Abstract
Many underlying causes of human infertility have been overcome by using in vitro fertilization (IVF) and embryo transfer (ET) techniques. Nevertheless, implantation rates in IVF programs remain low despite the transfer of apparently healthy embryos. This suggests that there are problems with the differentiation of the uterus to the receptive state in response to the ovarian hormones estrogen and progesterone. The molecular basis of this receptive state when the uterine environment is conducive to blastocyst acceptance and implantation remains poorly understood. Normally, the “window” of uterine receptivity lasts for a limited time. Using ETs and the progesterone-treated delayed-implantation model in mice, we demonstrate here that levels of estrogen within a very narrow range determine the duration of the window of uterine receptivity. Although estrogen at different physiological concentrations can initiate implantation, we find that the window of uterine receptivity remains open for an extended period at lower estrogen levels but rapidly closes at higher levels. The uterine refractoriness that follows the receptive state at high estrogen levels is accompanied by aberrant uterine expression of implantation-related genes. These results suggest that careful regulation of estrogen levels is one of the important factors for improvement of female fertility in IVF/ET programs.
Synchronized development of the embryo to the blastocyst stage, differentiation of the uterus to the receptive state, and cross-talk between the blastocyst and uterine luminal epithelium are essential to the implantation process (reviewed in refs. 1–3). In mice and rats, estrogen is essential for preparation of the progesterone (P4)-primed uterus to the receptive state when the uterine milieu becomes favorable to blastocyst acceptance and implantation (1, 4). Normally, the “window” of uterine receptivity is maintained for a limited period. In mice, the uterus becomes receptive on day 4 of pregnancy or pseudopregnancy and proceeds to the refractory state on day 5 (3). However, the mechanism by which estrogen prepares the P4-primed uterus to the receptive state is not clearly defined yet. It is also unknown how the uterus, after achieving the receptive state for a limited period, proceeds to the refractory state. Various factors including cytokines, growth factors, homeobox transcription factors, and cyclooxygenase (COX)-derived prostaglandins participate in these processes through autocrine, paracrine, and/or juxtacrine mechanisms (reviewed in refs. 3, 5, and 6). For example, the gene encoding leukemia inhibitory factor (LIF) is expressed in the mouse uterine glands in response to estrogen stimulation (7, 8), whereas P4 regulates the gene for amphiregulin in the epithelium and Hoxa-10 in the stroma (9, 10). Furthermore, LIF and Hoxa-10 are considered to be critical to uterine preparation for implantation and decidualization, respectively (11, 12). The genes encoding HB-EGF, COX-2, and LIF are also considered critical to implantation, because these genes are expressed in the uterus at the site of blastocyst apposition before and during the attachment reaction (3). However, their interactions with respect to estrogen and uterine receptivity are not clearly understood.
We hypothesized that a critical level of estrogen is crucial in regulating the window of uterine receptivity for implantation in a P4-primed uterus by altering gene expression. We provide evidence from physiological and molecular gene-expression studies for this idea. There is evidence from other studies that ovarian hyperstimulation leads to implantation failure and embryonic resorption in mice (13, 14). Recent evidence also suggests that “on-time” implantation is crucial to successful pregnancy establishment in both humans and mice (15, 16). Thus, uterine receptivity established by coordinated interactions between ovarian P4 and estrogen is critical to successful implantation and pregnancy establishment. One prediction of our present investigation is that the reduced pregnancy rate in human in vitro fertilization (IVF)/embryo-transfer (ET) programs is the result of uterine refractoriness due to higher estrogen levels arising from ovarian hyperstimulation by exogenous gonadotropin administration for retrieving multiple eggs (17–19).
Methods
Animal Models, ET, and Treatments.
