gail model (see below), and women were categorized as having a Gail risk above the median or a Gail risk below the median. Eight to ten aspirations were performed per breast, and the aspirates were pooled for analysis. Samples were classified as nonproliferative, proliferative, or proliferative with atypia. At a median follow-up time of 45 months, women with a Gail risk above the median and evidence of proliferation with atypia had a fivefold increased risk for the development of breast cancer compared with women having a Gail risk above the median but with no evidence of proliferation with atypia. Women with a Gail risk below the median and no evidence of proliferation with atypia had no incidence of breast cancer in this time period.
Similar findings have been reported with cytologic samples acquired from nipple aspirate fluid (NAF). Wrensch et al. 42 performed nipple aspiration on 2,343 women, who were subsequently followed for an average of 12.7 years. Compared to women from whom no fluid was obtained, the relative risk for cancer development was 2.5 for women with hyperplasia, 4.9 for women with atypical hyperplasia, and 18.1 for women with atypical hyperplasia and a first-degree relative with breast cancer.
Analysis of NAF has attracted increasing interest with the recent introduction of ductal lavage. This technique was designed to resolve earlier problems in obtaining a large enough cell sample from simple nipple aspiration. In ductal lavage, a two-port catheter (one for infusion and one for aspiration) is introduced into the ductal openings at the nipple. A saline solution is introduced into the duct to rinse out cells, which are then aspirated into a collection chamber. A study by Dooley et al. 43 reported that adequate material for analysis was obtained from 78% of subjects using ductal lavage, compared with only 27% of subjects using nipple aspiration. Further, ductal lavage collected significantly more cells (a median of 13,500 epithelial cells per duct vs. 120 per breast with nipple aspiration). Although very promising, there are still important questions to be answered about the usefulness of this technique. For example, what are the clinical implications of negative findings by ductal lavage in a high-risk woman? Should such a woman be discouraged from receiving tamoxifen as a chemopreventive agent because of these findings? Until such questions are answered, ductal lavage is not recommended as a screening technique and should not be considered as a substitute for routine screening mammography. For women at very high risk for breast cancer, this technique may provide additional information that could be considered in planning a management strategy.
There is controversy over whether the atypical hyperplasia detected histologically on biopsy specimens is equivalent to the proliferation with atypia detected in cytologic specimens. It is suggestive that the reported increase in cancer risk associated with atypical cell proliferation is quite similar for both histologic and cytologic approaches (four- to fivefold), and that positive family history has a similar effect in increasing that risk (Fig. 2). This is supported by the results of King et al., who reported a significant correlation between histologic and cytologic atypical hyperplasia in cases with an underlying malignancy. 44
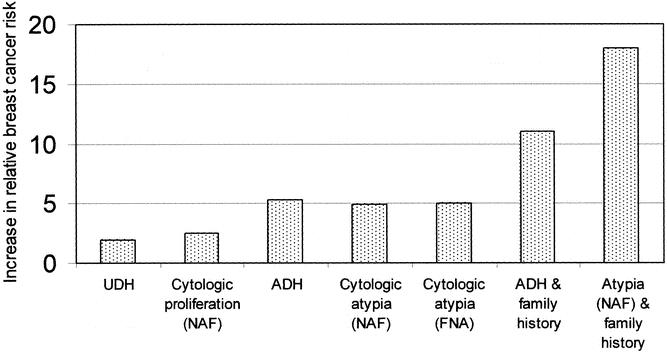
Figure 2. Increase in relative breast cancer risk in women with biopsy-proven usual ductal hyperplasia (UDH), cytologic proliferation in nipple aspiration fluid (NAF), biopsy-proven atypical ductal hyperplasia (ADH), cytologic proliferation with atypia in NAF or fine-needle aspiration biopsy, ADH with positive family history, and cytologic proliferation with atypia (NAF) and positive family history.
