Abstract
Objective
To test a hypothesis that definitive management of pseudocyst associated with chronic pancreatitis is predicated on addressing pancreatic ductal anatomy.
Summary Background Data
The authors have previously confirmed the impact of pancreatic ductal anatomic abnormalities on the success of percutaneous drainage of pancreatic pseudocyst. The authors have further defined a system to categorize the pancreatic ductal abnormalities that can be seen with pancreatic pseudocyst. The authors have published, as have others, the usefulness of defining ductal anatomy when managing pancreatic pseudocysts associated with chronic pancreatitis.
Methods
Beginning in 1985, all patients with pseudocyst who were candidates for intervention (operative, percutaneous, or endoscopic) have undergone endoscopic retrograde cholangiopancreatography (ERCP). An associated diagnosis of chronic pancreatitis was established by means of ERCP findings. Patients were candidates for longitudinal pancreaticojejunostomy (LPJ) if they had a pancreatic ductal diameter greater than 7 mm. In a nonrandomized fashion, patients were managed with either combined simultaneous LPJ and pseudocyst drainage or with LPJ alone.
Results
Two hundred fifty-three patients with pseudocyst have been evaluated. Among these there have been 103 patients with chronic pancreatitis and main pancreatic duct (MPD) dilatation (>7 mm). Among these 103 patients, 56 underwent combined LPJ/pseudocyst drainage and 47 had LPJ alone. Compared to combined LPJ/pseudocyst drainage, the patients undergoing LPJ alone had a shorter operative time, slightly less transfusion requirement, slightly reduced length of hospital stay, and slightly reduced complication rate. Long-term pain relief was achieved in 90%, and pseudocyst recurrence was less than 1%. Rates of each of these long-term outcomes were nearly incidental among the two groups.
Conclusions
Ductal drainage alone (LPJ) is sufficient in patients with chronic pancreatitis (MPD > 7 mm) and an associated pseudocyst. Simultaneous drainage of pseudocyst is not necessary.
For decades the literature regarding the management of pancreatic pseudocyst has focused almost entirely on the pseudocyst itself (maturity of the wall, obstruction of surrounding structures, infection, or hemorrhage). In the past two decades we and others have identified the unique characteristics seen and strategies employed when pseudocyst is associated with a diagnosis of chronic pancreatitis. 1 We have further established the value of defining ductal anatomy when approaching these patients. 2 Prinz established the management principle of combined simultaneous pseudocyst drainage and longitudinal pancreaticojejunostomy (LPJ), and we and others have demonstrated the safety and effectiveness of this strategy. 1,3–10
Our studies have led us to the conclusion that a pseudocyst represents a fistula between the main pancreatic duct (MPD) and the cystic fluid collection. As such, one would anticipate that the pseudocyst would be governed by the same precepts that have governed all other forms of fistula that are routinely managed by gastrointestinal surgeons. Seen in this light, the decades-long focus on the characteristics of the cyst itself is ill conceived. The pertinent focus of evaluation in the management of pseudocyst is the anatomic abnormalities in the MPD. We thus hypothesize that definitive and permanent resolution of ductal abnormalities may be expected to result in permanent resolution of pseudocyst. We have established a system for categorizing all possible ductal abnormalities seen in association with pseudocyst. 2 We have established that this system of defining ductal anatomy predicts success of percutaneous drainage of pseudocyst. We have previously published the usefulness of defining ductal anatomy as it directs therapy in patients with chronic pancreatitis. 1
Based on this foundation of data, we undertook an evaluation of the effectiveness of LPJ alone in the definitive management of pseudocyst associated with chronic pancreatitis, thus avoiding the need for simultaneous pseudocyst drainage.
METHODS
Since 1985 we have established a standard practice protocol of evaluating all patients with pseudocyst of the pancreas. Each is followed either in the hospital or in a clinic devoted to patients with diseases of the pancreas. A period of observation was given for patients to permit spontaneous resolution of pseudocyst. Patients who had spontaneous resolution of pseudocyst did not undergo endoscopic retrograde cholangiopancreatography (ERCP) unless indicated for other reasons, such as choledocholithiasis. Although patients were evaluated for pain or any other symptoms associated with pseudocyst, our philosophy has been to offer intervention (operative, percutaneous, or endoscopic) to all patients with persistent pseudocyst whose diameter was greater than 6 cm. In recent years consideration has also been made of the possible diagnosis of mucinous cystic neoplasm or intraductal papillary mucinous neoplasm (IPMN). Once a patient was deemed to be a candidate for intervention a preprocedure ERCP was performed in all patients. All patients had one or several CT scans, and recently magnetic resonance imaging (MRI) and endoscopic ultrasound have also been used in selected individuals. Ultrasound of the abdomen was used if gallstones were the suspected etiology of pancreatitis.
Using the CT scan, it was possible to define the characteristics of pseudocyst: diameter, maturity of the wall, single or multiple cysts, and evidence that the cyst was or was not causing compression of contiguous structures, including the duodenum, common bile duct, colon, or stomach. CT scanning also provided details that are associated with cystic neoplasms, including septations, apparent invasion of the wall of the cyst into surrounding structures, and identification of masses growing within the cyst. If suspicion existed, percutaneous aspiration was performed to obtain cytology and to stain for mucin.
CT scan also provided information that could be used to establish the diagnosis of chronic pancreatitis. This included the presence of calcifications in the parenchyma of the pancreas as well as calculi in the MPD. Characteristic dilation of the MPD was also identified in patients, and this finding was used to support the diagnosis of chronic pancreatitis.
MRI was primarily used to evaluate ductal anatomy when ERCP could not be done because of the distortion in the duodenal anatomy seen in association with an episode of acute pancreatitis. Endoscopic ultrasound was used primarily on patients with suspected malignancy and allowed us to obtain simultaneous needle biopsy of a mass or of aspiration of cyst fluid.
