Abstract
Objective
To assess the significance of axillary lymph node status and tumor size for predicting locoregional recurrence (LRR) and overall survival after mastectomy for breast cancer and to discuss the utility of postmastectomy radiation therapy.
Summary Background Data
Patients with locally advanced breast cancer require multimodality treatment combining chemotherapy (and/or hormonal therapy), surgery, and radiation. Randomized trials have demonstrated that postmastectomy radiation reduces LRR, but no overall survival benefit has been established.
Methods
Criteria for accrual to the Alabama Breast Cancer Project (1975–1978) were female gender and T2–3 breast cancer with M0 status. Patients underwent a radical or a modified radical mastectomy. Node-positive patients received adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy or adjuvant melphalan. Patients were evaluated for LRR and overall survival based on the number of positive axillary lymph nodes and (in N0 patients) pathologic tumor size. Significance was determined using chi-square analysis. Survival curves were generated using the Kaplan-Meier method and were compared by log-rank analysis.
Results
After median follow-up of 15 years, neither type of surgery nor chemotherapy was shown to affect locoregional disease-free or overall survival. LRR rates were higher and overall survival rates were lower in patients with nodal involvement, while tumor size was not shown to significantly affect these rates.
Conclusions
Patients with axillary lymph node metastases may benefit from postmastectomy radiation, but the use of postmastectomy radiation in N0 patients is not supported when it is based on tumor size alone.
Five percent to 10% of breast cancers are locally advanced (Table 1) at the time of diagnosis. 1 In the past, treatment of locally advanced breast cancer with mastectomy alone resulted in high rates of locoregional and distant failure. 2,3 Today, it is generally accepted that patients with locally advanced breast cancer require a multimodality approach combining chemotherapy (and/or hormonal therapy), surgery, and radiation. Randomized trials have demonstrated that postmastectomy radiation (PMR) reduces locoregional recurrence (LRR) after both radical and modified radical mastectomies. However, no consistent overall survival benefit has been established. 4–6
Table 1. LOCALLY ADVANCED BREAST CANCER
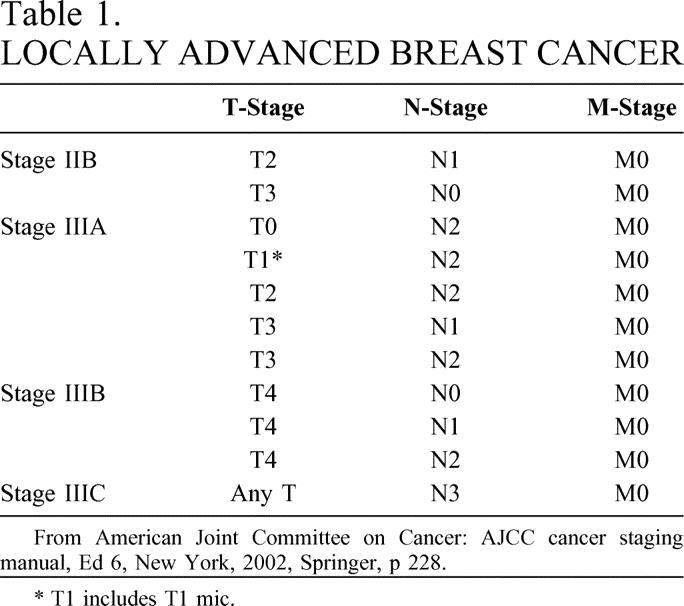
From American Joint Committee on Cancer: AJCC cancer staging manual, Ed 6, New York, 2002, Springer, p 228.
* T1 includes T1 mic.