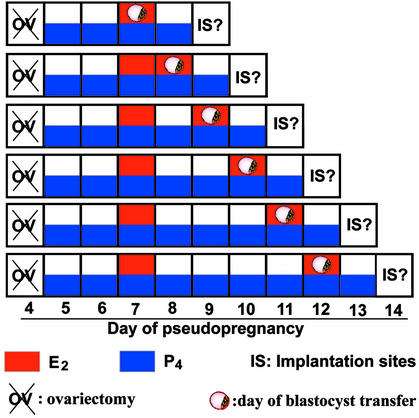
Adult CD-1 mice were purchased from Charles River Breeding Laboratories. Mice were housed in the Animal Care Facility according to National Institutes of Health and institutional guidelines for laboratory animals. Conditions of delayed implantation were induced by ovariectomizing pseudopregnant (day 1 = vaginal plug) mice on the morning of day 4 (0830–0900 hours) and maintained by daily injections of P4 (2 mg per mouse) from day 5 until the mice were killed. Day-4 blastocysts recovered from normal pregnant donors were transferred into uteri of these recipients on specific days and under specific treatments as shown in the Fig. 1 schematic diagram. Implantation sites were examined 24 or 48 h after the transfers of blastocysts by the blue-dye method (20). If no implantation sites are noted, uteri were flushed to recover blastocysts, and their gross morphology was examined microscopically. Statistical analysis was performed by ANOVA followed by Fisher's test.
Figure 1.
Schematic outline of experimental designs.
Probes and in Situ Hybridization.
Sense or antisense 35S-labeled cRNA probes were generated by using appropriate polymerases from cDNAs to Lif, Hoxa10, Hegf1, Areg, Ptgs1, and Ptgs2 for in situ hybridization as described (21). In situ hybridization was performed as described (21). Frozen sections (11 μm) were mounted onto poly-L-lysine-coated slides, fixed in cold 4% paraformaldehyde solution in PBS, acetylated, and hybridized at 45°C for 4 h in hybridization buffer containing the 35S-labeled antisense cRNA probes. After hybridization, the sections were treated with RNase A (20 μg/ml) at 37°C for 20 min. RNase A-resistant hybrids were detected by autoradiography. Sections hybridized with the sense probes served as negative controls.
Results
Estrogen Modulates the Window of Uterine Receptivity for Implantation.
Uterine preparation to the receptive state by P4 and estrogen is critical to implantation in mice. Our first objective was to determine the minimum dose of estrogen that is required to initiate implantation in a P4-primed uterus. Pseudopregnant recipient mice were ovariectomized on the morning of day 4 to induce the condition of delayed implantation, and this condition was maintained with daily injections of P4 (2 mg per mouse) from day 5. Day-4 normal blastocysts (hereafter, blastocysts) were transferred into P4-treated recipient uterine lumens on day 7 followed immediately by injection of different doses of estradiol-17β (E2, 1.5, 3.0, 10.0, or 25.0 ng per mouse). Implantation sites were examined 48 h later by the blue-dye method. We observed that E2 at 3.0, 10.0, or 25.0 ng was effective in inducing implantation. By contrast, 1.5 ng was suboptimal and ineffective in this response. These results demonstrate that E2 at all doses between 3.0 and 25.0 ng are equally effective in inducing implantation in P4-primed uteri (Table 1).
Table 1.
E2 at 3 ng is the minimal dose for the induction of implantation in P4-treated delayed uteri
| Doses of E2, ng | No. of recipients | No. of blastocysts transferred | No. of recipients with ISs (%) | No. of ISs (%) | No. of blastocysts recovered from mice without ISs (%) |
|---|---|---|---|---|---|
| 1.5 | 5 | 56 | 2 (40) | 2 (2) | 14 (40) |
| 3.0 | 5 | 70 | 5 (100) | 30 (43) | |
| 10.0 | 5 | 70 | 4 (80) | 34 (49) | 4 (29) |
| 25.0 | 5 | 58 | 5 (100) | 20 (34) |
Recipient mice were ovariectomized on day 4 of pseudopregnancy (0900 hours) and injected daily with P4 (2 mg per mouse) to induce the condition of delayed implantation. Day-4 normal blastocysts were transferred into uteri of these mice on day 7 at 1000 hours. The recipients received 1.5, 3.0, 10.0, or 25.0 ng of E2 immediately after blastocyst transfers. Implantation sites (ISs) were examined 48 h after embryo transfer by the blue-dye method. The uteri without implantation sites were flushed to recover unimplanted blastocysts. The rate of ISs in mice treated with 1.5 ng of E2 was significantly lower (P < 0.0001) from that of mice treated with 3.0, 10.0 or 25.0 ng of E2.
