Abstract
Objective
To compare the short-term results of the radiofrequency treatment of the gastroesophageal junction known as the Stretta procedure versus laparoscopic fundoplication (LF) in patients with gastroesophageal reflux disease (GERD).
Summary Background Data
The Stretta procedure has been shown to be safe, well tolerated, and highly effective in the treatment of GERD.
Methods
All patients presenting to Vanderbilt University Medical Center for surgical evaluation of GERD between August 2000 and March 2002 were prospectively evaluated under an IRB-approved protocol. All patients underwent esophageal motility testing and endoscopy that documented GERD preoperatively, either by a positive 24-hour pH study or biopsy-proven esophagitis. Patients were offered the Stretta procedure if they had documented GERD and did not have a hiatal hernia larger than 2 cm, LES pressure less than 8 mmHg, or Barrett’s esophagus. Patients with larger hiatal hernias, LES pressure less than 8 mmHg, or Barrett’s were offered LF. All patients were studied pre- and postoperatively with validated GERD-specific quality-of-life questionnaires (QOLRAD) and short-form health surveys (SF-12). Current medication use and satisfaction with the procedure was also obtained.
Results
Results are reported as mean ± SEM. Seventy-five patients (age 49 ± 14 years, 44% male, 56% female) underwent LF and 65 patients (age 46 ± 12 years, 42%, 58% female) underwent the Stretta procedure. Preoperative esophageal acid exposure time was higher in the LF group. Preoperative LES pressure was higher in the Stretta group. In the LF group, 41% had large hiatal hernias (>2 cm), 8 patients required Collis gastroplasty, 6 had Barrett’s esophagus, and 10 had undergone previous fundoplication. At 6 months, the QOLRAD and SF-12 scores were significantly improved within both groups. There was an equal magnitude of improvement between pre- and postoperative QOLRAD and SF-12 scores between Stretta and LF patients. Fifty-eight percent of Stretta patients were off proton pump inhibitors, and an additional 31% had reduced their dose significantly; 97% of LF patients were off PPIs. Twenty-two Stretta patients returned for 24-hour pH testing at a mean of 7.2 ± 0.5 months, and there was a significant reduction in esophageal acid exposure time. Both groups were highly satisfied with their procedure.
Conclusions
The addition of a less invasive, endoscopic treatment for GERD to the surgical algorithm has allowed the authors to stratify the management of GERD patients to treatment with either Stretta or LF according to size of hiatal hernia, LES pressure, Barrett’s esophagus, and significant pulmonary symptoms. Patients undergoing Stretta are highly satisfied and have improved GERD symptoms and quality of life comparable to LF. The Stretta procedure is an effective alternative to LF in well-selected patients.
Gastroesophageal reflux disease (GERD) is one of the most common disorders of the GI tract, accounting for 18.6 million cases per year in the United States. 1 It is responsible for the highest annual direct costs ($9.3 billion) related to all GI disorders, followed by gallbladder disease ($5.8 billion) and colorectal cancer ($4.8 billion). The largest component of the total direct costs for GERD is the cost of antireflux medications: $5.8 billion. 2 Historically, effective treatment options for GERD have included life-long antireflux medication and antireflux surgery. Although effective at controlling heartburn symptoms and healing esophagitis, 3 antireflux medications are expensive and do not correct the underlying mechanical and functional abnormalities that cause reflux.
Laparoscopic fundoplication (LF) has been shown to be safe and effective for the treatment of GERD, with 90% to 94% overall patient satisfaction at long-term follow-up. 4–6 In addition, there is objective evidence of normalization of acid exposure in 91% to 97% of patients more than 1 year after surgery. 6,7 However, an approach to the treatment of GERD that obviates the need for antireflux medications while presenting potentially less morbidity than surgery would be desirable. Recently, endoscopic approaches to the treatment of GERD have gained considerable interest. 8 The Stretta procedure (Curon Medical, Sunnyvale, CA), which endoscopically delivers radiofrequency energy to the smooth muscle of the gastroesophageal (GE) junction, has been shown to be safe, well-tolerated, and highly effective in the treatment of GERD. 9–13 In a randomized, double-blind, sham-controlled trial, there was a significant treatment-related reduction in GERD symptoms and esophageal acid exposure at 1 year, while the sham-treated group showed no improvement. 14
We present the results of 140 consecutive patients undergoing endoscopic (Stretta procedure) or surgical treatment (LF) of GERD.
METHODS
All patients presenting to Vanderbilt University Medical Center for surgical evaluation of GERD between August 2000 and March 2002 were prospectively evaluated under an IRB-approved protocol using validated GERD-specific and general quality-of-life (QOL) instruments. Manometry was performed in all patients using a station pull-through technique with Sandhill Scientific equipment, software, and a solid-state pressure catheter. Normal LES pressure (highest value recorded) measured in our lab is 15 to 30 mmHg. Patients were offered the Stretta procedure if they had documented GERD and did not have a hiatal hernia larger than 2 cm, LES pressure less than 8 mmHg, or Barrett’s esophagus. Patients with larger hiatal hernias, LES pressure less than 8 mmHg, or Barrett’s were offered LF.
Preoperative Evaluation
All patients had documented GERD, either by a positive 24-hour pH study or biopsy-proven esophagitis. Upper endoscopy and esophageal manometry were performed in all patients before surgical intervention.
GERD symptoms were scored according to the Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire, a previously validated disease-specific QOL instrument. 15,16 The QOLRAD was specifically developed to monitor changes in health-related QOL in patients suffering from heartburn and dyspepsia. The 25-item questionnaire is scored by a 7-point Likert response scale, where 7 is the best score and represents complete absence of symptoms. Our normal population has an average QOLRAD score of 6.1 (unpublished data). Generic health-related QOL was assessed using the 12-Item Short Form Health Survey (SF-12). 17,18 This survey was derived from the previously validated Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). 19,20 The survey can be divided into two components: Physical Component Summary (PCS) and Mental Component Summary (MCS). 21 Gas bloat, dysphagia, and asthma symptoms were evaluated by a questionnaire developed at Vanderbilt for evaluation of postoperative side effects after LF. Questions ask patients to report the amount and severity of symptoms as none, mild, moderate, or severe.
Stretta Procedure
The technique of performing Stretta has been previously described in detail. 12 Briefly, the Stretta procedure is performed on an outpatient basis in the same-day surgery center or endoscopy suite using intravenous conscious sedation. The Stretta catheter comprises a soft, flexible bougie tip and a balloon-basket assembly with four nickel-titanium needle electrodes (22-gauge, 5.5 mm in length) that are positioned radially around the balloon. A four-channel generator delivers temperature-controlled radiofrequency (RF) energy to the smooth muscle of the GE junction via the needle electrodes. Thermocouples at the base and tip of each needle allow for constant monitoring of temperature and impedance in the tissue. If the base of a needle (mucosal temperature) exceeds 50°C, the tip of a needle (muscle temperature) exceeds 100°C, or impedance exceeds 1,000 ohms, the generator shuts off energy to that particular needle. Simultaneous irrigation and suctioning of cooled sterile water maintains mucosal temperature below 50°C to prevent injury to the mucosa.
Diagnostic endoscopy is performed to identify the distance from the bite-block to the squamocolumnar junction (Z-line). A stiff, coated guidewire with a flexible tip is passed through the channel of the endoscope and left in the stomach while removing the scope. The Stretta catheter is inserted transorally over the guidewire and positioned 1 cm above the Z-line, and the guidewire is removed. The balloon is inflated to a pressure of 2.5 psi and the four needle electrodes are deployed into the muscle of the GE junction by advancing a thumb-control on the catheter handle. RF energy is delivered to each electrode for 90 seconds, aiming for a target temperature of 85°C. The catheter is repositioned 45°, and treatment is repeated at this level to create a ring of eight lesions. Endoscopy may be performed at this time to confirm proper positioning, and subsequent treatments can be adjusted accordingly. The catheter is then moved distally in 0.5-cm increments to create three more levels of rings in a similar fashion.