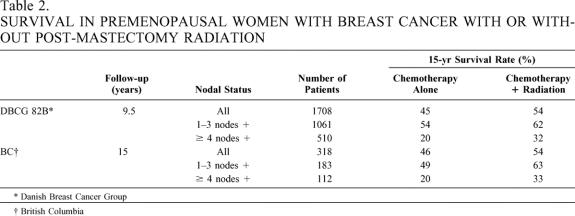
Controversy regarding PMR increased when an overview of eight postmastectomy trials (1949–1976) demonstrated that patients surviving 10 years or longer had significantly poorer survival thereafter if they had received PMR. 7 In 1994, a second overview of the same trials was presented, which detailed longer patient follow-up and cause-specific mortality data. 8 This overview did not demonstrate reduced survival after 10 years in the PMR patients but did show that cardiac-related mortality rates were significantly worse. Recently, two studies have shown improved survival for breast cancer patients after PMR. In both the Danish Breast Cancer Group 82B (DBCG 82B) trial 9 and the British Columbia trial, 10 premenopausal node-positive patients received cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy after mastectomy and were randomized to either observation or PMR. In both trials, patients with one to three and four or more positive axillary lymph nodes benefited from PMR (Table 2). No excess deaths from radiation toxicity were reported.
Table 2. SURVIVAL IN PREMENOPAUSAL WOMEN WITH BREAST CANCER WITH OR WITHOUT POST-MASTECTOMY RADIATION
* Danish Breast Cancer Group
† British Columbia
We have studied prospective clinical data for patients accrued to the Alabama Breast Cancer Project between 1975 and 1978. 11 This report examines the importance of axillary lymph node status and tumor size for predicting LRR and overall survival after standardized surgery for breast cancer. The utility of PMR is discussed in the context of our findings.
METHODS
Between 1975 and 1978, patients with operable breast cancer (T1–3, M0) were accrued to the Alabama Breast Cancer Project. Patients were randomized to undergo either a radical mastectomy or a modified radical mastectomy. Patients with metastases to axillary lymph nodes were also randomized to receive either melphalan (L-PAM) or CMF. Patient and disease variables such as age, tumor size, and number of axillary lymph nodes involved were recorded on entrance into the Alabama Breast Cancer Project. All human subject studies were conducted in accordance with the ethical standards of the Helsinki Declaration of 1975.
The median patient follow-up duration was 15 years (range 1–21). LRR of disease was defined as a new manifestation of disease near the site of the original breast cancer or in the ipsilateral axilla. Overall survival was calculated as the time from date of diagnosis to death.
LRR and overall survival rates were determined according to pathologic tumor size and the number of positive axillary lymph nodes and were compared using chi-square analysis. Survival curves were generated using the Kaplan-Meier method and compared by log rank analysis.
RESULTS
Demographics
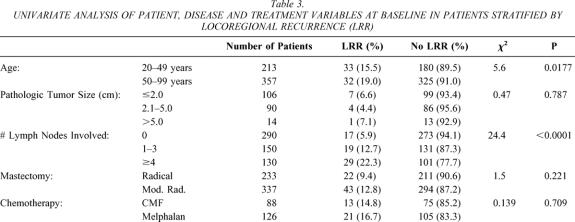