ERCP was the gold standard for confirming the diagnosis of chronic pancreatitis. The specific findings used to confirm the diagnosis of chronic pancreatitis were dilated MPD or irregularity with alternating areas of narrowing and dilatation (“chain of lakes”) with or without intraductal stones. Signs of early structural changes confirming the diagnosis of chronic pancreatitis were areas of dilatation of the secondary ductules (secondary ductular ectasia). Patients were characterized as “large duct” chronic pancreatitis when the widest MPD diameter was greater than 7 mm. Patients with “small duct” chronic pancreatitis had an MPD diameter less than 7 mm.
The additional ductal features evaluated by ERCP in patients with pseudocyst are designated in Figure 1. Of particular note for the present study were the diagnoses of chronic pancreatitis with or without direct communication with the pseudocyst (type VI or VII). ERCP was used to assess MPD anatomy in all patients with pseudocyst whose cyst persisted and who therefore represented candidates for intervention. ERCP was performed where possible within 24 hours of the planned intervention.
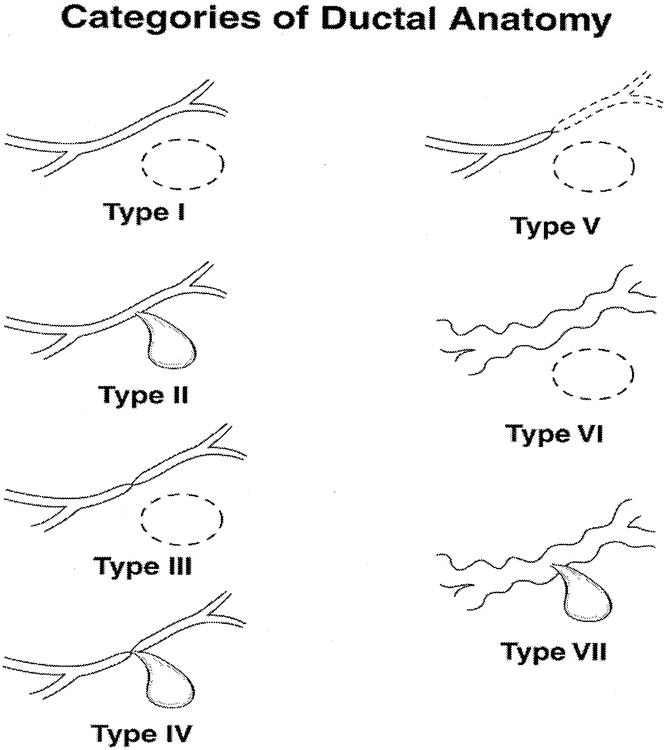
Figure 1. Categories of ductal abnormalities seen in patients with pseudocysts. Type I: normal duct with no communication with cyst. Type II: normal duct with duct–cyst communication. Type III: otherwise normal duct with stricture and no duct–cyst communication. Type IV: otherwise normal duct with stricture and duct–cyst communication. Type V: otherwise normal duct with complete cut-off. Type VI: chronic pancreatitis, no duct–cyst communication. Type VII: chronic pancreatitis with duct–cyst communication.
Standard clinical laboratory parameters were measured, including serum amylase, lipase, and liver function testing with emphasis on signs of biliary stenosis (alkaline phosphatase, gamma glutamyl transferase [GGT] and total bilirubin). Where suspicion of malignancy existed, a carbohydrate antigen 19-9 (CA19–9) was collected. Fasting blood sugar was measured and hyperglycemia, if detected, was fully defined. Complete blood count (CBC) was obtained in all patients and was helpful in evaluating pseudocyst complicated by hemorrhage or infection. Patients with chronic pancreatitis were evaluated for the presence of abdominal pain using visual analog scales, and by documenting dependence on narcotic analgesics to treat the pain. Patients were also evaluated for weight loss, nutritional status, and endocrine (glucose intolerance) or exocrine (steatorrhea) insufficiency states. When pancreatic functional deficits existed, patients were initially managed with appropriate replacement therapy.
Percutaneous Drainage
Percutaneous drainage of pseudocyst was already present in some patients who were either transferred from another hospital or from another service in our institution. In addition, certain patients were offered percutaneous drainage, often as a bridge to definitive operative management, particularly in cases of infected pseudocyst and severe nutritional deficit. Certain patients were managed with percutaneous drainage as part of an evaluation of the effect of ductal anatomy on the success of that modality in previously published data. 2 Patients with chronic pancreatitis who underwent percutaneous drainage were monitored for drain output and compared to patients who were managed operatively with LPJ and intraoperative placement of external drains in the pseudocyst.
Operation
Patients were considered candidates for LPJ if they had chronic pancreatitis and MPD diameter greater than 7 mm. The standard philosophy in the first 7 years of the study was to employ combined simultaneous LPJ and cyst-jejunostomy. Beginning in 1993, some patients were managed with the combined simultaneous LPJ and cyst-jejunostomy, while others underwent LPJ alone. In the initial experience with LPJ alone, an external closed suction drain was placed in the pseudocyst. In subsequent patients who were managed by LPJ alone, cysts were managed with simple aspiration at the time of operation. Candidates for each approach were not randomized.
The operative procedure is performed in a manner essentially identical to the description of Prinz et al. 10 A Roux limb is created 15 cm distal to the ligament of Treitz. For combined cyst-jejunostomy and pancreaticojejunostomy, a side-to-side cyst-jejunostomy is performed in one layer using 3-0 silk suture. The LPJ is also performed as a side-to-side anastomosis using a single layer of 3-0 silk suture.
The decision regarding simultaneous drainage of the biliary tree is made using an established formula previously described by us and by others. 1 We perform biliary drainage if the common bile duct is dilated (>12 cm) and the alkaline phosphatase level is persistently 2.5 times normal values. If there is bile duct narrowing without these parameters met, then no treatment is applied. It is vital to recognize that the classic appearance of the elongated stenosis seen in chronic pancreatitis is never a lesion amenable to endoscopic management. The stenosis of the distal common bile duct is always too long to be definitively managed by sphincterotomy. Stent placement simply commits the patient to permanent stent replacements and raises considerably the risk of recurrent cholangitis. Once the stent has been placed, it can never again be safely removed without a very high likelihood of cholangitis and sepsis.