Next, “pull-back” lesions are created by advancing the catheter into the stomach, inflating the balloon with 25 cc3 of air, and pulling back the catheter until resistance is met at the GE junction. The needles are deployed and RF energy is delivered for 90 seconds. This is repeated two more times at the same level by rotating the catheter 45° to the right and 45° to the left. A second pull-back technique is performed by inflating the balloon with only 22 cc3 of air. At the completion of the procedure, there are six sets of rings: four are created in the antegrade fashion and two using the pull-back technique. Immediately after the procedure, all patients undergo repeat endoscopy to assess the appearance of the mucosa and GE junction.
Laparoscopic LF Fundoplication
We use a five-trocar technique with complete mobilization of the gastric fundus, dissection of 3 cm of distal esophagus from the mediastinum, sutured closure of the diaphragm (± Gore-Tex pledgets), and performance of a 2-cm floppy (around a 60 French bougie) 360° fundoplication. A 270° posterior fundoplication (Toupet) was used in patients with impaired esophageal motility (<20 mmHg contraction amplitudes). Collis gastroplasty was performed when thorough mobilization of the distal esophagus from the mediastinum did not achieve a 3-cm length of intraabdominal esophagus without tension.
Postoperative Care and Follow-Up
Stretta patients are instructed to take liquids for 24 hours and then advance to a mechanical soft diet for 1 week. Medications are crushed for 1 month after the procedure. Patients are continued on antireflux medications for at least 3 weeks after the procedure and then are gradually weaned off as they feel improvement in GERD symptoms.
Stretta posttreatment management also includes patient education before discharge, as well as physician guidelines for diagnostic workup and early intervention in the event of a complication. A standardized patient discharge sheet provides the dietary and medication guidelines and summarizes warning signs for the initiation of physician contact. Patients are provided a Stretta Procedure Card with the name and contact information of their physician and are instructed to provide this to any healthcare professional should they seek care in the first 6 months after treatment.
Under an IRB-approved protocol, Stretta patients were asked to return 6 months postoperatively for esophageal manometry and 24-hour pH testing. Patients still requiring antireflux medications at 6 months were asked to discontinue use 1 week before pH testing.
All Stretta and LF patients completed QOLRAD and SF-12 questionnaires preoperatively while on maximal medical therapy and then postoperatively at 3, 6, and 12 months. Current medication use and overall satisfaction with the procedure were also obtained postoperatively in all patients.
Statistical Analysis and Financial Calculations
Data were analyzed with Stata statistical software (Version 7.0, Stata Corporation, College Station, TX). Continuous outcomes within groups (matched) were compared using the Wilcoxon signed-rank test. Outcomes between groups (unmatched) were compared using the Mann-Whitney test. Results are reported as mean ± SEM. Statistical significance is reported for P < .05.
Financial considerations were calculated from Vanderbilt University Medical Center databases (courtesy of Linda Kendall, Jim Dieterich, and Jeanne Potter). Hospital costs, which included disposable supplies, overhead, and administration, were averaged from five laparoscopic fundoplications and seven Stretta procedures.
RESULTS
Preoperative Assessment
According to our predetermined selection criteria, 140 consecutive GERD patients were offered surgical treatment. Sixty-five underwent the Stretta procedure (62 primary, 3 after failed Nissen) (Fig. 1) and 75 underwent LF (65 primary, 10 redo) (Fig. 2) at Vanderbilt University Medical Center between August 2000 and March 2002. In the LF group, 44% were male and 56% female, versus 42% male and 58% female in the Stretta group. The mean age was 49 ± 14 years for LF and 46 ± 12 years for Stretta. The average Body Mass Index (BMI) was 28.7 ± 1.0 for LF versus 30.3 ± 1.1 for Stretta.
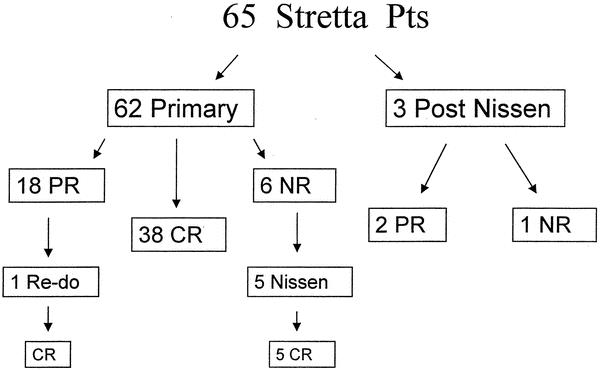
Figure 1. Results of 65 patients undergoing the Stretta procedure. Complete response (CR) is defined as no longer needing to take PPIs, partial response (PR) is defined as a reduction in PPIs to once per day or less, and no response (NR) is defined as remaining on preoperative twice-daily doses of PPIs. CR was achieved in 58%, PR was achieved in 31%, and NR was seen in 11% of patients after Stretta.
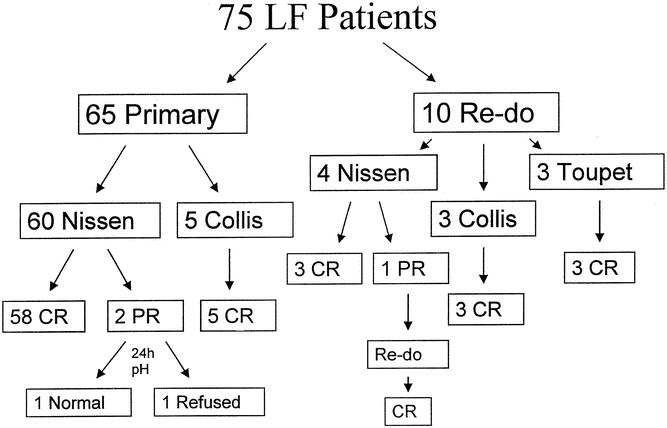
Figure 2. Results of 75 patients undergoing laparoscopic fundoplication (LF). CR was achieved in 97%, PR was achieved in 3%, and NR was not seen in any patient after LF.
Preoperative esophageal acid exposure time was higher in the LF group (11.3 ± 0.6% v. 8.5 ± 0.5%, P < .01). LES pressure was higher in the Stretta group (25.4 ± 2.9 mmHg v. 18.2 ± 1.5 mmHg, P < .01). All Stretta and primary LF patients had esophageal contraction amplitudes greater than 30 mmHg and complete relaxation of the LES.
Fifty-nine percent of the Stretta patients had no hiatal hernias, and 41% had small hiatal hernias (1–2 cm). Forty-two percent of the LF patients had large hiatal hernias (>2 cm), 15% had small hiatal hernias, and 43% had no hiatal hernias. Six of 75 (8%) patients in the LF group had Barrett’s esophagus.
Operative Information
All Stretta procedures were performed on an outpatient basis. The majority of the procedures were performed under intravenous conscious sedation; however, eight patients underwent general anesthesia secondary to patient choice, better pain control intraoperatively, or inability to cooperate with conscious sedation. The average operative time was 46.5 ± 0.9 minutes.
The LF procedures were tailored according to preoperative motility findings and intraoperative anatomic assessment. In the primary LF group, five patients required laparoscopic Collis gastroplasty secondary to inadequate length of intraabdominal esophagus. In the redo group, four Nissen, three Collis-Nissen, and three Toupet fundoplications were performed. The average length of stay for LF was 1.52 ± 0.1 days (range 0–6 days). The average operative time was 163 ± 5.3 minutes.
In the primary LF group, there was one (1.5%) conversion to laparotomy for an enterotomy made in a patient with a prior midline incision. In the redo group, 3 of 10 (30%) cases required conversion to laparotomy. All were secondary to dense adhesions around the GE junction.
