Abstract
Objective
To assess the utility of gracilis muscle transposition in the treatment of iatrogenic rectourethral fistula.
Summary Background Data
Iatrogenic rectourethral fistula poses a rare but challenging complication of treatment for prostate cancer. A variety of procedures have been described to treat this condition, none of which has gained acceptance as the procedure of choice. The aim of this study was to review the authors’ experience with gracilis muscle transposition in the treatment of iatrogenic rectourethral fistula.
Methods
A retrospective chart review of all patients who underwent gracilis muscle transposition for iatrogenic rectourethral fistula was performed, and follow-up was established by telephone interview. Successful repair was defined as absence of a fistula after reversal of fecal and urinary diversions.
Results
Eleven men, mean age of 62 years, underwent 12 gracilis muscle transpositions for rectourethral fistula between 1996 and 2001. Six patients had a history of pelvic radiotherapy, and five patients had previous failed attempts to repair the fistula. In nine patients, the fistula healed following gracilis muscle transposition. One patient developed a rectocutaneous fistula that healed with fibrin glue injection, and one developed perineal sepsis requiring debridement of the transposed gracilis. This patient underwent a second gracilis transposition, which uneventfully healed. Overall, all of the patients had closure of their diverting stomas and maintained healed rectourethral fistulas. There were no intraoperative complications, and the only long-term complication of this procedure was mild medial thigh numbness in two patients.
Conclusions
Gracilis muscle transposition is an effective surgical treatment for iatrogenic rectourethral fistula. It is associated with low morbidity and a high success rate.
Rectourethral fistula poses a difficult surgical challenge. Fistulas between the lower rectum and the urethra may be congenital, with the constellation of pelvic floor malformations, or acquired, due to inflammation, infection, neoplasia, or trauma. Iatrogenic rectourethral fistulas can follow the treatment of prostatic cancer. Although uncommon, these fistulas may occur following radical prostatectomy, 1 radiotherapy, 2 cryosurgery, 3 or seed implantation for the treatment of prostate carcinoma.
Although symptoms such as pneumaturia, fecaluria, and the passage of urine through the rectum are often alleviated by fecal and urinary diversion, these fistulas seldom spontaneously heal. 4 Even when diverted, patients may suffer from urinary tract infections, resistant to medical therapy. 5 Thus, most of these patients will eventually require surgical treatment.
Numerous surgical procedures have been described for the treatment of rectourethral fistula, 1,4,6–9 none of which has gained wide acceptance as the procedure of choice. The diversity in treatment methods, combined with the limited reported success rates, attest to the complexity of this difficult condition. After gaining significant experience with the harvest and transposition of the gracilis muscle for fecal incontinence, 10,11 we began to favor its use for the treatment of unhealed perineal wounds 12 and rectourethral fistulas.
The aim of this study was to review our experience with gracilis muscle transposition for the surgical treatment of iatrogenic rectourethral fistula resulting from treatment for prostatic carcinoma.
METHODS
A retrospective chart review of all patients who underwent repair of rectourethral fistula using gracilis muscle transposition was performed. Data on demographics, clinical presentation, operative information, and outcome were collected using a Microsoft Access database. Patients were contacted by telephone to establish follow-up when necessary.
Preoperatively, all patients had establishment of fecal diversion, usually by a laparoscopic stoma. 13 At the recommendation of a urologist, a suprapubic catheter may also have been placed.