Postoperatively, patients were evaluated for length of hospitalization (LOS), length of operation (minutes), transfusion requirement, and complication rate. Each was measured in patients who had LPJ alone and compared to those treated with LPJ and cyst-jejunostomy. These two groups were also compared for age, gender, pseudocyst size, and medical comorbidities. Patients with preoperative percutaneous drainage who had chronic pancreatitis were monitored for drain output (mL/24 hours), and these drain outputs were compared to drain outputs in patients managed with LPJ alone and external drainage of the cyst at the time of operation.
Finally, patients were evaluated for pain relief, defined as absence of pain (analog scale = 0) and as freedom from the need for narcotic analgesics. Recurrence of pseudocyst was evaluated by follow-up CT scan, MRI, or ultrasound.
RESULTS
We have evaluated 253 patients with pseudocyst, 187 men and 66 women (mean age 46 ± 4.1 years). Neither age nor gender distribution differed in patients managed by combined LPJ/cyst-jejunostomy when compared to LPJ alone. The cause of pancreatitis was ethanol abuse in 138 patients and biliary in 80. None of the 103 patients evaluated in this study had gallstones as the causative agent for chronic pancreatitis. Twenty-five patients had pseudocyst after abdominal trauma. The remaining 10 patients had a variety of less common causes of pancreatitis with consequent pseudocyst formation. The mean interval from initial presentation to intervention or spontaneous resolution was 22.7 ± 5.2 days. Spontaneous resolution was documented in 68 patients (all with acute pancreatitis). No patient with chronic pancreatitis had spontaneous resolution of pseudocyst.
Using clinical features alone to distinguish acute pancreatitis from chronic pancreatitis, an initial diagnosis of chronic pancreatitis was made in 86 patients, and 160 patients were diagnosed clinically as having acute pancreatitis. ERCP clarified the diagnosis of chronic pancreatitis in 103 patients. Seventeen patients were identified unexpectedly as having chronic pancreatitis after initially being designated as having acute pancreatitis.
ERCP
The diagnosis of chronic pancreatitis with an MPD diameter greater than 7 mm was confirmed by ERCP in 103 patients. Communication between the duct and the pseudocyst was confirmed in 74 of the 103 (72%). Only 9 of the 103 (9%) patients with chronic pancreatitis had multiple pseudocysts, compared to 73 of the 160 (46%) patients with acute pancreatitis who had multiple cysts. Associated common bile duct stenosis was seen in 28 of the 103 (27%) patients with chronic pancreatitis, 26 of which were caused by chronic pancreatitis-associated narrowing of the distal common bile duct. Only two patients had bile duct stenosis caused by compression of the pseudocyst. Each of these 28 patients had elevated alkaline phosphatase (mean 577.1 ± 17.2 u/L [normal 110 u/L]). Mean bilirubin was 1.7 mg/dL. Only the two patients with compression by pseudocyst had significant hyperbilirubinemia. Using ERCP, the mean diameter of the MPD was 9.2 ± 1.1 mm. There were no complications associated with ERCP in the 103 patients with chronic pancreatitis.
All 103 patients with chronic pancreatitis had chronic unremitting abdominal pain (mean visual analog scale 9.6 ± 0.7) and each required daily narcotic analgesics (mean 7.1 ± 1.1 tablets/daily). Mean weight loss from predebilitated body weight was 16.2 ± 2.2 lb. Exocrine insufficiency (18/103 [17%]) and endocrine insufficiency (12/103 [12%]) was identified and treated with replacement therapy as indicated. The distribution of each of those factors was not different in the group of patients treated with LPJ alone compared to patients treated with combined simultaneous LPJ/cyst-jejunostomy.
CT scan revealed gland calcification in 59 of the 103 (57%) patients. The mean diameter of pseudocyst in the 103 patients with chronic pancreatitis was 8.6 ± 1.3 cm. A wall thickness greater than 4 mm was identified in all patients, while only 33 of the 160 (21%) patients with acute pancreatitis had pseudocyst with thickness greater than 4 mm on initial imaging.
Percutaneous Drainage
Sixteen of the 103 patients with pseudocyst associated with chronic pancreatitis had percutaneous drainage before the operative management. Nine of these 16 had drains placed at an outside hospital or on another service in our institution. The remaining seven patients had percutaneous drains placed for suspicion of infection (five confirmed as infection). The mean daily drain output for these 16 patients was 322.6 ± 16.7 mL/24 hours. All patients with chronic pancreatitis had failure of percutaneous drainage and required subsequent operative drainage.
Preoperative Evaluation
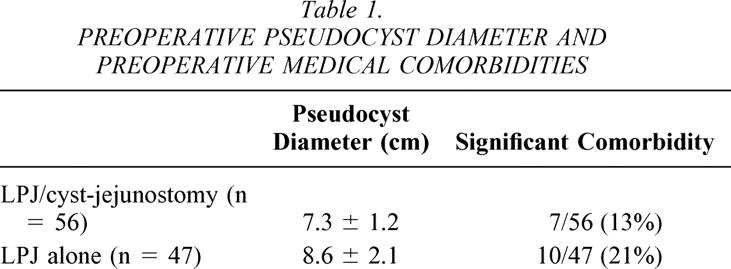
The mean diameter of pseudocyst in the 103 patients with chronic pancreatitis was 8.6 cm. This size was larger than cysts seen in patients with pseudocyst that developed as a consequence of acute pancreatitis. The mean diameter in patients who had combined LPJ/cyst-jejunostomy was 7.3 cm; for patients who had LPJ alone it was 8.6 cm (Table 1). Medical evaluation revealed significant medical comorbidities in 7 of the 56 (13%) patients who had combined LPJ/cyst-jejunostomy compared to 10 of the 47 (21%) patients who had LPJ alone (see Table 1).