Five hundred eighty-eight patients were accrued to the study. Three hundred eleven patients were randomized to undergo either radical or modified radical mastectomy, while 277 patients received either radical or modified radical mastectomy on a nonrandom basis. Overall, radical mastectomy was used to treat 237 patients, while 351 were treated by modified radical mastectomy. LRR was evaluated in 570 patients for whom the axillary lymph node status was documented (96.4%). Table 3 shows patient, disease, and treatment variables for these patients. Pathologic tumor size was documented for 210 of 290 N0 patients (72.4%). Sixty-two percent of patients were 50 years of age or older. Table 4
Table 3. UNIVARIATE ANALYSIS OF PATIENT, DISEASE AND TREATMENT VARIABLES AT BASELINE IN PATIENTS STRATIFIED BY LOCOREGIONAL RECURRENCE (LRR)
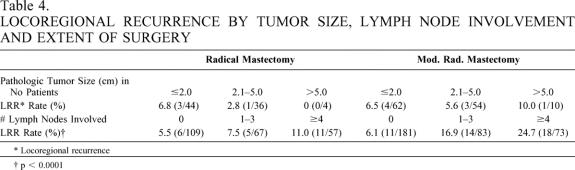
Table 4. LOCOREGIONAL RECURRENCE BY TUMOR SIZE, LYMPH NODE INVOLVEMENT AND EXTENT OF SURGERY
* Locoregional recurrence
† p < 0.0001
Locoregional Disease-Free Survival
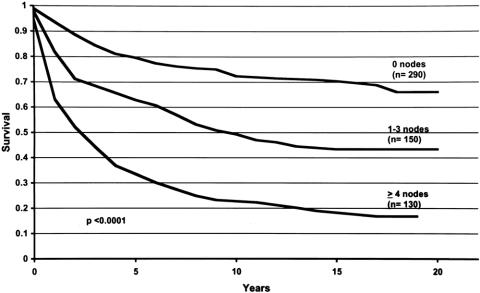
Five- and 10-year locoregional disease-free survival rates for the total population of 588 patients were 65.7 ± 2.0 and 55.8 ± 2.1, respectively. After median follow-up of 15 years (range 1–21 years), neither type of surgery nor type of chemotherapy significantly altered locoregional disease-free survival. Five hundred seventy patients were evaluated for LRR based on the number of axillary lymph nodes involved with disease. In both the radical and modified radical mastectomy groups, LRR rates were significantly higher in patients with axillary lymph node metastases (P = .0125 and P < .0001, respectively). Figure 1 shows locoregional disease-free survival in 570 patients based on the number of positive axillary lymph nodes. Locoregional disease-free survival was better when fewer nodes were involved (P < .0001). In N0 women, pathologic tumor size did not significantly alter LRR rates for either the radical or modified radical mastectomy groups. Figure 2 shows locoregional disease-free survival for these patients. Table 3 shows patient, disease, and treatment variables. LRR occurred more frequently in patients who were less than 50 years of age (P = .0177).
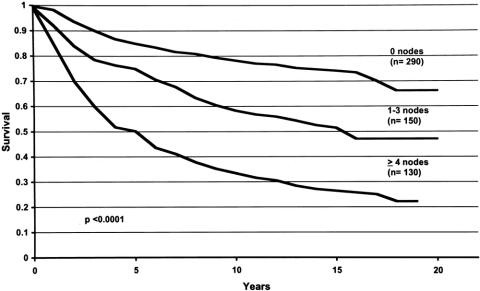
Figure 1. Locoregional disease-free survival curves for breast cancer patients with zero, one to three, and four or more positive nodes. For each time period (years, x-axis), the probability of not developing locoregional recurrence of disease is shown (y-axis). Locoregional disease-free survival was better when fewer nodes were positive.
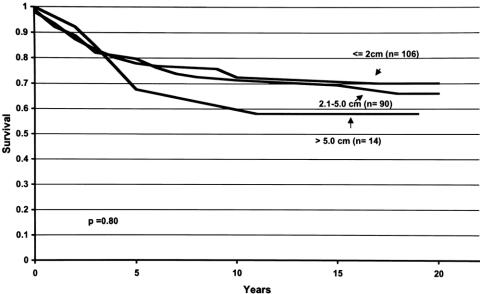
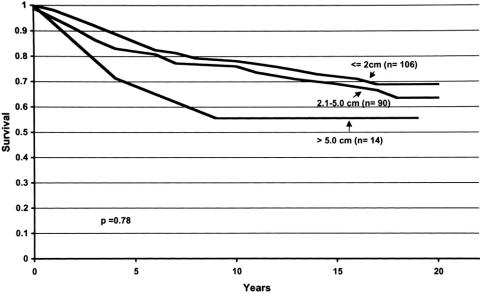
Figure 2. Locoregional disease-free survival curves for breast cancer patients with tumors measuring 2.0 cm or less, 2.1 to 5.0 cm, and more than 5.0 cm. For each time period (years, x-axis), the probability of not developing locoregional recurrence of disease is shown (y-axis). Tumor size was not shown to affect this probability.
