Abstract
Objective
To evaluate the Jarvik 2000 axial flow left ventricular assist sys-tem (LVAS) as a bridge to transplant and as destination therapy.
Summary Background Data
The Jarvik 2000 LVAS was implanted in 22 patients (16 men, 6 women; mean age 53 years) as a bridge to transplant (in the United States) and in 4 patients (all men; mean age 62.8 years) as destination therapy (in the United Kingdom). All patients in both of these initial feasibility studies were in NYHA class 4.
Methods
The pump was implanted through a thoracotomy or median sternotomy incision with the aid of partial cardiopulmonary bypass in bridge-to-transplant patients. A skull-mounted percutaneous power delivery was used for the patients who received the pump as destination therapy.
Results
Of the 22 bridge-to-transplant patients, 13 underwent transplant; 7 died during support; and 2 studies are ongoing. The surviving patients have an average follow-up of 15 months; one died at 2.6 months after transplant, and the remaining patients are all in NYHA class 1. Support averaged 67.1 days. Deaths were due to acute myocardial infarction in two patients and multiorgan failure in five patients. Hemodynamic function improved with LVAS support. The average cardiac index increased 70.6% by 48 hours after implant, pulmonary capillary wedge pressure decreased 44%, systemic vascular resistance decreased significantly, and inotropic support became unnecessary. Similar results have been seen in the patients who received the device as destination therapy. In that series, one patient died of subdural hematoma 380 days after implant. The other two patients are in NYHA class 1, 642 and 889 days after implant. The average cardiac index increased 89.5%, and pulmonary capillary wedge decreased 52.2%.
Conclusions
The Jarvik 2000 axial-flow LVAS can be used safely in selected patients to provide support until transplant or as destination therapy. In this series, the patients who most benefited from this device were those who required true left ventricular assistance rather than total capture of left ventricular output. Current experience indicates that continuous offloading of the ventricle is most effective when there is enough residual myocardial function to maintain pulsatility and aortic root ejection and to maintain, with nonpulsatile pump support, a normal cardiac index as well as reinstitution of the Frank-Starling response to the native ventricle.
Implantable, pulsatile ventricular assist systems (VAS) are widely used to support patients with chronic heart failure. The frequency of use of these systems continues to increase due to the growing numbers of patients with heart failure and expanded access to such devices. Thousands of patients worldwide have been successfully bridged to heart transplant, and a small group of patients have had a VAS removed following sufficient recovery of myocardial function. Clinical studies have demonstrated adequate safety and efficacy of VAS when applied as a temporary bridge to heart transplantation 1 or as destination therapy. 2 Studies are ongoing to assess whether chronic VAS support will allow sufficient myocardial function for device removal without heart transplantation. 3,4 Although the results of studies with currently available pulsatile pumps have shown that selected patients can benefit from this technology, serious complications inherent with their design persist. 5,6
Axial flow pumps have been developed in an effort to provide circulatory support in patients otherwise too small or not well suited for conventional support. 7 Preliminary reports indicate that the Jarvik 2000 (Jarvik Heart Inc., New York, NY) axial flow VAS can support patients for many months with minimal device-related complications. 8–10 Axial flow pumps are relatively simple in design and are intended to be easy to implant and reliable, and may be associated with fewer complications than their pulsatile cousins. We are involved in clinical studies to evaluate use of the axial flow VAS as a bridge to heart transplantation and for destination therapy. In this report, we present our experience with the Jarvik 2000 VAS.
METHODS
Patients
The clinical study for evaluation of the Jarvik 2000 axial flow VAS began in April 2000 with the first bridge-to-transplant implantation. In the United States, the pump is implanted as a bridge to transplant under an FDA investigational device exemption protocol. In this report, we also give the results of use of this device as a destination therapy, in conjunction with investigators at the Oxford Heart Centre (Oxford, UK). All study candidates must have New York Heart Association (NYHA) class 4 heart failure and a cardiac index (CI) less than 2.0 L/min/m2, must be receiving maximal medical support, and must not have significant comorbidities. Twenty-two patients have undergone bridge-to-transplant implantation with this system at the Texas Heart Institute (Houston, TX), and four have had the device implanted for destination therapy in Oxford. The studies were approved by each institution’s ethics committee and the appropriate U.S. and U.K. governmental agencies.