Complications
In the Stretta group, only one (1.5%) minor complication occurred secondary to the Stretta procedure. A 22-year-old man developed transient gastroparesis based on endoscopic findings of retained food within the stomach and esophagitis, likely due to cessation of acid-suppressing medications 12 days after the procedure. He required nasogastric decompression, intravenous fluids, acid-suppressive medications, and hospitalization for several days. Repeat endoscopy 4 weeks later confirmed the gastroparesis and esophagitis had completely resolved. He was off all antireflux medications and without symptoms 3 months later. Another complication that appeared unrelated to the Stretta procedure occurred in a 36-year-old patient with bipolar disorder taking antipsychotic medications. He presented 26 days after the procedure with acute pancreatitis and required hospitalization for 1 month. The pancreatitis has recurred several times after his hospitalization. At 6 months follow-up, he remained on PPIs for GERD.
In the LF group, there were seven (11%) major complications. There were two enterotomies, one pneumothorax, one slipped-Nissen, one paraesophageal hernia, and two incisional hernias. One enterotomy was identified and repaired intraoperatively. The other enterotomy was identified on POD 1 and repaired laparoscopically; the patient was discharged on POD 4. The pneumothorax occurred secondary to extensive mediastinal dissection in a patient who required a Collis-Nissen for a large hiatal hernia. The slipped-Nissen occurred on POD 2 after an episode of retching; the patient was returned to the operating room and repaired laparoscopically. The paraesophageal hernia occurred in one of the redo patients 18 months after surgery; the patient underwent an open paraesophageal hernia repair and was doing well 3 months later.
Outcome Assessment
At 6 months, GERD-specific QOL scores (QOLRAD) and general health scores (SF-12) were significantly improved in both groups (P < .01) (Figs. 3 and 4). Follow-up questionnaires were filled out in clinic or by mail by the patients. Stretta patients had a mean follow-up of 7.3 ± 0.6 months (range 3–15 months), and LF patients had a mean follow-up of 5.2 ± 0.5 months (range 2–10.5 months). There was an equal magnitude of improvement between pre- and postoperative SF-12 scores in the Stretta and LF patients (P > .05). The Stretta and LF patients had similar baseline GERD symptoms (QOLRAD) and the same magnitude of improvement in their GERD symptoms postoperatively (P > .05).
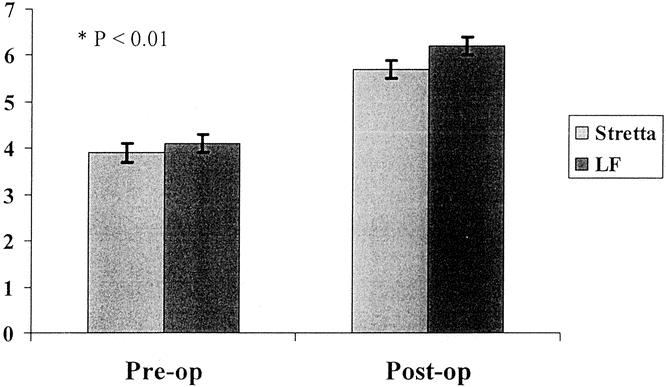
Figure 3. QOLRAD scores before and after Stretta and LF. Highest possible score is 7, representing complete absence of symptoms. Our normal population has an average QOLRAD score of 6.1. Preoperative QOLRAD scores were assessed while the patient was on maximal medical therapy for GERD. QOLRAD scores significantly improved to a similar degree after Stretta (3.9 ± 0.2 to 5.7 ± 0.2, P < .01) and LF (4.1 ± 0.2 to 6.2 ± 0.1, P < .01).
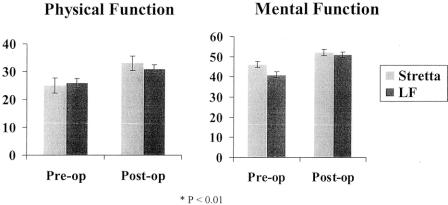
Figure 4. 12-Item Short Form Health Survey (SF-12) QOL scores before and after Stretta and LF. Preoperative SF-12 scores were assessed while the patient was on maximal medical therapy for GERD. Both physical (Stretta: 25 ± 2.4–33 ± 2.8, P < .01; LF: 26 ± 1.4–31 ± 1.5, P < .01) and mental (Stretta: 46 ± 1.2–52 ± 1.7, P < .01; LF: 41 ± 1.5– 51 ± 1.3, P < .01) functioning scores were significantly improved after both procedures.
Fifty-eight percent of Stretta patients were off PPIs, and 31% had reduced their dose significantly; 97% of LF patients were off PPIs. Twenty-two Stretta patients returned for esophageal manometry and 24-hour pH testing at a mean of 7.2 ± 0.5 months. There was a significant decrease in esophageal acid exposure time (8.2 ± 0.9% to 4.4 ± 0.5%, P < .01) (Fig. 5) and Johnson DeMeester score (39.4 ± 4.5 to 26.6 ± 5.2, P < .01). There was no significant change in mean LES pressure (22.8 ± 2.4 to 23.5 ± 2.5, P > .05). Eight of the 22 (36%) patients who underwent repeat pH testing had normalization of their acid exposure time. Ten of 22 (45.5%) patients were complete responders (no longer taking PPIs), and 8 of these 10 (80%) had normalization of their acid exposure time (pH < 4.0). None of the partial responders (PPI use decreased to QD or less) or nonresponders (PPI use unchanged) had normalization of their acid exposure time.
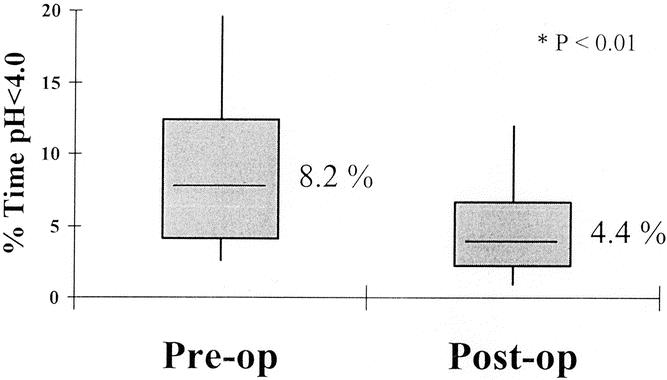
Figure 5. Results of 24-hour pH studies before and after Stretta. Twenty-two patients were studied at a mean of 7.2 ± 0.5 months after surgery. There was a significant decrease in percent esophageal acid exposure time after Stretta. Thirty-six percent of patients had normal acid exposure times in the distal esophagus postoperatively.
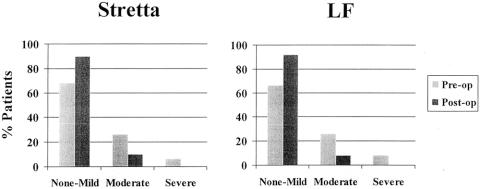
Both groups showed a similar improvement in dysphagia scores (Fig. 6). There was no requirement for dilation of residual dysphagia in either group. Neither group had any cases of severe dysphagia or new-onset dysphagia postoperatively. Gas bloat appeared to be much improved after Stretta, while there appeared to be a slight increase after LF (Fig. 7). No severe cases of gas bloat were identified after either procedure.
Figure 6. Dysphagia as assessed by patients before and after Stretta and LF. Dysphagia improved after both procedures to a similar degree. No cases of worsening dysphagia or new-onset dysphagia were identified after Stretta or LF.
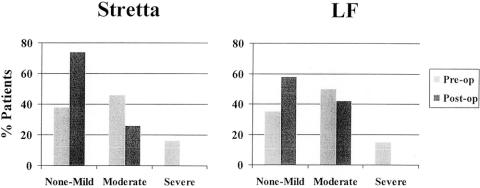
Figure 7. Gas bloat symptoms before and after Stretta and LF. Gas bloat symptoms were more frequent and severe after LF than after Stretta. No patient had increased gas bloat symptoms after Stretta.