Overall Survival
Five- and 10-year overall survival rates for the total population of 588 patients were 73.4 ± 1.8 and 63.5 ± 2.0, respectively. After median follow-up of 15 years (range 1–21 years), neither type of surgery nor type of chemotherapy significantly altered overall survival. Five hundred seventy patients were evaluated for overall survival based on the number of axillary lymph nodes involved with disease. Figure 3 shows overall survival in these patients. Overall survival was better when fewer nodes were involved (P < .0001). Figure 4 shows overall survival stratified for tumor size in the 210 N0 patients. Tumor size was not shown to alter overall survival.
Figure 3. Overall survival curves for breast cancer patients with zero, one to three, and four or more positive nodes. For each time period (years, x-axis), the probability of not dying of disease is shown (y-axis). Overall survival was better when fewer nodes were positive.
Figure 4. Overall survival curves for breast cancer patients with tumors measuring 2.0 cm or less, 2.1 to 5.0 cm, and more than 5.0 cm. For each time period (years, x-axis), the probability of not dying of disease is shown (y-axis). Tumor size was not shown to affect this probability.
DISCUSSION
With general acceptance of multimodality therapy for advanced breast cancer, the role of PMR has been carefully evaluated. The primary criticism directed toward the DBCG 82B trial and the British Columbia trial has been in regards to the surgical techniques employed, especially the number of axillary lymph nodes removed. In the DCBG 82B trial, the average number of axillary lymph nodes removed was 7, 9 while in the British Columbia trial, it was 11. 10 LRR rates in each trial were higher than expected (26% in the DBCG 82B trial and 23% in the British Columbia trial). It has been suggested that more aggressive clearance of the axilla in both of these trials would have resulted in lower LRR rates and better survival. On the other hand, a 10-year review of node-positive patients with breast cancer who were entered into four Eastern Oncology Cooperative Group (ECOG) trials revealed a 21% LRR rate for all patients and a 29% rate for patients with more than 4 positive axillary lymph nodes, despite a median of 15 nodes being resected. 12 In a similar analysis from the M.D. Anderson Cancer Center, a 19% LRR rate was reported for all patients, while the rate for breast cancer patients with more than 4 positive axillary lymph nodes was 26%, despite a median of 17 nodes being resected and all patients receiving anthracycline-based chemotherapy. 13
In contrast to the DBCG 82B and British Columbia trials, several other trials of PMR in combination with chemotherapy have demonstrated reduced LRR but no significant improvement in overall survival. 14–18 However, a recent meta-analysis by the Early Breast Cancer Trialists, which included these studies, showed a significant improvement in overall survival following PMR. 19 A recent NIH/NCI Consensus Conference reviewed all of the current literature concerning PMR and concluded that patients with four or more positive axillary lymph nodes benefit from PMR. 20
In keeping with the data presented above, our analysis of outcome data from the Alabama Breast Cancer Project after 15 years of follow-up shows that increased LRR and reduced overall survival correlate with an increasing number of positive axillary lymph nodes. While there is general agreement that breast cancer patients with four or more positive nodes benefit from PMR, consensus regarding optimal therapy for patients for one to three positive axillary lymph nodes awaits completion of an ongoing Southwest Oncology Group (SWOG)/Radiation Therapy Oncology Group (RTOG) trial, which is accruing patients with one to three positive axillary lymph nodes after mastectomy and chemotherapy for randomization to chest wall and regional node radiation versus observation.
