Abstract
Objective
To examine the authors’ experience with preoperative ipsilateral portal vein embolization (PVE) and assess its role in extended hepatectomy.
Summary Background Data
Extended hepatectomy (five or more liver segments) has been associated with higher complication rates and increased postoperative liver dysfunction than have standard hepatic resections involving lesser volumes. Recently, PVE has been used in patients who have a predicted (postresection) future liver remnant (FLR) volume less than 25% of total liver volume in an attempt to increase the FLR and reduce complications.
Methods
Sixty patients from 1996 to 2002 were reviewed. Thirty-nine patients had PVE preoperatively. Eight patients who had PVE were not resected either due to the discovery of additional unresectable disease after embolization but before surgery (n = 5) or due to unresectable disease at surgery (n = 3). Therefore, 31 patients who had PVE subsequently underwent extended hepatic lobectomy. A comparable cohort of 21 patients who had an extended hepatectomy without PVE were selected on the basis of demographic, tumor, and liver volume characteristics. Patients had colorectal liver metastases (n = 30), hepatocellular carcinoma (n = 15), Klatskin tumors (n = 9), peripheral cholangiocarcinoma (n = 3), and other tumors (n = 3). The 52 resections performed included 42 extended right hepatectomies, 6 extended left hepatectomies, and 4 right hepatectomies extended to include the middle hepatic vein and the caudate lobe but preserving the majority of segment 4. Concomitant vascular reconstruction of either the inferior vena cava or hepatic veins was performed in five patients.
Results
There were no differences between PVE and non-PVE groups in terms of tumor number, tumor size, tumor type, surgical margin status, complexity of operation, or perioperative red cell transfusion requirements. The predicted FLR was similar between PVE and non-PVE groups at presentation. After PVE the FLR was higher than in the non-PVE group. No complications were observed after PVE before resection. There was no difference in postoperative mortality, with one death from liver failure in the non-PVE group and no operative mortality in the PVE group. Postoperative peak bilirubin was higher in the non-PVE than the PVE group, as were postoperative fresh-frozen plasma requirements. Liver failure (defined as the development of encephalopathy, ascites requiring sustained diuretics or paracentesis, or coagulopathy unresponsive to vitamin K requiring fresh-frozen plasma after the first 24 hours postresection) was higher in the non-PVE patients than the PVE patients. The hospital stay was longer in the non-PVE than the PVE group.
Conclusions
Preoperative PVE is a safe and effective method of increasing the remnant liver volume before extended hepatectomy. Increasing the remnant liver volume in patients with estimated postresection volumes of less than 25% appears to reduce postoperative liver dysfunction.
The application of hepatic resection for the management of primary and secondary liver malignancies has increased in the last decade. Advances in patient selection, surgical technique, and perioperative management have resulted in increased safety. Most series of hepatic resections from experienced centers report operative mortality below 5% in all patients and a mortality of approximately 1% in patients with no underlying cirrhosis. 1–5 Extended hepatectomy, defined as resection of at least five hepatic segments as described by Couinaud, 6 is still associated with higher operative morbidity and mortality. 1,7 The increased morbidity and mortality rates seen with extended resections are largely due to complications associated with postoperative hepatic insufficiency such as cholestasis, coagulation abnormalities, fluid retention, and hepatic synthetic dysfunction. Hepatic insufficiency becomes an even more formidable problem when complex biliary or vascular reconstructions is required in addition to the extended hepatectomy. 7–9 Recently there has been an emphasis on linking complications after extended hepatic resection to the amount or volume of liver left after the resection rather than to the amount of liver resected. 10
Preoperative portal vein embolization (PVE) was first described by Kinoshita 11 and later used by Makuuchi in the setting of hepatic resection of hilar cholangiocarcinomas. 12 The underlying principle is to block the portal venous flow to the side of the liver ipsilateral to the lesion in order to cause hypertrophy of the contralateral side and increase the size of the future liver remnant (FLR). The assumption has been that the larger volume seen in the FLR after PVE correlates with increased function and that therefore postresection liver dysfunction can be minimized. The purpose of this study is to review our experience with preoperative PVE and assess its role in extended hepatectomy.
METHODS
Sixty patients considered for extended hepatic resections at the University of Florida, Gainesville, from 1996 to 2002 were reviewed; 52 of them underwent extended hepatectomy. Thirty-nine patients had PVE preoperatively. Six of these patients were included in an earlier publication on PVE. 13 Eight patients who had PVE were not resected either due to the discovery of additional unresectable disease after embolization but before surgery (n = 5) or due to unresectable disease at surgery (n = 3). Therefore, 31 patients who had PVE subsequently underwent extended hepatic lobectomy. A comparable cohort of 21 patients who had undergone extended hepatectomy without PVE was selected on the basis of patient, tumor, and liver volume characteristics (Table 1). Of the five patients who developed unresectable disease after PVE but before surgery, three developed additional disease within the segments of liver originally planned to be left in (FLR), while two patients developed pulmonary metastases. Patients who had PVE but were unresectable at the time of surgery were unresectable on the basis of unsuspected extrahepatic disease (two cases) and because of inability to achieve complete tumor clearance (one case). Tumor clearance could not be achieved in the one patient with a Klatskin tumor that involved both the left hepatic artery and the bifurcation of the portal vein in a case where a right trisegmentectomy had been planned; therefore, a segment 3 biliary bypass was performed. Patients had colorectal liver metastases (n = 30), hepatocellular carcinoma (n = 15), Klatskin tumors (n = 9), peripheral cholangiocarcinoma (n = 3), and other tumors (n = 3).