Genetic Background
Genetic predisposition is one of the most intellectually intriguing factors associated with increased risk for breast cancer. The growing knowledge base about the fundamental changes in gene structure and expression involved in tumorigenesis suggest that patterns of risk can be precisely defined on a person-by-person basis. Genetic predisposition is reflected in the approximately 20% of breast cancer patients who have a positive family history of breast cancer, and identified more specifically in the 5% of patients in whom a specific germline mutation has been identified. 45
Family History
A case of familial breast cancer was first described over 135 years ago, 46 and many subsequent studies have attempted to define levels of risk associated with varying degrees of positive family history. All such studies published between the years 1966 and 1996 were systematically reviewed and subjected to meta-analysis by Pharoah et al. in 1997. 47 They identified 52 case-control studies and 22 cohort studies that quantified the risk associated with a family history of breast cancer. As had been anticipated based on earlier studies, 48,49 they found that the degree of risk was a function of the type of relative affected (first or second degree), the age at which the relative developed cancer, and the number of relatives affected. Compared to individuals with no family history of breast cancer, they estimated a relative risk of 1.8 associated with a first-degree relative who developed breast cancer at 50 years of age or older compared with a relative risk of 3.3 for a first-degree relative who developed breast cancer at an age less than 50 years. The relative risk associated with having a second-degree relative with breast cancer was 1.5. If two first-degree relatives (e.g., mother and daughter) were affected, the relative risk was 3.6.
Specific Germline Mutations Associated With Breast Cancer
The BRCA1 and BRCA2 genes are associated with an inherited susceptibility to breast and ovarian cancers. 50 Initial studies indicated that the increased risk for carriers of BRCA mutations was very high, with an expected lifetime incidence of cancer approaching 90% by the age of 70. 51 Easton et al. 45 estimated a relative risk of more than 200 below the age of 40 and a relative risk of 15 in the 60-to-69-year age bracket. More recently, Colin Begg at Memorial Sloan-Kettering reassessed the risk associated with BRCA genes and suggested that earlier estimates may have been too high. 52 Because most BRCA mutation carriers were ascertained by membership in families with a high incidence of breast and ovarian cancers, the actual effects of the gene are likely to be confounded by environmental factors or by the contributory activity of other genes. This idea is supported by the wide variation in estimates of the lifetime incidence of breast cancer (26–74%) among eight studies reviewed in the Begg report. The idea that the impact of the BRCA genes on breast cancer susceptibility may be considerably less than previously believed is an important one, as BRCA-positive women often consider the possibility of prophylactic mastectomy to reduce their risk.
At least one (and possibly several) other major susceptibility gene is likely, since only a fraction of high-risk families have been demonstrated to have mutations in BRCA1 or BRCA2. 53 In addition, de Jong et al. 54 have identified 13 polymorphisms in 10 additional genes that are associated with breast cancer at a 5% significance level, some of which may be involved in breast cancer susceptibility seen in a number of rare genetic syndromes (e.g., Li Fraumeni syndrome, Cowden syndrome, ataxia-telangiectasia 50).
MODELS FOR ASSESSING BREAST CANCER RISK FROM MULTIPLE FACTORS
Studies examining single risk factors for breast cancer development have frequently found that the factor under consideration is synergistically affected by the presence of other risk factors. For example, the relative risk associated with alcohol consumption increases as a function of increased body mass, 10 while the relative risk associated with body mass increases as a function of patient age. 11 Several models have been developed to assess the interactive effect of multiple risk factors on overall patient risk.
Gail Model
The most widely applicable model for general risk assessment is the Gail model. Gail et al. 55 assessed a variety of potential risk factors using unstratified logistic regression analysis. The analysis used prospectively collected data from 2,852 white women with breast cancer and 3,146 white women controls, all of whom received yearly breast cancer screening as part of the Breast Cancer Detection Demonstration Project. They identified some factors that were not significantly associated with breast cancer risk (e.g., cigarette smoking or the use of oral contraceptives) and others that, although significant risk factors, affected only a small number of women (alcohol consumption). They found that the major determinants of risk in this population of women were: (1) family history in a first-degree relative, (2) late age at childbirth, (3) early menarche, and (4) multiple previous benign breast biopsies. The Gail model is based on these four factors, as modified by age (less than 50 vs. 50 or older) and calculates risk over a specific period. Although Gail et al. also recognized that the presence of atypical hyperplasia on biopsy was a significant risk factor, 40 pathology reports were not available for a significant number of patients in the study. For patients with available pathologic data and a positive finding of atypical hyperplasia, the effect is approximated by multiplying the calculated relative risk by 1.82.