Table 1. PREOPERATIVE PSEUDOCYST DIAMETER AND PREOPERATIVE MEDICAL COMORBIDITIES
Operation
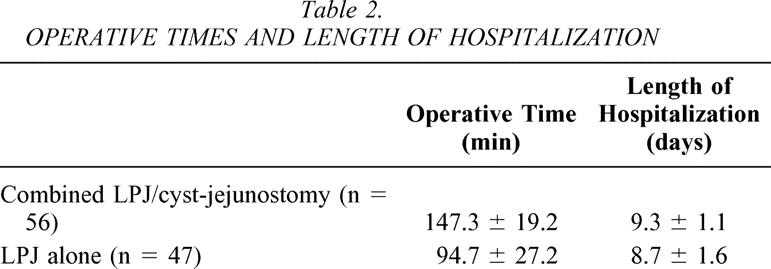
LPJ was performed in all patients. In the 56 patients who underwent combined simultaneous LPJ and cyst-jejunostomy, the mean operative time was 147.3 ± 19.2 minutes; the mean operative time for the 47 patients who underwent LPJ and cyst aspiration was 94.7 ± 27.2 minutes (Table 2). Length of hospitalization for patients who had combined procedure was 9.3 ± 1.1 days compared to 8.7 ± 1.6 days for patients with LPJ alone (see Table 2). There were no operative deaths.
Table 2. OPERATIVE TIMES AND LENGTH OF HOSPITALIZATION
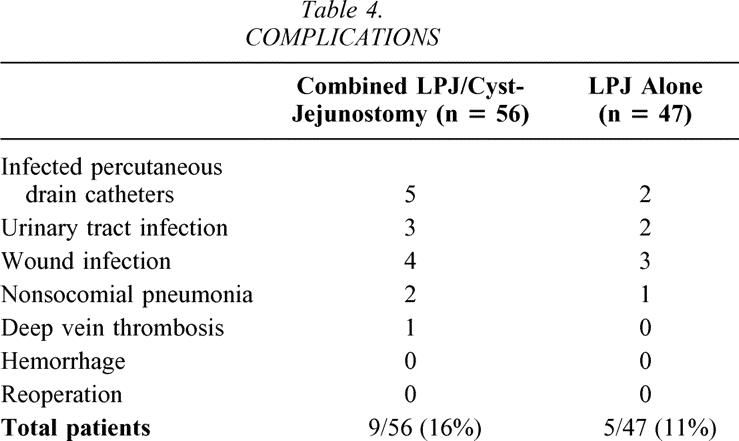
Transfusion requirements for the patients who had the combined procedure (0.5 ± 0.1 units per patient) were nearly identical to the transfusion requirements for the patients treated with LPJ alone (0.7 ± 0.2 units per patient). Nine of the 56 (16%) patients who had combined LPJ and cyst-jejunostomy required transfusion compared to 3 of the 47 (6%) patients treated with LPJ alone (Table 3). A total of 15 complications occurred in nine patients who had combined LPJ and cyst-jejunostomy (9/56 [16%]), and a total of 8 complications occurred in five patients who underwent LPJ alone (5/47 [11%]) (see Table 3). The specific complications are listed in Table 4. Infection of the percutaneous drainage catheters was common in the patients managed by this modality preoperatively in an attempt to treat the cyst definitively. The development of infection in these percutaneously drained cysts correlated with the number of days of catheter drainage. No patients had an episode of significant postoperative hemorrhage, and no patient required reoperation.
Table 3.TRANSFUSION REQUIREMENTS AND COMPLICATION RATE S
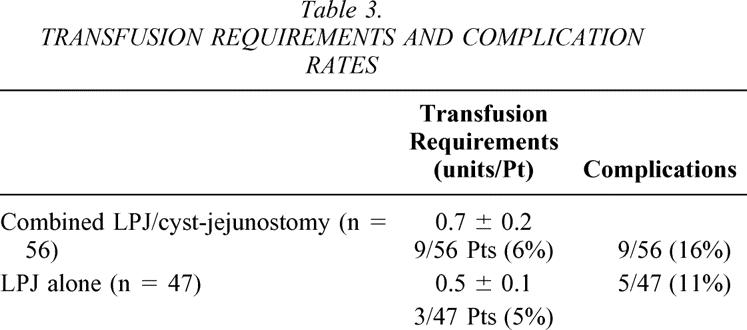
Table 4. COMPLICATIONS
External Pseudocyst Drainage Outputs
Among the 47 patients who were managed by LPJ alone, the associated pseudocyst was managed by intraoperative placement of external closed suction drainage in the initial 33 patients. Thereafter, the cysts were simply aspirated at the time of operation (14 patients). The mean daily drain output of enzyme-rich fluid was 3.1 ± 0.7 mL/24 hours in the patients who had intraoperative external drains placed. All of these patients had zero output after day 3. These drainage outputs are compared to patients who were all managed by percutaneous drainage preoperatively (16 patients): these drains recorded daily outputs of 322 ± 16 mL/24 hours (Fig. 2).
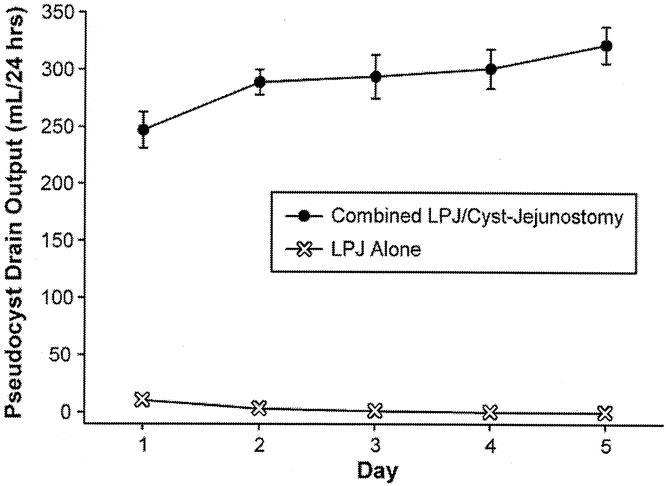
Figure 2. Mean daily drain output (mL) in patients who had preoperative percutaneous drainage of pseudocyst (n = 16) compared to mean daily drain output of closed suction drains placed intraoperatively in patients who underwent LPJ alone (n = 33).