In node-negative breast cancer patients, tumor size has been considered the most important single prognostic factor for LRR and overall survival. Tumor size 1 cm or smaller has been associated with a very favorable prognosis. 21,22 Tumor size also correlates closely with axillary lymph node metastases. Fifteen percent of patients with tumors less than 1.1 cm in diameter had axillary lymph node metastases, compared to 60% of patients with tumors more than 5.5 cm in diameter. 23
To avoid the confounding effect of axillary lymph node metastases, only N0 patients from the Alabama Breast Cancer Project were analyzed to determine the effect of tumor size on LRR and overall survival. No significant correlation between increasing size of the primary breast cancer and LRR or overall survival was seen. Since pathologic tumor size was recorded for only 210 of the 290 N0 patients studied (72.4%), the findings must be carefully interpreted because of possible biases in patient selection. In addition, since only a small number of patients with tumors greater than 5 cm were studied (n = 14), conclusions regarding the utility of PMR for tumors 5 cm or larger must be formulated with caution. It is reasonable to conclude that the prognostic significance of tumor size for both LRR and overall survival is minimal in the node-positive breast cancer population because of the overriding prognostic significance of axillary lymph node involvement.
In general, for node-positive breast cancer, the radiation therapy fields for PMR routinely cover the chest wall, the supraclavicular lymph node basin, and the axillary apex. The chest wall can be treated through medial and lateral tangents or with an electron en face field. There is significant controversy among radiation oncologists about the necessity of treating the ipsilateral internal mammary lymph nodes. These nodes can either be included in the chest wall fields or treated through a separate matched field. Typical doses to the chest wall and nodal sites are 45 to 50 Gy, with 1.8 to 2.0 Gy per day fractionation. A boost to the chest in the region of the mastectomy scar is indicated for T4 tumors or positive surgical margins, providing for a total dose of 60 Gy in the boost field.
Even with the prevalent use of mammography, 5% to 15% of breast cancers are locally advanced at diagnosis. 1 Neoadjuvant chemotherapy is becoming increasingly common in the treatment of locally advanced breast cancer. The goal of neoadjuvant chemotherapy is to reduce the tumor size before the institution of local therapy. In addition, the sensitivity of a tumor to a therapeutic regimen becomes known, and nonresponders can be switched to a different regimen for continued neoadjuvant or subsequent adjuvant therapy. Radiation therapy has been used in combination with neoadjuvant chemotherapy, allowing breast-conservation therapy in 24% to 85% of patients. 24–30 Locoregional failure rates after breast-conserving therapy in these series have ranged from 6.8% to 19%. In many of these series, radiation therapy alone without surgery was administered when a complete clinical response to chemotherapy was achieved. 24–27
Discussion
Dr. William C. Wood (Atlanta, GA): I would first compliment Drs. Beenken and Bland and their colleagues for another thoughtful check on our assumptions about breast cancer. You base this on a data set of patients managed on a prospective protocol that you now rush to publication with only 15 years of median follow-up. You address local/regional failure and confirm the significance of lymph node metastases number as a prognostic factor after first- or second-generation adjuvant chemotherapy, and you rightly relate this to considerations of postmastectomy radiation therapy.
Our meta-analysis of 52 randomized trials addressing this question in over 25,000 women was reviewed at the Early Breast Cancer Clinical Trial Collaborative Meeting at Oxford 2 years ago. I am sorry to say that it is still not published. At 25 years of follow-up, we see zero benefit on survival from postmastectomy radiation. Radiation reduces local failure, and thus deaths from breast cancer, leading to a small improvement in survival seen at 10 years. Beginning at about 8 to 10 years, there is an increased rate of death in the radiated patients that wipes out the initial benefit—for example, a 28% increase in cardiovascular deaths beginning at 8 to 10 years. This was suggested to be caused by radiation to the heart with left-sided breast cancer. But in fact we have found that this detriment is identical for right- and left-sided breast cancer.
As the magnitude of this radiation detriment is fixed, your demonstration that positive node number, not tumor size, can be used to select the group of patients at higher risk of local/regional failure allows us to tip the survival balance in their favor.
My questions: First, why did you see no association of local/regional failure with tumor size in a mature data set that confirms the nodal association? A couple of obvious possibilities. One is, with very good surgery and adjuvant chemotherapy, you achieved very good local control. You only had 12 patients who had local failure in your N0 group, and it may just be too few numbers. Or, do you think that by appropriately limiting your analysis of tumor size to the N0 patient, you biased your answer? The larger the tumor grows while remaining node-negative, the more you select for tumors of lower biologic aggressiveness. But the smaller tumors include those of higher aggressiveness, but benefit from early detection.