The technique of gracilis muscle transposition for the repair of rectourethral fistula has been previously described. 14 In brief, the patient is positioned either in the supine position with the legs abducted or in a modified lithotomy position using stirrups. Three 3- to 5-cm-long incisions are made alongside the inner part of the thigh (Fig. 1). The gracilis muscle is identified and its tendon is disconnected from its insertion near the tibial plateau. The muscle is then dissected free, creating a tunnel between the incisions, and delivered through the proximal incision. Care should be taken at this point to identify and preserve the neurovascular bundle (Fig. 2). A subcutaneous tunnel is then made towards the perineum, the muscle is placed in the pocket proximal to the upper incision, and the thigh incisions are closed. The patient is then turned to the prone jack-knife position. A horizontal incision is made between the anus and scrotum (Fig. 3) and is deepened in the space between the urethra and the rectum. A large urinary bladder catheter is helpful to identify and protect the remainder of the urethra not involved in the fistula. The dissection is undertaken to divide the fistula tract and reach cephalad to noninflamed tissue. The rectal defect is then closed primarily or with an advancement flap, and the urethral defect may be closed with interrupted absorbable sutures over the indwelling catheter. The subcutaneous tunnel between the perineum and thigh is then approached through the perineal side, and the gracilis muscle is rotated and placed in the space between the rectum and the urethra. Four to six polypropylene sutures are applied at the apex of the incision to hold the muscle in place (Fig. 4). Before skin closure, a small suction drain is placed in the perineal wound.
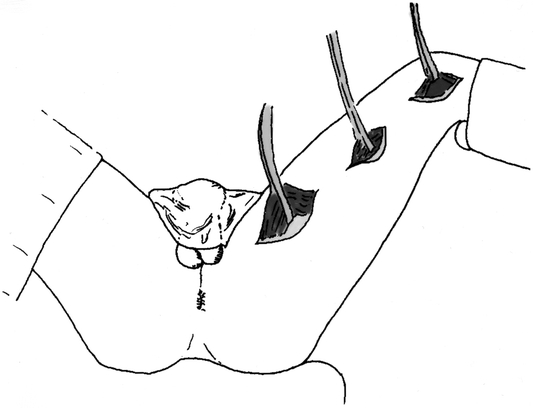
Figure 1. The thigh skin incisions and gracilis muscle mobilization.

Figure 2. The gracilis muscle mobilized through the superior thigh incision. Arrow points to the neurovascular bundle.
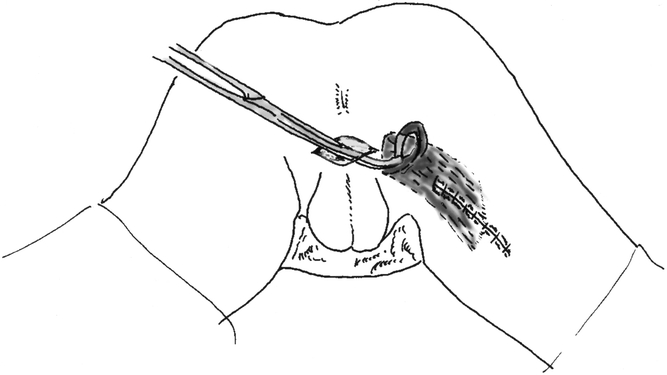
Figure 3. The perineal skin incision, and tunneling of the gracilis muscle from the thigh to the perineum.
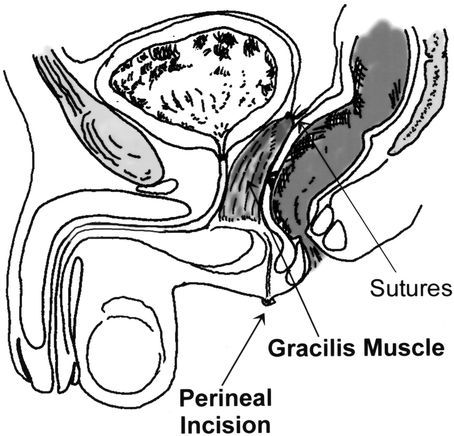
Figure 4. The gracilis muscle transposition separates between the rectum and the urethra.
Postoperatively, patients are permitted dietary intake ad libitum, are encouraged to ambulate, and are maintained on broad-spectrum antibiotics for 24 hours. The urinary bladder catheter is left in place, and 6 to 8 weeks later all patients undergo rectal contrast enema, voiding cystourethrography, cystoscopy, and proctoscopy. If the fistula has healed, the bladder catheter is removed and stoma reversal is scheduled.