Device Description
The Jarvik 2000 is an implantable VAS (Fig. 1) that produces blood flow by means of a single, rotating, vaned impeller. 11 This Jarvik 2000 consists of a blood pump, 16-mm outflow graft, percutaneous power cable, pump-speed controller, and a direct-current power supply. The blood pump is small (about the size of a common C-cell battery). All blood-contacting surfaces within the pump are made of smooth titanium. The power cable is constructed of pacemaker-type wires that are insulated with polyurethane and partially covered with Dacron. The impeller, which is composed of a neodymium-iron-boron magnet and hydrodynamic titanium blades, is held in position by two ceramic bearings. The hydrodynamic blades are located on the outer surface of the impeller, and outflow stator blades are located downstream from the impeller. The electromagnetic force created by the motor spins the impeller at 8,000 to 12,000 rpm, generating an average flow rate of 3 to 6 L/min at 4 to 6 W of power against physiologic pressure. Continuous power is provided to the controller and pump by rechargeable lead-acid or lithium-ion batteries.
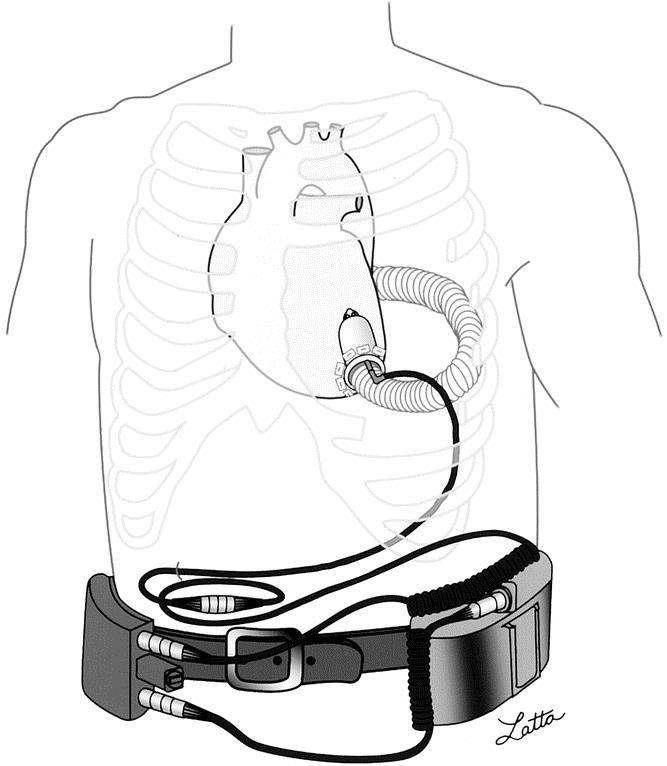
Figure 1. The Jarvik 2000 ventricular assist system. The blood pump is positioned in the left ventricle and is connected to the external power and control by the externalized power cable. Pump speed is manually adjusted on the analog controller, and continuous power is provided by portable batteries.
The pump is implanted through a left thoracotomy or sternotomy and with the aid of partial cardiopulmonary bypass. 12,13 The outflow graft may be placed either on the ascending or descending aorta. The pump is positioned within the left ventricle. A Silastic sewing cuff, placed on the left ventricular apex, secures the pump within the left ventricle. The percutaneous power cable is externalized through the right side of the abdomen or alternatively via a skull-mounted pedestal. 14 Thus far, the VAS for destination therapy has been used with the skull-mounted pedestal, and the bridge-to-transplant VAS has been used with a Dacron-covered percutaneous power cable.
Statistical Analysis
Statistical differences were calculated using the t test. Differences were considered significant at P < .05.
