Abstract
Objective
To analyze outcomes after liver transplantation (LT) in patients with fulminant hepatic failure (FHF) with emphasis on pretransplant variables that can potentially help predict posttransplant outcome.
Summary Background Data
FHF is a formidable clinical problem associated with a high mortality rate. While LT is the treatment of choice for irreversible FHF, few investigations have examined pretransplant variables that can potentially predict outcome after LT.
Methods
A retrospective review was undertaken of all patients undergoing LT for FHF at a single transplant center. The median follow-up was 41 months. Thirty-five variables were analyzed by univariate and multivariate analysis to determine their impact on patient and graft survival.
Results
Two hundred four patients (60% female, median age 20.2 years) required urgent LT for FHF. Before LT, the majority of patients were comatose (76%), on hemodialysis (16%), and ICU-bound. The 1- and 5-year survival rates were 73% and 67% (patient) and 63% and 57% (graft). The primary cause of patient death was sepsis, and the primary cause of graft failure was primary graft nonfunction. Univariate analysis of pre-LT variables revealed that 19 variables predicted survival. From these results, multivariate analysis determined that the serum creatinine was the single most important prognosticator of patient survival.
Conclusions
This study, representing one of the largest published series on LT for FHF, demonstrates a long-term survival of nearly 70% and develops a clinically applicable and readily measurable set of pretransplant factors that determine posttransplant outcome.
Fulminant hepatic failure (FHF), also referred to as hyperacute, acute, or subacute liver failure, is a life-threatening condition that occurs in approximately 2,000 individuals each year in the United States. 1 While the etiologies of FHF are multiple and varied, the prognosis is dependent on several factors, including the underlying cause of liver failure. For instance, it is well known that the spontaneous recovery rates from FHF from such etiologies as hepatitis A 1 and acetaminophen toxicity 2 are high, whereas those same rates for other types of viral hepatitis and idiosyncratic drug reactions are quite low. 3 Advances in intensive care and medical management as well as the development of artificial liver support systems 4,5 have no doubt led to some modest improvements in outcomes. Unfortunately, without liver transplantation (LT), the overall prognosis for patients with FHF is quite poor, with survival rates usually reported between 10% and 30%. 6 The most common cause of death from FHF is either cerebral edema or sepsis. 7
LT has not only revolutionized but remains the gold standard for the treatment of irreversible FHF. There are several series reported from transplant centers in North America 8–15 and Europe 16–23 in support of this statement. However, outcomes are limited by the timely availability of suitable donor organs, despite the fact that these patients are afforded the highest priority in organ allocation systems. 24,25 Still, overall survival rates reported after LT for irreversible FHF are superior to those reported with any form of medical management, demonstrating a 40% to 75% long-term survival.
Without a doubt, predicting whether the patient with FHF will require a life-saving transplant or will recover with medical management alone is difficult. Many studies have attempted to identify prognostic indicators in patients with FHF that will support this clinical decision. The two most widely applied predictive criteria are those from Kings College Hospital in London 3 and Hopital Beaujon in Clichy, France. 26,27 The utility of these criteria has been validated by other investigators, 28–30 thus supporting the use of these prognostic indices as an adjunct in the clinical decision as to the potential for spontaneous recovery with medical management versus the need for LT in patients with FHF.
In contrast, few investigations have examined predictive factors for patients who have irreversible FHF awaiting LT. The King’s College group 21 reviewed 100 transplant recipients and determined that the serum creatinine and APACHE score was the most important prognosticator. Bismuth et al., 22 after reviewing 116 transplant recipients, reported that the grade of coma and the use of high-risk donor liver grafts predicted outcome. Our group, 14 in a review of 57 pediatric transplants for FHF, determined that age and ventilator dependence were predictive factors for outcome. Study size was a potential limiting factor in the statistical analysis for each of these investigations. Therefore, the aim of this investigation was to analyze 35 pretransplant variables in a large group of patients who had undergone LT for irreversible ALF in an effort to determine prognostic indices in this patient population.
METHODS
A retrospective analysis was undertaken of all patients undergoing LT at the Dumont-UCLA Transplant Center from the initiation of the program on Feb. 1, 1984, until Dec. 31, 2001. Patients were identified from a transplant database that encompasses all such patients. Diagnoses were cross-referenced through the anatomic pathology database maintained in the Department of Pathology at the David Geffen School of Medicine at UCLA using the keyword searches “submassive necrosis,” “massive necrosis,” “acute liver failure,” and “fulminant liver failure.” Only those patients meeting the following criteria for acute liver failure were included in the investigation: 1) absence of a clinical history of liver disease, 2) absence of physical findings consistent with chronic liver disease, 3) explant pathology demonstrating predominantly necrosis in the absent of cirrhosis. Our early experience with this group of patient has been reported previously. 14,31,32
Data collection was then undertaken using several available medical records sources, including UCLA hospital medical records, UCLA transplant services database, Dumont-UCLA transplant center records and database, and the United Network for Organ Sharing (UNOS) database. The primary endpoints used in this investigation were patient and graft survival. The 35 pretransplant predictive factors are shown in Table 1, divided into pretransplant recipient variables, pretransplant recipient laboratory variables, donor variables, and operative variables. As most variables are self-explanatory, only those requiring definition are discussed here.
