Abstract
Objective
To assess whether lack of enteral feeding significantly impairs generation of specific immune responses to an acute viral infection.
Summary Background Data
Parenteral feeding provides adequate nutrients to meet metabolic needs, but lack of enteral stimulation creates a defect in mucosal immunity characterized by loss of IgA-mediated defenses in the respiratory tract.
Methods
The enzyme-linked Immunospot (ELISPOT) assay was used to determine accumulation of immunologic cells in the nasal passages after diet manipulation. Viral shedding and nasal IgA levels were measured in additional groups of mice.
Results
After determining the time course of antibody-forming cells (AFCs) via ELISPOT to an active infection with the A/PR8 influenza virus, a significant reduction was found in total AFCs, IgA-producing AFCs, and IgG-producing AFCs over the course of a 13-day experiment with significant depression in viral-specific respiratory IgA levels. Eight days following an active infection, seven of nine total parenteral nutrition-fed animals continued to have viral shedding in the nasal passages compared to one of nine chow-fed mice and one of six animals fed a complex enteral diet.
Conclusions
Lack of enteral stimulation significantly impairs the generation of IgA-mediated mucosal immunity.
It is estimated that 50% of the body’s immunity resides within the human lamina propria, accounting for 70% of total antibody production. 1 Sensitization of most B cells and T cells for mucosal immunity occurs within the Peyer’s patches for distribution throughout the body. 2 Over the past 6 years, our laboratory has focused on how route and type of nutrition influence mucosal immunity to define the mechanisms by which parenteral feeding of patients increases susceptibility to pneumonia, while enteral feeding reduces this risk. 3,4 Experimentally established immunity against A/PR8 (H1N1) influenza virus 5 and Pseudomonas aeruginosa6 is severely impaired with parenteral feeding compared to chow or intragastric feeding of a complex enteral diet. This respiratory defect occurs simultaneously with reductions in lamina propria T- and B-cell populations, 7 decreases in the Th2 IgA-stimulating cytokines IL-4 and IL-10, 8 and drops in intestinal and respiratory total IgA levels. 9
The nasal mucosa is the first site of contact for inhaled pathogens. The nasal-associated lymphoid tissue (NALT) of rodents generates local immune responses that protect the upper respiratory tract. Following immunization, cells sensitized in NALT 10–14 migrate to cervical lymph nodes for distribution to the nasal passages, where antibody-forming cells (AFC) generate a specific immune response to clear an active infection. For the A/PR8 influenza virus, a specific IgA-mediated defense results. 14–16 Although analogies between human Waldeyer’s ring and rodent NALT have been speculated, a separate upper respiratory antigen-processing point in humans is unproven. The rodent respiratory tract, however, provides a model to study responses to active infection.
Since lack of enteral feeding impairs established mucosal immunity against the A/PR8 virus, 5 we speculated that parenteral feeding would impair the generation of specific defenses to new infection. We examined the time course of appearance of AFCs in nasal passages and cervical lymph nodes after viral inoculation, generation of respiratory viral-specific antibody levels, the effects of nutrition on AFCs in the nasal passages and superficial cervical lymph nodes, and the effect of lack of enteral stimulation on elimination of the active infection at 8 days. Parenteral feeding significantly impairs this AFC response within the nasal passages and reduces viral-specific IgA. We conclude that lack of enteral feeding impairs the immunologic response to new infectious challenges.
METHODS
Animals
These studies conformed to the guidelines for the care and use of laboratory animals established by the Animal Care and Use Committee of the University of Tennessee Health Science Center. All protocols were approved by that committee. Six- to eight-week-old male mice from the Institute of Cancer Research (Harlan Bioproducts for Science, Indianapolis, IN) were housed in an American Association for the Accreditation of Laboratory Animal Care Facility. Conditions of temperature and humidity were controlled with a 12-hour light/12-hour dark cycle. Mice were quarantined and fed commercial mouse chow (RMH3200, Agway, Syracuse, NY) and water ad libitum for 2 weeks before protocol entry while in quarantine. During the experiments, mice were housed in metal metabolism cages with wire grid bottoms to eliminate coprophagia and ingestion of bedding.
Experiment 1: Time Course for AFC Generation in Chow-Fed Mice
To determine optimal time points for identification of AFCs by enzyme-linked Immunospot (ELISPOT) assay, 10 chow-fed mice were infected intranasally with the A/PR8 (H1N1) influenza virus as described below and sacrificed 3, 6, 10, 13, and 20 days postinfection for nasal AFC studies.