RESULTS
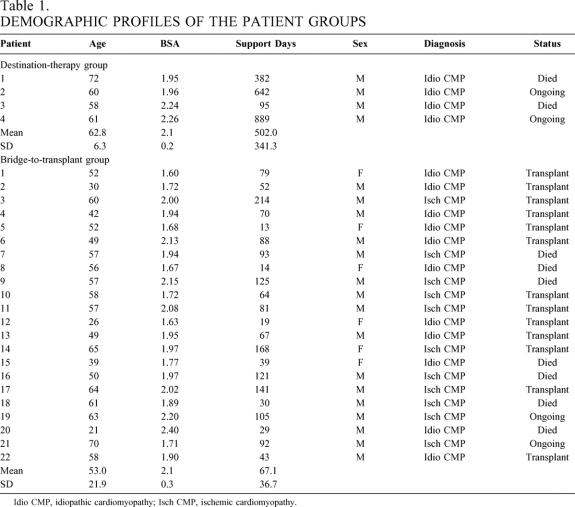
The demographic profiles of the two patient groups are listed in Table 1. At implantation, all patients were in NYHA class 4 heart failure and were receiving maximal medical therapy. The 4 destination therapy patients were all men; the bridge-to-transplant group comprised 16 men and 6 women. The average age of the bridge group (53 years) was approximately 10 years younger than that of the destination therapy group (62.8 years). The average duration of support was significantly different (P < .001). The destination therapy patients were supported for an average of 502 days, while support in the bridge group averaged 67 days (range 13–214). In the destination therapy group, two patients died at 382 and 95 days. Thirteen patients (59%) in the bridge group underwent successful heart transplants, and 12 remain alive and well with an average posttransplant follow-up of 15 months (range 0.8–29 months). One patient died of allograft rejection 2.6 months after transplant. Seven patients (32%) died waiting for heart transplant, and two patients (9%) continue to be supported at 105 and 90 days.
Table 1. DEMOGRAPHIC PROFILES OF THE PATIENT GROUPS
Idio CMP, idiopathic cardiomyopathy; Isch CMP, ischemic cardiomyopathy.
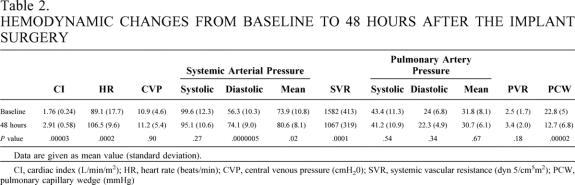
Hemodynamic function improved immediately with VAS support, and all patients were successfully weaned from cardiopulmonary bypass. In three patients, implantation was done without cardiopulmonary bypass. In the bridge-to-transplant group, the changes in CI, heart rate, mean and diastolic arterial blood pressure, systemic vascular resistance, and pulmonary capillary wedge pressure were statistically significant (Table 2). At 48 hours after implantation, the average CI had increased 70.6% (from 1.76 to 2.91 L/min/m2), the average pulmonary capillary wedge pressure decreased 44% (from 22.8 to 12.7 mmHg), and the systemic vascular resistance decreased 33% (from 1,582 to 1,067 dynes). A significant increase in the mean and diastolic arterial blood pressure (8% and 20%, respectively) occurred by 48 hours after the implant, and only minimal inotropic support was needed.
Table 2. HEMODYNAMIC CHANGES FROM BASELINE TO 48 HOURS AFTER THE IMPLANT SURGERY
Data are given as mean value (standard deviation).
CI, cardiac index (L/min/m2); HR, heart rate (beats/min); CVP, central venous pressure (cmH20); SVR, systemic vascular resistance (dyn 5/cm5m2); PCW, pulmonary capillary wedge (mmHg)
Of the four patients supported for destination therapy, three were rehabilitated and discharged from the hospital between 3 and 8 weeks after the implant surgery. Hemodynamic changes similar to the bridge-to-transplant group were observed in the destination-therapy patients. Two patients died during support (at 95 and 382 days). One patient died secondary to right-heart failure; the other died of complications from a subdural hematoma. The skull-mounted pedestal was well healed and free of infection in three of the four patients. Two patients have lived for extended periods; both are alive and well at 642 and 889 days after implant. One patient travels extensively in Europe and the United States, hikes, and participates in long-distance walk-a-thons.