Table 1. PATIENT CHARACTERISTICS
PVE was performed at the discretion of the operating surgeon when volumetric measurements suggested that the FLR volume would be less than 25% of the calculated total liver volume (TLV). PVE was also used in cases where FLR was predicted to be less than 40% but there was underlying liver dysfunction such as fibrosis/cirrhosis for some of the patients with hepatocellular carcinomas, or cholestasis in patients with hilar cholangiocarcinoma. The standardized FLR was calculated using the equation: FLR = FLR volume/TLV × 100. FLR volume was directly measured from computed tomography, while TLV was calculated from the patient’s body surface area using a formula described previously described by Vauthey et al.: TLV = 706.2 × BSA + 2.4. 14
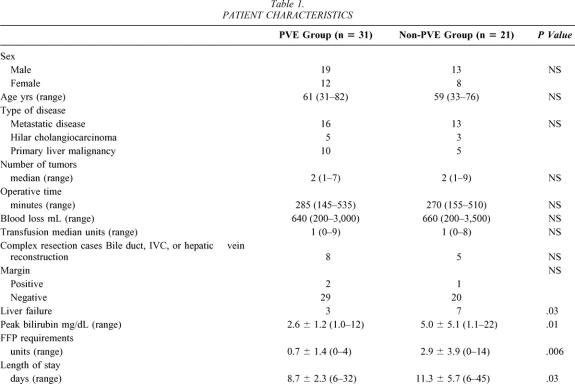
Percutaneous PVE was performed using an ipsilateral approach and has been described in detail elsewhere. 15,16 In brief, percutaneous access to the ipsilateral portal vein to the lesion was achieved under light sedation with both ultrasound and fluoroscopic control. After portography and an assessment of portal venous anatomy, selected portal vein segments were embolized using polyvinyl alcohol particles and microcoils. Patients underwent repeat volumetric CT assessment and restaging at 4 to 6 weeks after PVE (Figs. 1 and 2).
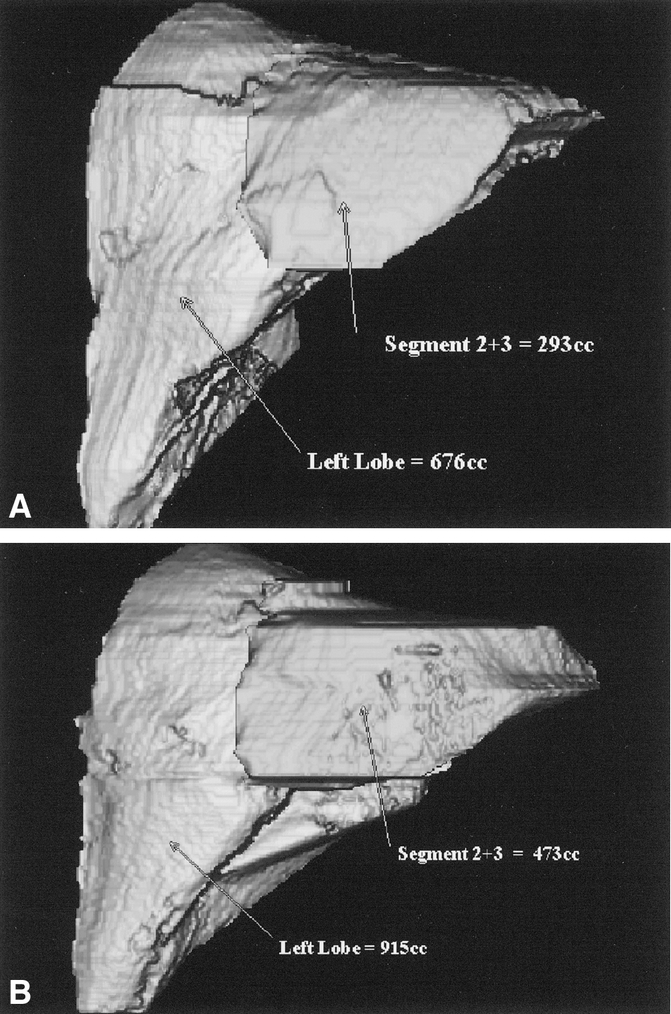
Figure 1. CT volumetry of the liver before and after PVE in a patient with cholangiocarcinoma.