Successful repair was defined as the absence of rectourethral fistula symptoms, such as urinary tract infection, pneumaturia, fecaluria, or passage of urine through the rectum, following reversal of fecal and urinary diversions. In addition, success was measured by the absence of a fistula by direct visualization and radiographic studies.
RESULTS
Twelve gracilis muscle transpositions were performed in 11 men (mean age 62.4 years, range 46–74) with rectourethral fistulas between 1996 and 2001. In all of these patients, the fistula was a result of treatment for carcinoma of the prostate. Eight patients developed fistula following radical prostatectomy; in one, radioactive beads were concomitantly implanted, and in another, the fistula was a result of transurethral surgery for stricture complication of radical prostatectomy. In one patient, the fistula occurred during cryosurgery of the prostate; two patients had radiotherapy alone, one of them through radioactive bead placement. Overall, six patients had a history of external or internal pelvic radiation therapy.
Gracilis muscle transposition has been the principal treatment for rectourethral fistula in our department in the past 5 years. Five patients, however, underwent previous surgical attempts to repair the rectourethral fistula, most of them before referral to our institution.
In all of the patients, the fecal stream was diverted until healing of the fistula was demonstrated by physical examination and contrast studies. In two patients, the diverting stoma was performed laparoscopically concomitant with the gracilis muscle transposition. A urinary Foley catheter was kept in place for at least 6 weeks and was removed after healing was demonstrated by voiding cystography or cystoscopy.
In all 11 patients, the rectourethral fistula eventually healed, and fecal diversion was reversed (Table 1). Urinary diversion was reversed in all but one patient with a severe urethral stricture. One patient had a urinary leak through the perineal wound, leading to wound infection. The gracilis flap was debrided 5 weeks later and the wound was left open. The rectourethral fistula persisted and the patient had a second gracilis muscle transposition 5 months later. This time the rectourethral fistula healed well, and the diversion was successfully reversed. A second patient developed a rectoperineal fistula to the surgical incision following reversal of the fecal diversion, which was successfully treated with application of fibrin glue to the fistula tract. In a third patient, an asymptomatic persistent rectourethral fistula was suspected on office physical examination 6 weeks after surgery. Closure of the stoma was deferred and the fistula spontaneously healed. Overall, 10 (83%) of the 12 transposition flaps resulted in complete healing of the rectourethral fistula; in only two cases were further surgical procedures required, with ultimate complete healing.
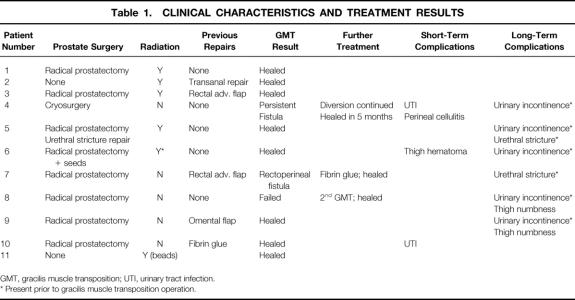
Table 1. CLINICAL CHARACTERISTICS AND TREATMENT RESULTS
GMT, gracilis muscle transposition; UTI, urinary tract infection.
* Present prior to gracilis muscle transposition operation.