Second question, did any of these women receive postmastectomy radiation?
Dr. R. Phillip Burns (Chattanooga, TN): I appreciate being asked by the Alabama group to comment on this well-done study with long-term follow-up in a disease that affects our patients and exposes them to a lifetime risk.
Among other things to appreciate in this report is the fact that it confirms the bias that many of us have regarding the sometimes confusing but practical biology of breast cancer. It challenges the prevailing sentiment of some that the most significant prognostic dilemma in the patient with breast cancer is tumor size by showing that both local/regional recurrence and overall survival are more importantly affected by axillary nodal status than tumor size. The paper does give rise to several questions.
Your study appropriately looks at tumor size as a sole predictor of outcome in the 290 patients who were node-negative, of which you were able to measure only 210. But in looking further at the tumor size issue, did you evaluate it in regards to the different categories of node status (i.e., one to three positive nodes rather than four), and can you suggest any additive prognostic effect for local recurrence from both large tumor and node-positive status?
In the discussion you mentioned that multiple studies evaluating the effectiveness of postmastectomy radiation have analyzed the total number of axillary nodes removed at the time of mastectomy, with a range of 7 to 17 nodes in those studies. I did not, however, discover the number of nodes that you removed and would like to know what was the average number of resected nodes and was there any difference in total number of removed nodes between the node-negative and node-positive group.
Since your results somewhat reduce concern about tumor size in breast cancer, have the results of this study had any effect on your willingness to further stratify patients to neoadjuvant therapy in an effort to downstage the disease and make more patients candidates for breast-conserving treatment? I would assume that would be the case.
You made the point of the 2001 NIH Consensus Conference clearly recommending postmastectomy radiation in patients with greater than four positive nodes. But what about the one-to-three category that you reference? I know you are participating in cooperative trials regarding this issue. But what is your current practice and recommendation to nonstudied patients in your practice, or what do you recommend to the practicing surgeon regarding this patient category of one to three positive nodes?
Dr. Charles E. Cox (Tampa, FL): Thank you for the opportunity to discuss this paper. I haven’t had a chance to review the manuscript, and actually the questions that I had were pretty much preempted by the discussants today.
The main question I wish to ask: Did patients with four or more nodes have radiation therapy in this group, or were there any that did, since that is the standard that has been applied for as much as 20 years? I think, again in discussion, as Dr. Wood pointed out, the attendant long-term morbidities of radiation therapy to the chest wall are critical to the outcomes of this paper. And I would certainly ask the authors if they are planing to include that in the paper. It would be nice if Dr. Wood’s data were published; I think that would be very helpful.
Dr. Edward M. Copeland, III (Gainesville, FL): I am interested, Dr. Beenken, in the subset of patients that had local/regional failure. There are those who have thought that after mastectomy local/regional failure without distant disease at the time of presentation represents stage 4 disease. You gave the survival of the entire group. What happened to those patients who had local/regional failure? Did they all die?
Many years ago at the M.D. Anderson Hospital we looked up a group of patients who had local/regional failure after mastectomy and without distant disease. We found 60 patients. All 60 of these patients died, unfortunately, of metastatic breast cancer. That local/regional failure is stage 4 disease has been challenged on actually very few occasions, because it is studied infrequently in patients who have had mastectomy. You have the data. Have you mined it to find out what happened to those women who failed?
Dr. Samuel W. Beenken (Birmingham, AL): The conventional wisdom of the last few decades has been that breast cancer is a systemic disease, that involvement of regional nodes is a sign of distant spread, and that treatment of locoregional sites will not affect overall survival. However, we know that not all node-positive breast cancer patients die of their disease. Two series reported in the literature—one by Adair from Memorial Sloan-Kettering in 1974 and another by Veronesi in Milan in 1985—showed that disease-free and overall survival rates at 10 years were approximately 20% and 50% in patients with involved axillary and/or internal mammary lymph nodes.