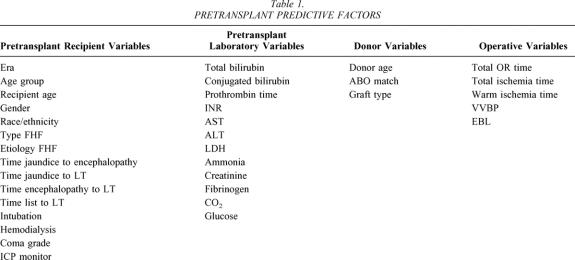
Table 1. PRETRANSPLANT PREDICTIVE FACTORS
The era of transplant was delineated by dividing the entire experience into three equal blocks of 6 years (1984–1989, 1990–1995, 1996–2001). For age group, 18 years was used as the cutoff between adults and children. Age was used as both a continuous variable based on the median and as a block variable (<1 year old, 1–6 years old, >6–18 years old, >18–50 years old, or >50 years old) in an effort to identify at-risk groups by age. The nomenclature for the type of FHF has been controversial. 33–36 For the purpose of this manuscript, FHF was used as a generic term to encompass all patients meeting the inclusion criteria. Forty-two days from the onset of jaundice to encephalopathy serves as the division between those patients classified as having acute liver failure (ALF) and those classified as having subacute liver failure (SALF). From those patients with ALF, a separate group was delineated due to the rapid onset of liver failure within 7 days of jaundice (hyperacute liver failure [HALF]). The etiology of liver failure was stratified into acetaminophen, other drugs, hepatitis A, other viruses, toxins, metabolic, and other, including mostly cryptogenic causes. The time from list to LT was the time from listing the patient with UNOS to transplantation. The coma grade was grouped into no coma at the time of LT (grade 0) versus grades 1 plus 2, or grades 3 plus 4. 37 The donor variable “ABO blood group match” was stratified into identical, compatible, or incompatible, as was the donor graft type (cadaveric whole grafts, living related segmental grafts, ex vivo reduced segmental grafts, and in situ split segmental grafts). The operative variables examined were total operative (OR) time, defined from abdominal skin incision to closure, total ischemia time, defined from donor cross-clamp to recipient portal reperfusion, warm ischemia time, defined from removal of the donor liver from ice storage to portal reperfusion, the use of venovenous bypass (VVBP) during the anhepatic phase, and intraoperative blood loss (EBL), as estimated by the volume of blood transfused during the transplant.
Patient confidentiality was maintained by removing all patient-specific identifiers. Institutional review board approval was obtained. Patient follow-up was concluded on July 1, 2002, or at patient death, thereby ensuring a minimum 6-month follow-up of all survivors. The median patient follow-up time was 41.1 months (range 0–191.1 months).
Statistical analysis was undertaken using patient and graft survival as endpoints. Survival was calculated using the method of Kaplan-Meier. The log-rank test was used to compute the impact of each of the 35 pretransplant variables delineated above on patient and graft survival, ignoring the other 34 factors. The results are reported as event (patient death or graft lost) per 1,000 patient or graft months of follow-up. P < .05 was considered significant, although P values = .05 to 1 were considered for further analysis.
Based on the univariate analyses previously reported and a clinical knowledge of what factors are useful preoperatively, 11 potential predictors were chosen for multivariate consideration: age (<1 year, 1–6 years, actual age if >6 years), time from jaundice to encephalopathy, race/ethnicity, intubation, hemodialysis, coma grade (3–4 vs. 1–2), donor age, conjugated bilirubin, INR, ALT, and creatinine. The models are based on finding subsets of these 11 factors that simultaneously predict the outcome (death or graft failure). Factors were not retained in a model if they were not simultaneously significant after accounting for factors already in the model. Of the 204 persons, data were complete on all 11 factors above in 178. The other 26 had missing data on at least one of the 11 factors.
These 11 factors were simultaneously assessed using tree structured survival analysis (TSSA) methods, which do not assume that the factors are additive. 38 Once a subset of the 11 predictive factors was identified by TSSA and the data were partitioned into risk groups (nodes), relative risk was computed empirically and also under a proportional hazard (Cox) model. The TSSA model uses the method of recursive binary partitioning to choose a set of variables that can define and identify high-, medium-, and/or low-risk subsets of subjects. The TSSA method looks at all values of all 11 candidate variables and then chooses a critical value C, the best value of the best variable, such that the survival is as significantly different as possible between those whose values are lower than C compared to those whose values are greater than or equal to C, splitting the subjects into two non-overlapping risk groups or tree “nodes.” The log-rank test is used to determine if splitting using the value C results in statistically significant differences. For a given variable, all possible values of C are tried and the best one is selected. The subjects are thus partitioned into a finite set of mutually exclusive groups (terminal nodes) where the risk of mortality (or graft failure) is as different as possible between the groups and is similar within a group.
RESULTS
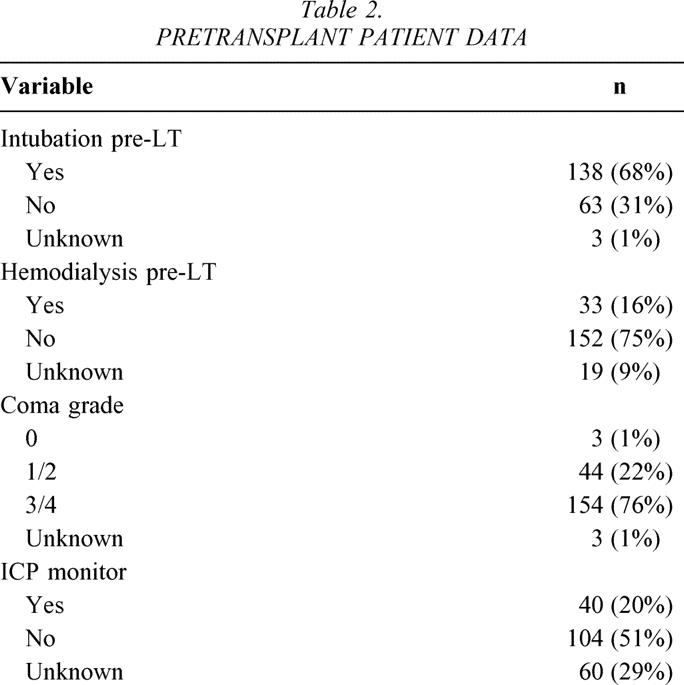
Of the 3,205 LTs undertaken during the study interval, 204 (6.4%) patients underwent LT for FHF. Figure 1 demonstrates the number of transplants performed per year for FHF, with the highest number performed in 2000 (n = 21) and the lowest in 1984 (n = 0). The number of patients transplanted per era was 38 in 1984 to 1989, 82 in 1990 to 1995, and 84 in 1996 to 2001. Median age was 20.2 years (range 0.2–72.1 years), 55% of patients were adults, 60% were women, and the predominant racial groups were white (40%), Latino/Hispanic (43%), African American (9%), Asian (8%), and other (<1%). The majority of patients had either ALF (48%) or HALF (45%), with a smaller number classified as SALF (7%). The median time from onset of jaundice to encephalopathy was 9 days (range 0–109 days), median time from onset of jaundice to LT was 15 days (range 0–152 days), and median time from onset of encephalopathy to LT was 4 days (range 0–72 days). Median time from listing to LT was 2 days (range 0–88 days). By far the majority of patients were the equivalent of status 1 using current UNOS listing criteria. 39 The etiologies of FHF are shown in Figure 2, with the majority of patients classified as “other” consisting mostly of cryptogenic FHF. The pretransplant clinical condition of the patients is shown in Table 2.
