Abstract
Objective
To evaluate the early results of endovascular grafting for high-risk surgical candidates in the treatment of abdominal aortic aneurysms (AAA).
Summary Background Data
Since the approval of endoluminal grafts for treatment of AAA, endovascular repair of AAA (EVAR) has expanded to include patients originally considered too ill for open AAA repair. However, some concern has been expressed regarding technical failure and the durability of endovascular grafts.
Methods
The University of Alabama at Birmingham (UAB) Computerized Vascular Registry identified all patients who underwent abdominal aneurysm repair between January 1, 2000, and June 12, 2002. Patients were stratified by type of repair (open AAA vs. EVAR) and were classified as low risk or high risk. Patients with at least one of the following classifications were classified as high risk: age more than 80 years, chronic renal failure (creatinine > 2.0), compromised cardiac function (diminished ventricular function or severe coronary artery disease), poor pulmonary function, reoperative aortic procedure, a “hostile” abdomen, or an emergency operation. Death, systemic complications, and length of stay were tabulated for each group.
Results
During this 28-month period, 404 patients underwent AAA repair at UAB. Eighteen patients (4.5%) died within 30 days of their repair or during the same hospitalization. Two hundred seventeen patients (53%) were classified as high risk. Two hundred fifty-nine patients (64%) underwent EVAR repair, and 130 (50%) of these were considered high-risk patients (including four emergency procedures). One hundred forty-five patients (36%) underwent open AAA repair, including 15 emergency operations. All deaths occurred in the high-risk group: 12 (8.3%) died after open AAA repair and 6 (2.3%) died after EVAR repair. Postoperative length of stay was shorter for EVAR repair compared to open AAA.
Conclusions
High-risk and low-risk patients can undergo EVAR repair with a lower rate of short-term systemic complications and a shorter length of stay compared to open AAA. Despite concern regarding the durability of EVAR, high-risk patients should be evaluated for EVAR repair before committing to open AAA repair.
Abdominal aortic aneurysm (AAA) remains the 13th leading cause of death for males in the United States. Operative therapy for this disease has been well established and can reduce the mortality from aneurysm disease so that it becomes equivalent to the background population. 1 Sometimes patients are refused operative therapy because of the morbidity associated with a formal open operation and aortic cross-clamping. Other times, patients avoid operative therapy because they fear an operation of this magnitude and the potential consequences. A patient’s decision to undergo operative therapy may be affected by a relative or friend who had a poor outcome after AAA repair.
In the wave of minimally invasive surgical options, an endovascular approach for AAA has recently been approved. In September 1999 the Food & Drug Administration (FDA) approved two different endoluminal stent-grafts for the treatment of AAA. The endovascular repair of AAA (EVAR), done via limited access and incisions in the femoral arteries, has been shown to have reduced morbidity compared to open surgical controls. 2,3 There has been widespread application of this new technology, but there remains uncertainty about its long-term benefits. Specifically, some authors consider EVAR a “failed experiment.”4 Subsequently, the FDA issued a warning about the implantation of these devices in a letter sent to clinicians involved in their use. 5 In addition, there have been scattered reports about intermittent failure of these devices when patients present with ruptured aneurysms after implantation of endoluminal grafts. 6 Thus, while the long-term results are not clear, the clinician is faced with a quandary of the appropriate application for EVAR.
Additionally, patients are often driven to choose a minimally invasive approach for surgical problems. While the less invasive procedures may carry an increased risk, the perception of the shortened hospitalization and the shortened recovery time have influenced many patients to seek minimally invasive approaches. Thus, it has become the responsibility of the practicing physician to consider all therapies and counsel the patient toward the appropriate one. However, at times surgical decisions may be influenced by a patient’s desire for a shortened recovery time and quicker return to normal activity despite the potential impact on the durability of treatment. The question then remains, “What is the appropriate application for minimally invasive approaches for aortic surgery?” While some authors advocate these minimally invasive approaches only for the high-risk patient, 7 we found that many patients sought this less invasive EVAR therapy so that they could avoid the major morbidity associated with an open aortic operation. Since EVAR has become approved, we sought to balance these two opinions of durability of an established open AAA repair against a patient’s preference for less invasive therapy that may not be as durable.
In this study we reviewed our early experience with EVAR after approval of the procedure by the FDA. We sought to understand the short-term complications and mortality associated with this minimally invasive approach in both high- and low-risk patients.