Experiment 2: Effect of Route and Type of Nutrition on AFC Response in Nasal Passages and Superficial Cervical Lymph Nodes During Active Infection
Of 192 cannulated animals, 157 (81.8%) completed the experiments. Four animals escaped from their tail restraint and became disconnected from their catheters. The remaining animals (primarily total parenteral nutrition [TPN]-fed animals) developed neck swelling and technical loss of their catheter in the first 4 days of TPN. Only 10 animals developed technical problems after the first 5 days. The experiment was completed in mice randomized to three diet groups: chow (n = 53), IV-TPN (n = 51), or a complex (i.e., nonelemental) enteral diet consisting of intact protein, fat, and complex carbohydrates (CED; n = 53). IV-TPN and chow mice received intravenous catheters, while CED mice underwent gastrostomies according to our previously published protocols. 5–9 Mice were partially immobilized by tail restraint, which does not induce physical or biochemical stress. 17
Catheterized mice were given 0.9% NaCl solution of 4 mL/d for 48 hours (Fig. 1). Mice had ad libitum access to chow and water until the third postoperative day, when they were started on their respective diets and underwent intranasal inoculation of the A/PR8 influenza virus. The chow group continued to receive 0.9% NaCl at 4 mL/d with access to chow and water. The IV-TPN mice were advanced from 4 mL/d of TPN solution to a goal rate of 11 mL/d by the third day. The TPN solution contained 4.1% amino acids and 34.3% glucose (6,455.4 kJ/L), with a nonprotein calorie/nitrogen ratio of 663.6:1 kJ/g nitrogen. CED mice received Nutren 1.0 (Nestle, Deerfield, IL) increased to a goal rate of 15 mL/d by the third day. Nutren contains 12.7% carbohydrate, 3.8% fat, and 4% protein (4,200 kJ/L); the nonprotein calorie/nitrogen ratio of Nutren was 665.2:1 kJ/g nitrogen. Animals received 63 kJ energy and 95 mg nitrogen to meet the requirements for 25- to 30-g mice. They were sacrificed at 6, 9, and 13 days after immunization and beginning diet. They were exsanguinated via the retro-orbital plexus under anesthesia, and nasal passages and superficial cervical lymph nodes cells were harvested and prepared for the ELISPOT assay.
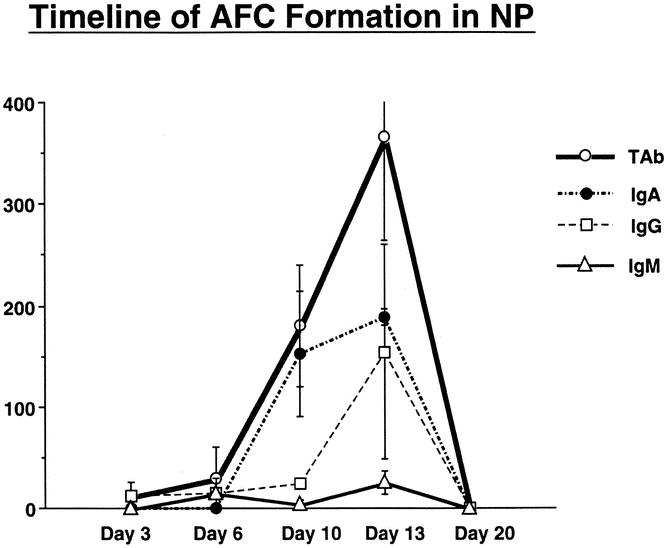
Figure 1. Timeline showing the number of AFCs (IgG-, IgA-, IgM-, and total Ab-producing cells) appearing after nasal inoculation with virus in nonimmune mice on days 3, 6, 10, 13, and 20.
Experiment 3: Effect of Route and Type of Nutrition on Viral-Specific Respiratory IgA
Three weeks after A/PR8 immunization, animals were randomized to chow, IV-TPN, or CED. Two days later, the animals began their diets. On the fifth day of diet, animals were anesthetized and the distal trachea was clamped for upper respiratory tract lavage with 1 mL phosphate-buffered saline (PBS) for nasal wash IgA. The lower respiratory tract was lavaged with 1 mL PBS. Samples were combined, frozen, and analyzed for antiviral IgA.
Experiment 4: Effect of Route and Type of Nutrition on Viral Detection in Nasal Washes During Active Infection
Chow-fed mice (n = 20) were inoculated with A/PR8 virus and sacrificed at days 6, 7, 8, and 9 (n = 5/d) for nasal washes to determine viral clearance from the nasal passages. Mice were then randomized to the three diet groups (chow, n = 9; IV-TPN, n = 9; CED, n = 6), and the protocol detailed in experiment 2 was followed, with sacrifice at 8 days. Nasal washes were assayed for the A/PR8 influenza virus.
A/PR8 Infection
Mice were inoculated intranasally with A/PR8 (H1N1) influenza virus, while awake, to ensure only an upper respiratory infection according to our previously published protocol. 5,9
Superficial Cervical Lymph Node Preparation
In experiments 1 and 2, lymph nodes from mice were removed and individually placed in 15-mL centrifuge tubes containing 4 mL PBS (pH 7.4). Contents were transferred to individual culture dishes and the superficial cervical lymph nodes (SCLNs) were pressed between frosted glass slides to obtain a single cell suspension, filtered through a 90-μm nylon filter, and transferred back into 15-mL tubes. Cells were centrifuged at 1,000 rpm for 10 minutes and resuspended in 1 mL Iscove’s modified Dulbecco’s medium (IMDM) containing 10% fetal bovine serum (FBS). Lymphocytes were counted and processed for use in the ELISPOT assay.