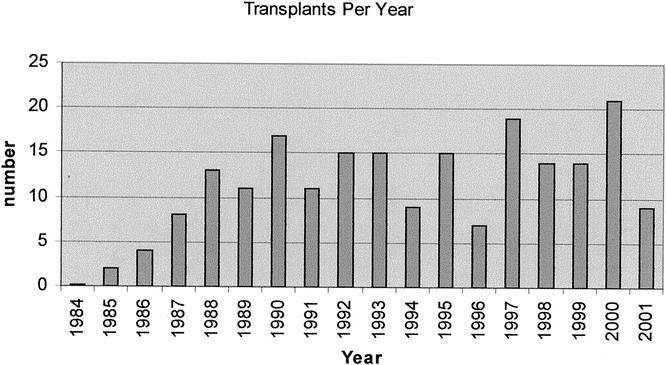
Figure 1. Transplants per year.
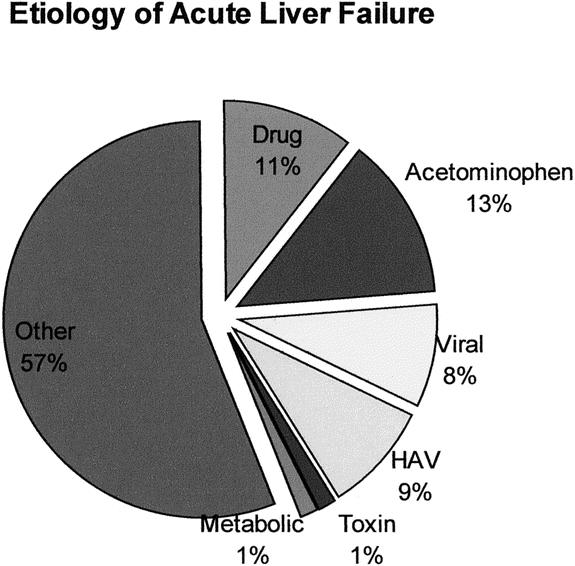
Figure 2. Etiology of acute liver failure.
Table 2. PRETRANSPLANT PATIENT DATA
Twelve pretransplant laboratory values were assessed. The median (range) of the serum total bilirubin was 24.05 mg/dL (2.4–60.7 mg/dL), conjugated bilirubin 11.9 mg/dL (0.4–48.5 mg/dL), prothrombin time 25.8 seconds (10.3–100 seconds), INR 2.6 (1.06–1.55), AST 439.0 U/L (18–17,567 U/L), ALT 543.0 U/L (20–12,820 U/L), LDH 444.5 U/L (164–23,140 U/L), serum ammonia 147.0 mcg/dL (29–488.0 mcg/dL), serum creatinine 0.9 mg/dL (0.1–12.5 mg/dL), serum carbon dioxide (CO2) 23.0 mmol/L (5–35.0 mmol/L), fibrinogen 138.0 mg/dL (15.0–432.0 mg/dL), and serum glucose 113.0 mg/dL (6.0–44.0 mg/dL).
Three donor characteristics were analyzed. The analysis was limited to the donor used for the primary transplant, thereby excluding all donors used for retransplantation. The median donor age was 24.0 years (range 0.2–78 years). Donor–recipient ABO blood group matching was identical in 61%, compatible in 22%, incompatible in 13%, and unknown for 4%. The majority (80%) of liver grafts were procured as whole organs. Living donors of left lateral segments and ex vivo reduced cadaveric grafts accounted for 5% each of total grafts used. The remaining grafts were procured using the cadaveric in situ split liver technique previously described. 40
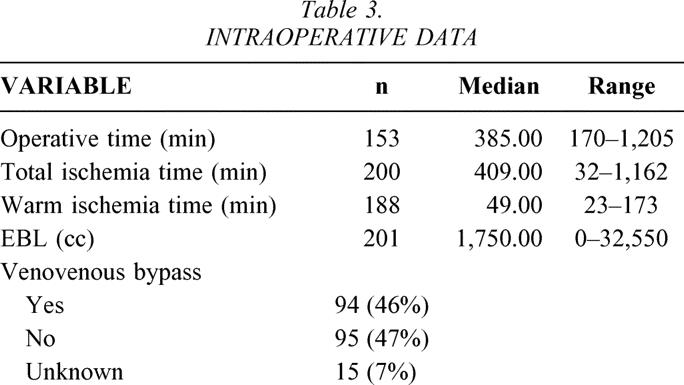
Five intraoperative variables were examined (Table 3).
Table 3. INTRAOPERATIVE DATA
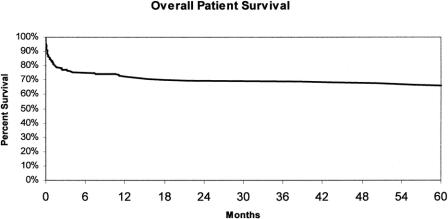
Overall patient survival is shown in Figure 3. Survival at 1, 3, and 5 years after LT was 72.5% ± 3.1%, 69.8% ± 3.2%, and 66.9% ± 3.4%, respectively. There were 72 deaths; the predominant cause of death was sepsis and multisystem organ failure (Fig. 4). Interestingly, 22% (n = 16) of the deaths were due to neurologic complications, of which 13 occurred within 1 month of the transplant and resulted directly from FHF. There were nine (14%) deaths caused by cardiac failure, eight of which occurred within 1 month of transplantation. There were three intraoperative deaths included as cardiac deaths for an overall intraoperative mortality of 1.5%.
Figure 3. Overall patient survival.
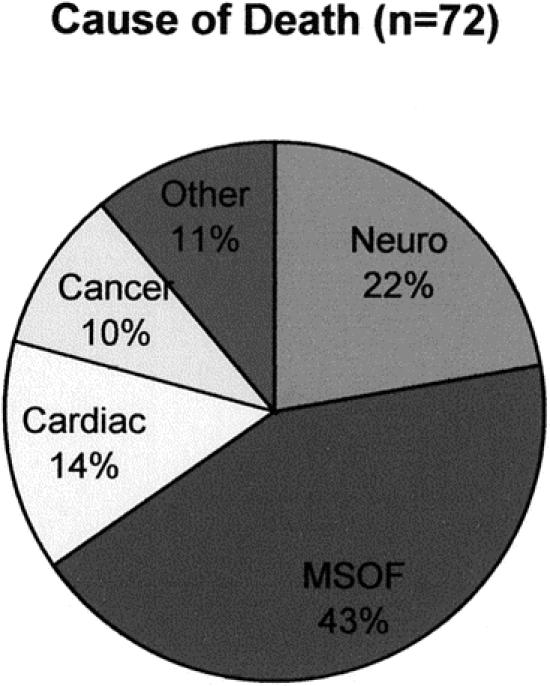
Figure 4. Causes of death.