Although the overall rate of complications has been acceptable, serious patient-related complications that have been experienced include postoperative bleeding, left ventricular thrombus, coronary thrombosis, subdural hematoma, and gastrointestinal bleeding. There have been no pump failures or pump-related complications. Three patients have been treated successfully with tissue plasminogen activator for lysis of ventricular thrombi. The amount of hemolysis has been acceptable. There have been no significant device-related infections in any patient. There have been a small number of technical difficulties with batteries and connectors, but there have been no system failures, and support has been continuous in all patients.
DISCUSSION
In this study, the Jarvik 2000 VAS proved safe and effective both for bridge-to-transplant and for destination-therapy patients. We learned that the Jarvik 2000 functions best when used to assist native left ventricular function (i.e., by partially unloading the ventricle, thus allowing native ventricular ejection to make up the remainder of the cardiac output). Experience with pulsatile left ventricular assist devices has shown that complete unloading of the left ventricle occurs in the properly functioning system. Partial unloading can result in normalization of CI by improving native ventricular function, and allowing the failed ventricle to have a normal Frank-Starling response results in the abolition of the isometric phase of ventricular contraction, thus ensuring reduction of ventricular stress and normalization of left ventricular end diastolic pressure. 15,16
Unloading the left ventricle with partial assist also optimizes the ratio between myocardial oxygen delivery and consumption. Earlier animal studies have shown that continuously unloading the left ventricle with an axial-flow pump decreases perfusion to nonischemic myocardium while increasing perfusion to the ischemic myocardium. 17,18 The decrease in coronary flow to the nonischemic myocardium may be due to the reduced metabolic demand in the presence of systolic and diastolic unloading and less cardiac work.
The Jarvik 2000 normally augments left ventricular function rather than captures the entire cardiac output. Continuous blood flow through the pump ensures that the left ventricle is continuously unloaded, even to the degree that the same patients have had short periods of nonpulsatile flow that have been well tolerated physiologically. The Jarvik 2000 can provide a cardiac output of up to 6 L/min, depending on the preload, afterload, and pump speed. The main determinants of the amount of blood flow through the pump are the impeller speed and the pressure difference across the pump. When the impeller rotates at a constant speed, the amount of blood flow through the pump primarily depends on the difference between the left ventricular and aortic pressures. Accordingly, as the pressure within the left ventricle increases during systole, blood flow through the pump also increases during systole. Pump flow continues during diastole, thus converting the normally passive flow of diastole to active, positive-pressure flow. The cyclical increase and decrease in flow through the pump is augmented by the pumping action of the native ventricle. As the pump speed increases, the pulse pressure decreases because of left ventricular unloading by the pump. Improvement in native left ventricular function can be detected by an increase in the average pulse pressure. This method of circulatory assist is sufficient to allow complete physical rehabilitation and recovery from the implant surgery.
We advise anticoagulant therapy for patients supported by the Jarvik 2000. This is not needed for the pump, as there is no stasis or negative-pressure flow (suction) within the properly functioning apparatus. However, because of poor ventricular function, hypovolemia, or both, stasis can occur. Thrombosis within the blood pump was not observed in this series of patients. Thrombus formation, likely due to areas of stasis around the back of the pump, has occurred. This can propagate and cause partial inflow obstruction and hemolysis. Three patients have required tissue plasminogen activator to treat this. At present, the goal of anticoagulation therapy is to maintain an INR greater than 2.0, or a partial thromboplastin time greater than 65 seconds. Maintaining a pump speed that allows some aortic outflow reduces stasis in the left ventricle and should minimize thrombotic complications.