Nasal Passage Lymphocyte Preparation
After removing SCLNs, mice were decapitated and the facial skin, cheek muscles and bones, and palate with the NALT were removed according to the method of Asanuma et al. 10 The remaining sections, consisting of nasal turbinates, septa, and lateral walls, were cut into pieces in culture dishes containing 2 mL IMDM with 4 mg/mL collagenase (Sigma Chemical, St. Louis, MO). The tissues from each mouse were transferred to individual 15-mL centrifuge tubes and incubated for 30 minutes at 37°C. Dissociated cells were filtered, washed twice in IMDM, and resuspended in 2 mL IMDM containing 75% Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden). Four milliliters of a 40% Percoll solution was layered on top of the 75% layer and the cells in the interface were removed after centrifugation at 600g for 20 minutes at 25°C. Cells were washed and resuspended in IMDM containing 10% FBS and held on ice for counting. Cell separation resulted in greater than 90% viability of nasal passage lymphocytes determined by the trypan blue exclusion test.
ELISPOT Assay
Goat antimouse kappa and lambda unlabelled affinity-purified polyclonal antibodies (Southern Biotechnology, Birmingham, AL) coated 96-well nitrocellulose bottom multiscreen hemagglutination (HA) filtration plates (Millipore Corp., Bedford, MA). After incubation overnight at 4°C, wells were washed with PBS and blocked with 1% bovine serum albumin (BSA) in PBS. Lymphocytes suspended in IMDM containing 10% FBS were added to duplicate wells (100 μL/well) and incubated at 37°C in a humidified atmosphere containing 5% CO2. Plates were washed with PBS-Tween and incubated overnight at 4°C with 50 μL goat antimouse IgM, IgG, or IgA conjugated with alkaline phosphatase (Southern Biotechnology) diluted 1:500 in PBS. Plates were washed with PBS and spots representing single AFCs were developed with 5-bromo-4-chloro-3-indolyl phosphate (Sigma Chemical) in diethanolamine buffer. Optimal spot development generally required 1 hour at room temperature, after which plates were washed with tap water and allowed to dry overnight. Blue spots representing AFCs were counted with a dissecting microscope.
Antibody Quantification
IgA was measured in nasal secretions and bronchoalveolar lavage in a sandwich enzyme-linked immunoabsorbent assay using a polyclonal goat antimouse IgA (Sigma) to coat the plate, a purified mouse IgA standard, and a horseradish peroxidase-conjugated goat antimouse IgA. Combined nasal and bronchoalveolar lavage specimens were reported as total respiratory IgA levels.
Nasal Wash Sample Collection for Viral Assay
Nasal washes were collected after exsanguination to prevent blood contamination. Through a midline incision, the trachea was clamped at the thoracic inlet and 500 μL Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics was injected into the tracheal lumen cephalad to the obstruction using a 25-gauge B-bevel needle. Wash fluid draining from the nostrils was collected in a microcentrifuge tube, placed on ice, and immediately assayed for influenza virus.
Viral Assay
Viral samples were serially diluted (10-fold) in DMEM supplemented with 2.5 μg/mL amphotericin B (Sigma), 50 μg/mL gentamicin, and 10% FCS. Triplicate 100-μL samples of each dilution were placed into 96-well round-bottom tissue culture plates. To each well was added 100 μL of a 2 × 105 cells/mL suspension of Madin-Darby Canine Kidney cells in the antibiotic-supplemented DMEM-10% FCS. The plates were incubated 24 hours at 34°C in 5% CO2. The culture fluid was removed and replaced with DMEM (150 μL/well) containing 2.5 μg/mL amphotericin B, 50 μg/mL gentamicin, and 2 μg/mL trypsin. The plates were incubated 4 days longer at 34°C in 5% CO2. Assay for viral growth was determined by hemagglutination. To each well was added 50 μL of a 0.5% suspension of chicken red blood cells (Charles River). Hemagglutination was read after 1 to 2 hours in the cold.
Statistical Analysis
Analysis of variance (ANOVA) with multiple preplanned comparisons using least-squared means was performed to determine if differences existed between or within groups over time. The Fisher exact test was used to compare differences in viral shedding between groups at day 8 following inoculation with the A/PR8 influenza virus.
RESULTS
Experiment 1: Time Course for AFC Generation in Chow-Fed Mice
Spot-forming cells were not detected until day 6 and increased on days 10 and 13, decreasing by day 20 (see Fig. 1). For logistic reasons, days 6, 9, and 13 were used as the time points for identification of immunoglobulin spot-forming cells in subsequent experiments.
Experiment 2: Effect of Route and Type of Nutrition on AFC Response in Nasal Passages and Superficial Cervical Lymph Nodes During Active Infection
Weight Changes
There were no significant differences between groups in precannulation weight (Table 1). In both the 6- and 9-day animals, chow-fed mice weighed significantly more than CED or IV-TPN animals, but there were no significant differences between CED and IV-TPN fed mice. In day 13 animals, chow mice weighed significantly more than CED or IV-TPN animals, and CED animals weighed significantly more than IV-TPN animals. Two variables confound the interpretation of these weight data. First, chow animals retained approximately 1.4 g of food and feces in the intestine at the end of the experiment, while CED animals withheld approximately 0.5 g. The gastrointestinal tract was empty in IV-TPN animals and the weight differences between groups was exaggerated in the chow and CED mice.