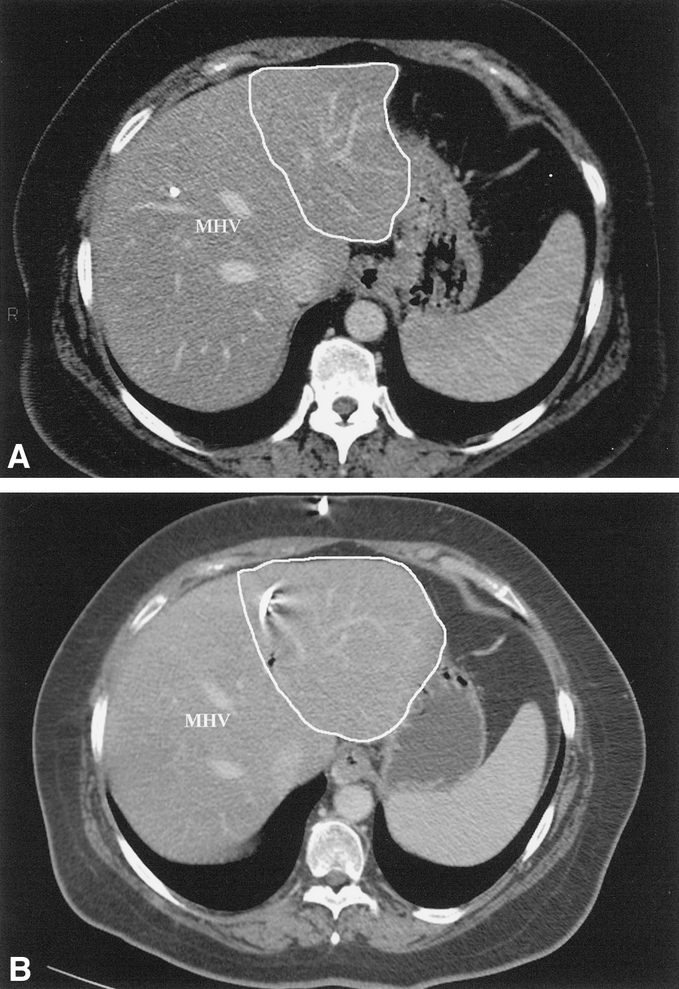
Figure 2. CT scans demonstrating the size of the left lateral segment before and after PVE in a patient with cholangiocarcinoma (same patient as in Fig. 1).
The 52 resections comprised included 42 extended right hepatectomies, 6 extended left hepatectomies, and 4 right hepatectomies extended to include the middle hepatic vein and the caudate lobe while preserving most of segment 4. 17 Concomitant vascular reconstruction of either the inferior vena cava or hepatic veins was performed in five patients, while portal vein resection/reconstruction was performed in three patients with Klatskin tumors.
All liver resections were performed with central venous pressure at or below 5 cm H2O. Inflow and outflow control was generally obtained before hepatic parenchymal transection. In cases where vascular reconstruction was not planned, parenchymal transection was performed with continuous inflow occlusion (Pringle maneuver). In cases that required vascular reconstruction, inflow occlusion was not used during the parenchymal transection portion of the resection, but these cases required either total vascular isolation or varying degrees of vascular control during the vascular reconstruction portion of the procedure. Details of the technique used for vascular reconstruction have been presented previously. 8,9,18
Operative mortality and morbidity were defined as death or complication within 30 days of the procedure or within the same hospital stay. Operative blood transfusion requirements were defined as blood transfused intraoperatively or within 24 hours postprocedure. Liver failure was defined as the development of encephalopathy, ascites requiring sustained diuretics or paracentesis, or coagulopathy unresponsive to vitamin K requiring fresh-frozen plasma (FFP) after the first 24 hours postresection. The indication for giving FFP is standardized at our institution. Patients who have an International Normalized Ratio (INR) of 2.0 or greater are given 2 units FFP. INR is assessed approximately 8 hours after the end of the procedure and then daily.
Statistical analysis was performed using SPSS 10.0 software (SPSS Inc., Chicago, IL). Parametric analysis of characteristics of patients who did or did not undergo PVE were performed using Student t tests, while nonparametric analysis was performed using the Fisher exact or chi-square test where appropriate. Actuarial 1-year survival was calculated using the Kaplan-Meier method. Significance was specified as α = 0.05. Results are reported as mean ± 1 standard deviation unless otherwise specified.
RESULTS
PVE was performed successfully in all 39 patients in whom it was attempted. There were no complications associated with the procedure. Twenty-two patients had right portal vein embolizations, 15 patients had the right portal vein plus segment 4 portal vein branches embolized, and 2 patients had left portal vein embolizations. Before PVE the FLR was 22 ± 6%, which was not different than the FLR of 23 ± 4% observed in the 21 patients who did not undergo PVE (P = .4). After PVE the FLR increased to 31 ± 7%, which was significantly higher than both the pre-PVE FLR and the FLR of patients not undergoing PVE (P = .001) (Fig. 3). The mean increase in standardized FLR (i.e., when compared to total calculated liver volume) was 8.4 ± 2.7% (range 4–15%), which represents an increase of about 35% in the functional liver mass to be left in after resection (Fig. 4).
Figure 3. After PVE, future liver remnant (FLR) volumes were higher than before PVE and when compared to the group of patients not undergoing PVE.