METHODS
We reviewed the University of Alabama at Birmingham (UAB) Computerized Vascular Registry to identify all patients who underwent AAA repair between January 1, 2000, and June 12, 2002. Patients were stratified according to the type of repair (open AAA vs. EVAR) and then were classified as low risk or high risk. Patients were considered high risk based on the classifications listed in Table 1. Death, systemic complications, and length of stay were tabulated for each group. Systemic complications were categorized according to the following criteria: respiratory complications requiring ventilation or extending the hospitalization beyond 14 days; cardiac complications related to atrial fibrillation or heart block or enzymatic evidence of myocardial necrosis; renal failure causing elevation of creatinine more than 1.0 mg/dL above baseline or requiring an additional intervention (dialysis); wound complications, defined as requiring an additional procedure for management or additional antibiotics for care; miscellaneous complications, including patients who required readmission without definite cause, or hospitalization beyond 14 days related to a medical condition that was exacerbated by the surgical procedure. EVAR was accomplished with either commercially available grafts (Ancure, Guidant Corporation, Menlo Park, CA; or AneuRx, Medtronic Corp., Sunnyvale, CA) or a custom-made graft (Talent, World Medical, Sunrise, FL; or a surgeon-made stent-graft using Dacron grafts and self-expanding stents). Results were tabulated based on intention-to-treat analysis. Late conversions were tabulated as EVAR for the first procedure and open AAA repair for the second procedure.
Table 1. HIGH-RISK CRITERIA
Statistical analysis was done with the following: Fisher exact tests were used to test for associations between two-level categorical variables (e.g., respiratory complications and repair method). If the direction of the expected association was known a priori, one-tailed tests were used. Multivariable logistic regression models were fit to model complications by risk group and repair method. Odds ratios were used to describe the relationship. Wilcoxon rank-sum tests were used to test for differences in the continuous measure length of stay between groups. The nonparametric test was used instead of t tests because the length-of-stay values were not normally distributed. All analyses were performed using SAS 8.2 (Cary, NC). P < 0.05 was considered significant.
RESULTS
During this 28-month period, 404 patients underwent AAA repair at UAB or at the Birmingham Veterans Administration Hospital. Patient characteristics and endograft types used are listed in Table 2. Two hundred seventeen patients (53.7%) were classified as high risk based on one of the seven criteria listed. One hundred thirty (32.2%) patients underwent EVAR and 87 (21.5%) patients underwent open AAA repair (Table 3). Seventy-one of the 87 open AAA patients had only one high-risk classification, 10 had two high-risk classifications, and 6 had three high-risk classifications. In the 130 high-risk EVAR patients, 99 had a single high-risk classification, 27 had two high-risk classifications, and 4 had three high-risk classifications.
Table 2. PATIENT CHARACTERISTICS AND ENDOGRAFT TYPES
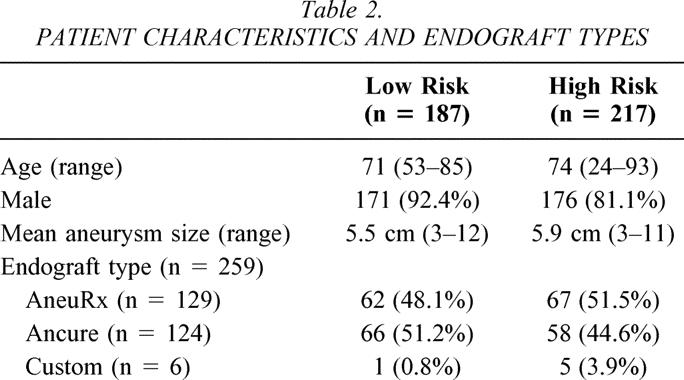
Table 3. HIGH-RISK CATEGORIES COMPARED BY PROCEDURE TYPE
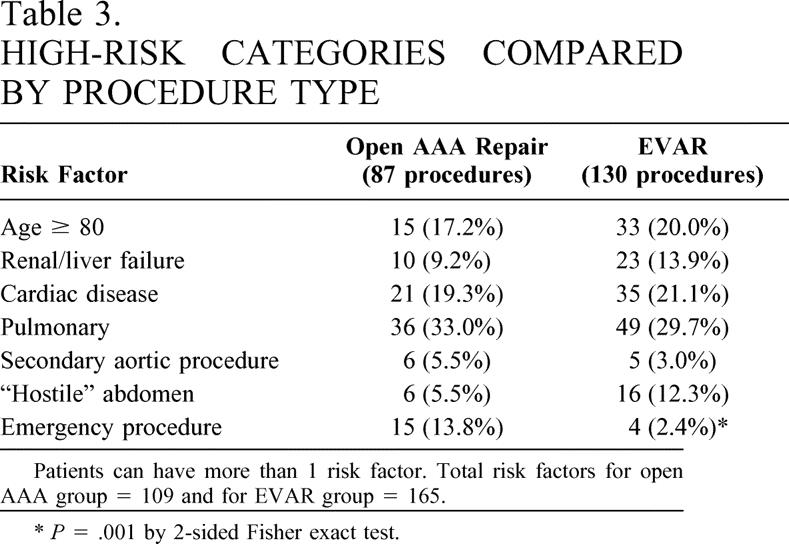
Patients can have more than 1 risk factor. Total risk factors for open AAA group = 109 and for EVAR group = 165.