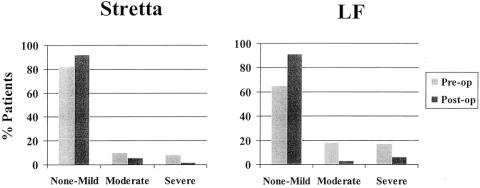
There appeared to be a trend in the LF patients having greater asthma symptoms preoperatively and showing a slightly better improvement postoperatively, as compared to Stretta. However, both procedures improved both the severity and frequency of symptoms (Fig. 8).
Figure 8. Asthma symptoms before and after Stretta and LF. The LF group had more frequent and severe symptoms that appeared to improve slightly better than in the Stretta group. However, both procedures reduced the frequency and severity of asthma symptoms.
Both groups were highly satisfied with their procedure, 89% Stretta versus 96% LF.
Financial Comparison
The average hospital cost of the Stretta procedure was $1,808, approximately one-third the cost of LF ($5,715) (Fig. 9).
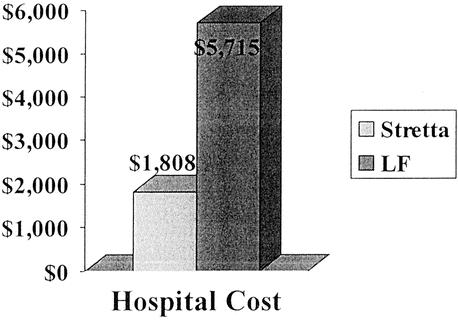
Figure 9. Mean hospital costs for performing Stretta and LF.
DISCUSSION
GERD is a common disorder affecting many Americans on a daily basis. 1 Chronic reflux may cause serious complications, such as ulceration, stricture, and Barrett’s esophagus. 22 The main goals of GERD therapy are to relieve symptoms and heal esophagitis, thereby preventing complications. Effective medical and surgical treatment options for GERD are available, but controversies exist. Medical therapy with PPIs relieves GERD symptoms and heals esophagitis in the majority of patients, but long-term therapy is costly and difficult to maintain. 2,3 In addition, discontinuation of medication results in recurrence of symptoms in up to 80% of patients. 23
LF has become the most common operation performed for GERD in the United States. In short-term follow-up, we have shown it to be very effective in improving GERD symptoms and GERD-specific QOL, while having high patient satisfaction (96%). Others have shown a significant improvement in esophageal acid exposure, with 91% to 97% of patients having normalization of acid exposure times more than 1 year after surgery. 6,7 We, as well as other institutions, have also shown excellent long-term results with LF. Eighty percent of patients are free of significant reflux symptoms, and greater than 90% of patients are satisfied with their surgery at 5 or more years follow-up. 5,24,25
However, an approach to the treatment of GERD that has less morbidity than surgery and obviates the need for antireflux medications is desirable. Our series, as well as reports from the literature, confirms that Stretta is a safe procedure. 9,10,12–14,26–28 Our only complication related to the Stretta procedure was a case of transient gastroparesis and esophagitis. The mechanism of the gastroparesis is not well understood. There was no fever or leukocytosis present, so we believe it was unlikely to represent a small perforation with adynamic ileus. A thermal injury to the vagal trunks might explain the gastroparesis; however, it was transient and resolved completely. This patient has no symptoms of gastroparesis and does not take any GERD medication at this time. The esophagitis was probably related to the emesis and reduction in oral intake of acid-suppressive medications. The other complication reported is thought to be completely unrelated to Stretta. Since the attack of acute pancreatitis 26 days after the procedure, the patient has had at least two other episodes of pancreatitis. We believe the pancreatitis occurred coincidentally after the Stretta procedure and may have been caused by a reaction to one of his antipsychotic medications. In comparison, LF was associated with several significant complications, three of which required major operative intervention to repair.
Side effects of the Stretta procedure are very mild and usually limited to the very early postprocedure period. Dysphasia and gas bloat, which were common complaints preoperatively, were lessened after the Stretta procedure. There were no cases of increased dysphagia or gas bloat symptoms after Stretta. No patients undergoing Stretta have required endoscopic dilation for dysphagia, and no patients have developed postoperative motility disorders. LF patients typically had more frequent and intense gas bloat symptoms in the first 6 months as compared to Stretta. Our long-term data suggests that dysphagia resolves, but gas bloat symptoms continue to bother a small number of patients after LF. 25
We have shown that the Stretta procedure is moderately effective in the treatment of GERD. We had significant improvement in GERD symptoms and GERD-specific QOL that paralleled that seen after LF. We also had a significant decrease in esophageal acid exposure time (8.2 ± 0.9% to 4.4 ± 0.5%, P < .01) and eliminated PPI use in the majority of patients (58%). An additional 31% of the Stretta patients have reduced their PPI use significantly. Eighty-nine percent of our Stretta patients were highly satisfied with their procedure and would do it again, compared to 96% of the LF patients.
There are many reports in the literature that confirm the effectiveness of the Stretta procedure. 9,10,12–14,26–28 The U.S. open label trial of 118 GERD patients demonstrated significant improvements after 12 months in heartburn scores, GERD-specific QOL scores, PPI use (fell from 88% to 30%), and acid exposure time (10.2% to 6.4%). 10 The only sham-controlled trial in the emerging field of endoscopic therapy for GERD has recently been reported. 14 Sixty-four GERD patients were randomly assigned to the Stretta procedure (n = 35) or to a sham procedure (n = 29). At 6 months, the active treatment group had significantly and substantially improved GERD symptoms and QOL, as compared with sham treatment. These improvements were sustained at 12 months. At 6 months, 20 sham patients crossed over to active treatment. The sham group had no improvement in symptoms between baseline and 6 months; however, after being crossed over to active treatment at 6 months, these patients also significantly improved their GERD symptoms and QOL. Esophageal acid exposure time was significantly improved in the active group at 12 months and in the sham crossover group at 6 months (same magnitude of improvement).
Wolfsen and Richards recently reported a registry of 558 patients treated with the Stretta procedure at multiple centers. 11 There were 33 institutions, and mean follow-up was 8 months. The percent of patients with satisfactory GERD control (absent or mild) improved from 26% at baseline while on PPIs to 77% after Stretta. Baseline patient satisfaction on PPIs was 23%, compared to 87% at follow-up. Median drug requirement improved from twice-daily PPIs to prn antacids. Ninety percent of the patients would recommend the Stretta procedure to a friend.
The mechanism responsible for the therapeutic effect of RF energy delivery to the GE junction for GERD is partially understood. One possible mechanism of action is mechanical alteration of the GE junction. Thickening of the GE junction musculature in response to RF energy has been demonstrated in canine esophagectomy specimens. 26 This effect is attributed to heat-induced tissue contraction, followed by fibroblast and collagen deposition. As a result, there is a reduction in tissue compliance and an increase in tensile strength of the GE junction. This may be responsible for the increase in basal and postprandial LES pressure demonstrated in both animal and human studies. 28,29 In addition, an increase in gastric yield pressure (resistance to reflux during gastric distention) has been demonstrated following the delivery of RF energy in a porcine model. 29
The second possible mechanism of action is a reduction in the frequency of transient LES relaxations (TLESRs), the primary cause of GERD in most patients. 30 Three recent studies, animal and human, have demonstrated a significant reduction in TLESRs following treatment with RF energy. 13,26,28 The major neural elements controlling TLESRs in humans are thought to consist of mechanoreceptors residing in the cardia of the stomach. A reflex arc involving vagal afferent fibers transmits information from the activated mechanoreceptors to the brain stem. The motor pattern generator, located in the brain stem, then relays inhibitory signals via vagal efferent fibers back to the LES, esophageal body, and diaphragmatic hiatus. 30,31 Ablation of these vagal afferent pathways by RF energy may account for the decrease in TLESRs seen after the Stretta procedure.