We next determined the effects of different levels of estrogen on the duration of the window of receptivity. To address this, we again used P4-treated delayed recipient mice that were injected with various doses of E2 (1.5, 3.0, 10.0, or 25.0 ng per mouse) on day 7. On day 8, blastocysts were transferred immediately followed by a second injection of E2 at 3.0 ng, and implantation sites were examined 48 h later (see Fig. 1). We observed that most of the mice that received 1.5 or 3.0 ng of E2 showed implantation after receiving the second injection of E2 at 3.0 ng, whereas those that received 10.0 or 25.0 ng as the first injection had very few or no implantation sites after the second E2 injection (Table 2). Furthermore, increasing the dose of the second E2 injection to 10.0 or 25.0 ng did not improve implantation rates. These results demonstrate that lower doses of E2 maintain the P4-primed uterus in a state such that a second estrogen exposure readily induces implantation if blastocysts are present in the uterus. In contrast, the uterus becomes refractory at higher doses of estrogen (10.0 or 25.0 ng) and does not support implantation after a second exposure to estrogen at either a low or high dose (Table 2).
Table 2.
Effects of E2 on the duration of the window of uterine receptivity for implantation
| Treatment (E2, ng per mouse)
|
No. of recipients | No. of blastocysts transferred | No. of mice with ISs (%) | No. of ISs (%) | No. of blastocysts recovered from mice without ISs (%) | |
|---|---|---|---|---|---|---|
| Day 7 (1st injection of E2, ng) | Day 8 (2nd injection of E2, ng) | |||||
| 1.5 | 0 | 5 | 74 | 0 | 0 | 19 (26) |
| 1.5 | 4 | 49 | 3 (75) | 19 (39) | 0 | |
| 3.0 | 7 | 92 | 7 (100) | 28 (30) | ||
| 3.0 | 0 | 4 | 46 | 1 (25) | 8 (17) | 0 |
| 3.0 | 10 | 146 | 9 (90) | 49 (34) | 0 | |
| 10.0 | 4 | 56 | 4 (100) | 15 (27) | ||
| 10.0 | 0 | 3 | 37 | 1 (33) | 1 (3) | 1 (7) |
| 3.0 | 6 | 84 | 0 | 0 | 0 | |
| 10.0 | 8 | 94 | 2 (25) | 6 (6) | 3 (19) | |
| 25.0 | 3.0 | 6 | 84 | 0 | 0 | 14 (17) |
| 25.0 | 4 | 48 | 0 | 0 | 5 (10) | |
The recipient mice were ovariectomized on day 4 of pseudopregnancy (0900 hours) and injected daily with P4 (2 mg per mouse) to induce the condition of delayed implantation. On day 7, the recipients received the first injection of E2 at 1.5, 3.0, 10.0, or 25.0 ng. On day 8, day-4 normal blastocysts were transferred into these recipients immediately followed by a second injection of the vehicle (oil) or E2 at 1.5, 3.0, 10.0, or 25.0 ng. Implantation sites (ISs) were examined 48 later. The uteri without implantation sites were flushed to recover unimplanted blastocysts. The IS rate in mice receiving the first E2 injection at 1.5 or 3.0 ng followed by the second injection of 1.5, 3.0, or 10.0 ng of E2 was significantly higher (P < 0.001) than those receiving the first E2 injection at 10.0 or 25.0 ng.
These results prompted us to examine whether estrogen at a lower dose can prolong the receptive state for an extended period. P4-treated ovariectomized pseudopregnant recipients were given an injection of 3.0 ng of E2 on day 7 followed by blastocyst transfers 24, 48, 72, 96, or 120 h later. Immediately after the ET, the recipients were given a second injection of 3.0 ng of E2, and implantation sites were examined 48 h later. The results show that uterine refractoriness is postponed in the majority of the mice at this low dose of 3.0 ng of E2 for at least 4 days (Table 3). In contrast, when the first injection of E2 was 25.0 ng the uterus became refractory within 24 h and remained refractory for the next 72 h examined (data not shown).