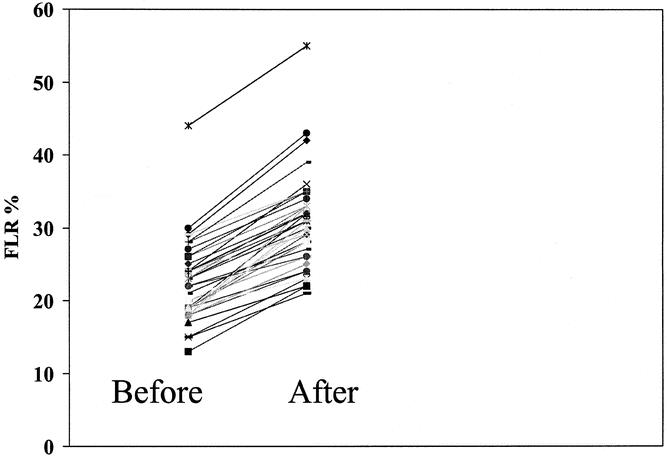
Figure 4. The mean increase in standardized FLR (i.e., when compared to total calculated liver volume) was 8.4 ± 2.7% (range 4–15%), which represents an increase of about 35% in the functional liver mass after resection.
At operation no identifiable problems arose secondary to the use of PVE. Although frequently coils were identified at the transection line of the right portal pedicle when it was divided, there was no difficulty in applying and firing a vascular stapler across the pedicle and the coils. There was no difference in intraoperative blood loss, perioperative transfusion requirement, or operative time between patients who received PVE and those who did not. Operative mortality was similar between groups, with one patient death from liver failure in the non-PVE group and none in the PVE group. Postoperative peak bilirubin was higher in the non-PVE than the PVE group (5.0 ± 5.1 mg/dL vs. 2.6 ± 1.2 mg/dL, P = .01), as were postoperative FFP requirements (2.9 ± 3.9 units vs. 0.7 ± 1.4 units, P = .006). Liver failure was higher in the non-PVE patients than the PVE patients (33% vs. 10%P = .03). There were three bile leaks, two of which required endoscopic sphincterotomy and biliary stent insertion for management. An additional two patients underwent percutaneous drainage of subphrenic abscess/fluid collections that did not communicate with the biliary tree. Two patients required right-sided pleurocentesis for management of pleural effusions that were thought to be compromising ventilatory status. One patient required temporary dialysis and one patient developed a pneumonia, which was successfully treated with antibiotics. There was no significant difference in the complication rate between PVE patients and non-PVE patients if liver failure was excluded as a complication. With the exception of two bile leaks, however, all other complications occurred in patients who manifested some degree of liver failure. The hospital stay was longer in the non-PVE than the PVE group (11.3 ± 5.7 days vs. 8.7 ± 2.3 days, P = .03).
With limited median follow-up of 13 months (range 3–54 months), the overall actuarial 1-year survival was 83% and was not different between patients who had PVE and those that did not.
DISCUSSION
Liver resection has become a relatively standardized, safe procedure in the last decade. Extended hepatectomy, however, continues to have an increased operative morbidity and mortality of 4% to 8%, depending on the extent and complexity of the procedure, when compared to liver resections of lesser magnitude. 1,2 The volume of functional liver mass left after resection is an important factor in the development of postoperative complications and subsequent mortality. 7,10 Liver volume measurement for adult living donor liver transplantation is standardized to achieve a graft weight to recipient weight of at least 1% because of the clear link between adequate functional hepatic mass of the donor graft to recipient weight and postoperative complications. 19 In the non-transplant liver resection setting, the minimum acceptable liver volume remaining postresection has not been well assessed but is generally thought to be about 25% of the normal liver volume. In cases where there is liver dysfunction such as cirrhosis or cholestasis, 40% of the normal volume is acceptable. 20 The advent of PVE allows optimization of the remnant liver volume in cases where it is projected to be less than ideal.
PVE blocks portal flow to the side of the liver ipsilateral to the lesion to be resected and causes an increase in size of the FLR. The increase in size is due to both clonal expansion and cellular hypertrophy. 21 The assumption that an increase in liver volume correlates with increased function post-PVE has been demonstrated in studies that show an increase in asialoglycoprotein receptor binding sites in the FLR before resection. 22 Asialoglycoprotein receptor binding site density has been shown to correlate with both the number of viable hepatocytes and liver function after resection. 23–25
Calculation of future liver volume in the resection setting has not been standardized. One widely used method is to use CT volumetric analysis to calculate the liver remnant as a percentage of total liver volume by attempting to subtract the tumor volume. 20 In the present study we calculated the remnant liver volume using CT volumetry and calculated the total liver volume using a formula based on body surface area. We feel that this reduces some of the difficulties in accurately assessing total liver volume in patients whose liver morphology changes with tumor size and location, as well as with PVE. This method of “standardized future remnant liver volume” has been previously demonstrated to correlate FLR volume to outcome after hepatic resection. 16
Five of 39 patients who had PVE developed additional disease either locally in the liver or distantly in the lung after PVE, precluding curative resection. Although this may represent disease that would have manifested in any event, it raises the concern that PVE may be promoting tumor growth. Partial hepatectomy has been demonstrated to accelerate local tumor growth in the liver 26,27 but not to increase experimental tumor burden in the lung. 28 Recent retrospective studies on PVE yield differing results in terms of long-term survival. The majority of studies show an equivalent survival between standard resection patients and patients who would otherwise have been unresectable without PVE. 13,29 Two recent retrospective studies, however, have demonstrated an increased risk of disease recurrence after PVE. 30,31 With limited follow-up, our own study could not, nor was it designed to, identify a difference in long-term survival. It is plausible that the local or systemic increase in growth factors or cytokines caused by PVE would also be caused by hepatectomy without PVE and have a similar effect on tumor growth. The clinical import of PVE on tumor growth is currently only speculative.