Another potential explanation of the reduced frequency of TLESRs resides in the progressive tissue remodeling observed after Stretta. Remodeling may result in a decrease in compliance in the region of the GE junction responsible for triggering TLESRs, thereby resulting in fewer TLESRs when the stomach is distended by air or ingested material. 13
Is the effect of the Stretta long term? Our range of follow-up is 3 months to 15 months, so we are limited in our ability to quantify length of time beyond 15 months that patients are improved. Our limited data suggest that there continues to be improvement in symptoms beyond the first year after treatment. In the Stretta registry, subgroup analysis of patients less than 1 year versus greater than 1 year follow-up showed a superior effect on symptom control and drug use in those patients beyond 1 year follow-up, thus supporting the durability of the procedure. 11 Follow-up of the open-label trial and the sham study suggests that treatment effect improves from 6 months to 12 months after Stretta. Longitudinal follow-up of patients enrolled in these studies will be needed to assess the long-term outlook for the Stretta procedure.
Long-term durability of antireflux surgery has been questioned by some authors as well. Spechler et al. reported that 62% of surgical patients were taking antireflux medications on a regular basis at 10 years follow-up. 32 However, these data are difficult to interpret, since not all patients back on medications were studied for objective evidence of recurrent reflux. In addition, several investigators have demonstrated that most patients who use acid-suppression medications after antireflux surgery do not have abnormal esophageal acid exposure, and the use of these medications is thus often inappropriate. 33,34 Furthermore, the only randomized trial comparing antireflux surgery with modern medical therapy (omeprazole) showed operative treatment to be more effective in controlling GERD at 5 years follow-up. 35
There is some concern that the Stretta procedure works by desensitizing the esophagus; however, analysis of our postoperative pH data showed that all of the patients studied who were failures of the Stretta procedure (continued on maximal PPI therapy) had pathologic acid exposure in their distal esophagus. Eighty percent of the patients who had a complete response to Stretta (no longer taking PPIs) normalized their pH scores. No patients have returned after Stretta with esophagitis while improving their GERD symptom scores. While we cannot completely rule out some contribution of esophageal desensitization, our data strongly refute this theory of symptom improvement.
Technical considerations have affected the results of both Stretta and LF. Outside this study, 2 years ago four cases of esophageal perforation occurred shortly after introduction of the Stretta procedure into clinical use. Since that time, rigorous physician training, guidelines for postoperative care, and physician education have reduced complications across the country to less than 0.15%, and no Stretta-related major complication has occurred in the last 12 months (1,600 cases). 36 The Stretta registry indicated there was no difference in outcomes between the physicians who had performed 10 or more cases and physicians who had performed fewer than 10 cases. This short learning curve indicates that the Stretta procedure can be promulgated to a larger group of physicians who can achieve results similar to those obtained by institutions treating a larger volume of patients.
One indication of the complexity of laparoscopic fundoplication is the current rate of redo operations that experienced surgeons are performing. Reports have shown anatomic failure rates ranging from 3.6% to 7%. 37,38 There are also concerns regarding the differences in patterns of failure between patients at academic centers and those referred from outside institutions. 37 The majority of failures from academic centers resulted from transdiaphragmatic herniation of the wrap. Diaphragmatic stressors (retching, straining, heavy lifting), large hiatal hernias (>3 cm), and advanced GERD (esophageal stricture, Barrett’s esophagus) appeared to play a significant role in these anatomic failures. 37,38 Fundoplication failures referred to academic institutions appeared more related to operative technique, where a twisted or slipped fundoplication accounted for the majority of failures. 37 In our study, 10 of the 75 (13%) LF cases were redo procedures. Six (60%) of the redo cases were the result of a slipped (n = 2) or twisted (n = 4) wrap, whereas four cases (40%) were the result of wrap disruption with or without hiatal herniation. Unfortunately, these redo procedures have less satisfactory results from the second operation than those achieved from the primary one. 37–40
Financial considerations may play an important role in the decision for the type of treatment. Analysis of the hospital costs associated with performing Stretta and LF shows that Stretta costs are about one-third the cost of LF. This reflects the increased amount of time required to perform LF, complexity of the technology involved, and the longer hospitalization. Also, a large percentage of LF patients underwent additional, more complex procedures. Eight of 75 (11%) LF patients underwent Collis gastroplasty secondary to shortened esophagus, which contributed to longer operative times and hospital stays. Twelve LF procedures were performed on an outpatient basis, but the majority still require at least an overnight stay. The Stretta procedure remains a true outpatient procedure that is well tolerated, and because of a standard technique each case can be performed in a remarkably similar amount of time.
While several insurance companies in Tennessee are reviewing new data for Stretta to determine their coverage policy, several continue to deny payment for Stretta because they have ruled it as experimental therapy. In these cases of denied coverage, several patients elected to pay for the procedure out of pocket. This stresses the value to the patient of a less invasive surgical treatment of reflux that does not require any incisions, and the patient can return to work immediately.
Future studies need to elucidate the role of Stretta in the treatment of specific subsets of patients. Although we have not treated patients after gastric resection or gastric bypass surgery, the endoscopic treatment of these patients may prove to be very beneficial. Stretta was partially effective in two of three patients who had recurrent reflux after previous Nissen fundoplication. As stated earlier, reoperation in this group of patients is known to result in poorer outcomes than the primary surgery. 37–40 Therefore, to study this patient population objectively, we have joined other institutions as part of a multicenter trial investigating Stretta treatment in patients who have recurrent reflux after Nissen fundoplication.
The morbidly obese population may be another subset of refractory GERD patients who could benefit from Stretta. A number of obese patients simply do not want to undergo gastric bypass surgery but desire surgical treatment of their GERD. Rattner et al. found that morbidly obese patients have a higher rate of failure after fundoplication than do patients of normal weight. 41 They speculated that abdominal pressure contributes to the breakdown of the repair and leads to recurrent reflux. Performance of the Stretta in a morbidly obese patient is no more time-consuming or technically demanding than in a patient with a normal BMI.
Can the Stretta be performed in patients with Barrett’s esophagus or larger hiatal hernias? There are several important considerations that we believe limit the usefulness of Stretta in these patients. Patients with long segment Barrett’s have altered mucosal landmarks at the GE junction, making it difficult to identify the proper site of treatment. In addition, many of the Barrett’s patients have severe disease in which normalization of acid and non-acid refluxate is paramount. 42,43 The Stretta procedure does not normalize esophageal acid exposure in all patients; therefore, we believe it should be considered inadequate therapy for this population.
Patients with small hiatal hernias will have some diaphragm around the area where the needle electrodes are positioned for treatment. This tissue barrier provides a safety zone, preventing RF thermal injury to vital structures in the mediastinum, so patients who have hiatal hernias larger than 2 cm are not offered the Stretta procedure. Patients with large hiatal hernias and inadequate LES barriers (LESP < 8 mmHg) are better suited for LF, where the anatomic defect and the mechanical barrier to reflux can be restored. As better techniques of treatment using RF energy are developed, indications and patient selection may change.
It is unclear whether the Stretta procedure is as effective as LF in improving the symptoms of extra-esophageal reflux (e.g., cough, hoarseness, asthma). In this study, there appeared to be a trend in the LF patients having greater asthma symptoms preoperatively and showing a slightly better improvement postoperatively, as compared to Stretta. However, both procedures seemed to improve the severity and frequency of symptoms. Other trials have shown that the best predictor of success in relieving extra-esophageal symptoms is proper patient selection (positive dual-probe 24-hour pH study) and a good response to preoperative PPI therapy. 44 We have ongoing studies comparing medical and surgical (LF and Stretta) treatment of extra-esophageal manifestations of GERD.
What about the patients who have an incomplete or no response to Stretta? We wait at least 6 to 12 months before offering any further therapy, because patients may continue to see improvement in reflux symptoms up to 1 year. 10,11 Patients who had a partial response to Stretta but continue with significant GERD symptoms are offered either repeat Stretta or LF. One patient in this study underwent repeat Stretta and has significant relief of symptoms 2 months posttreatment. Patients who have no response to Stretta are only offered LF, because we believe only patients with a partial response would benefit from a second Stretta treatment. Five patients underwent LF after Stretta, and all had a complete response. No anatomic abnormalities suggestive of scar or injury secondary to Stretta could be detected at surgery.