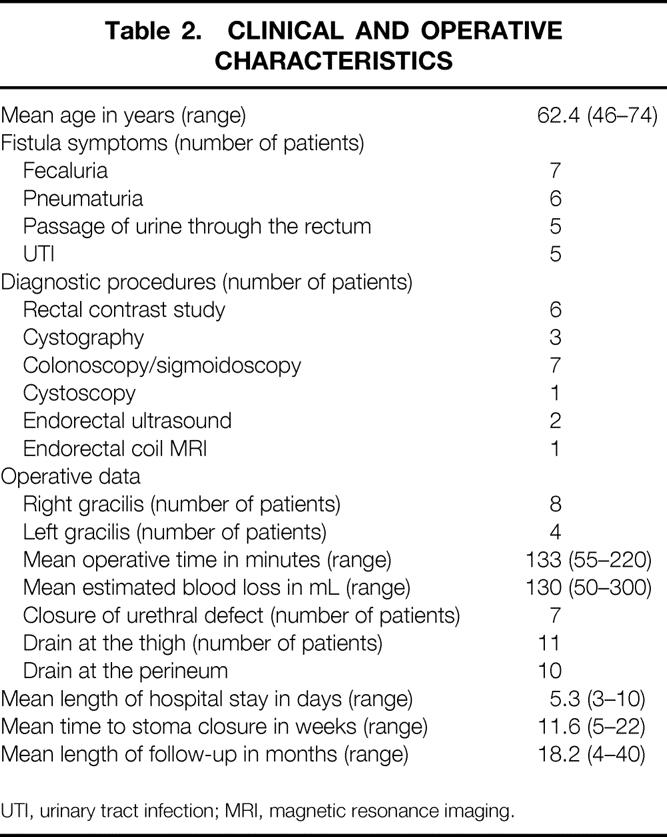
There were no intraoperative complications. The only long-term postoperative complication was mild thigh numbness in two patients. Five patients who suffered from urinary incontinence before the fistula repair, and two who had urethral strictures, did not improve in this regard following the gracilis muscle transposition. At a mean follow-up of 18.2 (range 4–40) months from diverting stoma closure, none of the patients developed a recurrent rectourethral fistula. Clinical and operative characteristics of the patients and treatment results are summarized in Tables 1 and 2.
Table 2. CLINICAL AND OPERATIVE CHARACTERISTICS
UTI, urinary tract infection; MRI, magnetic resonance imaging.
DISCUSSION
Iatrogenic rectourethral fistula is a rare but debilitating complication that can follow invasive treatment for prostate carcinoma. These fistulas tend not to heal spontaneously and are challenging to repair. Various surgical procedures have been suggested for the repair of these fistulas, which may be divided into two main categories. In the first, the luminal side of the anterior rectal wall is approached and the rectal defect is closed, with or without an advancement flap. The rectal lumen may be reached through the anus or through the posterior wall of the rectum, either via a transsphincteric plane or using a transsacral incision. 1,15,16 The major potential drawback of these approaches is that they treat only the rectal side of the fistula. Unlike rectovaginal fistulas, where the rectal side is the higher-pressure side of the fistula, and thus repair of the rectal side may be superior, 17,18 repairing a rectourethral fistula through the rectum does not close the high-pressure side, which is the urethral side in this condition.
Therefore, in the second category of repair, the principal aim is to bring viable tissue to interpose between the rectum and the urethra. The plane between these two organs is dissected, the fistula is divided, and both the rectal and urethral defects are repaired. A viable tissue flap is then transposed to separate the rectum from the urethra. Several types of tissue flaps may be used. The greater omentum is one option, 19 but this involves a major laparotomy with deep anterior pelvic dissection and may not be feasible in patients who have had previous abdominal surgeries. Transposition of the gracilis muscle as a viable flap provides a well-vascularized muscle rotation flap, avoiding the need for laparotomy.
The gracilis muscle has been used as a rotation flap or for various purposes without a significant effect on lower limb strength and range of motion. In colorectal surgery, it has been used to construct a neosphincter around the anus, with or without electrical stimulation in patients with fecal incontinence. 10,11,20 It has also been used for reconstruction of large perineal wounds following abdominoperineal resection, 12 for the treatment of ileal pouch anal anastomotic fistulas, 21 and for the repair of unhealed wounds after proctectomy for Crohn’s disease. 22 The use of the gracilis transposition for the treatment of rectourethral fistula was described by Ryan et al. in 1979. 14 Nyam and Pemberton 4 suggested that gracilis muscle transposition had a better success rate than did other types of repair of rectourethral fistula, but their experience was based on three cases, with a 100% success rate, over a period of 15 years.