While we could not demonstrate a difference in operative mortality with the use of PVE, there was a significant difference in parameters of postoperative liver dysfunction, such as peak bilirubin, FFP utilization, and liver failure. Hospital stay was also shorter. Other published series on PVE have largely focused on patients who were otherwise unresectable due to small liver volumes in whom PVE was performed to increase the FLR. 13,20,29 These studies have demonstrated that patients with small projected FLRs could successfully undergo PVE followed by liver resection with similar results in both operative mortality and postoperative liver dysfunction compared to patients with adequate FLRs not requiring PVE. In contrast, our study compares patients with marginal FLRs (<25%) who underwent extended hepatectomy to a similar group of patients who had PVE performed in order to increase the FLR above 25%. We believe this is why a difference in postoperative liver dysfunction was seen in our study but not in many others. It may also be reasonable to extrapolate that with less liver failure, there will be less operative mortality, but that conclusion requires a larger study to validate.
One of the limitations of this study is its design. Patients were retrospectively selected to match liver volume and patient characteristics. As PVE became more easily available over the study period, it was more frequently used in “marginal cases,” with the majority of non-PVE cases being done before 2000, while the majority of PVE cases were done after 2000. Experienced liver surgeons did all resections, with no significant changes in operative technique over the time period; however, some bias is obviously introduced by the selection process.
Every experienced liver surgeon who deals with hilar cholangiocarcinoma by extended hepatic resection and bile duct reconstruction has struggled with the severe liver dysfunction that can follow curative resection. While the numbers in our current study do not support a separate analysis of these patients, a personal observation is the remarkable improvement in postoperative course that PVE makes in this subgroup of patients. Deciding which side of the liver to embolize preoperatively can be difficult in this particular group of patients.
In summary, preoperative PVE is a safe and effective method of increasing the remnant liver volume before extended hepatectomy. Increasing the remnant liver volume in patients with estimated postresection volumes of less than 25% reduces postoperative liver dysfunction and its associated complications.
Discussion
Dr. Leslie H. Blumgart (New York, NY): Thank you very much for your paper. There are several questions that I raise here.
There is no question that you have demonstrated, as have others, that you can increase the size of the liver remnant by carrying out portal vein embolization. However, there is no evidence either in this paper or in anything else that has been published that it improves final outcome. Minor differences in bilirubin level and so on do not constitute a necessarily better outcome or a failure to regenerate.
You quoted a paper that Jarnagin produced from our series as showing a higher mortality in extended resections. That is quite true. But I have to point out that in that series of 1,800 liver resections, we only had liver failure as the primary cause of death in six patients, and it was not always due to failure to regenerate. I am concerned about your conclusion that portal vein embolization necessarily obviates liver failure.
There are one or two specific questions that I would like to ask.
Firstly, the number of patients in whom you carried out portal vein embolization (PVE) is very high, and most of them had colorectal cancer. We have been very wary about using PVE in this setting. I think it may be valuable, and I think further studies may prove it to be valuable in patients with an abnormal liver or a patient who has been deeply jaundiced. There is little or no evidence, as pointed out by Prof. Belghitti from Paris, that this is a valid procedure in metastatic colon cancer.
The questions I want to ask are the following:
Firstly, did you do laparoscopy before you did portal vein embolization? You had a fair number of patients who were found to be inoperable because of extrahepatic disease. To put a patient through PVE and then through a period of waiting without demonstrating first that there is no extrahepatic disease is probably unjustified.
Secondly, there is evidence that portal vein embolization may lead to increase in growth of tumor, both in the liver and possible extrahepatic sites. Did any of your patients who proved to be inoperable have tumor growth in the remnant of the liver as a reason for inoperability?
Dr. William Chapman (St. Louis, MO): I too would have a few comments that I would like to make on this paper. The concept that these authors have brought forth today is based on previous research demonstrating that ligation of a major portal venous branch leads to atrophy of the supplied segment or segments of the liver, with compensatory hypertrophy in the remaining liver. This group has taken advantage of this concept to increase remnant size prior to planned resection. In the current report, the authors have convincingly demonstrated that this technique will lead to hypertrophy of the nonembolized liver. In their hands there were no embolization-associated complications. However, I would agree with the previous comment that this is a retrospective study of nonrandomized patients. Furthermore, the authors note that more of the patients underwent preoperative embolization in the latter period of this study. So from this point of view, it is difficult to know if their improved results were solely due to the embolization or if they were influenced by improved techniques and/or a selection bias over time. Having said this, this appears to be a safe technique and one that I believe should be considered in patients with a predicted marginal remnant liver volume.
I have three general questions for the authors: What adjustments do you make in the non-cirrhotic setting but when there is abnormal liver parenchyma; for example, the patient with underlying steatosis or fibrosis? There was mention made of a 40% minimum remnant volume in the setting of cirrhosis. But I wonder what you do with lesser degrees of abnormal liver. Do you ever biopsy the non-tumor-bearing liver and utilize this information; for example, subtract out the amount of steatosis from the calculations utilized for your projected remnant liver volume?
Number 2. What approach have you taken if the liver doesn’t hypertrophy to the greater than 25% volume calculation that you have mentioned during the waiting phase? Do you wait longer? Or have you repeated the embolization in any circumstances?