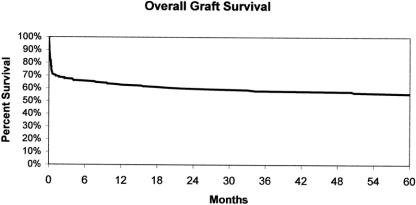
The overall graft survival is shown in Figure 5. Survival at 1, 3, and 5 years after LT was 63.2% ± 3.4%, 58.0% ± 3.5%, and 56.6% ± 3.6%, respectively. There were 95 grafts lost after LT, with the predominant cause of loss being patient death (that is, the recipient died with a functioning allograft) (Fig. 6). Twenty-seven grafts were lost to primary nonfunction, representing 29% of all grafts lost or 13.2% of the total number of grafts transplanted. The overall rate of hepatic artery thrombosis was 4.4% of all transplants or 9% of all grafts lost.
Figure 5. Overall graft survival.
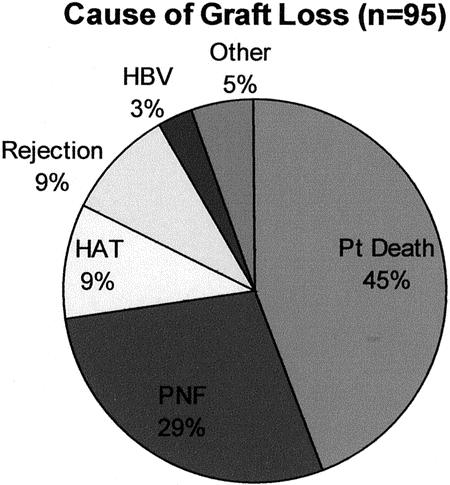
Figure 6. Cause of graft loss.
Univariate Analysis
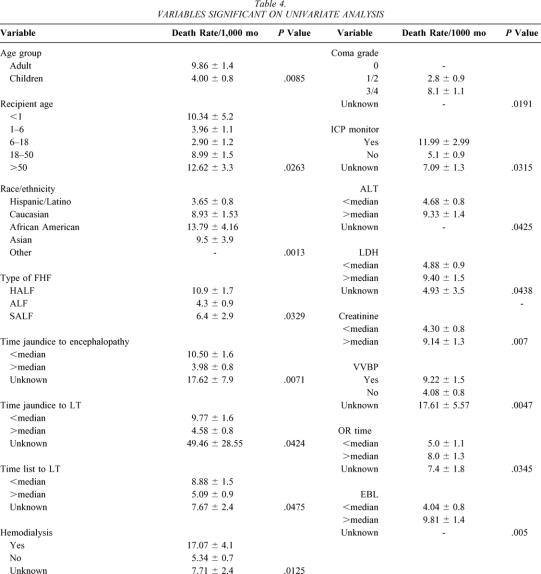
Table 4 shows the variables that had a significant impact on patient survival. The variables intubation, conjugated bilirubin, and INR were found to have some influence on patient survival (P > .05 and P < 0.1). The following variables were found in this analysis not to be predictors of patient survival: era, gender, etiology of FHF, time from encephalopathy to LT, total bilirubin, prothrombin time, AST, ammonia, carbon dioxide, fibrinogen, glucose, donor age, ABO blood group match, donor graft type, total ischemia time, and warm ischemia time.
Table 4. VARIABLES SIGNIFICANT ON UNIVARIATE ANALYSIS
For graft survival, the variables that were found to have a significant (P < .05) impact on outcome are shown in Table 5. The variables ethnicity/race, time from jaundice to encephalopathy, ICP monitor, VVBP, and donor age were found to have some influence on graft survival (P > .05 and P < 0.1). The following variables were found in this analysis not to be independent predictors of graft survival: era, age group, recipient age, gender, type of FHF, etiology of FHF, time encephalopathy to LT, time list to LT, intubation, coma grade, total bilirubin, prothrombin time, INR, AST, ALT, LDH, ammonia, carbon dioxide, fibrinogen, glucose, ABO blood group match, graft type, operative time, total ischemia time, and warm ischemia time.
Table 5. VARIABLES WITH A SIGNIFICANT IMPACT ON GRAFT SURVIVAL

Multivariate Analysis
Variables were chosen based on univariate statistical strength as well as potential clinical impact. Only one of several overlapping or potentially redundant variables such as age group and recipient age was considered for the analysis. Furthermore, any variable that could not be predicted pretransplant was avoided, such as EBL, operative time, warm ischemia time, and total ischemia time. As shown in Figure 7, the multivariate analysis revealed creatinine as the strongest predictor of post-LT patient mortality and identified the critical value as 1.45 mg/dL. The time from jaundice to encephalopathy, with a critical value of 3.5 days, and INR, with a critical value of 2.48, were also predictors of outcome. A strongly significant survival difference was seen in the mathematical model when patients were separated into the nodes based on the critical values of these three factors. Notably, although the other eight factors did not change the significance of the mathematical model beyond that of these three, these factors are not insignificant. As seen in Figure 8, multivariate analysis revealed the conjugated bilirubin as the strongest predictor of post-LT graft failure, with the critical value 5.65 mg/dL. The serum creatinine, with a critical value as above, and the donor age, with a critical value of 23.05 years, were also important predictors. Again, the predictive ability of these three factors was strongest for graft survival, and adding the other eight values to the model did not improve the predictability.
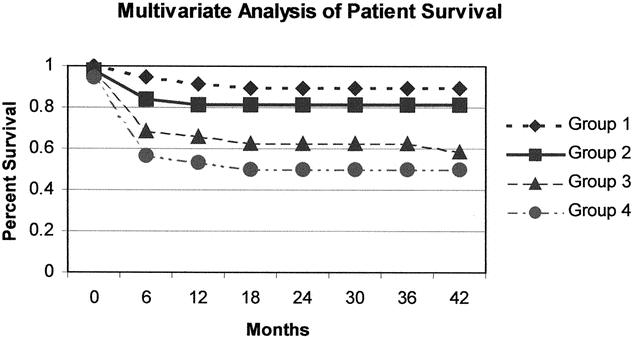
Figure 7. Group 1: Creatinine <1.45 mg/dL and time Jaundice to encephalopathy >3.5 days and INR >2.48; Group 2: Creatinine <1.45 mg/dL and time Jaundice to encephalopathy >3.5 days and INR <2.48; Group 3: Creatinine <1.45 mg/dL and time Jaundice to encephalopathy <3.5 days; Group 4: Creatinine >1.45 mg/dL regardless of other 2 values. P < 0.0001.