Based on our experience with dissection and mobilization of the gracilis muscle for neosphincter procedures and unhealed perineal wounds, we have adopted the gracilis muscle transposition as the procedure of choice for rectourethral fistula. Since 1996, we have offered this procedure to all patients presenting to our department with rectourethral fistula. Our results suggest that this technique has a high success rate and a low morbidity.
A few technical aspects related to this procedure should be emphasized. First, since all of these patients have a history of prostatic malignancy, a preoperative biopsy of the fistula tract may be indicated to exclude recurrent cancer. Second, care should be taken to rotate the muscle to the perineum without any tension on the neurovascular bundle, which may then lead to ischemia of the flap. The gracilis muscle length required to fill the dissected rectourethral space is significantly shorter than the length needed for wrapping around the anus in neosphincter procedures. Only minimal traction is required, as it is not used to anchor the muscle to bony structures, as in a neosphincter procedure. In our experience, none of the procedures was abandoned due to a short gracilis muscle or distal insertion of the neurovascular bundle, as has happened in gracilis neosphincter formation; there was no tension in any of these repairs.
The use of a diverting stoma to protect the repair until the fistula is healed is controversial. There are no data to support higher rates of success using a protective stoma. However, our personal bias is that these patients may have a limited number of attempts to successfully dissect the plane between the rectum and the urethra and thus should have the best possible conditions in the first repair. Most of the patients in this series had their fecal stream diverted before referral to our institution, and all others underwent a diverting stoma concomitant with the gracilis muscle transposition.
Recently, gracilis muscle harvest using an endoscopic approach for plastic surgery procedures was described. 23,24 Although we currently harvest the muscle using three small vertical skin incisions of approximately 3 to 5 cm each, the endoscopic approach may further reduce invasiveness and perhaps obviate upper medial thigh numbness.
CONCLUSIONS
Gracilis muscle transposition is a useful and effective method for the treatment of rectourethral fistula, providing a viable tissue flap between the rectum and the urethra. This procedure is associated with low morbidity and high success. However, preoperative urinary incontinence and urethral stenosis related to the initial treatment of the prostatic cancer may persist following successful repair of iatrogenic rectourethral fistula.
Footnotes
Presented at the annual meeting of the Association of Coloproctology of Great Britain and Ireland, Harrogate, UK, June 24–27, 2001.
Correspondence: Eric G. Weiss, MD, Department of Colorectal Surgery, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, FL 33331.
E-mail: mcderme@ccf.org
Accepted for publication July 18, 2002.
References
- 1.Boushey RP, McLeod RS, Cohen Z. Surgical management of acquired rectourethral fistula, emphasizing the posterior approach. Can J Surg. 1998; 41: 241–244. [PMC free article] [PubMed] [Google Scholar]
- 2.Dinges S, Deger S, Koswig S, et al. High-dose rate interstitial with external beam irradiation for localized prostate cancer-results of a prospective trial. Radiother Oncol. 1998; 48: 197–202. [DOI] [PubMed] [Google Scholar]
- 3.Izawa JI, Ajam K, McGuire E, et al. Major surgery to manage definitively severe complications of salvage cryotherapy for prostate cancer. J Urol. 2000; 164: 1978–1981. [PubMed] [Google Scholar]