Table 1. WEIGHT CHANGES IN ELISPOT STUDY
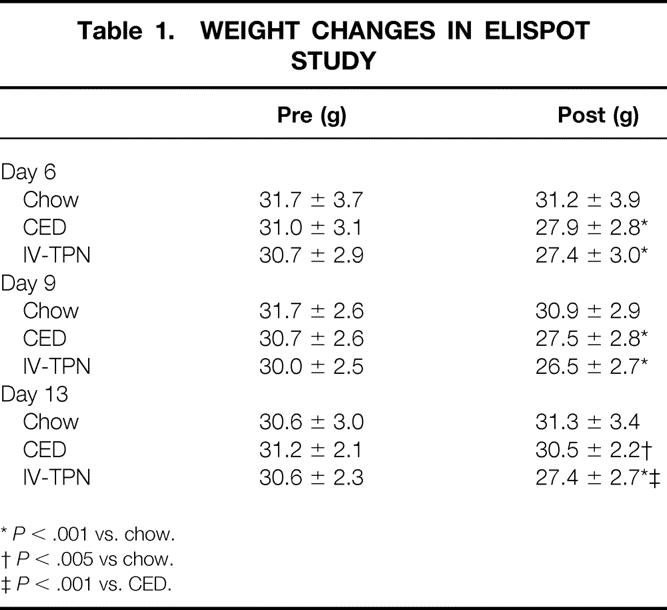
*P < .001 vs. chow.
†P < .005 vs chow.
‡P < .001 vs. CED.
Superficial Cervical Lymph Nodes
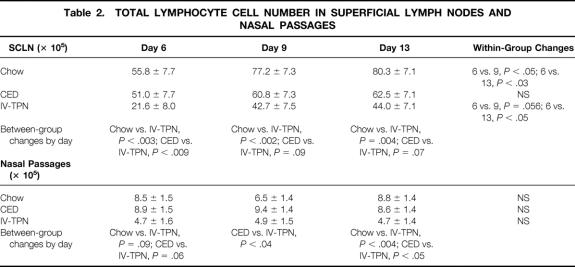
Total lymphocyte cell numbers in SCLN were significantly lower at day 6 in IV-TPN mice than in chow-fed or CED-fed mice (Table 2). SCLN total cell numbers increased in chow and IV-TPN mice from day 6 to day 9 and day 13. Significantly more lymphocytes were harvested from chow-fed animals than IV-TPN animals on days 9 and 13. CED lymphocyte numbers increased compared with IV-TPN animals, approaching statistical significance on days 9 (P = .09) and 13 (P = .07).
Table 2. TOTAL LYMPHOCYTE CELL NUMBER IN SUPERFICIAL LYMPH NODES AND NASAL PASSAGES
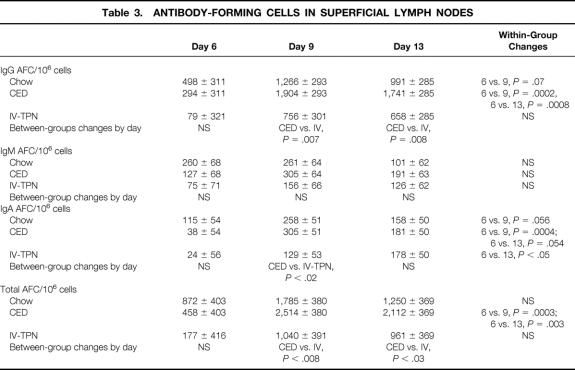
IgG AFCs (Table 3) increased in chow- and CED-fed animals over time, approaching statistical significance between days 6 and 9 in chow-fed animals (P = .07) and reaching significance between days 6 versus 9 and 6 versus 13 in CED mice. There were no significant increases in IgG AFCs in IV-TPN animals. IgG AFCs increased significantly in CED animals versus IV-TPN animals on days 9 and 13. The active infection did not affect IgM AFCs.
Table 3. ANTIBODY-FORMING CELLS IN SUPERFICIAL LYMPH NODES
IgA AFCs (see Table 3) increased in chow-fed animals from day 6 to 9 but then dropped. IgA AFCs increased in CED animals from day 6 to 9, approaching significance on day 13 (P = .054). IgA AFCs increased significantly between day 6 and 13 in IV-TPN animals. The only significant difference between groups occurred at day 9 between CED and IV-TPN groups.
Total AFCs (see Table 3) did not significantly increase in chow-fed animals over the experiment. CED mice significantly increased total AFCs between days 6 and 9 and days 6 and 13. There were no significant increases in total AFCs in IV-TPN animals. At both 9 and day 13, total AFCs were significantly greater in CED than IV-TPN animals.
AFC Response in Nasal Passages
Total lymphocyte cell recovery remained stable over time in chow-fed, CED, and IV-TPN animals (see Table 2). However, cell recovery was lower in IV-TPN mice at day 6, approaching statistical significance versus the chow (P < .09) and CED (P < .06) groups. By day 9, lymphocyte numbers increased significantly in CED mice compared with IV-TPN animals, and by day 13 cell recovery increased significantly in chow-fed and CED animals compared with IV-TPN mice.