On the other hand, many patients who are at high risk for locoregional recurrence harbor occult distant metastases. Without systemic chemotherapy, most of these patients will succumb to their disease. If the locoregional tumor burden is greater than that at distant sites or more resistant to systemic therapy, additional locoregional therapy could improve both disease-free and overall survival. In fact, as the efficacy of systemic therapy improves, the ability of additive locoregional radiation therapy to improve survival may become increasingly apparent.
In answer to Dr. Wood’s question, the primary reason that large tumors did not recur locally was good surgical technique. The Alabama Breast Cancer Project carefully instructed the involved surgeons as to the surgical techniques to be employed, thereby providing for uniformity as to the nature and extent of surgery.
Our analysis of tumor size in only N0 patients allowed us to focus on those patients and those clinical circumstances that were most important in predicting locoregional recurrence. There is a significant correlation between tumor size and the presence of axillary lymph nodes, so N0 patients only were studied to avoid the confounding influence of axillary lymph node status. While I did not present this data, a separate analysis of the total study population did not show tumor size to significantly influence locoregional recurrence regardless of lymph node status.
Finally, postoperative radiotherapy was strongly discouraged in this study, but for a variety of reasons, some patients did receive such therapy. Documentation regarding adjuvant radiotherapy was available for 343 patients. Two of 36 patients (5.6%) developing locoregional recurrence had adjuvant radiotherapy, while 20 of 285 patients (7.0%) who did not develop locoregional recurrence had adjuvant radiotherapy. There was no significant difference between these rates.
In answer to Dr. Burns’ question, the mean number of lymph nodes removed at the time of surgery was 18, and there was no difference in the number of lymph nodes removed when comparing the node-positive and node-negative populations.
As mentioned previously in this discussion, while tumor size was not shown to influence locoregional recurrence regardless of lymph node status, it did influence overall survival (P = .018). However, when multivariate analysis was performed, this effect was no longer seen.
In practice, I recommend that the surgeon speak to the patient with one to three positive axillary lymph nodes before that patient sees the radiotherapist and to very honestly explain the dilemma that we have. It is very reasonable to tell the patient that there is a conflict of opinion. Enrolling the patient in a clinical trial designed to answer the question is a reasonable alternative to making a decision based on inadequate data.
Finally, for patients with four or more positive lymph nodes, adjuvant radiotherapy was not recommended.
Dr. Copeland, the Alabama Breast Cancer Project only documented the first recurrence of breast cancer.
References
- 1.Osteen RT, Karnell LH. The National Cancer Data Base Report on breast cancer. Cancer. 1994; 73: 1994–2000. [DOI] [PubMed] [Google Scholar]
- 2.Donegan WL, Perez-Mesa CM, Watson FR. A biostatistical study of locally advanced breast carcinoma. Surg Gynecol Obstet. 1966:529–540. [PubMed]
- 3.Valagussa P, Bonadonna G, Veronesi U. Patterns of relapse and survival following radical mastectomy. Cancer. 1978; 41: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 4.Host H, Brennhovd IO, Loeb M. Postoperative radiotherapy in breast cancer: long-term results from the Oslo study. Int J Radiat Oncol Biol Phys. 1986; 12: 727–732. [DOI] [PubMed] [Google Scholar]
- 5.Palmer MK, Riberio GG. Thirty-four year follow-up of patients with breast cancer in a clinical trial of postop irradiation. Br Med J. 1985; 291: 1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallgren A. A controlled study: preop vs. postop irradiation. Int J Radiat Oncol Biol Phys. 1977; 2: 1167–1169. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987; 71: 15–25. [PubMed] [Google Scholar]
- 8.Cuzick J, et al. Cause-specific mortality in log-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994; 12: 447–453. [DOI] [PubMed] [Google Scholar]