In summary, we present a new paradigm for the surgical management of patients with refractory GERD (Fig. 10). While not all patients may have a complete response to Stretta, we truly have an effective, less invasive therapy for GERD that does not burn any bridges. Stretta is a reasonable procedure to use in well-selected patients. It should not be used in patients with Barrett’s esophagus, LES pressure less than 8 mm Hg, or large hiatal hernia (>2 cm). It may have specific utility in morbidly obese patients, patients with previous gastric resection or gastric bypass, or after failed fundoplication. The adoption of an endoscopic treatment for GERD has allowed us to stratify (Stretta v. LF) the management of our reflux patients according to size of hiatal hernia, LES pressure, and the presence or absence of Barrett’s esophagus or significant pulmonary symptoms.
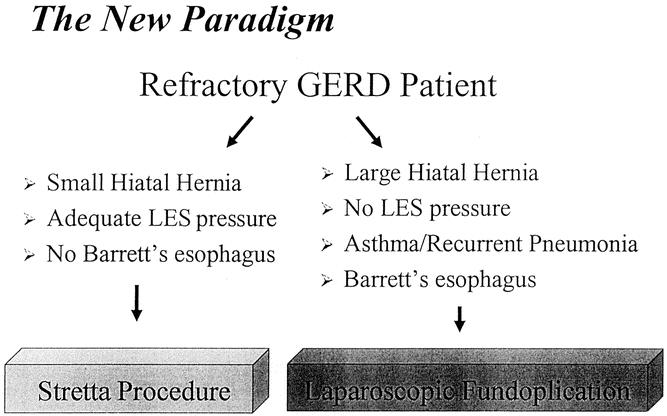
Figure 10. Our proposed paradigm for the surgical treatment of GERD. Patients are stratified to treatment with Stretta or LF according to size of hiatal hernia, LES pressure, and presence or absence of significant pulmonary symptoms or Barrett’s esophagus.
Discussion
Dr. Bruce D. Schirmer (Charlottesville, VA): I wish to congratulate Dr. Richards and his colleagues from Vanderbilt for their excellent work in helping promote a new, even more minimally invasive alternative for the treatment of selected patients with gastroesophageal reflux disease. They have been kind enough to furnish me with a copy of their manuscript, and I can assure you that it is of the same high quality as the presentation which you have just heard. The group has had the courage to try a procedure that could potentially decrease the number of laparoscopic antireflux operations done by us—clearly on the surface, a not popular notion with minimally invasive surgeons. They have also served as a site for the instruction of other physicians interested in performing the Stretta procedure and have thereby helped ensure its safety. Clearly their data justify the use of this procedure for certain selected patients with GERD. I have two questions and several comments for Dr. Richards.
First, the questions: Dr. Richards, in treating patients post-Stretta, your recommendation is to continue proton pump inhibitors, tapering them off only after at least 3 weeks postop. Why is this—especially in view of one of the plausible theories of Stretta’s efficacy, that of destroying the neural reflex arc responsible for transient lower esophageal sphincter relaxations? If this is the mechanism, then the resolution should be immediate post-procedure, and postoperative medical therapy should not be needed. The second question is a continuation of the first. That is, if the mechanism of Stretta’s efficacy is, on the other hand, from thickened scarring of the LES, as suggested by your medical regimen, then how do you explain there has been no difficulty from excess scarring when post-Stretta procedural patients have undergone laparoscopic fundoplication?
My comments are three: First, Stretta does offer potential therapy for GERD for patients who are currently poor candidates for antireflux surgery. These include reoperative situations and those rare patients with GERD after Roux-en-Y gastric bypass, for whom there is no good currently available treatment. Second, despite the potential for using Stretta to treat patients who are otherwise poor operative candidates, one must take careful note of the fact that the Vanderbilt group clearly documented GERD by standard objective testing criteria before any patient underwent Stretta. We must continue to observe the tenet of adhering to careful preoperative documentation of disease no matter how minimally invasive the therapy. This must not be disregard by potential therapists, whether surgical or nonsurgical, who perceive the minimally invasive and familiar endoscopic nature of the procedure to justify its use for the symptomatic problematic patient with undocumented disease.
Finally, Stretta reemphasizes the need for surgeons to know and perform flexible endoscopy. If we do not, we will continue to see the erosion of surgeon input into the treatment of traditional surgical diseases which now have a flexible minimally invasive therapeutic option. This has been the case with choledocholithiasis and in centers outside of Galveston recently with pancreatic pseudocysts. If pediatric surgery and treatment of the burn-injured patient are to now be essential components of the training of the general surgeon, it is even more important that the same status be given to flexible endoscopy and that every surgical resident should become proficient in flexible endoscopy during residency training. I hope my colleagues on the American Board of Surgery will soon share this view.
Dr. Nathaniel J. Soper (St. Louis, MO): I would like to congratulate Dr. Richards and his colleagues from Vanderbilt. And thanks for allowing me to look at the manuscript, which is excellent. I think we need to put this paper in context. There is truly an epidemic of gastroesophageal reflux disease (GERD) associated with the fastest-growing cancer, adenocarcinoma of the distal esophagus and proximal stomach, and there is much interest in treatment alternatives for GERD. There has also been an evolution of treatment over the last 10 years from open fundoplication to laparoscopic fundoplication, and now we are seeing endoluminal techniques, which are really only in their infancy, coming to the fore. It is critical for surgeons to anticipate this trend, and I applaud Dr. Richards and his colleagues for reporting their experience. Ideally, we would like to find a very cheap, noninvasive, nonmorbid, and repeatable procedure for GERD therapy. This report was a comparison, in a nonrandomized way, of course, of laparoscopic and endoluminal RF treatment of patients with documented GERD and relatively short-term outcomes. They were not randomized, but stratified, with the Stretta device being used for the less severe patients with reflux, and it should be noted that many of the patients undergoing fundoplication were either redo’s or had a short esophagus requiring lengthening procedures. Yet despite this, in those patients undergoing the Stretta procedure, about 40% of them in the short term were still on PPIs post-treatment, compared to only 3% of those undergoing laparoscopic fundoplication. I have several questions and comments, therefore, for the authors.
The exclusion criteria that were enunciated included an LES pressure less than 8 millimeters of mercury and asthma. Now, are there any data supporting these exclusion criteria? And is there any information regarding the use of Stretta in patients based on motility characteristics of the esophagus itself? These GERD patients, as stated in the manuscript, were “offered” these therapies. Was there any crossover? That is, were any patients who were eligible for the Stretta treated by laparoscopic fundoplication? If so, have these patients been compared to those who had the Stretta procedure? That is, comparing apples to apples? We have previously shown in our center that patients who were candidates for a Stretta procedure undergoing laparoscopic fundoplication had excellent outcomes, similar to those GERD patients who were not candidates for the Stretta procedure (Desa KM, et al. Symptomatic outcomes of laparoscopic antireflux surgery in patients eligible for endoluminal therapies. Surg Endosc., in press).
The next question is the issue of costs. The hospital costs are shown to be about three times greater for the laparoscopic fundoplication. Have the authors looked at the additional costs for those requiring retreatment and for the proton pump inhibitors that were used in 40% of these patients?
About one third of the patients undergoing Stretta had postoperative pH tests. Now, it is unclear to me from the manuscript if there was any difference in the outcomes of patients who had the pH tests versus those that did not. I wish you could comment on that. And was there any difference in the pH results in those patients whose responses were complete vs. partial vs. absent?
Both groups of patients had high mean BMIs. Did you look at the Stretta patients specifically to see whether those who had the highest BMIs did less well or equally well? We have recently shown and presented data at the AAS showing no difference in obese versus nonobese patients undergoing a laparoscopic fundoplication.