There were no significant differences in IgM-producing ELISPOTs present (Fig. 2). Total IgA-producing ELISPOTs (Fig. 3) in the nasal passages were similar in all groups on day 6 but increased significantly in chow-fed mice between days 6 and 13 and 9 and 13. CED significantly increased IgA ELISPOTs between days 6 and 13, with no significant increase with IV-TPN. Although there were no significant differences between IV-TPN animals and chow-fed animals (P = .16) or CED animals (P = .2) on day 9, IgA ELISPOTs increased significantly in chow versus IV-TPN groups and chow versus CED groups at day 13. IgA ELISPOTs were increased in CED versus IV-TPN mice at day 13, but this failed to reach significance (P = .14).
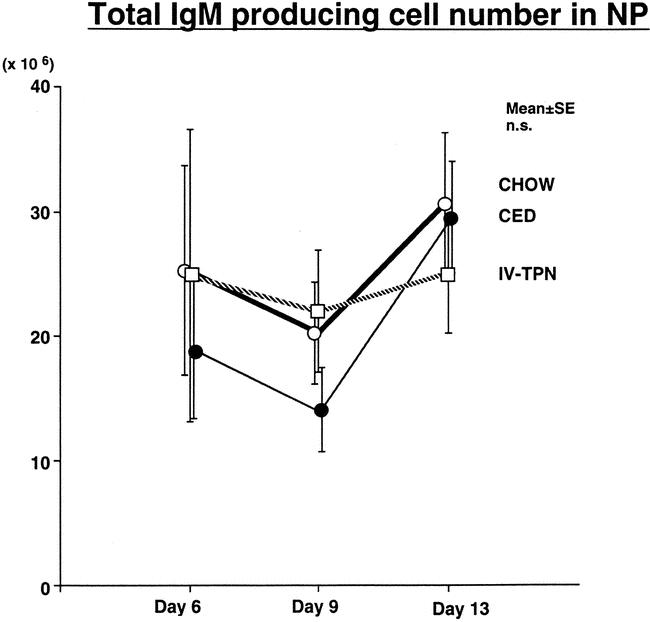
Figure 2. Total IgM AFC numbers in nasal passages. Mice were inoculated with virus on day 0 and fed chow, CED, or IV-TPN for 6, 9, or 13 days. There was no statistically significant difference between groups at any time points.
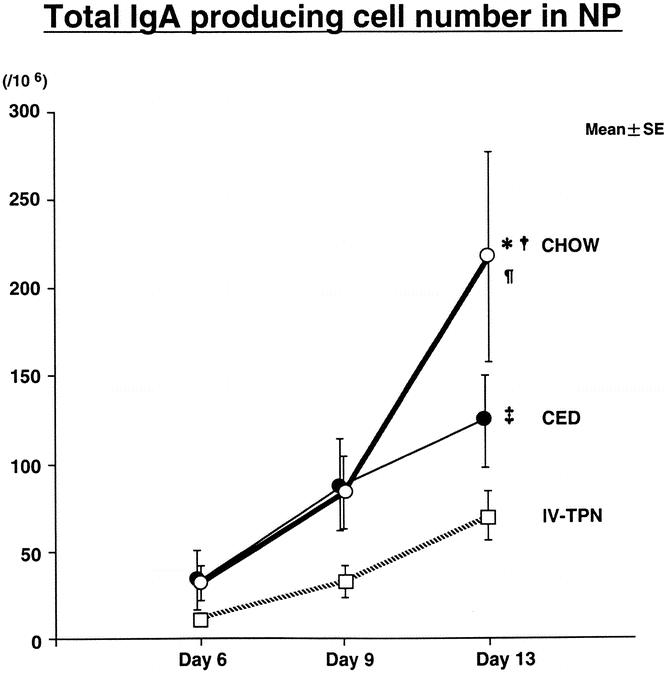
Figure 3. Total IgA AFC numbers in nasal passages. Mice were inoculated with virus on day 0 and fed chow, CED, or IV-TPN for 6, 9, or 13 days. *P < .05 versus IV-TPN day 13; †P < .05 versus CED day 13; ¶P < .007 versus chow days 6 and 9; ‡P < .03 versus CED day 6.
The most profound differences occurred in IgG-producing cells in response to the active infection. While IgG ELISPOTs did not increase in IV-TPN mice over the course of the experiment (Fig. 4), IgG ELISPOTs increased significantly in the chow group between days 6 and 13 and in the CED group between days 6 and 9 and 9 and 13. Although no significant differences occurred between groups on day 6, IgG ELISPOTs increased significantly between chow and IV-TPN groups at days 9 and 13. Similarly, there was a trend toward an increase in IgG ELISPOTs in the CED group versus IV-TPN animals at day 9 (P = .19); this reached statistical significance by day 13.

Figure 4. Total IgG AFC numbers in nasal passages. Mice were inoculated with virus on day 0 and fed chow, CED, or IV-TPN for 6, 9, or 13 days. *P < .05 versus IV-TPN day 13; †P < .009 versus chow day 6; ¶P < .05 versus IV-TPN day 9; ‡P < .02 versus CED day 6.