- 9.Overgaard M, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 2000; 337: 949–955. [DOI] [PubMed] [Google Scholar]
- 10.Ragaz J, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 2000; 337: 956–962. [DOI] [PubMed] [Google Scholar]
- 11.Maddox MA, Carpenter JT, Laws HL, et al. A randomized prospective trial of radical (Halstead) mastectomy versus modified radical mastectomy in 311 breast cancer patients. Ann Surg. 1983; 198: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recht A, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999; 17: 1689–1700. [DOI] [PubMed] [Google Scholar]
- 13.Katz A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18: 2817–2827. [DOI] [PubMed] [Google Scholar]
- 14.Olson JE, et al. The role of radiotherapy in the management of operable locally advanced breast carcinoma; results of a randomized trial by the Eastern Cooperative Oncology Group. Cancer 1997; 79: 1138–1149. [PubMed] [Google Scholar]
- 15.Blomqvist C, et al. The combination of radiotherapy, adjuvant chemotherapy and tamoxifen in stage II breast cancer: long-term follow-up of results of a randomized trial. Br J Cancer. 1992; 66: 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velez-Garcia E, et al. Postsurgical adjuvant chemotherapy with or without radiotherapy in women with breast cancer and positive axillary nodes: a South-Eastern Cancer Study Group (SEG) trial. Eur J Cancer. 1992; 28: 1833–1837. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 18.Griem KL, et al. The 5-year results of a randomized trial of adjuvant radiation therapy after chemotherapy in breast cancer patients treated with mastectomy. J Clin Oncol. 1987; 5: 1546–1555. [DOI] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists’ Collaborative Group. Favorable and unfavorable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials. Lancet. 2000; 355: 1757–1770. [PubMed] [Google Scholar]
- 20.National Institutes of Health Consensus Development Conference Statement. Adjuvant therapy for breast cancer, Nov. 1–3, 2000. J Natl Cancer Inst. 2001;93:979–989. [DOI] [PubMed]
- 21.Ries LA, Henson, DE, Harras A. Survival from breast cancer according to tumor size and nodal status. Surg Oncol Clin North Am. 1994; 3: 35–52. [Google Scholar]
- 22.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. The Surveillance, Epidemiology, End Results (SEER) program of the National Cancer Institute. Cancer. 1989; 63: 181–187. [DOI] [PubMed] [Google Scholar]
- 23.Rosen PP, Groshen S, Saigl PE, et al. A long-term follow-up study of survival in Stage I (T1N0M0) and Stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989; 7: 355–366. [DOI] [PubMed] [Google Scholar]
- 24.Eltahir A, Heys SD, Hutcheon AW. Treatment of large and locally advanced breast carcinomas using neoadjuvant chemotherapy. Am J Surg. 1998; 175: 127–132. [DOI] [PubMed] [Google Scholar]
- 25.Merajver SD, Weber BL, Cody R. Breast conservation and prolonged chemotherapy for locally advanced breast cancer: the University of Michigan experience. J Clin Oncol. 1997; 15: 2873–2881. [DOI] [PubMed] [Google Scholar]
- 26.Touboul E, Buffat L, Lefranc JP. Possibility of conservative local treatment after combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer. Int J Radiat Oncol Biol Phys. 1996; 34: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 27.Calais G, et al. Conservative treatment feasibility with induction chemotherapy, surgery, and radiotherapy for patients with breast carcinoma larger than 3 cm. Cancer. 1994; 74: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 28.Bonadonna G, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of 3 centimeters or more. J Natl Cancer Inst. 1990; 82: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 29.Bonadonna G, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GF, Birchansky CA, Komarnicky LT. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994; 73: 362–369. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Samuel W. Beenken, MD, Department of Surgery, University of Alabama at Birmingham, Suite 620 WTI, 1530 3rd Avenue South, Birmingham, AL 35294.
E-mail: samuel.beenken@ccc.uab.edu
Accepted for publication December 2002.