- 4.Nyam DC, Pemberton JH. Management of iatrogenic rectourethral fistula. Dis Colon Rectum. 1999; 42: 994–997. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Marx AC. Conservative therapy of rectourethral fistula: five-year follow-up. Urology. 1990; 35: 533–536. [DOI] [PubMed] [Google Scholar]
- 6.Vidal Sans J, Palou Redorta J, Pradell Teigell J, et al. Management and treatment of eighteen rectourethral fistulas. Eur Urol. 1985; 11: 300–305. [DOI] [PubMed] [Google Scholar]
- 7.Tiptaft RC, Motson RW, Costello AJ, et al. Fistulae involving rectum and urethra: the place of Park’s operations. Br J Urol. 1983; 55: 711–715. [DOI] [PubMed] [Google Scholar]
- 8.Martelli A, Bercovich E, Lo Cigno M, et al. Transcoccygeal approach for rectourethral fistula repair. Urol Int. 1984; 39: 292–297. [DOI] [PubMed] [Google Scholar]
- 9.Wilbert DM, Buess G, Bichler KH. Combined endoscopic closure of rectourethral fistula. J Urol. 1996; 155: 256–258. [PubMed] [Google Scholar]
- 10.Mander BJ, Wexner SD, Williams NS, et al. Preliminary results of a multicenter trial of the electrically stimulated gracilis neoanal sphincter. Br J Surg. 1999; 86: 1543–1548. [DOI] [PubMed] [Google Scholar]
- 11.Mavrantonis C, Billotti VL, Wexner SD. Stimulated graciloplasty for treatment of intractable fecal incontinence: critical influence of the method of stimulation. Dis Colon Rectum. 1999; 42: 497–504. [DOI] [PubMed] [Google Scholar]
- 12.Shibata D, Hyland W, Busse P, et al. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999; 6: 33–37. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira L, Reissman P, Nogueras J, et al. Laparoscopic creation of stomas. Surg Endosc. 1997; 11: 19–23. [DOI] [PubMed] [Google Scholar]
- 14.Ryan JA, Beebe HG, Gibbons RP. Gracilis muscle flap for closure of rectourethral fistula. J Urol. 1979; 122: 124–125. [DOI] [PubMed] [Google Scholar]
- 15.al-Ali M, Kashmoula D, Saoud IJ. Experience with 30 posttraumatic rectourethral fistulas: presentation of posterior transsphincteric anterior rectal wall advancement. J Urol. 1997; 158: 421–424. [DOI] [PubMed] [Google Scholar]
- 16.Bukowski TP, Chakrabarty A, Powell IJ, et al. Acquired rectourethral fistula: methods of repair. J Urol. 1995; 153: 730–733. [PubMed] [Google Scholar]
- 17.Kodner IJ, Mazor A, Shemesh EI, et al. Endorectal advancement flap repair of rectovaginal and other complicated anorectal fistulas. Surgery. 1993; 114: 682–689. [PubMed] [Google Scholar]
- 18.Lee PY, Fazio VW, Church JM, et al. Vaginal fistula following restorative proctocolectomy. Dis Colon Rectum. 1997; 40: 752–759. [DOI] [PubMed] [Google Scholar]
- 19.Trippitelli A, Barbagli G, Lenzi R, et al. Surgical treatment of rectourethral fistulae. Eur Urol. 1985; 11: 388–391. [DOI] [PubMed] [Google Scholar]
- 20.Baeten CG, Geerdes BP, Adang EM, et al. Anal dynamic graciloplasty in the treatment of intractable fecal incontinence. N Engl J Med. 1995; 332: 1600–1605. [DOI] [PubMed] [Google Scholar]
- 21.Gorenstein L, Boyd JB, Ross TM. Gracilis muscle repair of rectovaginal fistula after restorative proctocolectomy. Report of two cases. Dis Colon Rectum. 1988; 31: 730–734. [DOI] [PubMed] [Google Scholar]
- 22.Rius J, Nessim A, Nogueras JJ, et al. Gracilis transposition in complicated perianal fistula and unhealed perineal wounds in Crohn’s disease. Eur J Surg. 2000; 166: 218–222. [DOI] [PubMed] [Google Scholar]
- 23.Hallock GG. Minimally invasive harvest of the gracilis muscle. Plast Reconstr Surg. 1999; 104: 801–805. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel JH, Lee C, Trabulsy PP, et al. Endoscopic harvest of the gracilis muscle flap. Ann Plast Surg. 1998; 41: 384–389. [DOI] [PubMed] [Google Scholar]