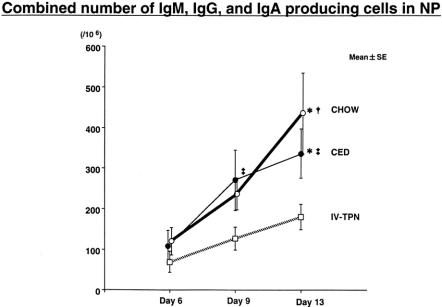
Total Ig ELISPOTs (Fig. 5) increased significantly over time in both chow (day 6 vs. 13, and 9 vs. 13) and CED (day 6 vs. 9, and 6 vs. 13) groups, with no significant increases in IV-TPN mice. There were no significant differences between groups on day 6, but there was a trend toward an increase in Ig ELISPOTs between IV-TPN and either CED (P < .08) or chow (P = .17) at day 9; this reached statistical significance by day 13 versus chow-fed and CED mice.
Figure 5. Total number of AFCs (IgG, IgA, IgM) in nasal passages. Mice were inoculated with virus on day 0 and fed chow, CED, or IV-TPN for 6, 9, or 13 days. *P < .05 ver-sus IV-TPN day 13; †P < .02 versus chow days 6 and 9; ‡P 0.05 versus CED day 6.
Experiment 3: Effect of Route and Type of Nutrition on Viral-Specific Respiratory IgA
Viral-specific IgA was significantly reduced in IV-TPN mice compared with chow-fed or CED mice (Fig. 6).
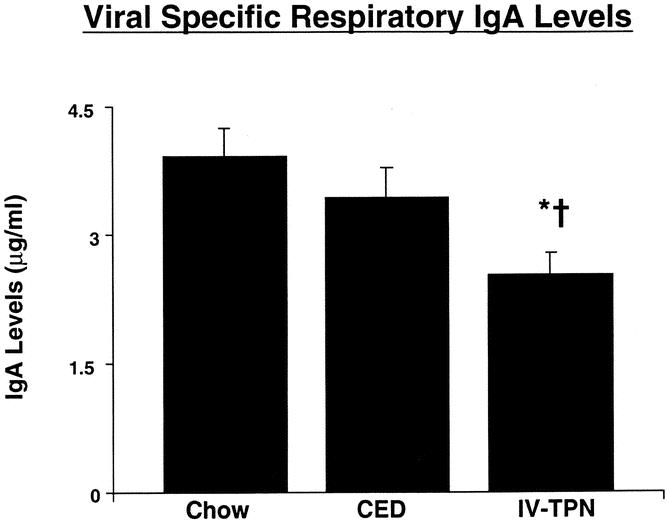
Figure 6. Effect of route of nutrition on viral-specific respiratory IgA levels. Mice were nasally inoculated with virus 3 weeks before entry into the experimental protocol. After 5 days of feeding with chow, CED, or IV-TPN, viral-specific respiratory IgA levels were measured. *P < .003 versus chow; †P < .04 versus CED.
Experiment 4: Effect of Route and Type of Nutrition on Viral Detection in Nasal Washes During Active Infection
All but one chow-fed mice cleared the virus by day 8 (Fig. 7). This day was chosen for evaluation after diet manipulation. Eight days following the active infection, seven of nine IV-TPN animals had continued viral shedding compared to one of six CED mice (P < .05) and one of nine chow-fed mice (P < .02).
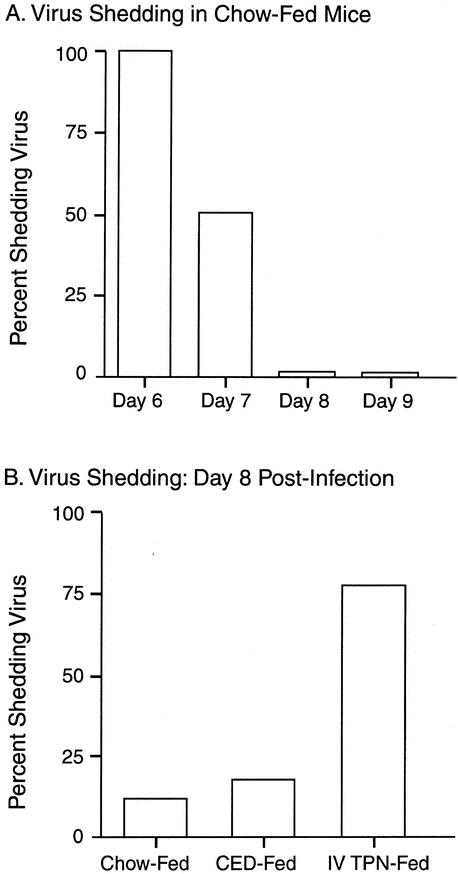
Figure 7. (A) Virus was cleared from the nasal passages of most animals by day 8. (B) Viral shedding at day 8 was significantly greater in IV-TPN-fed mice than chow-fed or CED-fed animals (P < .05).
DISCUSSION
The integrity of mucosal defenses against invasive pathogens is influenced by route and type of nutrition. This work demonstrates that parenteral feeding reduces viral-specific IgA in convalescent animals and blunts the immunologic response of acutely infected animals, indicated by a reduction in AFCs within the nasal passages. These data are consistent with the observed increase in infectious complications occurring in patients who are not fed enterally.