Four of the bridge-to-transplant patients developed chronic gastrointestinal bleeding during support. In three of these patients, the source of the bleeding was confirmed to be an arteriovenous malformation. Arteriovenous malformations usually remain a subclinical anomaly, but an increased incidence of bleeding has been noted in patients with aortic stenosis. 19 A characteristic common to aortic stenosis and patients supported by the Jarvik 2000 is a reduced pulse pressure. To minimize this complication, we advise using the lowest pump speed to allow for the maximal pulse pressure and a normal CI.
The Jarvik 2000 has a number of potential advantages over conventional pulsatile VAS devices, including small size and ease of implantation and operation. Although the pump has typically been implanted through a left thoracotomy or median sternotomy incision with the aid of partial cardiopulmonary bypass, we have implanted the pump without cardiopulmonary bypass or with limited cardiopulmonary bypass times (<10 minutes). Minimizing or avoiding cardiopulmonary bypass altogether during the implant should significantly reduce serious complications, such as bleeding secondary to coagulopathy. The smaller foreign material surface area and the minimal movement of the device inside the body also reduce the possibility of device-related infection. In fact, no serious device-related infections occurred in the patients in this series.
The skull-mounted pedestal for power-cable delivery, which was used for the four destination-therapy patients, has proved to be very reliable for extended periods. 20 The two long-term patients have not experienced infections related to the pedestal. The pedestal is similar to that used for artificial ear implants, which is a reliable method for long-term percutaneous access.
The Jarvik 2000 is simple to operate. Hospital staff have found the system to be very user-friendly. Few technical difficulties have been encountered, and no failures of the system have occurred during up to 502 days of continuous support. Ambulatory patients can easily maintain the system themselves. Pump speeds can be adjusted for increased output by means of a simple dial knob, and changing batteries is simple. In Europe, eight patients have been discharged from the hospital supported by the Jarvik 2000. In the U.S., a home-discharge program has just begun.
In summary, these initial clinical results indicate that the Jarvik 2000 can safely and effectively support patients either for a few months while they await heart transplantation or as destination therapy when they are not heart transplant candidates. Further studies are needed to better define the patient population that will benefit most from the Jarvik 2000 and to better understand patients’ physiologic responses to the VAS for optimized patient management. It seems that the best indication is for homebound or outpatient, inotrope-dependent class 3 or 4 heart failure patients.
Discussion
Dr. Thomas H. Schwarcz (Lexington, KY): I rise on behalf of Robert Mentzer from Kentucky, who was unable to attend today. We would like to compliment Dr. Frazier and his colleagues on this most interesting presentation and to commend him and his colleagues for their life-long dedication to the basic research of circulatory support devices and the artificial heart. Dr. Frazier has inspired numerous young investigators in the fields of biology, engineering, and surgery to devote their energies to the development of devices that benefit patients with end-stage heart disease.
This morning’s report of the successful implementation of the Jarvik 2000 clearly demonstrates the feasibility of using a compact axial-flow impeller pump to improve hemodynamic function in patients with failing hearts. The implications should not be underestimated. Now, of course, we are anxious to learn more about the efficacy of the device compared to devices currently used. Will it be superior to the current systems in use in terms of more flexibility in patient selection, postimplant survival, and quality of life?
At the University of Kentucky, we use the HeartMate device as a bridge to transplantation. In some cases, the device has been used de facto as destination therapy. Our experience has been the same as that reported by others. At least one in two patients experience one or more life-threatening complications, and one third to one half experience a drive-line or peri-implant infection. Without the device, however, many of our patients simply would not be alive. Thus, we are in great need of a new system that will reduce our infection, thromboembolism, and bleeding complication rates. From your presentation, it appears that the Jarvik 2000 holds great promise in this regard.
Another area of clinical interest is the use of the left ventricular assist device as a bridge to recovery. Long-term unloading has been purported to result in partial myocardial recovery. In fact, there are reports of changes in myocardial gene expression, and you and others have reported successful device explantation. The evidence is still lacking, however, that this occurs frequently enough to warrant the use of this device for that purpose.
With these comments in mind, I have several questions.
First, what are your thoughts regarding the use of the Jarvik 2000 not only as a bridge to transplant or destination therapy, but also as a bridge to recovery?