The original Gail model, as described above, estimated the risk of invasive or in situ breast cancer for white women undergoing yearly screening. The modified Gail model, as adapted for use with the NSABP P1 trial, was changed to include African-American patients and to estimate risk for invasive breast cancer only. 56 A subsequent modification introduced corrections for use with Hispanic women. A computerized version of the modified Gail model is available on the Internet and has been widely distributed on disc by the National Cancer Institute.
Claus Model
Although the Gail model is widely used, it has several important deficits. It uses only a limited amount of family history information. It does not consider breast cancer in second-degree relatives, family history of contralateral cancer, or the age at which relatives developed breast cancer. It also does not consider findings of ovarian cancer or lobular carcinoma in situ. The Claus model was developed to address some of these deficits. 57 This model was based on data obtained from 4,730 patients aged 20 to 54 with histologically confirmed breast cancer and 4,688 controls matched for age (by 5-year age category) and geographic location. Risk assessment was based on the number and type of relatives affected and on the ages at which they became affected. The authors emphasize that this model is appropriate only for a particular high-risk subset of patients with breast cancer: those who have at least one female relative also diagnosed with breast cancer.
Other Models
Other models of breast cancer risk assessment are also aimed at high-risk subsets of patients and thus are less widely applicable than the Gail model. The BRCAPRO model calculates the probability that a particular set of family history criteria is related to a mutation in a BRCA gene. 58 This model takes into account the ages of all affected first- and second-degree relatives, and whether they have been diagnosed with unilateral breast cancer, bilateral breast cancer, or ovarian cancer. The Bodian model calculates the risk of invasive or in situ breast cancer based on the occurrence of LCIS and the age at which LCIS was diagnosed. 59
FUTURE STUDIES
In the process of tumorigenesis, genetic material is mutated, deleted, amplified, and expressed according to a complex program leading ultimately to the development of a malignant phenotype. This process involves a genetic mosaic of dozens, perhaps hundreds, of genes. 54,60 While the powerful tools of molecular biology have allowed researchers to examine some of the genes involved in this process on a one-by-one basis, they have not yielded an accurate picture of the multilevel interactions involved.
Microarray technology is a new approach that allows a comprehensive assessment of the entire genome at the DNA or RNA level. Microarrays are composed of microscopic grid patterns containing genetic material from up to 25,000 genes at the same time. DNA-based microarrays screen for both qualitative and quantitative variations in genomic DNA, using comparative hybridization. RNA-based microarrays can measure the expression of the 3% to 5% of genes that are active at any one time.
DNA-based microarrays containing probes for 600 genetic loci distributed across the entire genome have been used to measure loss of heterozygosity (LOH), a marker for genetic instability. 61 Euhus et al. have proposed that the earliest events in breast carcinogenesis are those that induce genomic instability. 62 In a study of 30 asymptomatic women sampled by random fine-needle aspiration biopsy of the upper outer quadrant, they demonstrated that LOH is associated with the presence of cytologic proliferation (both usual and atypical) and with increased risk for breast cancer, as assessed with the Gail model.
Van ’t Veer et al. 63 have used RNA-based microarrays to investigate the relationship between gene expression profiles and breast cancer prognosis. Using RNA isolated from 98 primary breast tumors, they hybridized onto microarrays containing probes from 25,000 genes. The abundance of the RNA transcripts was quantified by measurement of fluorescence intensities. They found that 5,000 genes were significantly expressed, of which 231 were ultimately found to be related to disease outcome. A poor prognosis was associated with upregulation of genes associated with the cell cycle, invasion, metastasis, angiogenesis, and signal transduction.