Table 3.
E2 at 3 ng prolongs uterine receptivity beyond 24 h
| Time of blastocyst transfer after 1st E2 (3 ng) injection, h | No. of recipients | No. of blastocysts transferred | No. of mice with ISs (%) | No. of ISs (%) | No. of blastocysts recovered from mice without ISs (%) |
|---|---|---|---|---|---|
| 24 | 10 | 146 | 9 (90) | 49 (34) | 0 |
| 48 | 4 | 51 | 4 (100) | 10 (20) | |
| 72 | 5 | 67 | 4 (80) | 26 (40) | 3 (21) |
| 96 | 4 | 49 | 3 (75) | 16 (33) | 0 |
| 120 | 5 | 70 | 2 (40) | 7 (10) | 0 |
The recipients were ovariectomized on day 4 of pseudopregnancy at 0900 hours and injected daily with P4 (2 mg/ml) to induce the condition of delayed implantation. On day 8, the recipients received the first injection of E2 at 3 ng. Day-4 normal blastocysts were transferred into these recipients 24, 48, 72, 96, or 120 h after the first injection of E2 followed by a second injection of E2 at 3 ng immediately after blastocyst transfers. Implantation sites (ISs) were examined 48 later. The uteri without ISs were flushed to recover unimplanted blastocysts. The IS rate was statistically insignificant (P > 0.05) among mice receiving blastocyst transfers 24, 48, 72, or 96 h after the first 3-ng E2 injection; the IS rate, however, was lower (P < 0.05) in mice receiving blastocyst transfers at 120 h than in those receiving transfers 24 or 72 h after the first 3-ng E2 injection.
We next examined whether uterine refractoriness induced at higher doses of E2 is reversed by increasing the dose of P4. Increasing P4 to 4 mg per mouse did not rescue the implantation failure in recipients that received E2 at 10.0 or 25.0 ng for the first injection (data not shown). Taken together, these results show that specific levels of estrogen determine the duration of the window of uterine receptivity for implantation in mice. In other words, uterine refractoriness occurs much faster at higher estrogen levels than at lower doses. Furthermore, higher doses of P4 fail to neutralize estrogen-induced refractoriness.
Implantation-Specific Gene Expression Is Aberrant at the Blastocyst Site After a High Dose of Estrogen.
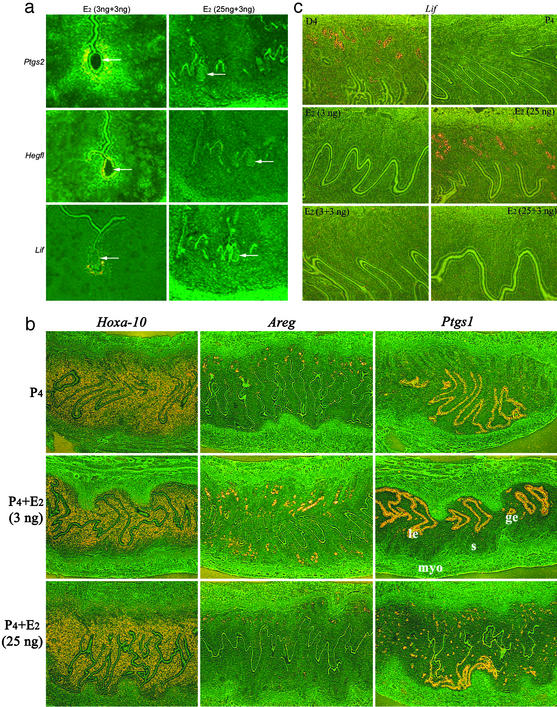
The above results suggested that uterine refractoriness induced by higher doses of E2 is due to aberrant expression of specific genes in the uterus. We therefore asked whether implantation-specific genes are expressed correctly at the implantation sites of P4-primed delayed recipients given E2 at 3.0 or 25.0 ng immediately after blastocyst transfer on day 7. After 24 h, we examined the expression of Lif, Hegfl (HB-EGF), and Ptgs2 (COX-2), because of their known roles in implantation in mice (reviewed in ref. 3), and found that expression was normal (data not shown). We next asked whether these genes are expressed correctly when uterine receptivity is sustained by a lower dose of E2 or when uterine refractoriness is induced by a higher dose. To address this issue, P4-treated recipients with conditions of delayed implantation received an injection of either 3.0 or 25.0 ng of E2 on day 7. Blastocysts were transferred on day 8 immediately followed by a second injection of 3.0 ng of E2. After 24 h, sections of uteri with or without implantation sites but containing blastocysts were processed for in situ hybridization. We observed that Lif, Ptgs2, and Hegfl genes were expressed correctly at the implantation sites when the mice received the first and second injections of E2 at 3.0 ng. In contrast, mice receiving 25.0 ng of E2 as a first injection completely lacked any sign of implantation or the expression of these genes adjacent to blastocysts after the second injection of E2 at 3.0 ng (Fig. 2a). These results suggest that uteri maintained in a receptive state at the lower E2 dose behaved normally with respect to implantation and gene expression, whereas implantation fails and gene expression becomes aberrant in the uterus proceeding to refractoriness in response to E2 at 25.0 ng.