Second, for over 50 years, we have debated the importance of pulsatile flow. While the Jarvik 2000 circumvents the chronic nonpulsatile flow issue, what is the evidence that this is really important?
Third, despite the efforts of yourself and others, it took the FDA almost 20 years to approve the left ventricular assist device as a therapeutic modality. Would you speculate on what needs to be done and how long it will take to determine whether the Jarvik 2000 will be available for use as a bridge to recovery, a bridge to transplant, or destination therapy?
Finally, the FDA recently approved the HeartMate left ventricular assist device for destination therapy. Should the Centers for Medicare/Medicaid Services approve reimbursement for this application, the implantation of the device may increase dramatically at hospitals without transplant programs. Likewise, the indications may broaden, say, for post-cardiotomy cardiogenic shock. With a device such as the Jarvik 2000, what are your thoughts regarding the development of guidelines as to who, or what institutions, should be implanting this device, or any device, that is designed to function as permanent therapy?
Dr. Timothy J. Gardner (Philadelphia, PA): Let me also add my compliments to Dr. Frazier and to the team at Texas Heart, with Dr. Cooley standing behind them, for 25 years of dedicated work in this area. Dr. Frazier and his colleagues have devoted 25 years of hard work, not only in the experimental laboratory, but also caring for an extremely difficult group of patients.
Mechanical heart assist devices aren’t “Star Wars” material. Dr. Haller’s excellent summary of the history of pediatric surgery and the many new surgical developments over the past 30 to 40 years may portend a similar development in mechanical cardiac assistance over the next 5 to 10 to 15 years. It is likely that these devices will be available for implantation in community hospitals without transplant facilities. As Dr. Frazier pointed out, there are more than 200,000 patients with severe chronic congestive heart failure each year and only 2,000 available hearts for transplantation. The DRG for congestive heart failure is the fastest-growing Medicare DRG. We may be at the threshold of significant advances in mechanical assist devices. And Dr. Frazier and his group should be complimented for their pioneering work.
The NIH, by the way, set up an RFA that was nearly impossible to achieve, perhaps because the Heart Institute Advisory Committee had little confidence in mechanical cardiac assist devices for destination use. Likewise, the FDA seems to be resisting the acceptance of mechanical assistance, perhaps believing that treating patients with chronic heart failure with mechanical devices is inappropriate.
Putting such philosophical points aside, this particular device really does provide an intermediate VAD approach without complete unloading of the heart. What we have had until now has been the balloon pump for limited assistance versus near complete unloading with first-generation VADs.
I was disappointed to hear Dr. Frazier’s comment that this is not a device that he believes will be useful in the acute situation, because this is where I think we are really challenged today. You have two choices: Either the patient succumbs, or you place a complete left ventricular assist device that is very difficult to remove in many patients without moving on to transplantation. I would ask Dr. Frazier to comment further on what he sees as the best device for use as an acute rescue situation.
I enjoyed this paper, and I believe that Dr. Frazier should be acknowledged and congratulated for his impressive personal persistence and leadership in this difficult field.
Dr. Walter H. Merrill (Cincinnati, OH): Dr. Frazier and his colleagues have brought to our attention an innovative and exciting treatment option for patients with heart failure due to poor left ventricular performance. Many of these patients are seriously ill despite extensive efforts to help them with multiple medications, including inotropic agents. They have a very poor prognosis, with a probability of survival beyond 2 years approaching zero.
The Jarvik 2000 assist system has many characteristics that are favorable. It is simple in concept and design, reliable, and reasonably easy to insert. The technique of insertion can be modified to suit individual patient requirements. The device can be used in quite small or in large patients. There have been no device failures. In addition, it is easy to control and can be utilized in an outpatient setting, thereby facilitating physical rehabilitation.
I have several questions for the authors.
First, it seems that the best results for this device are obtained when the patient’s left ventricle is capable of maintaining forward output and thus pulsatile flow. Is it possible to determine preoperatively if the patient’s left ventricular performance is so poor that insertion of this device would not likely result in maintenance of pulsatile flow? If so, would such a determination suggest that the patient might be better treated with a pulsatile assist system?