*P = .001 by 2-sided Fisher exact test.
High-risk classification predicted an increased complication rate (odds ratio [OR] 2.4, 95% confidence interval [CI] 1.4–4.1). Eighteen patients (4.5%), all in the high-risk group, died either during their hospitalization or within 30 days of their procedure. Twelve patients (8.7%) died after open repair and six patients (2.3%) died after endovascular repair (P = .01). Specifically, two patients died during the operation (emergency procedures) and eight patients died more than 30 days after their procedure. One patient died 3 days after discharge. Additionally, 64 patients suffered complications that either extended their hospitalization or necessitated additional medical attention soon after their operation, for a total death and complication rate of 20.4%. Other complications are categorized in Tables 4 and 5 according to the high-risk and low-risk groups. The EVAR patients in both the high- and low-risk classifications had fewer complications than the open repair patients, though the difference was only marginal in the low-risk group. Also, the complications after EVAR were associated with shorter hospitalization compared to similar complications in the open group (Table 6). Ten of the 15 miscellaneous complications in the open group were related to gastrointestinal dysfunction (most commonly an extended ileus) requiring parenteral nutrition and nasogastric compression. In the EVAR group, one patient developed an ileus and two patients had contained gastrointestinal bleeding.
Table 4. COMPLICATIONS IN HIGH-RISK PATIENTS COMPARED BY REPAIR METHOD
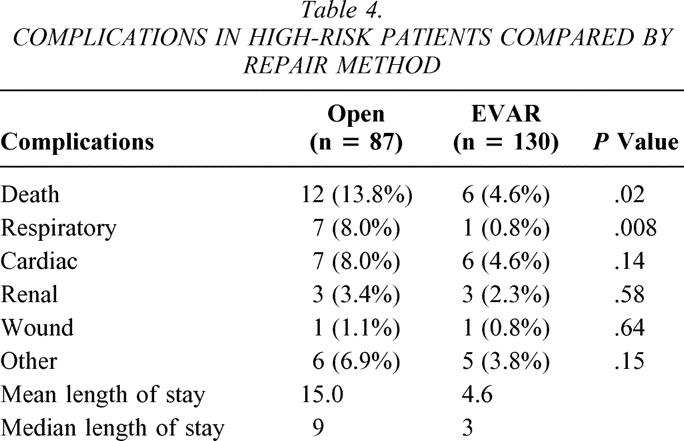
Table 5. COMPLICATIONS IN LOW-RISK PATIENTS COMPARED BY REPAIR METHOD
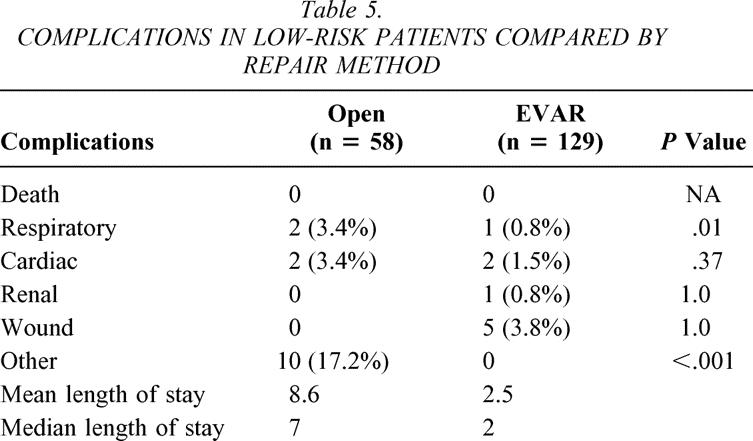
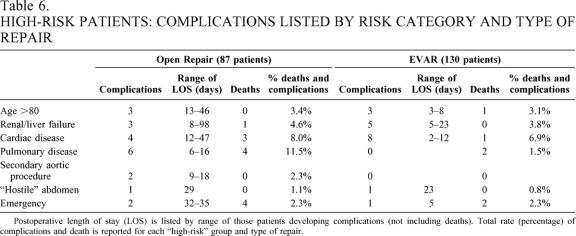
Table 6. HIGH-RISK PATIENTS: COMPLICATIONS LISTED BY RISK CATEGORY AND TYPE OF REPAIR
Postoperative length of stay (LOS) is listed by range of those patients developing complications (not including deaths). Total rate (percentage) of complications and death is reported for each “high-risk” group and type of repair.