Although much of the recent research in this area has used material obtained from tumor samples, the technology is now being adapted for use with cell samples from fine-needle aspiration and core biopsy. 64,65 These relatively noninvasive biopsy techniques could reasonably be used on selected nonsymptomatic women (e.g., those with a positive family history) as part of a definitive risk assessment strategy. Likewise, it should be possible to adapt this technology to use with the epithelial cell samples obtained through ductal lavage. Microarray technology has great promise for developing a molecular portrait of genetic interactions that may be used to determine an individual’s risk for breast cancer. An ability to detect the earliest changes associated with breast tumorigenesis years or decades before the appearance of measurable tumor may allow the introduction of more effective chemopreventive strategies.
Footnotes
Correspondence: S. Eva Singletary, MD, FACS, Department of Surgical Oncology, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 444, Houston TX 77030-4095.
E-mail: esinglet@mdanderson.org
Accepted for publication November 26, 2002.
References
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998; 90: 1371–1388. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Eisner MP, Kosary CL, et al. (eds). SEER Cancer Statistics Review, 1973–1997, National Cancer Institute. NIH Pub. No. 00–2789. Bethesda, MD, 2000.
- 3.Vogel VG. Breast cancer risk factors and preventive approaches to breast cancer. In: Kavanagh JJ, Singletary SE, Einhorn N, et al. (eds). Cancer in women. Malden, MA: Blackwell Science, 1998: 58–91.
- 4.Harvey EB, Schairer C, Brinton LA, et al. Alcohol consumption and breast cancer. J Natl Cancer Inst. 1987; 78: 657–661. [PubMed] [Google Scholar]
- 5.Manisto S, Virtanen M, Kataja V, et al. Lifetime alcohol consumption and breast cancer: a case-control study in Finland. Public Health Nutr. 2000; 3: 11–18. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Kreger BE, Dorgan JF, et al. Alcohol consumption and risk of breast cancer: the Framingham study revisited. Am J Epidemiol. 1999; 149: 102–104. [DOI] [PubMed] [Google Scholar]
- 7.Ellison RC, Zhang Y, McLennan CE, et al. Exploring the relation of alcohol consumption to risk of breast cancer. Am J Epidemiol. 2001; 154: 740–747. [DOI] [PubMed] [Google Scholar]
- 8.Vachon CM, Cerhan JR, Vierkant RA, et al. Investigation of an interaction of alcohol intake and family history on breast cancer risk in the Minnesota breast cancer family study. Cancer. 2001; 92: 240–248. [DOI] [PubMed] [Google Scholar]
- 9.Ursin G, Useng C-C, Pagganini-Hill A, et al. Does menopausal hormone replacement therapy interact with known factors to increase risk of breast cancer? J Clin Oncol. 2002; 20: 699–706. [DOI] [PubMed] [Google Scholar]
- 10.Royo-Bordonada MA, Martin-Moreno JM, Guallar E, et al. Alcohol intake and risk of breast cancer: the EURAMIC study. Neoplasma. 1997; 44: 150–156. [PubMed] [Google Scholar]
- 11.Tretli S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int J Cancer. 1989; 44: 23–30. [DOI] [PubMed] [Google Scholar]
- 12.Clemens M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001; 344: 276–285. [DOI] [PubMed] [Google Scholar]
- 13.Verkasalo PK, Thomas HV, Appleby PN, et al. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control. 2001; 12: 47–59. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002; 20: 42–51. [DOI] [PubMed] [Google Scholar]
- 15.Del Giudice ME, Fantus IG, Ezzat S, et al. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998; 47: 111–120. [DOI] [PubMed] [Google Scholar]
- 16.Suga K, Imai K, Eguchi H, et al. Molecular significance of excess body weight in postmenopausal breast cancer patients in relation to expression of insulin-like growth factor I receptor and insulin-like growth factor II genes. Jpn J Cancer Res. 2001; 92: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoll BA. Perimenopausal weight gain and progression of breast cancer precursors. Cancer Detect Prev. 1999; 23: 31–36. [DOI] [PubMed] [Google Scholar]
- 18.Keating NL, Cleary PD, Rossi AS, et al. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med. 1999; 130: 545–553. [DOI] [PubMed] [Google Scholar]