Finally, in the current report you measured the predicted liver remnant volume as a percentage of the calculated ideal liver volume. Why not measure the actual total liver volume and actual remnant liver volume and subtract out the tumor mass? What about determining the projected amount of liver remnant as a percentage of body weight, as is currently done for live donor liver transplantation?
I enjoyed this paper very much and would like to thank the authors for sending me the manuscript in advance of the meeting. The authors have brought our attention to a technique that I believe should be considered in patients with predicted small remnant liver volumes.
Dr. Douglas Farmer (Los Angeles, CA): I would first like to thank Dr. Howard and his co-authors as well as the Association for the invitation to review this manuscript, which we received and read in advance. We feel that this study represents an important addition to the growing body of literature on the use of portal vein embolization prior to resection. We also feel that this technique has been championed by several surgical groups in Asia, Europe, and the United States as a mechanism to reduce postsurgical liver failure after extended partial hepatectomies. It represents an attempt to safely perform extended resections in patients who otherwise are not candidates for resection due to inadequate remnant liver volumes. We do have several specific questions after reviewing this manuscript.
The first series of questions relates to the study design. Specifically, how were the patients selected for embolization? The manuscript states that they were selected “at the discretion of the operating surgeon.” Was there some standardized criteria used amongst the surgeons?
The second is, how was the control group selected? Are these just contemporaneous resections that happened during the study interval? Or were they patients that were for whatever reason deemed unsuitable for this embolization technique? And if the latter is true, why? Clearly, the best control group would be a randomized group of patients, which to my knowledge has not been performed in any study related to this embolization procedure, and I wondered if you could comment on that. Is that practical? Or has it been done and I just didn’t find it in my review?
The next questions relate to cirrhosis. It was unclear from my review of the manuscript how many of your patients actually had cirrhosis. Although the response to this procedure may be somewhat blunted in the cirrhotic patient, it actually may be the cirrhotic patient that could benefit most from this procedure.
What do you feel is the most optimal time between embolization of the portal vein and resection, and why? In other studies, regeneration of the liver has been dependent on a number of factors, including growth factors, TNF, and IL-6. Have you systematically analyzed any of these factors in your patients? Technically, was there any difference in the portal inflow occlusion times in your group and did you employ any precondition maneuvers prior to Pringling?
We also had issue with the definition of postoperative liver failure, which we feel was somewhat vague, and would like a clarification of the term “coagulopathy unresponsive to vitamin K” and what is “sustained diuretic use”? We would also like to put forth the thought that perhaps a more objective assessment of liver failure, such as that achieved with the M.E.L.D. scoring system, might better differentiate your groups of patients. I don’t know if you have or haven’t looked at that.
Finally, as it relates to the subgroup of patients who have cirrhosis and have hepatocellular carcinoma. At what cut-off do you feel these patients should actually be considered for transplantation, even if they do not meet the Mazaferro Milan criteria? There have been some recent publications from the University of California at San Francisco as well as Barcelona to suggest that these patients may have a better survival than that originally predicted by this original publication from Italy. If could you comment on that.
Dr. Alan W. Hemming (Gainesville, FL): Dr. Blumgart had two specific questions—a few comments, but two specific questions.
Do we do laparoscopy in all of our patients prior to portal vein embolization because of the risk of us not going on to surgery in all patients? We perform laparoscopy in most patients, and not necessarily before the portal vein embolization. The yield for laparoscopy for us has not been as high as in other people’s hands. Others report a 30% pickup of new information from laparoscopy, but with the latest generation of scanners we have been down more around 10%. We have had some difficulty with the colorectal metastases patients in getting adequate visualization at laparoscopy, because of the adhesions from previous colectomy. On the other hand, there is no published evidence that portal vein embolization by itself carries any long-term implications. With portal vein embolization, patients come in, have their portal vein embolization one day and go home the next day. To be frank, if patients do not go on to curative surgery, then the impact on long-term outcome is likely to be minimal. We think doing portal vein embolization is worth the possible benefit and has a relatively low downside risk.
The next question, Dr. Blumgart, was tumor growth in the remnant liver? Yes, we had two patients that developed new tumors that we could not see on the initial scan that became evident during the interval after portal vein embolization. That would be one of our concerns, and it is partially addressed in the paper. When you direct growth factors up one side of the liver by portal vein embolization you are stimulating growth in both the liver and whatever else happens to be there. If you have a few cells sitting on the left side of your liver and stimulate the left liver to grow, you will stimulate the tumor cells to grow as well. In fact, there have been studies to show that the tumors will grow at a faster rate than the liver is regenerating. So this is certainly a concern. There are animal models on postresection tumor growth in both lung and in liver, and there is no particular reason to suspect it would be any different in portal vein embolization versus resection. There is also no evidence that we are not doing something we wouldn’t do at operation when the ipsilateral portal vein is divided anyway.
Dr. Chapman asked about the use of portal vein embolization in the noncirrhotic but otherwise injured liver; for instance, steatosis or the cholestatic liver. Actually, one of the things portal vein embolization seems to be the most useful for is in Klatskin tumors in patients that have quite severe cholestasis and in whom you are planning on doing an extended resection. Those patients have been relatively difficult in terms of the amount of liver dysfunction they have postoperatively. Although I think there are only five or six patients that had Klatskin tumors that were embolized in this series, the difference in their postoperative course they had with portal venous embolization was quite remarkable. One of the problems with that particular set of patients is deciding which side to embolize up front, since you are not necessarily sure preoperatively which side of the liver you are going to be resecting. Certainly we would recommend not embolizing if you are not sure what side of the liver you are going to be resecting.