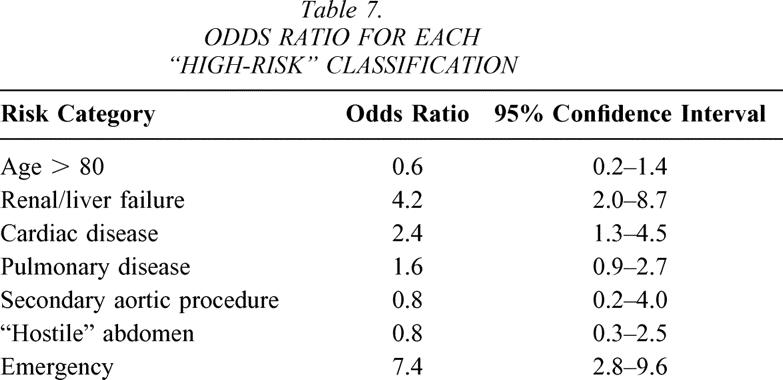
Open AAA predicted a higher complication rate and increased length of stay (LOS; OR 4.0, 95% CI 2.4–4.6). In the 145 open patients, the LOS ranged from 3 to 98 days (mean 12.5 days, median 8 days). In the 259 EVAR patients, the LOS ranged from 1 to 63 days (mean 3.6 days, median 2 days). Tables 4 and 5 show mean and median LOS after each procedure based on the risk classification. Table 7 defines OR for all risk categories.
Table 7. ODDS RATIO FOR EACH “HIGH-RISK” CLASSIFICATION
The impact of complications was particularly important for the high-risk patients. Specifically, complications after open repair increased the median LOS from 7 to 14.5 days, while the impact of complications in the EVAR group increased the median LOS from 2 to 5 days. One EVAR patient required 23 days because of complex wound necrosis in the groin, where an iliofemoral bypass was required. However, most of the wound complications did not require additional hospitalization but were managed with additional outpatient visits. By contrast, complications after open AAA significantly extended the LOS. Specifically, two patients required 98 hospital days after their open repair: one after removing a failed endograft required tracheostomy and prolonged ventilator wean; the other patient developed renal failure and respiratory problems that extended his postoperative stay. In summary, complications after open repair were more deleterious to the patient and the LOS was substantially longer than that for the EVAR group (see Table 6).
The risk classification predicted increased LOS and complications (see Table 7). Emergency operation, renal or liver failure, severe cardiac disease, and severe pulmonary disease predicted a higher rate of complications and deaths. There were similar distributions of complications in the most risk categories between the open and EVAR patients. First, 6 of 15 emergency operations resulted in either intraoperative death or death during the same hospitalization. Sixteen of 31 patients in the cardiac high-risk group developed complications, including four deaths. Three of these deaths occurred in the open AAA group and one death occurred in the EVAR group. Nine of 15 patients with high-risk renal or liver disease developed complications. One death occurred after open AAA repair, and no deaths occurred after EVAR repair. Forty-six patients were older than 80, and seven of these patients developed complications, including one patient who died after endovascular repair. However, the pulmonary-risk patients more commonly suffered complications and death after the open repair (11.5%) compared to EVAR (1.5%). Ten high-risk pulmonary patients developed complications after open AAA repair. Four of those resulted in death. In the 49 high-risk pulmonary patients in the EVAR group, there were two deaths secondary to respiratory complications.
While risk categories predicted complications after either repair method, respiratory complications were most pronounced after open repair. High-risk patients developed respiratory complications after 8.0% of open AAA and 0.8% of EVAR (P = .008). The difference was more subtle in the low-risk group (see Tables 4 and 5). While severe cardiac disease also predicted more complications (OR 2.4, 95% CI 1.3–4.5), the difference was not statistically significant in the open AAA versus EVAR groups (8.0% vs. 4.5% high risk, 3.4% vs. 1.5% low risk).