Why does the Stretta procedure fail in a subset of these patients? The mechanism of action is not clear to me. According to the manuscript, the authors will retreat patients who are partial responders to the Stretta, but not those who are nonresponders. What is the rationale for this approach? Are there any data regarding the mechanical, anatomical, and functional outcomes of those patients who were subjected to multiple treatments with Stretta? We have treated several failures of the Stretta and have seen no apparent evidence of anybody having been in that area before. So I am still a little unsure of how this device works.
The authors showed that there was a slight decrease in the incidence of gas bloat syndrome in patients undergoing the Stretta compared to those undergoing laparoscopic fundoplication. Can the authors explain why this might be, based on the mechanisms of action?
Finally, there are now many endoluminal techniques described for treatment of GERD. These include injecting various polymers into the LES region, and a number of different endoscopic plicators have been developed. Dr. Richards, in your assessment, which of these has the opportunity of being the next great endoluminal technique?
Dr. Bill Richards (Nashville, TN): Thank you for your very kind comments and the questions. To answer Dr. Schirmer first.
The first question is, why continue PPI for 3 weeks after Stretta? It is important to recognize patients undergoing the Stretta procedure do not respond immediately. It takes about 2 to 3 weeks before we start seeing improvement in symptoms. In about a third of the patients, it takes 3 months or longer before we see a treatment effect. And the treatment effect is compounded over time. Clearly, we don’t fully understand the mechanism of action for the Stretta procedure.
A corollary to the question was, if the Stretta works because it destroys nerves that control transient LES relaxations, why doesn’t the Stretta work immediately? To answer that, we have an alternative hypothesis that treatment in the gastroesophageal junction and the cardia of the stomach works through collagen contraction, which alters the basic mechanoreceptive mechanism by which transient LES relaxations are triggered back to the brain. I personally think this is a more plausible hypothesis rather than the destruction of nerves, given the fact the Stretta procedure does seem to work better over time.
The second question is sort of a continuation of the first question. That is, if there is no scarring when operating on the post-Stretta patient, why does the Stretta work? I would point out the Stretta catheter has 5.5-millimeter needles. You are inserting these needles into the muscularis of the gastroesophageal junction and upper stomach. So you are really inserting them in the thickest part of the GE junction and upper stomach. I believe we are not getting treatment effect beyond the muscularis, and muscularis temperatures are very, very tightly controlled by the computer that delivers the RF energy. So we just don’t see any effect in the mediastinum or in the peritoneal cavity.
I appreciate the very kind comments about the procedure and would agree with Dr. Schirmer wholeheartedly on the three points that you make. Specifically, I think there are patient groups that are going to be much better treated with Stretta; we just don’t understand who makes up those groups quite yet. I will expand on this later.
Dr. Soper asked about different motility aspects, preoperative motility, are there patients that should be excluded or included? I particularly think patients who have low amplitude of the esophageal contractions may be better suited for the Stretta. But we have not treated that group of patients as yet. There are other patients, as you point out, Dr. Schirmer, that are ideal candidates for the Stretta—post-Nissen failures, post-gastric resection, post-gastric bypass patients—that may not be eligible for a laparoscopic fundoplication. I agree with the comment that endoscopic treatment of GERD should be held to the same degree and high level of preoperative evaluation that we subject our laparoscopic Nissen patients to.
Dr. Soper asked, what about other endoluminal techniques, which are the new, hot techniques? My personal choice after looking at all of these is the Stretta procedure. I would look at it as the glass being half full right now and that it is very promising.
We should be able to find areas and amounts of RF energy treatment that will yield better results in the future. Clearly we do not understand why some patients respond so well and other patients, 11% of our patient population, did not respond at all.
I agree wholeheartedly with the comments from Dr. Schirmer about training of surgical residents in flexible endoscopy. And parenthetically, I think as we see increasing numbers of patients being treated with endoscopic techniques that surgical training in this area is paramount.
To answer Dr. Soper’s questions. The first question was about exclusion criteria; do we have data for exclusion criteria for the LES pressure less than 8 millimeters of mercury? Part of that was taken from the original open-label trial Stretta treatment protocol. They had recognized an LES pressure less than 5 millimeters of mercury as the cutoff for not performing Stretta. In our lab normals are a little bit higher than the normals taken by the water-perfused manometry used in that study. So we just continued the exclusion/inclusion criteria promulgated by the open-label trial.
I believe patients with pulmonary symptoms (cough, asthma, recurrent pneumonias) need to be treated with a modality of treatment that has been shown to absolutely reduce esophageal acid exposure. Until proven otherwise, the Stretta should be considered potentially inadequate therapy for that patient group.
Dr. Soper asked if patients selected Nissen over Stretta when presented with all the data. Yes, a number of our patients did select Nissen over Stretta. A lot of this was due to their insurance coverage and our inability to convince the insurance company in a timely manner that they could be treated effectively by Stretta.
We did not specifically compare apples to apples, as Dr. Soper suggests. This is clearly a study which compares apples to oranges, where we have two separate groups. One group has much more disease and is more suited for the laparoscopic fundoplication.
Dr. Soper asked a question about the hospital costs. Our hospital costs also include costs for patients undergoing Collis gastroplasty, larger hiatal hernias, and other procedures, so that is not fair to compare Stretta costs to the laparoscopic fundoplication costs. In addition, we did not include the additional patient costs for PPI use or additional treatment. We also did not include the time away from work that is seen after laparoscopic fundoplication and the time available for work in the Stretta group. But I think the hospital cost data gives you some idea that the Stretta will always be less costly than the fundoplication.
Dr. Soper asked me to expound on the patient outcomes for 22 patients undergoing postprocedure 24-hour pH studies. We had 10 patients that were considered complete responders out of the 22. That is less than 50%, but we had nearly 60% complete responders in our total group. That means that our complete responders were underrepresented in our pH data, and therefore our pH data may underreport the magnitude of improvement after Stretta. Eight out of the 10 patients who had a complete response off all PPIs had normalization of their pH.
Dr. Soper asked about the effect of BMI on treatment. As you noted, our Stretta patients had a slightly greater BMI. We have not broken out the analysis to look at BMI effect on the Stretta procedure. I can tell you that in your own data you have presented about the effect of BMI on laparoscopic fundoplication that you recognize that it is more difficult and more time-consuming to perform a fundoplication in a morbidly obese individual. In a morbidly obese individual it is no more time-consuming or difficult to do the Stretta than in a patient with a normal BMI.
Why does the Stretta fail, and why not treat complete failures? We don’t know why patients fail Stretta. I suspect areas of RF energy treatment sometimes do not hit the sweet spot or critical area for success. I believe if you fail at a modality, retreatment is only an exercise in futility, but since partial responders have shown some response, it makes sense to treat them again with the same modality. Therefore, we have treated one patient with a partial response who now has a complete response to the Stretta procedure.
Why do gas bloat symptoms seem to improve more in the Stretta patients than in the fundoplication patients? The Nissen fundoplication increases lower esophageal sphincter pressure and thereby provides a much greater barrier to reflux of both air and liquid within the stomach than the Stretta procedure. That is reflected in the higher scores we see post-fundoplication for gas bloat symptoms.
Again, I would like to amplify which endoluminal techniques may become more important in the future. Obviously I don’t have a crystal ball, but I can say that there are only two techniques that currently have FDA approval. Of those two techniques, the Stretta procedure is the only one that has been subjected to a sham versus treatment trial. There is a significant treatment-related response to the Stretta as compared to the sham patients, who received no benefit from sham treatment. So I think right now, the front runner is the Stretta procedure. I think that future investigation can 1) elucidate the mechanism of action, 2) improve the 36% complete response rate for acid normalization, and finally determine which patient characteristics predict good results.
I’d like to think the Southern Surgical Association for the privilege of the floor.