- 19.Maddison J. Hormone replacement therapy for menopausal symptoms. Lancet. 1973; 1: 1507. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Stampfer MJ, Willett WC, et al. Prospective study of estrogen replacement therapy and risk of breast cancer in postmenopausal women. JAMA. 1990; 264: 1648–1653. [PubMed] [Google Scholar]
- 21.Steinberg KK, Thacker SB, Smith SJ, et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA. 1991; 265: 1985–1990. [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997; 350: 1047–1059. [PubMed] [Google Scholar]
- 23.Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002; 288: 872–881. [DOI] [PubMed] [Google Scholar]
- 24.Land CE, Boice JD Jr, Shore RE, et al. Breast cancer risk from low dose exposure to ionizing radiation: results of parallel analysis of three exposed populations. J Natl Cancer Inst. 1980; 65: 353–368. [PubMed] [Google Scholar]
- 25.Boice JD Jr, Preston D, Davis FG, et al. Frequent chest X-ray fluoroscopy and breast cancer incidence among tuberculosis patients in Massachusetts. Radiat Res. 1991; 125: 214–222. [PubMed] [Google Scholar]
- 26.Clemons M, Loijens L, Goss P. Breast cancer risk following irradiation for Hodgkin’s disease. Cancer Treatment Review. 2000; 26: 291–302. [DOI] [PubMed] [Google Scholar]
- 27.Brinton LA, Schaiere C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988; 6: 145–154. [DOI] [PubMed] [Google Scholar]
- 28.Brinton LA, Hoover R, Fraumeni JF Jr. Reproductive factors in the aetiology of breast cancer. Br J Cancer. 1983; 47: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinton LA, Hoover R, Fraumeni JF Jr. Epidemiology of minimal breast cancer. JAMA. 1983; 249: 483–487. [PubMed] [Google Scholar]
- 30.Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972; 48: 605–613. [PubMed] [Google Scholar]
- 31.White E. Projected changes in breast cancer incidence due to the trend toward delayed childbearing. Am J Public Health. 1987; 77: 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharpe CR. A developmental hypothesis to explain the multicentricity of breast cancer. Can Med Assoc J. 1998; 159: 55–59. [PMC free article] [PubMed] [Google Scholar]
- 33.Hankey BG, Curtis RE, Naughton MD, et al. A retrospective cohort analysis of second breast cancer risk for primary breast cancer patients with an assessment of the effect of radiation therapy. J Natl Cancer Inst. 1983; 70: 797–804. [PubMed] [Google Scholar]
- 34.Rosen PP, Groshen S, Dinne DW, et al. Contralateral breast carcinoma: an assessment of risk and prognosis in stage I (T1N0M0) and stage II (T1N1M0) patients with 20-year follow-up. Surgery. 1989; 106: 904–910. [PubMed] [Google Scholar]
- 35.Bellamy CO, McDonald C, Salter DM, et al. Noninvasive ductal carcinoma of the breast: the relevance of histologic categorization. Hum Pathol. 1993; 24: 16–23. [DOI] [PubMed] [Google Scholar]
- 36.Singletary SE. Lobular carcinoma in situ of the breast: a 31-year experience at the University of Texas M.D. Anderson Cancer Center. Breast Dis. 1994; 7: 157–163. [Google Scholar]
- 37.Frykberg ER, Santiago F, Betsill WL, et al. Lobular carcinoma in situ of the breast. Surg Gynecol Obstet. 1987; 164: 285–301. [PubMed] [Google Scholar]
- 38.Stybo TM, Wood WD. The management of ductal and lobular breast cancer. Surg Oncol. 1999; 8: 67–75. [DOI] [PubMed] [Google Scholar]
- 39.Page DL, Dupont WD, Rogers LW, et al. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982; 49: 751–758. [DOI] [PubMed] [Google Scholar]
- 40.Dupont WD, Park FF, Hartmann WH, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993; 71: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 41.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000; 92: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 42.Wrensch MR, Petrakis NL, King EB, et al. Breast cancer incidence in women with abnormal cytology in nipple aspirates of breast fluid. Am J Epidemiol. 1992; 135: 130–141. [DOI] [PubMed] [Google Scholar]
- 43.Dooley WC, Ljung B-M, Veronesi U, et al. Ductal lavage for detection of cellular atypia in women at high risk for breast cancer. J Natl Cancer Inst. 2001; 93: 1624–1632. [DOI] [PubMed] [Google Scholar]
- 44.King EB, Chew KL, Petrakis NL, et al. Nipple aspirate cytology for the study of breast cancer precursors. J Natl Cancer Inst. 1983; 71: 1115–1121. [PubMed] [Google Scholar]
- 45.Easton DF, Bishop DT, Ford D, et al. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Am J Hum Genet. 1995; 56: 265–271. [PMC free article] [PubMed] [Google Scholar]
- 46.Broca PP. Traité des tumeurs. Paris: Asselin, 1866.