Figure 2.
Uterine gene expression in receptive and refractory uteri. (a) Lif, Ptgs2, and Hegfl expression is aberrant at the site of a blastocyst in a refractory uterus induced by 25 ng of E2. Recipient mice were ovariectomized on day 4 of pseudopregnancy and injected daily with 2 mg of P4 to induce the condition of delayed implantation. On day 7, the recipients received the first injection of E2 at 3 or 25 ng. On day 8, blastocysts were transferred into these recipients immediately followed by a second injection of 3 ng of E2. Uterine sections containing blastocysts were processed for in situ hybridization 24 h later. (Magnification, ×100.) Arrows indicate the location of blastocysts. (b) Uterine expression of Areg and Ptgs1 becomes aberrant at a higher E2 level. Pseudopregnant mice ovariectomized on day 4 were injected daily with P4 to induce the condition of delayed implantation. On day 7, they received an injection of 3 or 25 ng of E2. Uteri were processed for in situ hybridization 24 h later. (Magnification, ×40.) Note that although Hoxa-10 expression is similar at 3 or 25 ng of E2, the expression of amphiregulin and COX-1 is aberrant at 25 ng of E2. le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium. (c) Uterine Lif expression is different at higher and lower doses of E2. As stated above, pseudopregnant mice ovariectomized on day 4 were treated with P4 daily to induce the condition of delayed implantation. On day 7, they received 3 or 25 ng of E2 or received the first injection of E2 at 3 or 25 ng followed by a second injection of 3 ng of E2 24 h later. Uteri were processed for in situ hybridization at different times. Results of Lif expression at 24 h are shown. (Magnification, ×100.) E2 at 3 ng as one or two injections failed to detect glandular Lif expression, whereas 25 ng of E2 as a single injection induced this expression. The expression was undetectable when the first E2 injection was at 25 ng followed by a second injection at 3 ng. Representative sections of day 4 (D4) pregnant uterus showing glandular Lif expression and of P4-treated uteri showing the absence of Lif expression were used as positive and negative controls, respectively.
Genes Associated with Uterine Preparation to the Receptive State Are Aberrantly Expressed After a High Dose of Estrogen.
Expression of Ptgs1 (COX-1), Lif, Hoxa10, and Areg (amphiregulin) is normally associated with uterine preparation to the receptive phase (3). We observed that 24 h after an injection of E2 at 3.0 or 25.0 ng, the expression pattern of Hoxa10 was normal. However, the expression of Ptgs1 became aberrant, whereas the expression of Areg was undetectable in the uterus after an injection of 25.0 ng of E2. The uterine expression of Ptgs1 became mostly confined to glands at 25.0 ng of E2 (Fig. 2b). These results suggest that the aberrant expression of Ptgs1 and down-regulation of Areg at higher E2 levels are indicative of uterine refractoriness. All these genes are expressed in a cell-specific manner throughout the mouse uterus in the morning and afternoon on day 4 of pregnancy before or at the time of the attachment reaction (8, 9, 12, 22). Overall, these results suggest that a rapid onset of uterine refractoriness at a higher estrogen level is due to a failure in maintaining the correct expression of genes associated with uterine receptivity and attachment reaction. A similar observation was noted when a receptive uterus on day 4 proceeds to a refractory state in the afternoon of day 5 in intact pseudopregnant mice (data not shown).