Follow-Up
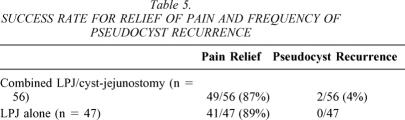
The mean length of follow-up for the entire group of chronic pancreatitis patients with associated pseudocyst was 73 ± 11months. For patients treated with combined LPJ/cyst-jejunostomy the mean follow-up was 89 ± 16 months, and for patients treated with LPJ alone the mean follow-up was 61 ± 9 months. Follow-up CT scanning was performed in all patients due to ongoing management and evaluation of chronic pancreatitis. There were two recurrences of pseudocyst in the follow-up period, both in patients with combined LPJ/cyst-jejunostomy. Pain relief, defined as pain-free (analog scale 0) and no need for narcotic analgesics, was achieved in 90/103 (87%). All patients had improvement in pain symptoms. Pain relief was comparable in the two groups of patients (49/56 [87%] in patients who had combined LPJ/cyst-jejunostomy and 41/47 [89%] in patients who has LPJ alone;Table 5).
Table 5. SUCCESS RATE FOR RELIEF OF PAIN AND FREQUENCY OF PSEUDOCYST RECURRENCE
DISCUSSION
We have spent years exploring the mechanism involved in the development and persistence of pancreatic pseudocysts by examining various clinical and structural aspects of these entities. We embarked on these studies with the postulate that pseudocysts are simply fistulae between the main pancreatic duct and the cyst. We have proposed that pseudocysts therefore may be expected to be governed by the same precepts as fistulae in other areas of the intestinal tract. By understanding pseudocyst in this way, one’s focus immediately shifts from the pseudocyst itself to the source of the fistula, the MPD. Despite this, for decades the literature pertaining to pseudocysts has uniformly emphasized the cyst itself, with little or no attention to the impact of ductal anatomy on the natural course. In 1989 we reported the usefulness of assessing pancreatic ductal anatomy in planning strategies for the operative management of pseudocysts. 1 We have recently reported data confirming that pancreatic ductal anatomy effectively predicted the likelihood of success and the likely failure of percutaneous drainage of pseudocysts. 2 We have established a system for categorizing the various anatomic abnormalities that can be found in patients with pseudocysts (see Fig. 1). The current study is an extension of the prior study and is intended to further confirm that ductal anatomy, and not the characteristics of the cyst, determines the course of pseudocysts by specifically examining patients with chronic pancreatitis- associated pseudocysts. This category of ductal anatomy is defined in type VI and VII ducts in our system of categorization.
Our data in the present study confirm that ductal drainage alone in chronic pancreatitis patients with pseudocyst is sufficient and effective in completely resolving associated pseudocysts. We infer from these data that the dynamics of the ductal system completely determine the course of pseudocysts in patients with chronic pancreatitis. We placed intraoperative external drains in the pseudocysts of 33 patients who had LPJ alone, and the output of these drains was essentially zero immediately after operation. The fact that our external drain outputs dropped so promptly further illustrates the dominant role that ductal pressures play in pseudocyst dynamics (see Fig. 2). Without gland and ductal pressures favoring flow of pancreatic juice through the fistulous tract into the pseudocyst, the cyst output essentially ceased. When we recognized this phenomenon, we felt that the data supported our decision to no longer place external drains at the time of operation. We now simply aspirate easily accessible cysts.
Several conclusions may be drawn from our data. In addition to the effectiveness in resolving pseudocyst and the safety in performing this procedure, we have demonstrated a possible advantage in operative time (see Table 2). We have not established a difference in transfusion requirements; however, we did note that certain patients, particularly those with pseudocysts in the tail of the pancreas or in the hilum of the spleen, when approached to perform a cyst-jejunostomy required extensive dissection and increased risk of hemorrhage. The patients who had LPJ alone did not require this added dissection. Thus, although this subset was too small to draw firm conclusions, there is a suggestion that eliminating dissection of the pseudocyst may reduce morbidity and reduce total transfusion requirements.
Another question that we anticipated to be raised regarding this strategy was the long-term efficacy both in terms of pain relief and recurrence of pseudocyst. Our data convincingly establish that both factors have a high likelihood of success using LPJ alone, but they also confirm that these outcomes are essentially identical to outcomes achieved with the combined procedure. In addition to our long-term efficacy measures, the safety and efficacy of operative management of pancreatic pseudocyst managed by either LPJ alone or by a combined procedure are reflected in our zero mortality, our lack of reoperation, and our low complication rates. These data should serve as a gold standard to which both endoscopic and interventional radiographic techniques must be compared.
Although there are no prior reports of operative management of pseudocyst by means of ductal decompression alone, this concept is similar to the use of transpapillary stents placed at the time of ERCP. This technique has been available and therefore evaluable for more than a decade. Despite this, there are insufficient data to ascertain its place in the management of patients with pseudocysts. The significant feature of this literature is the fact that the ductal decompression can achieve resolution of pseudocysts. This phenomenon provides further support for the concept that pseudocysts of the pancreas represent fistulae and the concept that addressing the ductal environment will facilitate pseudocyst regression.
Among the many papers that have evaluated transpapillary stent placement in the management of pseudocysts, patient populations have ranged from 23 to 9 patients. 3–7 The range of duration of follow-up (10–19 months) has been short, and success rates (30–78%) have covered a wide spectrum. Pain relief has been temporary in most. A factor that argues against using these techniques in chronic pancreatitis is the fact that effective therapy likely will require years of repeat stent placements. Attendant on this fact is the added risk of infection, particularly when stent occlusion takes place. With pseudocysts associated with acute pancreatitis, there may be a more definitive role for transpapillary stents; however, the success of stenting is thought to depend on radiographically established communication between the MPD and the cyst. This communication is far less commonly demonstrated in patients with acute pancreatitis (17–31%) compared to cyst–duct communication rates seen radiographically in chronic pancreatitis (49–74%). Finally, it is imperative to consider that extensive endoscopic management of a pseudocyst is not reasonable in a patient who will subsequently require operation to manage the pain associated with chronic pancreatitis.