We don’t biopsy the liver to assess steatosis and don’t utilize that in our calculations, although alternatively when we are transplanting we do correct for the amount of steatosis.
Another question was, if the volume doesn’t change with portal vein embolization, what would we do? In this particular group of patients we didn’t have that problem. These are mostly noncirrhotics and for extended hepatectomy. So we saw in all of these patients—if you remember the graph, all of them had an upward shift from pre-embolization to post-embolization. The group that isn’t included in this study but sometimes have very little regeneration is the cirrhotics that you are planning on doing less than an extended hepatectomy on; something like a right lobectomy or a left lobectomy. I have had a few patients who just didn’t regenerate at all. In my mind that probably tells you that you might not want to be doing full right lobectomy or left lobectomy in these patients.
Some people suggest that if you wait longer in cirrhotics, you will get more regeneration. I haven’t really seen that. It has been our experience that either they are regenerating by 6 weeks or they are not. But if the volume doesn’t improve—in these particular patients, let’s say we were at a volume around 23—24% where our cut-off is supposedly 25%, I don’t think it would stop me. We used to do these resections all the time with projected volumes of 20 to 21%. And actually going back to one of the things Dr. Blumgart said, certainly patients don’t die of being yellow. They may look yellow, but that is not necessarily a concern. Some of the other complications to do with liver failure are a concern.
Dr. Chapman asked why did we use a calculated total liver volume versus an actual volumetric analysis by CT. One of the problems with doing CT analysis is there is certain error inherent each time you calculate a volume. If there are multiple tumors and you have to calculate both the liver volume and the tumor volume and then subtract it, you are magnifying your errors. The other thing is, you have to decide which volume you are going to use. After portal vein embolization, the total liver volume changes by CT, and basically we want to use a standardized ideal liver weight. That correlates with what we do for transplant in that function seems to be related in terms of graft versus body weight, and a standardized liver volume based on body surface area is a similar concept.
Dr. Farmer, how were patients selected? Well, the portal vein embolization patients were simply all patients that had their portal vein embolized and had extended hepatectomies. The control group is a mixed bag. Some are from
earlier in the time period where we weren’t so eager to use portal vein embolization. Those patients would have borderline liver volumes close to 25% here. There is another group of patients that went to the operating room, initially planned to have a non-extended operation but had an extended operation because of the findings at operation. Obviously those patients hadn’t had portal vein embolizations. Retrospectively we went back to the pre-op CTs and these patients had volumes less than we would like.
In terms of the criteria for embolization, I stick pretty much to 25% of the calculated volume if they have normal liver. The other patients have either injured liver, and almost all of those are Klatskin tumors. Additionally, if I am adding either a vena cava resection or a hepatic vein reconstruction where I may or may not have increased ischemic time to the liver, then I think portal vein embolization is prudent.
Comments about a randomized trial. I would love to do a randomized trial. We wouldn’t have enough patients to do it alone so it would have to be done as a multicenter trial. However, if you believe portal vein embolization works, you are going to have a hard time taking a patient that you think needs embolization and telling them that you are just going to go ahead and operate on them anyway.
Cirrhosis. In this particular group none of the patients had frank cirrhosis. We were using a fibrosis scoring system such that no patient had a fibrosis score more than 4, with 5–6 being cirrhosis. So a few patients were fibrotic, but not cirrhotic.
The optimal time to wait after embolization? It is a little bit taken from everybody else’s experience. We wait 4 to 6 weeks. It has been said in cirrhotics that you might want to wait a little longer. Also, the other group of patients this is becoming more and more applicable to is the group of patients that receive neoadjuvant chemotherapy. The whole idea of what neoadjuvant chemotherapy is going to do to the liver and its ability to regenerate is a little bit up in the air. With patients receiving neoadjuvant chemotherapy, we are waiting a little bit longer. But I can’t tell you how long we have to wait to see the optimal regeneration.
The issues about Pringling and whether we use ischemic preconditioning. For the last 2 years I have been using ischemic preconditioning with no definitive proof that it works, but I like the concept and certainly its benefits have been demonstrated in animal models. Most patients, for instance, a right trisegmentectomy would be probably about 15 minutes worth of Pringle time, a left trisegmentectomy would be more like 40 minutes of Pringle time. And we do it continuously, not intermittently.
The definition of liver failure I agree is arbitrary. The definition of liver failure that we used was encephalopathy, INR > 2, uncontrollable without FFP after 24 hours, ascites requiring paracentesis or sustained diuretics, sustained diuretics meaning on diuretics to control ascites for 1 week. And we purposely did not include things like bilirubin tripling or anything like that, because, like I said, we don’t believe that being yellow really matters that much, it is more other parameters like synthetic function.
In terms of did we use the M.E.L.D. score to try and define our liver failure a little more closely. M.E.L.D. has really been shown to correlate well with mortality in chronic liver failure. It really hasn’t been utilized much in acute liver failure, which is pretty much the definition of what happens after liver resection. In fact, I think to talk about using M.E.L.D. in acute liver failure and fulminant liver failure may be premature.