Second, are there patient comorbidities, such as advanced pulmonary hypertension, or right ventricular dysfunction, or the presence of an aortic valve prosthesis, that might serve as relative contraindications to this procedure?
Finally, in the face of changing preload and afterload conditions, could outpatients be taught to adjust the pump flow settings in order to optimize their overall hemodynamic state? Could they utilize either systolic and diastolic blood pressure or pulse wave characteristics from an oxygen saturation meter as a guide to adjusting the device?
Finally, I congratulate the authors for their excellent presentation and for the outstanding results they have obtained in treating patients with a complex and high-risk clinical problem.
Dr. O. Howard Frazier (Houston, TX): Dr. Garrison pointed out the possible use of this device as a bridge to recovery. Although we didn’t have time to describe this use, I believe that, with time, myocardial improvement can occur. In fact, I think nearly all patients with idiopathic cardiomyopathies could have enough improved heart function to have the pump removed. The Jarvik pump could probably be removed through a thoracoscopy, simply by clipping the graft. I do think this and all ventricular assist devices have great potential clinical importance as a bridge to recovery.
Most complications with this and other assist devices are related to patient factors. The problem is that most of the patients who receive the pump are already too sick, and I think we implant this technology too late, which contributes to the poor results.
With regard to pulsatile flow, one of the points I have tried to make is that the problem has been mainly with the circulatory system and cardiac function, not the end-organ function. Whether flow is pulsatile or nonpulsatile does not appear to affect the kidneys or liver the way it does the heart. It may be that with the use of a right-sided pump, however, we can balance the flow to overcome the impaired right ventricular function and inadequate pump filling.
I appreciate Dr. Gardner’s remarks. In my experience, the best device for acute use is the Hemopump, which was previously available. We used the Hemopump in three patients with acute decompensation after coronary bypass and in three patients after failed heart transplants; five of those six patients survived. The company wanted to use the Hemopump as the cardiologist would use it, and that is what led to the eventual discontinuance of its use in the United States. However, the Hemopump is coming back in another form, and I hope this technology can eventually be utilized for the purpose for which it was designed: acute perioperative heart failure.
Pertaining to Dr. Merrill’s questions, we, of course, try to assess patients preoperatively. Clearly, the ideal patients are those with idiopathic cardiomyopathy with some preservation of ventricular function. That being said, one of our longest survivors on the pump was a patient with a severe ischemic cardiomyopathy. When these patients do not have to go on the heart-lung machine, they have done quite well. It is, I think, very important that there be some better way to assess patient selection for the Jarvik. We are now exploring the use of the dobutamine stress test to assure adequate cardiac reserve before implanting the continuous flow pump as a true assist device.
As for outpatient support, the patients in England are all outpatients. Their INR is checked about once a month, just as it would be with a mechanical mitral valve. Otherwise, these patients are on their own, and they have done quite well as outpatients. They are also able to regulate the speed of the pumps on their own. One of the patients I described hikes a lot. If he starts hiking up a hill and he gets a little tired, he just turns up his pump flow, which improves his exercise tolerance. There are currently eight outpatients in Europe.
In closing, I think our chief obstacle has been, as I have alluded to, regulatory. We have had so many regulatory barriers that unless something is done soon, it is going to be hard to get any of these valuable technologies approved for widespread use in the United States. On the other hand, the FDA has approved the internal cardiac defibrillator for use in biventricular pacing without clear evidence of efficacy. Of five of my recent patients—three of whom underwent heart transplantation and two of whom needed an LVAD inserted—all had undergone biventricular pacing.
I think a small nonpulsatile pump could be of enormous benefit, and even community hospitals could implant the pump with good outcomes. Most importantly, it would benefit this large group of outpatients with chronic heart failure currently limited to minimal activity while undergoing maximal medical management.