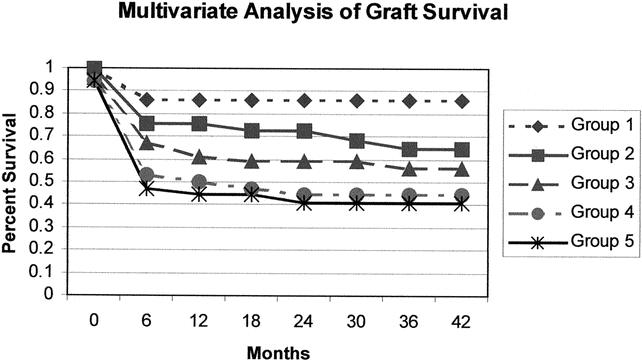
Figure 8. Group 1: Conjugated Bilirubin >11.95 and creatinine <1.45 and donor age <23.05; Group 2: Conjugated Bilirubin >11.95 and creatinine <1.45 and donor age >23.05; Group 3: Conjugated Bilirubin >5.65 and <11.95 and creatinine <1.45; Group 4: Conjugated Bilirubin >5.65 and creatinine >1.45; Group 5: Conjugated Bilirubin <5.65. P = 0.0005.
DISCUSSION
To our knowledge, this study represents the largest single-center series of patients undergoing LT for irreversible FHF. Furthermore, it represents one of the most comprehensive investigations to date examining the impact of pretransplant factors on patient and graft survival after LT for FHF. The objective of this investigation was not to identify predictive variables to aid in the identification of patients with irreversible FHF as compared to those with a strong likelihood of spontaneous recovery with medical management. Instead, we sought to examine the factors that predict poor outcome in patients already deemed to have irreversible FHF and awaiting LT. The results of this analysis may serve to aid the clinician in assessing the prognosis for patients with irreversible FHF undergoing LT.
The survival outcomes reported herein reinforce the impact that LT has had on the treatment of this acute variant of liver disease. Five-year patient and graft survivals of 66.9% and 56.6% support the life-saving potential of LT, as has been reported in the literature. 10,13,21,22,24,41 These results are even more dramatic when considering that the majority of these patients were extremely ill, with multiorgan involvement. Most of the deaths in this series were due to sepsis/multisystem organ failure, as has been reported by other investigators. 21,22 There was notably a high incidence of neurologic deaths after LT in this series. Although one group did not report any neurologic deaths after LT for FHF in a very selected group of 35 patients, 13 most others 21,22 have also noted a significant incidence of neurologic deaths, ranging from 4% to 11%. These findings reflect the difficulty that exists in predicting neurologic outcomes after LT. Our management strategy for neurologic protection is similar to those commonly reported in the literature. 1,42 Although ICP monitoring was applied in only 20% of the patients in this series, it has been universally considered in all patients with grade 3 or 4 coma since 1990. However, based on our analysis presented herein, the routine placement of ICP monitors in every patient with grade 3 or 4 coma does not appear to be supported. Between 1984 to 1989 there was essentially no ICP monitoring and there were four (10.5%) early neurologic deaths. From 1990 to 1995, 14 patients had ICP monitors placed and there were four (4.9%) early neurologic deaths, including 1 patient with an ICP monitor. In contrast, in our most recent experience after 1995, ICP monitors were placed in 26 patients, and there were five (6.1%) early neurologic deaths; 4 of these 5 patients had ICP monitors in place. These results demonstrate that ICP monitoring is being used more frequently and that high-risk patients are also being targeted more accurately. Furthermore, despite monitoring and treatment, neurologic death could not be prevented in some patients. These data have prompted a more detailed analysis of the neurologic evaluation, monitoring, and outcomes in this patient population.
The rate of primary nonfunction of the transplanted liver in this population was significant. Other reports on LT for patients with FHF noted incidences of primary nonfunction between 0% and 16%. 13,21,22,41 These rates are notably higher than those reported after LT for other indications (6–11%). 12,17,43 The well-known contributions of both donor and recipient factors are no doubt applicable here. First, with the highly urgent nature of the recipient’s liver failure, the first available organ was used in many instances. Second, the advanced, and many times extreme, nature of the recipient’s organ failure can negatively affect the early function of the transplanted allograft. This observation is supported in this series, where 19 of 27 (70.4%) patients with primary nonfunction died a median of 1.1 months after LT. The interplay between these two variables—acutely ill recipient with multisystem organ failure and marginal donor quality—cannot be overemphasized. While this investigation did not focus on donor quality and outcome, several donor factors were examined. Donor age did appear in the univariate analysis to have a modest effect on graft survival. In contrast to Bismuth et al.’s work, 22 the graft type (whole, split segment, reduced segment, living donor segment) in this study did not significantly affect patient or graft survival, although close scrutiny of the data reveals that recipients of in situ split liver grafts had 24.7 graft failures per 1,000 graft months, whereas recipients of whole, living donor, or ex vivo split grafts had 6.3 to 11.9 graft failures per 1,000 patient months (P = .58). Our data also did not show a statistically significant negative impact on outcome with ABO matching, as reported by other groups. 22,44 However, a clinical difference was observed, with ABO-incompatible grafts developing 14.6 graft failures per 1,000 graft months as compared to 9.4 to 12.6 graft failures per 1,000 patient months for identical and compatible grafts, respectively. Again, as a result of these data, we are initiating an investigation into donor quality as it relates to outcome in this patient population.
The univariate analysis performed in this investigation confirms the well-recognized tenet that the sickest patients fare the worst after LT for FHF. This observation is supported by the significantly poorer outcome seen in those patients with HALF, shorter times between the onset of jaundice and encephalopathy, shorter times between the onset of jaundice and LT, intubation, hemodialysis, deeper coma, and higher creatinine. We consider several of the univariate pretransplant variables biased and therefore must caution against over-interpretation. For example, the patients with shorter times from listing with UNOS to LT fared worse than those with longer times. This variable is biased toward the most severely ill patients in that these patients were more likely to receive a marginal donor in an effort to achieve liver replacement quickly. The variable ICP monitor is also somewhat biased by our selective monitoring practice, described above. The variable VVPB also contains bias as we do not routinely place children on VVBP and transplant the better adult candidates off bypass. Due to these inherent trends in these data points, we obviously did not choose to include these in the multivariate analysis.