The issue of MRCP as an alternative to ERCP in the management of these patients is pertinent. As a collective policy encompassing all patients with pseudocyst (see Fig. 1), we continue to believe that we derive important observations that as yet are not typically defined by MRCP, such as distinguishing stricture and complete cut-off of the MPD and such as communication demonstrated by ERCP between the duct and the cyst. Although these observations play a role in our decision-making in patients who do not have a diagnosis of chronic pancreatitis, these factors play no role in our choices in the management of patients with chronic pancreatitis. Once the diagnosis of chronic pancreatitis is established and ductal dilatation is confirmed, we proceed to LPJ regardless of the relationship between the duct and the cyst. Thus, MRCP would be a perfectly acceptable option to evaluate duct anatomy in the subset of patients with a diagnosis of chronic pancreatitis.
Combining definitive drainage of the pancreatic duct (LPJ) to treat chronic pancreatitis with a simultaneous drainage of an associated pseudocyst was advocated by Prinz et al. in several studies. 8–10 These papers made note of the fact that many patients with chronic pancreatitis who were candidates for LPJ had previously undergone operative pseudocyst drainage. They made the rather obvious but often neglected observation that chronic pancreatitis and pancreatic pseudocyst present with essentially identical clinical features (pain, weight loss, and a known history of pancreatic disease). Thus, they documented the failure to recognize and treat chronic pancreatitis simultaneously during the operative procedure performed to drain a pseudocyst. This failure ensures that the patient’s symptoms will not resolve. These studies from Prinz and Aranha as well as our own and other reports have established the safety and efficacy of combined LPJ and simultaneous cyst-jejunostomy.
Unfortunately, despite countless articles in the literature addressing the management of pseudocysts. there remains no uniformly accepted algorithm to direct the choice of modality in their management. Percutaneous drainage has many advocates. 11–13 Endoscopic management has many advocates. 3–7,14,15 Neither of these has achieved the success rates (pain relief/permanent resolution of pseudocyst) of operative management. These modalities have the appeal of avoiding an operative procedure, and certainly none of us can question the logic of this motivation. The data that are yet to be produced must attempt to resolve issues such as how many complications and how many repeat procedures in a nonoperative modality are too many. Our data in this report and many similar recent operative series have confirmed low rates of complications associated with operative management. Complications of both endoscopic and percutaneous management of pseudocyst are well documented. The risk of converting a sterile pseudocyst to an infected abscess, and the consequent conversion of an elective procedure to an urgent or emergent one, remains a very immediate issue encountered several times per year by any busy pancreatic surgeon.
Both endoscopic and percutaneous techniques provide vital information regarding the anatomy of a pseudocyst and its associated ductal anatomy. Endoscopic and percutaneous interventions often provide a vital bridge to definitive therapy. Clearly, there are selected patients for whom nonoperative measures are ideally suited. We have provided data to aid in the choice of modality based on ductal anatomy, 2 and we have developed a system for categorizing ductal changes seen in patients with pseudocyst (see Fig. 2). These previously reported data and this study serve to establish further information that may be applied to a more logical approach to pseudocyst of the pancreas.
Discussion
Dr. Charles J. Yeo (Baltimore, MD): I rise to congratulate Drs. Nealon and Walser for clearly advancing our knowledge about one of my favorite topics, that of pancreatic pseudocysts. This paper may well portend a paradigm shift: that is, the drainage of the main pancreatic duct alone, a modified Puestow-Partington type procedure, in patients with chronic pancreatitis and main duct dilatation may be the answer.
In brief, their data analyze 103 selected patients with chronic pancreatitis, mean duct diameter greater than 7 mm, and a pseudocyst. All of these patients took analgesics, all had pain, and all had a pseudocyst wall greater than 4 mm in size. 72% of these patients had a radiographically defined communication between the main pancreatic duct and the pseudocyst.
The data as presented show no significant differences between ductal drainage alone versus ductal drainage plus pseudocyst drainage when comparing complications, transfusions, and length of stay. Of course, eliminating operative pseudocyst drainage saved about 1 hour of operative time. Both procedures had excellent outcomes over a 5- to 7-year follow-up. Likely the key here is that both groups had longitudinal main pancreatic duct drainage, thereby reducing ductal and parenchymal pressure.
I would like to pose several questions for Dr. Nealon.
First, the groups were not randomly allocated to longitudinal pancreaticojejunostomy (LPJ) alone versus LPJ plus pseudocyst drainage. How did you choose? Or is this simply a historical transition, as I expect it is? What bias might the nonrandomized nature bring into the analysis?
Number 2. Is ERCP necessary in the year 2002? Or do you think modern MRCP techniques can substitute for defining the ductal anatomy and the duct–pseudocyst communication?
Number 3. For those of you in the group who are non-pancreatophiles, the finding of a markedly dilated main pancreatic duct, a spherical pancreatic cyst, and increased serum amylase and lipase can also be seen in patients with an intraductal papillary mucinous neoplasm (IPMN). This entity appears to be increasing to epidemic proportions. Beyond EUS, FNA, and tumor markers, how can you avoid the error of performing ductal drainage for an IPMN?
Number 4. Could you expand for us exactly how you perform the longitudinal pancreaticojejunostomy? Do you use a Roux-en-Y limb? What if you also, as your data show, have a 28% incidence of biliary stricture? Do you use two separate Roux-en-Y limbs? Or do you simply use one limb, and put the anastomosis in series?
Number 5. If you perform a subgroup analysis for the 28% of patients in your series who did not have documented pseudocyst–main duct communication, do their results differ? That is, does it matter that no ductal communication was seen by the ERCP?