Parenteral feeding is used in the treatment of critically ill and critically injured patients with gastrointestinal tract loss due to ileus, resection, or fistulas. While adequate nutrients can be provided parenterally, our work shows there are immunologic benefits gained at the mucosal surface when nutrients are delivered via the gastrointestinal tract that are not gained with parenteral feeding. In randomized, prospective studies, critically injured patients 3,4 randomized to enteral feeding have a significantly reduced incidence of pneumonia compared with patients fed parenterally, suggesting alterations in mucosal immunity induced by lack of enteral feeding. Experimentally, our prior work confirmed these alterations, demonstrating significant decreases in T- and B-cell populations within Peyer’s patches and the intestinal lamina propria with gut “starvation.”7 These alterations occur with decreases in the expression of mucosal addressin adhesion molecule-1 (MAdCAM-1) in the Peyer’s patches 18 and in the NALT (unpublished data). MAdCAM-1 directs naive L-selectin++, α4β7+ B and T cells into the Peyer’s patches and NALT for sensitization and the sensitized L-selectin±, α4β7++ cells into peripheral sites of function. Both intestinal and respiratory IgA levels drop concomitant with decreases in total intestinal levels of the Th2 IgA-stimulating cytokines IL-4 and IL-10 and in mRNA for these cytokines in isolated lamina propria cells. 8,19 These changes impair established respiratory immunity to either the A/PR8 influenza virus 5 or Pseudomonas aeruginosa. 6 Immunologic memory is not lost, since chow feeding recovers immunity against the A/PR8 virus within 5 days. 20
Our current work demonstrates that lack of enteral stimulation blunts generation of the immune response to an active infection. Established immunity against the A/PR8 virus is IgA-mediated. It can be generated passively by administering viral-specific IgA to nonimmune animals 15 and destroyed in immunized animals by administering anti-IgA, but not anti-IgG or anti-IgM antibody, with an infecting viral dose. 16 This study confirms that parenteral feeding reduces viral-specific IgA, consistent with our previous findings that parenteral feeding impairs established immunity to this influenza virus. It also shows alterations in the acute response to an active infection. In acute infection, both IgG- and IgA-producing cells increase in the nasal mucosa. 14 In contrast to a chronic infection, where IgG plays an insignificant role since it is not actively transported in significant quantities across the nasal mucosa, active infection increases mucosal permeability, allowing movement of both IgG and IgA across the mucosa for viral neutralization. Once the infection has been controlled and membrane integrity recovers, IgA serves as the primary immunologic defense against reinfection working with nonspecific defenses, such as lactoferrin, defensins, mucin, and other mechanisms, which eliminate attachment of bacteria to the mucosa. 21
The existence of NALT and bronchial-associated lymphoid tissue in humans has not been extensively studied. However, its well-delineated existence in rodent species allowed our examination of route and type of nutrition on cellular response to an active infection. The viral infection produced a predominately IgG response in the SCLNs and both an IgG and IgA response in the nasal passages. Our first experiment found few antibody-forming cells in normal noninfected mice and a peak in AFCs response by day 13, consistent with the work of Tamura et al. 14 The respiratory tract clears the active infection by 7 to 8 days so that by day 20, the AFC response is gone. Therefore, we focused on the generation and establishment of AFCs between days 6 and day 13 during dietary manipulation and showed an increase in SCLN response, which peaked at day 9 in enterally fed animals. This time course is consistent with processing of active cells within the NALT and migration of the sensitized cells through the SCLN in transit to the nasal passages for maturation and proliferation of the AFCs. As the active viral infection is cleared by day 7, the stimulus wanes for cell production in SCLNs. However, cells continue to increase in the nasal passages over time as both IgG- and IgA-producing cells accumulate.
Chow (as the control diet) and the complex enteral diet significantly enhanced the AFC response compared with parenteral feeding. Although we did not measure the viral-specific ELISPOT response, evidence that the cellular proliferation was directed against the infective agent is strongly implicated by the absence of any ELISPOTs at baseline; similarity in numbers of cells found by Tamura et al., 14 who measured viral-specific ELISPOTs; the significant increase in respiratory IgA levels in chow-fed and CED-fed mice; and the failure of IV-TPN mice to clear the virus from nasal passages by 8 days. The response to CED feeding was slightly blunted (especially the IgA AFC response) when compared with chow, but this could be due to factors such as intermittence of feeding, complexity of diet, altered hormonal response, and so forth. However, the increase in IgG-producing cells was comparable between the chow and CED mice, resulting in no differences in the total numbers of AFCs in the nasal passages by day 13 and significantly greater numbers than parenterally fed mice, who failed to mount a significant cellular response. Since the experiment was terminated by day 13, it was unknown whether the CED animals increased IgA-producing cells to chow levels.