Several univariate variables resulted in findings that are worthy of further elaboration. The age bias holds true in this investigation at the univariate level. Regardless of how it was analyzed, median, adult versus child, or age groups of less than 1, 1 to 6, 6 to 18, 18 to 50 and more than 50, age demonstrated an impact on outcome. In general, the older the recipient, the worse the outcome. The exception is the other extreme of age, the less-than-1-year-old child, where the outcomes were also inferior. This observation has been reported by others. 45 The observation that the recipient racial/ethnic background has an impact on outcome was also intriguing. In our series, being Hispanic/Latino was actually protective, as these patients had mortality rates approximately half those of whites and Asians. In contrast, African Americans demonstrated mortality rates 3.8 times higher than Hispanic/Latinos and 1.5 times higher than whites and Asians. The reasons behind these findings are unknown. The UNOS data reported in the SRTR confirms our findings that racial background does affect outcome. 39
The goal of the multivariate analysis was to develop a clinically applicable, easily measurable set of variables that could serve as an adjunct in the clinical decision to proceed with transplantation of a patient with irreversible FHF. The serum creatinine with a critical value of 1.45 mg/dL was the single most discriminating pretransplant variable predicting posttransplant outcome. When combined with a variable indicating the rate of onset of FHF (i.e., time from jaundice to encephalopathy), creatinine reliably discriminated between patients with a high probability of survival (>80%) versus those with a low probability of survival (50%). Likewise, pretransplant creatinine was a significant predictor of graft survival after LT. However, in the multivariate analysis of factors predicting graft survival, the conjugated bilirubin with a critical value of 5.65 was the most discriminating variable. We interpret these data to indicate that patient factors are playing a significant role in graft outcomes; that is, the conjugated bilirubin is interpreted to reflect the severity of the pretransplant FHF, as is the creatinine. Creatinine has been shown in other patient analyses to predict outcomes after LT for a variety of other indications. 46–48
Only two other similar studies with a large number of patients that have analyzed pretransplant variables are currently published. Devlin et al. 21 analyzed 100 adult patients undergoing LT for FHF. A battery of pretransplant factors was analyzed with the endpoint of 2-month mortality. For patients with non-acetaminophen-induced FHF, the univariate analysis revealed that the cause of FHF, admission serum creatinine, serum creatinine before LT, organ system failure score, and APACHE score were significant predictors. In the multivariate analysis, creatinine was the only significant predictor of outcome. Our data reinforce these findings and expand the results with a more statistically powerful investigation. For patients with acetaminophen-induced FHF, the time from ingestion to LT, the total bilirubin at LT, and the APACHE score were significant variables in the univariate analysis, whereas the APACHE score was the only significant prognosticator in the multivariate analysis. On the other hand, Bismuth et al. 22 analyzed 116 patients with FHF from a variety of causes. Multivariate analysis revealed that patient survival was independently predicted by the use of segmental donor grafts, steatotic donor grafts, and grade 3 coma on admission, whereas graft survival was independently predicted by the use of segmental donor grafts, ABO-incompatible grafts, and grade 3 coma on admission. Although our data did not support such a profound impact of donor variables, a more detailed analysis of graft quality in our series is warranted.
In conclusion, this investigation represents the largest single-center experience with LT for FHF. The overall experience reinforces the therapeutic effectiveness of LT for the treatment of irreversible FHF. Although FHF represents less than 10% of the indications for LT, long-term survival near 70% can be achieved in this severely ill group of patients. Sepsis and neurologic complications continue to account for the majority of deaths. One of the major aims of this study was to delineate pretransplant predictors of outcomes for patients awaiting LT with FHF. Nineteen variables were identified that, when analyzed to the exclusion of others, had an impact on both patient and graft survival. The majority of these variables were either direct or indirect indices for severity of patient illness. The pretransplant serum creatinine proved to be the single most powerful predictor of patient survival, and a mathematical model based on serum creatinine, time from onset of jaundice to encephalopathy, and INR was constructed that accurately predicts patient outcome after LT for irreversible FHF. Before widespread application of this model, validation using an independent data set is recommended. The results herein should serve as a useful clinical adjunct to predict patient outcome risk before transplantation.
Acknowledgment
The authors thank Dr. Jeffrey Gornbein for his expert statistical consultation and analysis.
Discussion
Dr. Andrew S. Klein (Baltimore, MD): I would like to congratulate Dr. Farmer and Dr. Busuttil and their colleagues from UCLA on this excellent review and analysis, which has further defined specific clinical factors predictive of outcome following liver transplantation for fulminant hepatic failure. The ever-widening gap between the number of patients in need of liver replacement and the relatively static pool of donated livers demands that the transplant community continue its critical assessment of these desperately ill patients, in part to determine the relative likelihood of a successful transplant and if indeed a liver should be allocated to this patient pool. I have several questions for the authors.
In your series, the liberalized use of intracranial pressure monitors following the year 1990 was associated with a decrease in early neurological death from over 10% to approximately 5% to 6%. During this time—this is my question—were there threshold cerebral perfusion pressures which if not maintained for a defined interval would result in your decision not to proceed with liver transplantation?
Also considered in the evaluation of a patient with acute liver failure is the likelihood that these patients will develop severe posttransplant neurological injury, which although perhaps not fatal is nonetheless a devastating development. So my second question is, were the ICP monitoring data that you analyzed predictive of this nonlethal neurological complication?
You indicated in the manuscript that the less sick adult patients were not placed on venovenous bypass during the transplant procedure. Although foregoing bypass may actually decrease time of surgery, others have suggested that the additional IV fluids that are required to support blood pressure and thereby tissue perfusion during the anhepatic phase of a non-bypassed patient may lead to increased cerebral edema and increased posttransplant neurological sequelae. So my third question is, what were the criteria used in your series to select patients to either receive or not receive bypass? Did you observe any difference in the incidence of nonlethal neurological complications in these bypass versus non-bypass patients?