An interesting observation was noted with respect to Lif expression. Lif is normally expressed in the mouse uterus in a biphasic manner: first in uterine glands on day 4 of pregnancy followed by transient expression in stromal cells surrounding the blastocyst at the time of attachment reaction at midnight of day 4 that persists through the morning of day 5 (8). However, in the ovariectomized or P4-primed ovariectomized mice, estrogen at higher doses rapidly induces Lif expression in uterine glands (7, 8). We sought to examine by in situ hybridization whether Lif expression is altered in the P4-primed uterus in response to a low or high dose of E2. We observed that E2 at 3.0 ng as a single injection or two injections at 24-h intervals failed to induce Lif expression in uterine glands at 1, 6, or 24 h after the last E2 injection. In contrast, E2 at 25.0 ng as a single injection, as expected, induced Lif expression in P4-primed uterine glands at these times. However, Lif expression was undetectable in uterine glands when the first injection of E2 was given at 25.0 ng followed 24 h later by a second injection of E2 at 3.0 ng (Fig. 2c). Recall that 3.0 ng of E2 as single or two injections can induce implantation with correct expression of Lif in stromal cells surrounding the implanting blastocyst (see Fig. 2a). These results suggest that stromal cell Lif expression at the site of blastocyst during the attachment reaction is more important than the glandular Lif expression.
Discussion
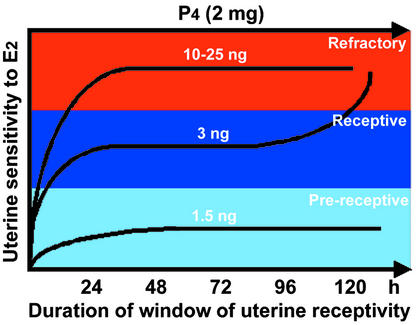
Preimplantation embryo development and uterine preparation for implantation are two major determinants of female fertility. Despite significant developments in IVF/ET technology in humans, the pregnancy success rates remain disappointingly low. The question of uterine receptivity for implantation is an important issue, because a low pregnancy success rate in IVF/ET programs is considered to be due to a higher incidence of implantation failure. This low rate of success is perceived as the result of transfer of IVF-derived embryos into the nonreceptive uterus. One cause of this low rate could be the high levels of estrogen resulting from hyperstimulation of the ovary by gonadotropin administration to retrieve multiple eggs (17–19, 23), thereby rendering the uterus refractory. Thus, uterine receptivity established by coordinated interactions of ovarian P4 with estrogen is critical to successful implantation and pregnancy outcome. However, critical levels of P4 and estrogen, their relative importance, and mechanism of actions in regulating uterine receptivity and refractoriness have not been examined in a physiologically defined system. Our results using a delayed-implantation model in mice provide evidence that the levels of estrogen within a very narrow range are critical determinants for transforming uterine receptivity to a refractory state, suggesting that the uterus is extremely sensitive to estrogen levels with respect to implantation (see Fig. 3). This remarkable sensitivity of the uterus to estrogen perhaps plays a significant role in ensuring on-time implantation, which is critical to successful pregnancy establishment and outcome.
Figure 3.
A scheme depicting modulation of the window of receptivity in the P4-primed uterus in response to changing estrogen levels. This scheme shows that estrogen at a low threshold level extends the window of uterine receptivity for implantation, but higher levels rapidly close this window, transforming the uterus into a refractory state.