Five patients required conversion from EVAR to open AAA repair during a follow-up of up to 28 months. One patient required conversion acutely due to inadequate approximation of the graft to the proximal aorta. She recovered and was discharged 7 days after the open operation. Another patient required conversion for acute expansion after treating an endoleak 4 months after the initial implantation. His graft was removed and replaced without incident, and he was discharged on day 7 after the open repair. One patient required conversion 1 year after implantation due to distal graft migration and aneurysm expansion. He required an extended postoperative stay (98 days) because of respiratory failure. The fifth patient required conversion after distal graft migration and aneurysm enlargement that failed treatment with a secondary proximal aortic cuff. He suffered a prolonged postoperative course secondary to exacerbation of preexisting liver failure, but he was eventually discharged after a 62-day hospitalization. There were no late deaths related to aneurysm rupture after EVAR or open AAA repair.
DISCUSSION
Three years after the approval of EVAR, there remains uncertainty about the application of this new technology in a standard vascular practice. Patients with AAA are often influenced by a personal experience or a report in the nonmedical literature and may pursue one particular treatment option. While the patient’s preference is important in the decision-making process of treating AAA, clinicians must also present balanced medical information to fully explain treatment options for a patient. The long-term durability of EVAR remains in question, leading to uncertainty about its application in patients with a good life expectancy. 8,9 We have used EVAR in a wide variety of patients based on both the surgeon’s and the patient’s preference during our practice in a tertiary referral center. Our experience started after the approval of EVAR, and we were not involved in premarket clinical trials with endoluminal grafts. This study was undertaken to analyze our “postmarket” experience in a tertiary medical center after the FDA approval of such devices. Therefore, our experience may offer additional insight into the application of EVAR. While we have gained from the experience of clinical trials by others, this series also represents our early “learning curve” with a new technology.
Our data support the premise that high-risk patients can undergo EVAR repair with a substantially lower LOS and early morbidity compared to open AAA repair. The patients who underwent EVAR had fewer complications and a shorter LOS. Additionally, when complications do occur, they are less morbid than with open AAA repair, and the patients have fewer additional hospital days after these complications compared to open AAA repair. Specifically, the gastrointestinal morbidity is reduced after EVAR compared to open repair. Only one patient after endovascular repair developed diarrhea thought to be related to ischemic changes of the colon. This was substantially different from the number of patients who had an extended ileus, colitis, or GI bleed after open AAA repair. Additionally, the wound complications after EVAR were minor, and only one patient in each group required additional operative intervention for control. There was a single wound dehiscence after open repair, and this patient required 16 days in the hospital. Myocardial infarctions after each group were somewhat similar, although two patients suffered congestive heart failure after EVAR. These patients likely would have been refused open repair because of their poor cardiac function. Additionally, respiratory complications were minimal in the EVAR group, even in the patients considered poor pulmonary risks.
Our results provide a more favorable opinion towards EVAR than some other clinical reports. Chaikof et al. reported little difference in patients with “increased” and “low” risk undergoing EVAR. 10 However, this series used slightly different criteria for “high-risk” patients and did not have an open AAA repair group for comparison. They found similar survival curves over the 5-year course of the study. Our series did not compare long-term outcomes due to the early experience with EVAR at this institution. Some reports have found that advanced age predicts a higher mortality and complication rate. 11,12 By contrast, in our series, patients older than 80 years did not have a different mortality or complication rate (OR 0.6, 95% CI 0.2–1.4).
Our experience is similar to clinical trials in that all patients who were anatomically suitable were offered EVAR. However, the high-risk patients were more often counseled toward EVAR, while the younger and healthier patients were more often counseled toward open repair. During the course of our experience, more of the younger, healthier patients chose EVAR because of this shortened LOS and reduced morbidity. Therefore, patients who underwent open AAA repair were more likely to have complicated aortic anatomy compared to EVAR patients. This more complicated aortic or iliac anatomy may imply that these patients may have other preexisting comorbidities that already skew them towards a high-risk classification, leading to a complication. Thus, the open AAA group may have a preexisting bias towards the patient with more advanced disease.
Additionally, this experience represents a tertiary referral center at an academic institution done after clinical trials proved the safety of EVAR. In the first year of this study, there was limited access to endovascular repair at multiple centers around our referral area. However, as more centers became trained for EVAR, our referrals shifted to the more complex anatomy. Likewise, as our experience grew, we were able to apply this technology to more complex aneurysm anatomy. Hence, while these data do reflect our learning curve for EVAR, the learning curve was built into our clinical experience of treating the patients with simpler anatomy initially and the more complex patients in the latter part of this study. Of note, we had fewer deaths in the earlier course of the study; however, as we applied this more broadly to high-risk patients, our death rate increased in the EVAR patients. Our report also includes treatment for ruptured aneurysms. Recent reports have suggested using EVAR for this high-risk patient to limit the physiologic stress after open repair of a ruptured AAA. 13 We classified ruptured AAA as an “emergency” procedure (high risk) if the procedure was done on the day of admission. Our inclusion of this group may increase our mortality rate, but we found no change in the conclusions due to a high rate of complications in both the emergency open (40%) and emergency EVAR (75%) groups.