Finally, your data provide a compelling argument that timely transplantation of patients with fulminant liver failure can result in excellent long-term survival. The intervals between jaundice and encephalopathy, jaundice and liver transplantation, and placement on the wait list and transplantation all significantly impacted patient survival. I believe it is important to note that the median time from listing to transplantation in your series was approximately 2 days. The most current UNOS national data suggest a median time from listing to transplantation in other geographic regions of the United States of 5 to 7 days for these same patients. So my final question is, would you interpret your data as supporting broader organ-sharing arrangements than currently exist for these types of patients?
Dr. J. Michael Henderson (Cleveland, OH): Very nice paper and a great contribution. You focused in on serum creatinine as the most important marker, so I have a couple of questions related to that. Is the creatinine value the level going into the operating room or at the time of listing? How did you handle patients on dialysis for this analysis? Was there a chance
Dr. Steven M. Steinberg (Columbus, OH): I am not a transplant surgeon; therefore, my question is probably going to be very naive. But it also deals with the creatinine. Were you able to distinguish any difference in outcome in those patients that have an elevated creatinine on the basis of acute tubular necrosis versus hepatorenal syndrome?
Dr. Ronald W. Busuttil (Los Angeles, CA): There is no doubt that transplanting a patient with fulminant hepatic failure is one of the most challenging endeavors that a transplant surgeon can undertake. These patients not only need a perfect operation, but they need even more perfect pre- and postoperative management to get them through it. Some of the questions have delved deeply in how we manage these patients.
Dr. Klein has inquired about ICP monitoring. We have experienced an evolution in the use of ICP monitoring for the years in our program. When we first started, we did not monitor patients at all. After 1980 or so, we placed ICP monitors in all patients because of an experience reported in the literature. In the last several years we have reached the conclusion that perhaps it is better to be selective in using ICP monitors because of the inherent hazards in these devices. We favor the extradural Camino catheter at our center. We have found that if we use the ICP monitor for only those patients that are in grade 3 or grade 4 coma, that we can probably help our anesthesiologists manage the patients during the procedure and avoid the large swings of intracranial pressure which precipitate herniation.
Regarding the use of ICP monitors to determine a patient’s candidacy, we use a cerebral perfusion pressure of less than 50 mmHg for greater than 2 hours as a cutoff. Unfortunately, we do not have an analysis for determining whether ICP monitoring can predict those patients which have not herniated but still have devastating neurological complications.
Venovenous bypass has also undergone somewhat of an evolution in our program. As you know from the early days when you were at UCLA, Dr. Klein, we would virtually always use venovenous bypass for adults. And we still use it in patients who we feel that excessive fluid loading during the anhepatic phase will be problematic. However, more frequently in well-selected patients in which the operation can done expeditiously, we will forgo venovenous bypass.
In regards to the short (2-day) period between listing and transplantation, that has increased in our most recent experience. I think the reason that we were able to usually get these patients transplanted in such an expeditious manner is that we use marginal donors liberally. We may be paying the penalty for that, and we are currently analyzing our data comparing the marginal versus non-marginal graft for fulminant failure. I do advocate broader sharing in these cases. However, one has to also be cognizant of the fact of acute liver failure that the marginal liver is not going to travel as well as the non-marginal one.
There is no question that creatinine in this study was the most robust factor predicting survival, as it has been for all types of end-stage liver disease. The issue which you bring up is whether the preoperative creatinine is affected by dialysis, and even for that matter by the fact that we had pediatric patients that we transplanted who will normally have a lower creatinine. This is an extremely important point. However, I think what we need to do, and which we are doing, is to separate out those patients that had dialysis, and only analyze the adult population. Using this more selective chart, I would predict that the serum creatinine threshold will be higher than the 1.45. Notwithstanding that, I think that it clearly will still be the most important parameter.
Dr. Steinberg was asking about differentiating hepatorenal versus acute tubular necrosis in these patients. The overwhelming majority of the patients had hepatorenal syndrome with recovery within a week or so after transplant. In those with ATN, recovery was usually delayed.
I would like to thank the Association for the privilege of the floor.
References
- 1.Lee WM. Acute liver failure. N Engl J Med. 1993; 329: 1862–1872. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Wendon J, Rela M, et al. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998; 27: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 3.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989; 97: 439–445. [DOI] [PubMed] [Google Scholar]
- 4.Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science. 2002; 295: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 5.Stockmann HB, Ijzermans JN. Prospects for the temporary treatment of acute liver failure. Eur J Gastroenterol Hepatol. 2002; 14: 195–203. [DOI] [PubMed] [Google Scholar]
- 6.Dhiman RK, Seth AK, Jain S, et al. Prognostic evaluation of early indicators in fulminant hepatic failure by multivariate analysis. Dig Dis Sci. 1998; 43: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 7.Gazzard BG, Portmann B, Murray-Lyon IM, et al. Causes of death in fulminant hepatic failure and relationship to quantitative histological assessment of parenchymal damage. Q J Med. 1975; 44: 615–626. [PubMed] [Google Scholar]
- 8.Iwatsuki S, Esquivel CO, Gordon RD, et al. Liver transplantation for fulminant hepatic failure. Sem Liv Dis. 1985; 5: 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peleman RR, Gavaler JS, Van Thiel DH, et al. Orthotopic liver transplantation for acute and subacute hepatic failure in adults. Hepatology. 1987; 7: 484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwatsuki S, Stieber AC, Marsh JW, et al. Liver transplantation for fulminant hepatic failure. Transplant Proc. 1989; 21: 2431–2434. [PMC free article] [PubMed] [Google Scholar]