Our results in mice raise the interesting possibility that the uterus becomes refractory after a considerably prolonged period of receptive state at a low dose of E2 (3.0 ng). These results also show that sharp changes occur with respect to uterine receptivity within a narrow range of E2 levels (3.0–25.0 ng). However, the mechanism by which E2 at 3.0 ng, but not at 10.0 or 25.0 ng, can prolong the receptive phase is not clearly understood. Doses of 10.0 and 25.0 ng of E2 as a first injection should not be considered nonphysiological, because both can induce implantation in a P4-primed uterus similar to 3.0 ng of E2. Therefore, we suggest that implantation-inducing changes in a P4-primed uterus produced by different doses of E2 during the initial phase, i.e., before 24 h, are similar, if not identical, to those changes occurring in the normal pregnant uterus with active blastocysts. This is consistent with our observation of normal expression of genes encoding LIF, COX-2, and HB-EGF at the site of implantation induced by either a low or high dose of estrogen. However, the duration of the window of uterine receptivity is drastically curtailed at higher estrogen levels. This is coincident with the absence of uterine expression of Lif, Ptgs2, and Hegfl at the site of the blastocyst when the mice received a first injection of E2 at 25.0 ng followed by a second injection of 3.0 ng of E2 immediately following blastocyst transfer. In contrast, these genes were expressed correctly at the implantation sites when the first and second injections were restricted to 3.0 ng. It could be postulated that the maintenance of the receptive state was altered at the molecular level in the presence of higher estrogen levels, leading to implantation failure. This postulation is supported by aberrant expression of genes encoding COX-1 and amphiregulin but not Hoxa-10 in the uterus 24 h after an injection of E2 at a higher dose. These changes could be due to alteration of nuclear receptors for estrogen (ER) and P4 (PR). Indeed, we observed that PR expression in the luminal epithelium but not in stromal cells was down-regulated 24 h after an injection of 25.0 ng of E2; little alteration in PR expression was noted at 3.0 ng of E2. In contrast, the ERα expression pattern in luminal and stromal cells was similar at both E2 concentrations (data not shown). Collectively, these results provide evidence that uterine gene expression conducive to blastocyst implantation is maintained at a lower E2 dose, whereas gene expression promptly becomes aberrant in the uterus, becoming refractory at higher E2 levels. These results also suggest that the molecular programming of the uterus with respect to specific gene expression is altered depending on the E2 levels within a very narrow range, regulating uterine receptivity and refractoriness.
An alternative possibility for prolonging the state of uterine receptivity could be due to differential responses of different regions of the uterus to a low dose of E2. For example, at 3.0 ng of E2 specific areas of the uterus may become receptive, whereas the remaining areas may still be in the neutral state. These remaining neutral areas then respond to a second injection of E2. In contrast, at 25.0 ng of E2 the entire uterus may respond rapidly to reach a maximally receptive state followed by a refractory state by 24 h. This is a possibility if the uterus is comprised of sensitive and less-sensitive areas that respond differentially to hormones and local factors. The validity of this possibility will require a closer examination of gene expression along the entire uterus under defined experimental conditions.
It is well documented that the effects of P4 and E2 are either synergistic or antagonistic with respect to various uterine functions and gene expression (5, 24). Therefore, it was obligatory to determine whether increasing the P4 doses would counteract the effects of higher doses of E2 in extending the uterine receptivity. Our observation of failure of P4 at an elevated level to reverse the adverse effects of higher doses of estrogen on uterine receptivity indicates that specific uterine functions are more sensitive to estrogen and independent of P4 levels. For example, the down-regulation of PR expression in the luminal epithelium at a higher E2 level suggests that the ineffectiveness of a higher dose of P4 apparently includes a change in the luminal epithelium. Because our results provide strong evidence that higher estrogen levels promptly transform the uterus to the refractory state, it may be possible to extend the state of uterine receptivity by neutralizing excess estrogen by the use of an aromatase inhibitor at the time of gonadotropin stimulation in human IVF/ET programs for correcting the cause of uterine refractoriness at higher estrogen levels. Indeed, decreasing estradiol levels during the preimplantation period by using a follicle-stimulating hormone/step-down regimen has been claimed to increase uterine receptivity in humans (18). However, there is also evidence that ovarian hyperstimulation does not adversely affect uterine receptivity for implantation in IVF/ET programs (25), suggesting that the range of estrogen levels is less restrictive in humans than in mice. Nonetheless, the information obtained from the present investigation in mice should provide valuable information for improving the implantation rate of the IVF-derived embryos in women. Complications associated with multiple embryo implantations also raise serious clinical and social concerns. Thus, prolonging the uterine receptive state may circumvent the necessity of multiple ET to improve pregnancy rates in humans.