- 47.Pharoah PDP, Day NE, Duffy S, et al. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997; 71: 800–809. [DOI] [PubMed] [Google Scholar]
- 48.Goldgar DE, Stratton MR, Eeles RA. Familial breast cancer. In: Eeles RA, Ponder BAJ, Easton DF, et al (eds). Genetic predisposition to cancer. London: Chapman and Hall, 1996: 227–238.
- 49.Kelsey JL, Gammon MD. Epidemiology of breast cancer. Epidemiol Rev. 1990;228–240. [DOI] [PubMed]
- 50.Brody L, Biesecker BB. Breast cancer susceptibility genes: BRCA1 and BRCA2. Medicine. 1998; 77: 208–226. [DOI] [PubMed] [Google Scholar]
- 51.Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995; 57: 1457–1462. [PMC free article] [PubMed] [Google Scholar]
- 52.Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002; 94: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 53.Serova OM, Mazoyer S, Puget N, et al. Mutations in BRCA1 and BRCA2 in breast cancer families: Are there more breast cancer susceptibility genes? Am J Hum Genet. 1997; 60: 486–495. [PMC free article] [PubMed] [Google Scholar]
- 54.de Jong MM, Nolte IM, te Meerman GJ, et al. Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet. 2002; 39: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989; 81: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 56.Prout MN. Breast cancer risk reduction: what do we know and where should we go? Medscape Women’s Health eJournal 2000; 5: 1–9. [PubMed] [Google Scholar]
- 57.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994; 73: 643–651. [DOI] [PubMed] [Google Scholar]
- 58.Berry DA, Parmigiani G, Sanchez J, et al. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst. 1997; 89: 227–238. [DOI] [PubMed] [Google Scholar]
- 59.Bodian CA, Perzin KH, Lattes R. Lobular neoplasia: long-term risk of breast cancer and relation to other factors. Cancer. 1996; 78: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 60.Singletary SE. A working model for the time sequence of genetic changes in breast tumorigenesis. J Am Coll Surg. 2002; 194: 202–216. [DOI] [PubMed] [Google Scholar]
- 61.Mei R, Galipeau PC, Prass C, et al. Genome-wide detection of allelic imbalance using human SNPs and high-density DNA arrays. Genome Res. 2000; 10: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Euhus DM, Cler L, Shivaparkar N, et al. Loss of heterozygosity in benign breast epithelium in relation to breast cancer risk. J National Cancer Inst. 2002; 94: 858–860. [DOI] [PubMed] [Google Scholar]
- 63.Van ’t Veer LJ, Dal H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002; 415: 530–536. [DOI] [PubMed] [Google Scholar]
- 64.Assersohn L, Gangi L, Zhao Y, et al. The feasibility of using fine needle aspiration from primary breast cancers for cDNA microarray analyses. Clinical Cancer Res. 2002; 8: 794–801. [PubMed] [Google Scholar]
- 65.Ellis M, Davis N, Coop A, et al. Development and validation of a method for using breast core needle biopsies for gene expression microarray analyses. Clinical Cancer Res. 2002; 8: 1155–1166. [PubMed] [Google Scholar]