This study did not address two other important factors regarding endovascular repair: cost and durability. The cost of the endovascular graft is substantially higher than the standard graft used for open AAA repair. The shortened LOS may offset the additional short-term cost for the device that is required for EVAR, but this does not consider the longer-term costs required for continued surveillance and, possibly, secondary intervention. Additionally, we did not address the long-term durability of this technique. As our experience covers only 3 years, we do not have enough data to establish durability. Yet, we have converted only five patients (1.9%) during the course of this study. Two patients were converted acutely to open AAA repair; three additional patients have been converted (6–24 months after the initial EVAR) and are included in the open AAA group for their second procedure. One patient had an uncomplicated course, while the other two were discharged after extensive hospitalizations for respiratory failure. Three additional patients in the EVAR group required additional EVAR procedures to treat endoleaks. None of them suffered complications after the secondary procedure. The minimal need for conversion and secondary procedures makes the EVAR procedure seem reasonably durable in our clinical practice. However, our study was designed to evaluate the early results of open AAA repair and EVAR in the face of specific risk classification. These data, then, offer a “real world” experience that will enable us to predict complication rates based on risk categories. Further understanding of these complication rates may sway some patients and physicians toward a particular repair type.
We did not undertake detailed analysis of the access problems that sometimes occur with EVAR. We used devices that required passage of catheters ranging from 21 to 24 French. At times, small iliac arteries hindered the progress of such devices, leading to the placement of an iliofemoral bypass graft to provide catheter access to the AAA. Other times, common femoral atherosclerotic plaque can interfere with EVAR access and local endarterectomy or interposition graft is required. However, we chose to report LOS or systemic complications rather than local technical complications due to the more realistic effect on the patient’s progress and the ultimate clinical impact that technical complications may have. For example, one patient required extensive postoperative care (23 days) because of wound failure after an iliofemoral bypass. Five of 16 other patients who required local vascular manipulation had complications, none of which were related to their local vascular access problems (respiratory failure, myocardial infarction, hip fracture after discharge, readmission for abdominal pain, and readmission for gastrointestinal failure). We cannot directly relate the local vascular problems to the systemic complications that occurred. It then appears that if these local vascular problems have an effect on their convalescence, these effects are better demonstrated in the LOS and system complication tables (see Tables 4 and 5).
CONCLUSIONS
Both high-risk and low-risk patients can undergo EVAR with a lower rate of short-term systemic complications and a shorter LOS when compared to open AAA repair. While we do not have complete understanding regarding the long-term durability of these endovascular grafts, our delayed intervention, thus far, has been minimal. For that reason and due to the lower rate of early complications, high-risk patients should be preferentially considered for EVAR over open AAA repair. Considering that low-risk patients also had fewer complications, patients who are anatomically suitable may be offered EVAR with a varying degree of emphasis, depending on their physiologic risk, and with cautious consideration about the unknown long-term durability. Further analysis of the long-term complications will ultimately affect EVAR application for our patients.
Discussion
Dr. Robert B. Smith, III (Atlanta): Dr. Jordan and his colleagues are to be congratulated for focusing our attention on one of the most controversial topics in vascular surgery currently, the preferred treatment for abdominal aortic aneurysms in this era when two effective methods compete. The authors have a large, contemporary experience with excellent clinical results, thoroughly analyzed, and well presented. They are aware of the two obvious weaknesses in this study: the fact that their patients were not randomized to the treatment arms, and that their follow-up is insufficient over time to demonstrate durability of the two competing therapies. Nevertheless, the data appeared to support their contention that high-risk patients tolerate endovascular repair better than open aneurysmectomy, and should be at least considered for the less invasive procedure. Not all will be found anatomically suitable, of course, but at this time the great majority can be treated with the endovascular method if it is felt to be advisable.
Endovascular repair was performed in almost two thirds of their total series; however, it was actually selected somewhat less often in high-risk than in low-risk candidates—60% versus 69%, respectively.
Dr. Jordan knows that I am a more traditional vascular surgeon with a healthy skepticism concerning the endovascular procedures currently available, especially in healthier patients with longer life expectancies. No one knows how these devices will perform 15 and 20 years down the road, nor can we predict the accumulated expense of lifetime periodic follow-up, plus any required reinterventions.