Acknowledgments
We thank Brigid Hogan for constructive insight and valuable advice and Takiko Daikoku and Haibin Wang for imaging and statistical analysis. This work was supported in part by National Institutes of Health Grants HD37830 (to S. K. Das), HD37394 (to B.C.P.), and HD12304 and HD33994 (to S. K. Dey) and a grant from the Mellon Foundation (to S. K. Dey). S. K. Dey is a recipient of a National Institute of Child Health and Human Development/National Institutes of Health MERIT Award.
Abbreviations
- P4
progesterone
- COX
cyclooxygenase
- LIF
leukemia inhibitory factor
- IVF
in vitro fertilization
- ET
embryo transfer
- E2
estradiol-17β
- PR
P4 nuclear receptor
References
- 1.Psychoyos A. In: Handbook Of Physiology. Greep R O, Astwood E G, Geiger S R, editors. Washington, DC: Am. Physiol. Soc.; 1973. pp. 187–215. [Google Scholar]
- 2.Carson D D, Bagchi I, Dey S K, Enders A C, Fazleabas A T, Lessey B A, Yoshinaga K. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 3.Paria B C, Reese J, Das S K, Dey S K. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga K. Prog Reprod Biol. 1980;7:189–199. [Google Scholar]
- 5.Lim H, Song H, Paria B C, Reese J, Das S K, Dey S K. Vitamins and Hormones. Vol. 64. New York: Academic; 2002. pp. 43–76. [DOI] [PubMed] [Google Scholar]
- 6.Norwitz E R, Schust D J, Fisher S J. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt H, Brunet L J, Stewart C L. Proc Natl Acad Sci USA. 1991;88:11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Lim H, Das S K, Paria B C, Dey S K. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 9.Das S K, Chakraborty I, Paria B C, Wang X-N, Plowman G, Dey S K. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 10.Benson G V, Lim H, Paria B C, Satokata I, Dey S K, Maas R L. Development (Cambridge, UK) 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 11.Stewart C L, Kasper P, Brunet L J, Bhatt H, Gadi I, Kontgen F, Abbondanzo S J. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 12.Lim H, Ma L, Ma W-G, Maas R L, Dey S K. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 13.Walton E A, Huntley S, Kennedy T G, Armstrong D T. Biol Reprod. 1982;27:847–852. doi: 10.1095/biolreprod27.4.847. [DOI] [PubMed] [Google Scholar]
- 14.Ertzeid G, Storeng R. Hum Reprod. 2001;16:221–225. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox A J, Baird D D, Weinberg C R. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Lim H, Paria B C, Matsumoto H, Swift L L, Morrow J D, Bonventre J V, Dey S K. Development (Cambridge, UK) 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 17.Pellicer A, Valbuena D, Cano F, Remohi J, Simon C. Fertil Steril. 1996;65:1190–1195. doi: 10.1016/s0015-0282(16)58337-x. [DOI] [PubMed] [Google Scholar]
- 18.Simon C, Garcia Velasco J J, Valbuena D, Peinado J A, Moreno C, Remohi J, Pellicer A. Fertil Steril. 1998;70:234–239. doi: 10.1016/s0015-0282(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 19.Ng E H Y, Yeung W S B, Lau E Y L, So W W K, Ho P C. Hum Reprod. 2000;15:250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 20.Paria B C, Huet-Hudson Y M, Dey S K. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das S K, Wang X, Paria B C, Damm D, Abraham J, Klagsbrun M, Andrews G, Dey S K. Development (Cambridge, UK) 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 22.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 23.Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Hum Reprod. 1999;14, Suppl. 2:107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 24.Huet-Hudson Y M, Andrews G K, Dey S K. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 25.Levy A J, Drews M R, Bergh P A, Miller B T, Scott R T. Fertil Steril. 2001;76:670–674. doi: 10.1016/s0015-0282(01)01988-4. [DOI] [PubMed] [Google Scholar]