In terms of cirrhotics and HCC and what would our cut-off for size be. We, like everybody else, have to live with the current tumor size definitions to get our increased M.E.L.D. scores. So right now we live with the definition of three tumors up to 3 cm in size or one tumor up to 5 cm in size. Do I think that there are patients out there with 12-cm tumors that benefit from transplantation? For sure. There are different tumor biologies. Basically what we are doing when we transplant folks is that as long as the tumor is confined to the liver, we are going to cure that patient with transplantation. So there are certainly patients that have a 12-cm tumor and no vascular invasion that will be cured by transplant. There are also patients with 2-cm tumors that have vascular invasion and circulating cells that we transplant, use immunosuppression, and will develop tumor recurrence. The key is going to be selecting those patients. So far we don’t have a good way of doing that. Because of organ unavailability, we err on the side of caution and try to maximize the use of organs.
I would like to thank the Association for the privilege of presenting our paper.
References
- 1.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000; 191: 38–46. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002; 236: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choti MA, Bowman HM, Pitt HA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998; 2: 11–20. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemming AW, Sielaff TD, Gallinger S, et al. Hepatic resection of noncolorectal nonneuroendocrine metastases. Liver Transpl. 2000; 6: 97–101. [DOI] [PubMed] [Google Scholar]
- 6.Couinaud C. Le Foie: Etudes Anatomicales et Chirurgicales. Paris: Masson & Cie; 1957: 187–208.
- 7.Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001; 192: 47–53. [DOI] [PubMed] [Google Scholar]
- 8.Hemming AW, Langham MR, Reed AI, et al. Resection of the inferior vena cava for hepatic malignancy. Am Surg. 2001; 67): 1081–1088. [PubMed] [Google Scholar]
- 9.Hemming AW, Reed AI, Langham MR, et al. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002; 235: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999; 188: 304–309. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986; 10: 803–808. [DOI] [PubMed] [Google Scholar]
- 12.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990; 107: 521–527. [PubMed] [Google Scholar]
- 13.Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002; 137: 675–681. [DOI] [PubMed] [Google Scholar]
- 14.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002; 8: 233–240. [DOI] [PubMed] [Google Scholar]
- 15.Madoff DC, Hicks ME, Vauthey JN, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002; 22: 1063–1076. [DOI] [PubMed] [Google Scholar]
- 16.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000; 127: 512–519. [DOI] [PubMed] [Google Scholar]
- 17.Strasberg SM, for the International Hepato-Pancreato-Biliary Association Terminology Committee Survey. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000; 2: 333–339. [Google Scholar]
- 18.Hemming AW, Cattral MS. Ex vivo liver resection with replacement of the inferior vena cava and hepatic vein replacement by transposition of the portal vein. J Am Coll Surg. 1999; 189: 523–526. [DOI] [PubMed] [Google Scholar]
- 19.Sugawara Y, Makuuchi M, Takayama T, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001; 192: 510–513. [DOI] [PubMed] [Google Scholar]
- 20.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000; 232: 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001; 88: 165–175. [DOI] [PubMed] [Google Scholar]
- 22.Kubo S, Shiomi S, Tanaka H, et al. Evaluation of the effect of portal vein embolization on liver function by (99m)Tc-galactosyl human serum albumin scintigraphy. J Surg Res. 2002; 107: 113. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Todo A, Ikekubo K, et al. Quantitative assessment of hepatocellular function through in vivo radioreceptor imaging with technetium 99m galactosyl human serum albumin. Hepatology. 1993; 17: 814–819. [PubMed] [Google Scholar]
- 24.Vera DR, Topcu SJ, Stadalnik RC. In vitro quantification of asialoglycoprotein receptor density from human hepatic microsamples. Methods Enzymol. 1994; 247: 394–402. [DOI] [PubMed] [Google Scholar]
- 25.Yumoto Y, Umeda M, Ohshima K, et al. Estimation of remnant liver function before hepatectomy by means of technetium-99m-diethylenetriamine-pentaacetic acid galactosyl human albumin. Cancer Chemother Pharmacol. 1994; 33: S1–6. [DOI] [PubMed] [Google Scholar]
- 26.Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999; 86: 784–788. [DOI] [PubMed] [Google Scholar]
- 27.Panis Y, Ribeiro J, Chretien Y, et al. Dormant liver metastases: an experimental study. Br J Surg. 1992; 79: 221–223. [DOI] [PubMed] [Google Scholar]
- 28.Picardo A, Karpoff HM, Ng B, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998; 124: 57–64. [PubMed] [Google Scholar]
- 29.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000; 231: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakabayashi H, Ishimura K, Okano K, et al. Is preoperative portal vein embolization effective in improving prognosis after major hepatic resection in patients with advanced-stage hepatocellular carcinoma? Cancer. 2001; 92: 2384–2390. [DOI] [PubMed] [Google Scholar]
- 31.Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001; 34: 267–272. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Alan W. Hemming, MD, MSc, FRCSC, FACS, University of Florida, Center for Hepatobiliary Disease, PO Box 100286, Gainesville, FL 32610.
E-mail: hemming@surgery.ufl.edu
Accepted for publication December 2002.