References
- 1.Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001; 122: 1186–1195. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;15:345:1435–1543. [DOI] [PubMed]
- 3.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999; 68: 734–741. [DOI] [PubMed] [Google Scholar]
- 4.Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002; 21: 516–521. [DOI] [PubMed] [Google Scholar]
- 5.Phillips WS, Burton NA, MacManus Q, et al. Surgical complications in bridging to transplantation: the Thermo Cardiosystems LVAD. Ann Thorac Surg. 1992; 53: 482–486. [DOI] [PubMed] [Google Scholar]
- 6.Myers TJ, Kahn T, Frazier OH. Infectious complications with ventricular assist systems. ASAIO J. 2000; 46: S28–S36. [DOI] [PubMed] [Google Scholar]
- 7.Frazier OH, Myers TJ, Jarvik RK, et al. Research and development of an implantable, axial-flow left ventricular assist device: the Jarvik 2000 heart. Ann Thorac Surg. 2001; 71: S125–S132. [DOI] [PubMed] [Google Scholar]
- 8.Frazier OH, Myers TJ, Gregoric ID, et al. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation. 2002; 105: 2855–2860. [DOI] [PubMed] [Google Scholar]
- 9.Siegenthaler MP, Martin J, van de Loo A, et al. Implantation of the permanent Jarvik 2000 left ventricular assist device: a single-center experience. J Am Coll Cardiol. 2002; 39: 1764–1772. [DOI] [PubMed] [Google Scholar]
- 10.Westaby S, Banning AP, Saito S, et al. Circulatory support for long-term treatment of heart failure: Experience with an intraventricular continuous flow pump. Circulation. 2002; 105: 2588–2591. [DOI] [PubMed] [Google Scholar]
- 11.Jarvik R, Scott V, Morrow M, et al. Belt-worn control system and battery for the percutaneous model of the Jarvik 2000 heart. Artif Organs. 1999; 23: 487–489. [DOI] [PubMed] [Google Scholar]
- 12.Siegenthaler MP, Martin J, Frazier OH, et al. Implantation of the permanent Jarvik 2000 left ventricular assist device: surgical technique. Eur J Cardiothorac Surg. 2002; 21: 546–548. [DOI] [PubMed] [Google Scholar]
- 13.Westaby S, Frazier OH, Beyersdorf F, et al. The Jarvik 2000 Heart. Clinical validation of the intraventricular position. Eur J Cardiothorac Surg. 2002; 22: 228–232. [DOI] [PubMed] [Google Scholar]
- 14.Westaby S, Frazier OH, Pigott DW, et al. Implant technique for the Jarvik 2000 heart. Ann Thorac Surg. 2002; 73: 1337–1340. [DOI] [PubMed] [Google Scholar]
- 15.Frazier OH, Myers TJ, Jarvik RK, et al. Research and development of an implantable, axial-flow left ventricular assist device: the Jarvik 2000 Heart. Ann Thorac Surg. 2001; 71: S125–S146. [DOI] [PubMed] [Google Scholar]
- 16.Frazier OH, Gregoric ID, Delgado RM, et al. Initial experience with the Jarvik 2000 left ventricular assist system as a bridge to transplantation: report of 4 cases. J Heart Lung Transplant. 2001; 20: 201. [DOI] [PubMed] [Google Scholar]
- 17.Merhige ME, Smalling RW, Cassidy D, et al. Effect of the Hemopump left ventricular assist device on regional myocardial perfusion and function. Reduction of ischemia during coronary occlusion. Circulation. 1989; 80: III-158–III-166. [PubMed] [Google Scholar]
- 18.Smalling RW, Cassidy DB, Barrett R, et al. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. Circulation. 1992; 85: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 19.Heyde EC. Correspondence. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958; 259: 196. [Google Scholar]
- 20.Westaby S, Jarvik R, Freeland A, et al. Postauricular percutaneous power delivery for permanent mechanical circulatory support. J Thorac Cardiovasc Surg. 2002; 123: 977–983. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: O. H. Frazier, MD, Texas Heart Institute, P.O. Box 20345, Houston, TX 77225-0345.
E-mail: mmallia@heart.thi.tmc.edu
Accepted for publication December 2002.