References
- 1.Frank L, Kleinman L, Ganoczy D, et al. Upper gastrointestinal symptoms in North America: prevalence and relationship to healthcare utilization and quality of life. Dig Dis Sci. 2000; 45: 809–818. [DOI] [PubMed] [Google Scholar]
- 2.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002; 122: 1500–1511. [DOI] [PubMed] [Google Scholar]
- 3.Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther. 2001; 15: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 4.Terry M, Smith CD, Branum GD, et al. Outcomes of laparoscopic fundoplication for gastroesophageal reflux disease and paraesophageal hernia. Surg Endosc. 2001; 15: 691–699. [DOI] [PubMed] [Google Scholar]
- 5.Lafullarde T, Watson DI, Jamieson GG, et al. Laparoscopic Nissen fundoplication: five-year results and beyond. Arch Surg. 2001; 136: 180–184. [DOI] [PubMed] [Google Scholar]
- 6.Hunter JG, Trus TL, Branum GD, et al. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg. 1996; 223: 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laine S, Rantala A, Gullichsen R, et al. Laparoscopic vs conventional Nissen fundoplication. A prospective randomized study. Surg Endosc. 1997; 11: 441–444. [DOI] [PubMed] [Google Scholar]
- 8.Triadafilopoulos G. Endoscopic therapies for gastroesophageal reflux disease. Curr Gastroenterol Rep. 2002; 4: 200–204. [DOI] [PubMed] [Google Scholar]
- 9.Triadafilopoulos G, Dibaise JK, Nostrant TT, et al. Radiofrequency energy delivery to the gastroesophageal junction for the treatment of GERD. Gastrointest Endosc. 2001; 53: 407–415. [DOI] [PubMed] [Google Scholar]
- 10.Triadafilopoulos G, Dibaise JK, Nostrant TT, et al. The Stretta procedure for the treatment of GERD: 6- and 12-month follow-up of the U.S. open label trial. Gastrointest Endosc. 2002; 55: 149–156. [DOI] [PubMed] [Google Scholar]
- 11.Wolfsen HC, Richards WO. The Stretta procedure for the treatment of GERD: a registry of 558 patients. J Laparoendosc Adv Surg Tech. 2002 (in press). [DOI] [PubMed]
- 12.Houston H, Khaitan L, Scholz S, et al. First year experience of patients undergoing the Stretta procedure. Surg Endosc. 2002 (in press). [DOI] [PubMed]
- 13.DiBaise JK, Brand RE, Quigley EM. Endoluminal delivery of radiofrequency energy to the gastroesophageal junction in uncomplicated GERD: efficacy and potential mechanism of action. Am J Gastroenterol. 2002; 97: 833–842. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Katz P, Wo JM, et al. Temperature-controlled radiofrequency energy delivery to the gastroesophageal junction for the treatment of GERD (the Stretta procedure): a randomized, double-blind, sham-controlled, multi-center clinical trial. Gastrointest Endosc. 2002; 55: AB19. [Google Scholar]
- 15.Wiklund IK, Junghard O, Grace E, et al. Quality of life in reflux and dyspepsia patients. Psychometric documentation of a new disease-specific questionnaire (QOLRAD). Eur J Surg Suppl. 1998; 583: 41–49. [PubMed] [Google Scholar]
- 16.Talley NJ, Fullerton S, Junghard O, et al. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001; 96: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 17.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 18.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998; 51: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 19.McHorney CA, Ware JE Jr, Lu JF, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994; 32: 40–66. [DOI] [PubMed] [Google Scholar]
- 20.Gandek B, Ware JE Jr, Aaronson NK, et al. Tests of data quality, scaling assumptions, and reliability of the SF-36 in eleven countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998; 51: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995; 33: AS264–279. [PubMed] [Google Scholar]
- 22.Kahrilas PJ. Gastroesophageal reflux disease. JAMA. 1996; 276: 983–988. [PubMed] [Google Scholar]
- 23.Chiba N, De Gara CJ, Wilkinson JM, et al. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997; 112: 383–387. [DOI] [PubMed] [Google Scholar]
- 24.Liu JY, Woloshin S, Laycock WS, et al. Late outcomes after laparoscopic surgery for gastroesophageal reflux. Arch Surg. 2002; 137: 397–401. [DOI] [PubMed] [Google Scholar]
- 25.Houston H, Khaitan L, Holzman M, et al. Laparoscopic Nissen fundoplication: 5 year follow-up [abstract]. Gastroenterology, 2002;122.
- 26.Kim MS, Dent J, Holloway R, et al. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation in a canine model. Gastrointest Endosc. 2002 (in press). [DOI] [PubMed]
- 27.Richards WO, Scholz S, Khaitan L, et al. Initial experience with the stretta procedure for the treatment of gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech A. 2001; 11: 267–273. [DOI] [PubMed] [Google Scholar]
- 28.Tam W, Shoeman MN, Zhang Q, et al. Delivery of radiofrequency energy to the lower esophageal sphincter inhibits transient LES relaxations in patients with gastroesophageal reflux disease: 12 months follow-up. Gut. 2002 (in press).
- 29.Utley DS, Kim MS, Vierra MA, et al. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: a porcine model. Gastrointest Endosc. 2000; 52: 81–86. [DOI] [PubMed] [Google Scholar]
- 30.Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995; 109: 601–610. [DOI] [PubMed] [Google Scholar]
- 31.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997; 336: 924–932. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001; 285: 2331–2338. [DOI] [PubMed] [Google Scholar]
- 33.Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg. 2002; 6: 3–10. [DOI] [PubMed] [Google Scholar]
- 34.Khajanchee YS, O’Rourke RW, Lockhart B, et al. Postoperative symptoms and failure after antireflux surgery. Arch Surg. 2002; 137: 1008–1014. [DOI] [PubMed] [Google Scholar]
- 35.Lundell L, Miettinen P, Myrvold HE, et al. Continued (5-year) followup of a randomized clinical study comparing antireflux surgery and omeprazole in gastroesophageal reflux disease. J Am Coll Surg. 2001; 192: 172–181. [DOI] [PubMed] [Google Scholar]
- 36.Gersin K, Fanelli R, Fleischer D. The Stretta procedure: technique optimization and incidence of complications. Surg Endosc. 2002 (in press).
- 37.Hunter JG, Smith CD, Branum GD, et al. Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg. 1999; 230: 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soper NJ, Dunnegan D. Anatomic fundoplication failure after laparoscopic antireflux surgery. Ann Surg. 1999; 229: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khaitan L, Bhatt P, Richards WO, et al. Comparison of patient satisfaction with redo versus primary fundoplication [abstract]. Surg Endosc, 2002. [DOI] [PubMed]
- 40.Horgan S, Pohl D, Bogetti D, et al. Failed antireflux surgery: what have we learned from reoperations? Arch Surg. 1999; 134: 809–817. [DOI] [PubMed] [Google Scholar]
- 41.Perez AR, Moncure AC, Rattner DW. Obesity adversely affects the outcome of antireflux operations. Surg Endosc. 2001; 15: 986–989. [DOI] [PubMed] [Google Scholar]
- 42.Kauer WK, Peters JH, DeMeester TR, et al. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995; 222: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberg S, Ritter MP, Crookes PF, et al. Gastroesophageal reflux disease and mucosal injury with emphasis on short-segment Barrett’s esophagus and duodenogastroesophageal reflux. J Gastrointest Surg. 1998; 2: 547–554. [DOI] [PubMed] [Google Scholar]
- 44.So JB, Zeitels SM, Rattner DW. Outcomes of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery. 1998; 124: 28–32. [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Supported by an educational grant from Ethicon Endosurgery, Cincinnati, Ohio (H.L.H., A.T., L.K.). W.O.R. was a consultant to and was supported by an educational grant from Curon Medical, Palo Alto, California.
Correspondence: William O. Richards, MD, D5219 MCN, Vanderbilt University Medical Center, Nashville, TN 37232.
E-mail: bill.richards@vanderbilt.edu
Accepted for publication December 2002.