Are we not at the same state of clinical equipoise that exists presently in carotid occlusive disease, with endarterectomy versus balloon angioplasty and stenting? A large NIH-sponsored multicenter trial is currently underway to address the treatment controversy regarding carotid disease. Is it not time for a similar examination of the relative merits of aneurysm procedures in both short- and long-term follow-up? Pending the results of such a trial, I would like for Dr. Jordan to say which method he would advise a close relative to choose if the individual in question was a low-risk, 65-year-old man with an aneurysm suitable for either procedure?
Dr. Thomas H. Schwarcz (Lexington, KY): The results of the study, particularly for the high-risk patients, are rather impressive. However, before we can definitively say that endovascular repair is the best treatment for the high-risk group, I think we need to compare the results in the high-risk group of patients to the previous experience when operation was denied. I would like to ask Dr. Jordan, has there been any retrospective analysis of his patients who were denied operation in the past, in terms of their long-term survival, compared to the current group of his patients?
And finally, as Dr. Smith has alluded, what is the cost-benefit analysis in terms of the frequent reinterventions and also the aortography that is often required in these endovascular grafts compared to the open repairs? And with regard to the aortography, how many complications were caused by the diagnostic evaluation to determine suitability for endovascular graft placement (specifically renal failure and other complications)?
Dr. Samuel R. Money (New Orleans, LA): Dr. Jordan, with pleasure I received your manuscript, the premise of which is that high-risk patients with abdominal aortic aneurysms do better with endovascular aortic aneurysm repair than open repair. I have a few comments for you.
First, I agree with the authors’ premise that endovascular grafting was originally created for the high-risk patient. And that is probably where it should be used. How did you define “high risk”? I know a lot of it was done on history alone. It is clear that a patient who is on home oxygen is at high risk. But what about a patient with a history of coronary artery disease? Did you get actual coronary artery angiography or stress testing before surgery or sestamibi scanning? Or was “high risk” simply defined on history alone?
Second, I believe your length of stay was excessive. You spent some time discussing this during your presentation. However, in the manuscript it sort of struck me that your overall length of stay was about 12 days for all open repairs. That is about 5 days greater than ours and most other tertiary care centers. In addition to that, your endovascular length of stay was approximately 3.5 days. A majority of our endovascular patients go home on day 1. And if we had guts we would probably send them home that night, but we are still a little frightened of the technology. Can you comment on that?
Finally, my last question sort of echoes what Dr. Smith asked. We realize you use this endovascular approach frequently. Approximately two thirds of your patients underwent endovascular repair. Almost 70% of them in the low-risk group underwent endovascular aneurysm repair. Do you think that it is appropriate to treat patients that way?
We have recently coined the term “irrational exuberance and endovascular enthusiasm” to define this approach to endovascular aneurysm repair. I guess translating that to the standard vernacular: Should someone who is a healthy 70-year-old undergo an endovascular repair? What about the healthy 75-year-old? Or let’s flip it the other way: What about the healthy 65-year-old?
Dr. William D. Jordan, Jr. (Birmingham, AL): Thank you, gentlemen, for those comments. I am glad to know I have friends in the audience. I would like to address them in reverse order.
First, Dr. Money, I think your question was related to the cardiac evaluation. Basically, every patient underwent a functional study that might be something as simple as an echocardiogram and a history. Those who had abnormalities were investigated, usually with a stress physiologic test, a nuclear study, and then a coronary angiography. Our goal was basically to treat anyone who had myocardium at risk before undergoing this procedure, even if it was an endovascular repair.
Our length of stay may seem excessive, but we track the endovascular repairs to be discharged in 2 days. Some go home at 1 day. But sometimes these elderly patients need more time. Our mean length of stay is longer because we included all of our complicated patients, and that is what skewed it in that direction.
I am going to save that “irrational exuberance” question for just a moment. I will address Dr. Schwarcz’ questions. What is the long-term survival of those patients who were denied repair in the past? How many died of ruptures, and how many that we would not have treated 5 years ago are we treating now?
I don’t have that data. But I would refer you to a manuscript that was published in JAMA in June of this year, with the lead author as Lederle, that looked at those who had aneurysms greater than 5.5 cm. They reported a 25% mortality in the first 2.5 years. That is a good paper that actually counteracts some of this concept of nonoperative therapy for aneurysms less than 5.5 cm. Dr. Lederle is also an author on that paper that tracks aneurysms less than 5.5 cm.
We also found that while we did aortography very early on in our experience with endovascular grafts, almost all of our patients, we now make that decision based on CT angiography. And we have had limited problems with dye contrast nephropathy after that procedure. In addition, our surveillance program is less intense in terms of CT scans. We have relied more on ultrasound and plain film to try to limit some of the radiation exposure and the cost of the surveillance.