There are several potential reasons for the reduction of the cellular response in parenterally fed animals. First, significant reductions could occur in adhesion molecule expression on the naive B and T cells (L-selectin++/α4β7+) or in the peripheral tissues (e.g., MAdCAM-1). We previously measured such reductions in Peyer’s patch MAdCAM-1 expression in parenterally fed mice, 18 as well as significant decreases in the nasal passages (unpublished data). A reduction in these adhesion molecules could impair naive T- and B-cell entry into the NALT. Second, an altered cytokine or chemokine environment could occur in the NALT, the SCLNs, or the nasal passages to inhibit proliferation and/or maturation of these cells with a reduction in antibody production. Such changes were noted in the intestine, where levels of the IgA-stimulating cytokines IL-4 and IL-10 dropped in proportion to decreases in intestinal IgA levels. 8 If similar decreases occurred in the upper respiratory tract cytokines, the cytokine milieu would not favor maturation of sensitized cells nor production of IgA, resulting in fewer AFCs. Finally, essential fatty acid (EFA) deficiencies may have occurred in TPN-fed mice, although this is unlikely for several reasons. First, 28 days of an EFA-deficient diet is necessary to impair humoral antibody responses in mice. 22 Second, 42 days of this diet is required to depress delayed-type cutaneous hypersensitivity reactions. 23 Since EFA deficiency usually occurs in animals receiving high caloric loads (which our animals were not) during growth and weight gain (and weights were stable in our TPN animals), EFA deficiency is unlikely. For consistency with our previous work and due to the known immunosuppressive effects of IV lipids, fat-free TPN was used in our experiment.
This work complements our previous studies into the vast network of immunocytes protecting the systemic circulation from the external environment. These mechanisms appear dependent on enteral stimulation to generate an optimal response to an active infection and maintain vigilance to previously known pathogens. The effects of gut “starvation” involve mechanisms of intestinal and extraintestinal protection at the mucosal surfaces. This work is consistent with a reduced rate of infectious complications in critically injured patients who receive postinjury enteral stimulation.
Acknowledgments
The authors thank Dana Marshall, PhD, and Mark Sangster, PhD, for their advice in the development of the ELISPOT model and gratefully acknowledge the help of Doris Parsons and Delight Utz in the preparation of this manuscript for publication.
Footnotes
Supported by NIH grant #R01 GM53439.
Correspondence: Kenneth A. Kudsk, MD, University of Wisconsin Medical School, Department of Surgery, H4/730 Clinical Science Center, 600 Highland Avenue, Madison, WI 53792-7375.
E-mail: kudsk@surgery.wisc.edu
Accepted for publication September 4, 2002.
References
- 1.Svanborg C. Bacterial adherence and mucosal immunity. In: Ogra PL, Mestecky J, Lamm ME, et al., eds. San Diego: Academic Press, 1974:71.
- 2.Underdown BJ, Mestecky J. Mucosal immunoglobulins. In: Ogra PL, Mestecky J, Lamm ME, et al., eds. Handbook of mucosal immunology. San Diego: Academic Press, 1994: 79.
- 3.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992; 215: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma reduced septic morbidity. J Trauma. 1989; 29: 916. [DOI] [PubMed] [Google Scholar]
- 5.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996; 223: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999; 229: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995; 39: 44. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999; 229: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997; 132: 1303. [DOI] [PubMed] [Google Scholar]
- 10.Asanuma H, Inaba Y, Aizawa T, et al. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Methods. 1995; 187: 41. [DOI] [PubMed] [Google Scholar]
- 11.Hiroi, T, Iwatani K, Iijima H, et al. Nasal immune system: distinctive Th0 and Th1/2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998; 28: 3346. [DOI] [PubMed] [Google Scholar]
- 12.Kuper CF, Koornstra PJ, Hameleers DMH, et al. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992; 13: 219. [DOI] [PubMed] [Google Scholar]
- 13.Sminia T, Kraal G. Nasal-associated lymphoid tissue. In: Ogra PL, Mestecky J, Lamm ME, et al., eds. Handbook of mucosal immunology. San Diego: Academic Press, 1999: 357.
- 14.Tamura S, Iwasaki T, Thompson AH, et al. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998; 79: 291. [DOI] [PubMed] [Google Scholar]
- 15.Renegar KB, Small PA. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991; 146: 1972. [PubMed] [Google Scholar]
- 16.Renegar KB, Small Jr PA. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991; 65: 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. JPEN. 1983; 7: 582. [DOI] [PubMed] [Google Scholar]
- 18.Fukatsu K, Zarzaur B, Johnson C, et al. Decreased MAdCAM-1 expression in Peyer’s patches: a mechanism for impaired mucosal immunity during lack of enteral nutrition. Surg Forum. 2000; 51: 211. [Google Scholar]
- 19.Fukatsu K, Kudsk KA, Wu Y, et al. TPN decreases IL-4 and IL-10 mRNA expression in lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001; 15: 318. [DOI] [PubMed] [Google Scholar]
- 20.Janu P, Li J, Renegar KB, et al. Recovery of gut-associated lymphoid tissue (GALT) and upper respiratory tract (URT) immunity following parenteral nutrition (TPN). Ann Surg. 1997; 225: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CD, Kudsk KA. Nutrition and intestinal mucosal immunity. Clin Nutr. 1999; 18: 337. [DOI] [PubMed] [Google Scholar]
- 22.DeWille JW, Fraker PJ, Romsos DR. Effects of essential fatty acid deficiency and various levels of dietary polyunsaturated fatty acids on humoral immunity in mice. J Nutr. 1979; 109: 1018. [DOI] [PubMed] [Google Scholar]
- 23.DeWille JW, Fraker PJ, Romsos DR. Effects of dietary fatty acids on delayed-type hypersensitivity in mice. J Nutr. 1981; 111: 2039. [DOI] [PubMed] [Google Scholar]