- 11.Gallinger S, Greig PD, Levy G, et al. Liver transplantation for acute and subacute fulminant hepatic failure. Transplant Proc. 1989; 21: 2435–2438. [PubMed] [Google Scholar]
- 12.Detre KM, Belle SH, Carr MA, et al. A report from the NIDDK Liver Transplantation Database. In: Terasasaki P, ed. Clinical Transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1989: 129–141. [PubMed]
- 13.Ascher NL, Lake JR, Emond JC, et al. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993; 128: 677–682. [DOI] [PubMed] [Google Scholar]
- 14.Goss JA, Shackleton CR, Maggard M, et al. Liver transplantation for fulminant hepatic failure in the pediatric patient. Arch Surg. 1998; 133: 839–844. [DOI] [PubMed] [Google Scholar]
- 15.Lu A, Monge H, Drazan K, et al. Liver transplantation for fulminant hepatitis at Stanford University. J Gastroenterol. 2002; 37: 82–87. [DOI] [PubMed] [Google Scholar]
- 16.Bismuth H, Samuel D, Gugenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med. 1987; 107: 337–341. [DOI] [PubMed] [Google Scholar]
- 17.Bismuth H, Castaing D, Ericzon BG, et al. Hepatic transplantation in Europe. First report of the European Transplant Registry. Lancet. 1987:674–676. [DOI] [PubMed]
- 18.Buckels JAC. Liver transplantation for acute fulminant hepatic failure. Transplant Proc. 1987; 19: 4365–4366. [PubMed] [Google Scholar]
- 19.Devlin J, Williams R, European FK506 Liver Study Group. Transplantation for fulminant hepatic failure. Comparing tacrolimus versus cyclosporine for immunosuppression and the outcome in elective transplants. Transplant. 1996; 62: 1251–1255. [DOI] [PubMed] [Google Scholar]
- 20.O’Grady JG, Alexander GJ, Thick M, et al. Outcome of orthotopic liver transplantation in the aetiological and clinical variants of acute liver failure. Q J Med. 1988; 68: 817–824. [PubMed] [Google Scholar]
- 21.Devlin J, Wendon J, Heaton N, et al. Pretransplantation clinical status and outcome of emergency transplantation for acute liver failure. Hepatology. 1995; 21: 1018–1024. [PubMed] [Google Scholar]
- 22.Bismuth H, Samuel D, Castaing D, et al. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg. 1995; 222: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devictor D, Desplanques L, Debray D, et al. Emergency liver transplantation for fulminant liver failure in infants and children. Hepatology. 1992; 16: 1156–1162. [PubMed] [Google Scholar]
- 24.Bismuth H, Samuel D, Castaing D, et al. Liver transplantation in Europe for patients with acute liver failure. Sem Liv Dis. 1996; 16: 415–425. [DOI] [PubMed] [Google Scholar]
- 25.McCashland TM, Shaw BW Jr, Tape E. The American experience with transplantation for acute liver failure. Sem Liv Dis. 1996; 16: 427–433. [DOI] [PubMed] [Google Scholar]
- 26.Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986; 6: 648–651. [DOI] [PubMed] [Google Scholar]
- 27.Bernuau J, Samuel D, Durand F, et al. Criteria for emergency liver transplantation in patients with acute viral hepatitis and factor V below 50% of normal: a prospective study. Hepatology. 1991; 14: 49. [Google Scholar]
- 28.Shakil AO, Kramer D, Mazariegos GV, et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transplant. 2000; 6: 163–169. [DOI] [PubMed] [Google Scholar]
- 29.Pauwels A, Mostefa-Kara N, Florent C, et al. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol. 1993; 17: 124–127. [DOI] [PubMed] [Google Scholar]
- 30.de Rave S, Tilanus HW, van der Linden J, et al. The importance of orthotopic liver transplantation in acute hepatic failure. Transplant Int. 2002; 15: 29–33. [DOI] [PubMed] [Google Scholar]
- 31.Brems JJ, Hiatt JR, Ramming KP, et al. Fulminant hepatic failure: the role of liver transplantation as a primary therapy. Am J Surg. 1987; 154: 137–141. [DOI] [PubMed] [Google Scholar]
- 32.Schiodt FV, Atillasoy E, Shakil AO, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg. 1999; 5: 29–34. [DOI] [PubMed] [Google Scholar]
- 33.Trey D, Davidson C. The management of fulminant hepatic failure. In: Popper H, Schaffner F, eds. Progress in Liver Disease. Vol 3. New York: Grune and Stratton; 1970: 292–298. [PubMed]
- 34.Tandon BN, Bernauau J, O’Grady J, et al. Recommendations of the International Association for the Study of the Liver Subcommittee on nomenclature of acute and subacute liver failure. J Gastroenterol Hepatol. 1999; 14: 403–404. [DOI] [PubMed] [Google Scholar]
- 35.O’Grady JG, Schalm SW, Williams R. Acute liver failure. Redefining the syndromes. Lancet. 1993; 342: 273–275. [DOI] [PubMed] [Google Scholar]
- 36.Bernuau J, Rueff B, Benhamou JP. Fulminant, subfulminant liver failure. Definitions and causes. Sem Liv Dis. 1986; 6: 97–106. [DOI] [PubMed] [Google Scholar]
- 37.Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997; 337: 473–479. [DOI] [PubMed] [Google Scholar]
- 38.Segal MR. Regression trees for censored data. Biometrics. 1988; 44: 35–47. [Google Scholar]
- 39.2001 OPTN/SRTR Annual Report, 2000. HHS/HRSA/OSP/DOT; UNOS; URREA; http://www.optn.org/data/annualReport.asp.
- 40.Busuttil RW, Goss JA. Split liver transplantation. Ann Surg. 1999; 229: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emond JC, Aran PP, Whitington PF, et al. Liver transplantation in the management of fulminant hepatic failure. Gastroenterology. 1989; 96: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 42.Hoofnagle JH, Carithers RL Jr, Shapiro C, et al. Fulminant hepatic failure. Summary of a workshop. Hepatology. 1995; 21: 240–252. [PubMed] [Google Scholar]
- 43.Bzeizi KI, Jalan R, Plevris JN, et al. Primary graft dysfunction after liver transplantation. From pathogenesis to prevention. Liver Transplant Surg. 1997; 3: 137–148. [DOI] [PubMed] [Google Scholar]
- 44.Farges O, Kalil AN, Samuel D, et al. The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplant. 1995; 59: 1124–1133. [PubMed] [Google Scholar]
- 45.Bonatti H, Muiesan P, Connolly S, et al. Liver transplantation for acute liver failure in children under 1 year of age. Transplant Proc. 1997; 29: 434–435. [DOI] [PubMed] [Google Scholar]
- 46.Ghobrial RM, Gornbein J, Steadman R, et al. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002; 236: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghobrial RM, Steadman R, Gornbein J, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001; 234: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markmann JF, Gornbein J, Markowitz JS, et al. A simple model to estimate survival after retransplantation of the liver. Transplant. 1999; 67: 422–430. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Supported in part by the Dumont Foundation, the Torino Foundation, and the JoAnn Barr Foundation.
Correspondence: Douglas G. Farmer, MD, FACS, Dumont-UCLA Transplant Center, Room 77-120 CHS, Box 957054, Los Angeles, CA 90095-7054.
E-mail: dgfarmer@mednet.ucla.edu
Accepted for publication December 2002.