And lastly, in the group with ductal drainage alone, you have transitioned from external catheter drainage to simple aspiration of the pseudocyst. Based on your theory, is it really necessary to aspirate the pseudocyst at all? Wouldn’t it simply disappear with ductal decompression alone?
I again congratulate Drs. Nealon and Walser for a thought-provoking presentation and manuscript.
Dr. Gary C. Vitale (Louisville, KY): This is really an excellent paper and really poses an interesting question as we are trying to become more minimalist in the approach to pancreatic disease. There are two aspects that I would like to focus on first. In this study, clearly the ductal anatomy is the key factor. Is ERCP essential at this point? Clearly anatomical evaluation is essential, but can MRCP be used in its place?
I think that it is not a foregone conclusion around the country that people get ERCP before pancreatic pseudocyst drainage. And although that is clearly the norm in Galveston and in Louisville, I think that needs to be preached.
In this series there were two important anatomical features noted, and I think they are critical for their results.
One is, the ducts were dilated above 7 mm and there was communication between bile duct and pseudocyst in most cases. I would like to know, do you think that there is a role for distal resection of the pancreas when the tail is clearly diseased? Do you think that there is better pain control if you can remove the calcified diseased pancreas, such as you showed in the one example that you had?
Another question: What do you recommend then for the patients with nondilated ducts, or ducts that did not communicate with the pseudocysts? The nondilated duct patients with pseudocysts are a special category. Is there a role for drainage there, or distal resection?
Also, does your data apply equally as well if you can’t demonstrate the ductal communication? Because I think that the drainage, either endoscopically or surgically, of the pancreatic ductal system is key to the improvement in these patients. I still think the long term probably requires resection.
We have two experiences in Louisville that interface with your work, and I would like your comments on these areas.
One is the endoscopic drainage of pancreatic pseudocysts. We have done now approximately 75 endoscopic pseudocyst drainages. We have looked at our data very carefully. We have an 85% long-term success rate with no downside; that is, the 15% that do not have successful drainage can go on to a surgical management without much problem.
The other experience is with pancreatic stenting by ERCP done in our surgical department in chronic pancreatitis. We recently reviewed our experience over about 8 or 9 years with 94 patients. We were able to get complete contact follow-up this summer with 84 of those 94, which is unusual in this group of patients. 80% feel the stents have helped them over time. In two thirds we were able to document actual decreased narcotic use or discontinuance of narcotic use using a Kentucky statewide narcotic reporting system.
My question is, do you have some experience or thoughts on the adjunctive use of ERCP with pancreatic drainage? It would seem to be necessary in those patients who have pancreatic duct strictures with nondilated ductal systems and pseudocysts in combination. This group ends up being a larger majority—not a large majority of those who are referred to surgeons, but if you are looking at pancreatitis as a whole. If you are doing ERCP, there are a lot of those patients out there in this category, and I want to know how you are managing them as well. Traditionally, we would use pancreatic resection since Peustow is not an option. We have found pseudocyst drainage plus pancreatic stenting is an alternative.
Thank you for an excellent study and all of your work, which helps all of us in this field.
Dr. John R. Potts, III (Houston, TX): I would like to thank the authors for providing this manuscript in advance of the meeting. It really is a provocative manuscript and one that I think will be referred to for many years to come.
In most series, including our own, about 95% of patients coming to operation for chronic pancreatitis have pain as at least one of their presenting complaints. And since we really can’t correct the endocrine or exocrine dysfunction associated with chronic pancreatitis with operation, it is pain relief that is usually our surgical goal for chronic pancreatitis.
Pseudocysts alone can cause pain. And many series have shown that drainage of the pseudocysts alone, even in patients with chronic pancreatitis, can give long-term pain relief in some patients. This being the case, I wonder if the authors have done a more extensive operation than is necessary in some of these patients.
I must admit that my own bias over the years has been to treat patients who have pseudocysts and chronic pancreatitis by first draining the pseudocyst alone, and then, if pain persists, approaching a more definitive operation for the chronic pancreatitis.
Lateral pancreaticojejunostomy is a definitive operation for pancreatitis, and the authors therefore imply that every patient with chronic pancreatitis and pseudocysts requires a definitive operation for the underlying chronic pancreatitis. Now, the next logical step in following that argument would be to say that if those patients with a pseudocyst and a small duct chronic pancreatitis would benefit from pancreatic resection at the time of operation for the pseudocyst. I don’t think that the authors really do that. But my question is, why add a definitive operation for a patient with a pseudocyst and chronic pancreatitis with a large duct and not add a definitive operation for those who have small ducts?
My other questions involve the Type 6 patients as illustrated in the manuscript. These are the patients who have a pseudocyst and a large pancreatic duct but no demonstrable communication between the two on ERCP. And my questions there are: Does this represent a failure of the ERCP to represent a communication that is there? If not, in what way does that drainage alone decompress those pancreatic pseudocysts?
Along those lines I would like to point out a simple observation, that the definition of a fistula is an abnormal communication between epithelialized surfaces, and since pseudocysts don’t have an epithelial surface it can’t truly be a fistula.
Again, I think this is a very provocative paper and one that we will see for a long time to come. I appreciate the opportunity to review the manuscript.
Dr. William H. Nealon (Galveston, TX): I thank all of the discussants for their provocative questions and kind words.
First, Dr. Yeo, you are correct that this is not a randomized controlled prospective study. You are correct that as we experienced success with ductal drainage alone, we essentially abandoned combined drainage. This concept arose with a mediastinal pseudocyst in a patient with chronic pancreatitis. It was not reasonable to attempt an internal drainage in the mediastinum, so I placed a drain. This combined with LPJ completely resolved the cyst. In attempting to explain this prompt resolution, we developed the concept that ductal dynamics determine the outcomes in the management of pseudocysts.