Finally, to answer Dr. Smith’s questions related to the issue of clinical equipoise. Carotid therapy is an entirely different problem, while this is an interest that I also have, and I am sure he knows that quite well. But to answer this issue about a randomized clinical trial for endovascular patients, the initial trial designs of both the Ancure and AneuRx stent grafts was to have randomization, but the patients basically would not accept it. They did not want to accept an open repair when they felt that they were suitable for an endovascular repair. So a randomized clinical trial was not suitable for endografts.
However, I should comment that there is a randomized trial going on in the Veterans Administration Hospital, the OVER trial, that has been initiated, and we are participating in that. I guess the veterans have a different approach than the other population that we deal with.
Finally, to address the “irrational exuberance,” or how would you advise the young patient? I can tell you that the younger patient that needs longer durability, I advise them more towards open repair without an absolute denial for endografts; I try to give them some risks and benefits, and also discuss this issue of the unknown durability of these devices. It is interesting that I have found that more and more patients want and demand the minimally invasive approach, accepting the unknown of the long-term durability of this new technology.
There is one particular patient that comes to mind that had his second episode of embolization from a small aneurysm. He had a 3.5-cm aortic aneurysm, also a 3-cm iliac aneurysm, that embolized a second time. I committed him to fix this aneurysm and advised open repair, with discussion of some of the complications that can occur. And after he heard both options, he reported to me that he was getting married in 12 days and requested endovascular approach. He returned at his 1-month postop visit and said it was the best honeymoon he ever had.
References
- 1.Szilagyi DE, Elliott JP, Berguer R. Coincidental malignancy and abdominal aorticaneurysm. Arch Surg. 1967; 95: 402. [DOI] [PubMed] [Google Scholar]
- 2.Moore WS, Rutherford RB. Transfemoral endovascular repair of abdominal aortic aneurysm: results of the North American EVT phase 1 trial. EVT Investigators. J Vasc Surg. 1996; 23: 543–553. [DOI] [PubMed] [Google Scholar]
- 3.Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: Multicenter prospective clinical trial. J Vasc Surg. 1999; 29: 292–308. [DOI] [PubMed] [Google Scholar]
- 4.Conners MS III, Sternbergh WC III, Carter G, et al. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: A cautionary note. J Vasc Surg. 2002; 36: 476–484. [DOI] [PubMed] [Google Scholar]
- 5.U.S. FDA. Warning letter for April 27, 2001, Public Health Notification: Problems with Endovascular Grafts for Treatment of Abdominal Aortic Aneurysm (AAA): Ancure® Endograft® System (Guidant Endovascular Solutions, Menlo Park, CA) and AneuRX® Stent Graft System (Medtronic AVE, Santa Rosa, CA).
- 6.Torsello GB, Klenk E, Kasprzak B, et al. Rupture of abdominal aortic aneurysm previously treated by endovascular stent graft. J Vasc Surg. 1998; 28: 184–187. [DOI] [PubMed] [Google Scholar]
- 7.Ohki T, Veith FJ, Shaw P, et al. Increasing incidence of mid-term and long-term complications after endovascular graft repair of abdominal aortic aneurysms: A note of caution based on a 9-year experience with 212 cases. Ann Surg. 2002; 234: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conners MS III, Sternbergh WC III, Carter G, et al. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: A cautionary note. J Vasc Surg. 2002; 36: 476–484. [DOI] [PubMed] [Google Scholar]
- 9.Collin J, Murie JA. Endovascular treatment of abdominal aortic aneurysm: a failed experiment. Br J Surg. 2001; 88: 1281–1282. [DOI] [PubMed] [Google Scholar]
- 10.Chaikof EL, Lin PH, Brinkman WT, et al. Endovascular repair of abdominal aortic aneurysms: risk-stratified outcomes. Ann Surg. 2002; 235: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dardik ALJW, Gordon TA, Williams GM, et al. Results of elective abdominal aortic aneurysm repair in the 1990s: A population-based analysis of 2,335 cases. J Vasc Surg. 1999; 30: 985–995. [DOI] [PubMed] [Google Scholar]
- 12.Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001; 33: 913–920. [DOI] [PubMed] [Google Scholar]
- 13.Veith FJ, Ohki T. Endovascular approaches to ruptured infrarenal aorto-iliac aneurysms. J Cardiovasc Surg. 2002; 43: 369–378. [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: William D. Jordan, Jr., MD, Associate Professor and Chief, Section of Vascular Surgery, UAB Department of Surgery, KB 430, 1922-7th Avenue South, Birmingham, AL 35294-0016.
E-mail: wdjordan@uab.edu
Accepted for publication December 2002.