Is ERCP a necessary part of our evaluation? It is an excellent question. Obviously, with high-quality MRCP, and even very high-quality spiral CT scanning, we get very good, clear information about the ductal anatomy. The only feature that I continue to find lacking is establishing the presence or absence of communication between the duct and the cyst. The questioners have already asked if that really makes a difference. We have previously demonstrated that this finding clearly makes a difference if percutaneous management is considered. Until I am otherwise persuaded, we will continue to use ERCP.
I perform ERCP at our institution. This may simplify some of this process, particularly as transpapillary placement of stents and endoscopic drainage of cysts is an option. The surgeon may well send the patients for an ERCP and never see them again. We are hopeful that our data will encourage all practitioners (surgeons, endoscopists, and interventional radiologists) to be more selective, and we believe ductal anatomy is the key to this selection. It is encouraging to hear that at Louisville the Department of Surgery also performing ERCP. As I listen to your description I am not certain that you have utilized ductal anatomy to select your patients. I cannot state more emphatically that our message is to provide a logical system for selecting appropriate modalities. Ideally, healthy interaction among the surgeon, interventional radiologist, and gastroenterologist, as we have at our institution, offers the best arrangement.
In the manuscript I discuss at some length the question of IPMN. I agree with Dr. Yeo that this entity has become common. Recognizing cysts and neoplasms is an important distinction. When we have a suspicion, we recommend biopsy and/or aspiration of fluid to see if it has mucin in it either using EUS or percutaneous access. There are a number of things we do intraoperatively as well. Most important, we advocate obtaining a biopsy of the cystic structure itself when we drain it.
Our reconstruction is a simple Roux-en-Y. We do use the same jejunal limb for both the biliary drainage and the pancreatic ductal drainage.
I have one additional comment regarding communication between the duct and the cyst since Dr. Yeo, Dr. Vitale, and Dr. Potts each mentioned this. I had an interesting question from John Cameron last year when I presented a paper on pseudocyst. He stressed that obviously there is some kind of a communication between the pseudocyst and the main pancreatic duct, or the cyst would simply be absorbed. I agree with Dr. Cameron. Pseudocysts are full of enzyme-rich material, and obviously there has to be some kind of communication. Thus, in our current manuscript, I describe “radiographically demonstrable communication,” which is more accurate than suggesting that some cysts have no communication. Once again, the only impact we have seen in patients who do or do not have communication demonstrated by ERCP are those treated nonoperatively either endoscopically or percutaneously.
Whether we need to aspirate the cyst is a good question. Occasionally multiple cysts or anatomically inaccessible ones have been managed without complete aspiration. These have uniformly resolved after drainage of the duct.
Dr. Vitale mentioned distal resection. I believe that our strategy is likely to offer considerably better outcomes compared to resection. A distal resection almost always involves a splenectomy, often necessitates transfusion, has associated endocrine loss, and raises the likelihood of pancreatic fistula. Success rates for distal resection in the treatment of pain in chronic pancreatitis have proved to be inferior to all other operations for chronic pancreatitis and are particularly inferior to drainage procedures. We would more emphatically advocate ductal drainage in this subset.
A question was raised regarding small duct disease. Jakob Izbicki in Germany has developed a procedure in which an excavation of the duct in a V-shaped manner facilitates using a variation on LPJ with, in his opinion, some preservation of functioning pancreas compared to a full resection. There is very little information on the long-term success of this procedure. Patients with small duct disease requiring surgical intervention are generally considered to be candidates for resection.
I would like to emphasize one more important factor regarding the question about endoscopic drainage. Chronic pancreatitis is a disease for a lifetime. The long-term effectiveness of stent drainage in a 43-year-old person with large duct disease is not good and certainly not a permanent solution. It is completely unreasonable to submit this person to repeated endoscopic procedures rather than definitive operative procedures. So in this disease especially, with or without pseudocysts, I am not supportive of temporary treatments with stent placement alone.
Dr. Potts, the basis for using combined cyst drainage was the result of several studies in the 1980s and ’90s by Richard Prinz and Jerry Aranha. They evaluated this question and came up with definitive data to support the concept of performing a combined drainage of the cyst and the duct.
You also asked about small duct disease. Yes, I would proceed to resectional therapy as the treatment of choice for these patients.
Just parenthetically, I will comment on the appropriateness of the term “fistula.” Technically a cyst without intervention is not a fistula. All therapies, however, are based on creating a fistula either with the gut (endoscopically or operatively) or with the skin (percutaneously). It is the behavior of the duct and cyst after intervention that is the focus of our report.
References
- 1.Nealon WH, Townsend CM, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg. 1989; 209: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery vs percutaneous drainage). Ann Surg. 2002; 235: 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozarek RA, Ball TJ, et al. Endoscopic transpapillary therapy for disrupted pancreatic duct and pancreatic fluid collection. Gastroenterology. 1991; 100: 1362–1370. [PubMed] [Google Scholar]
- 4.Barthet M, Bagallo M, et al. Management of cysts and pseudocysts complicating chronic pancreatitis. Gastroenterology. 1993; 17: 270–276. [PubMed] [Google Scholar]
- 5.Grimm H, Meyer WH, et al. New modalities for treating chronic pancreatitis. Endoscopy. 1989; 21: 70–74. [DOI] [PubMed] [Google Scholar]
- 6.Smits ME, Badiga SM, et al. Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc. 1994; 40: 449. [DOI] [PubMed] [Google Scholar]
- 7.Howell DA, Lehman GA, et al. Endoscopic treatment of pancreatic pseudocysts: a retrospective multicenter analysis. Gastrointest Endosc. 1995; 41: 424. [Google Scholar]
- 8.Aranha GV, Prinz RA, et al. Evaluation of therapeutic options for pancreatic pseudocysts. Arch Surg. 1982; 117: 717–721. [DOI] [PubMed] [Google Scholar]
- 9.Munn JS, Aranha GV, et al. Simultaneous treatment of chronic pancreatitis and pancreatic pseudocysts. Arch Surg. 1987; 122: 662–667. [DOI] [PubMed] [Google Scholar]
- 10.Prinz RA, Aranha GV, et al. Combined pancreatic duct and upper gastrointestinal tract and biliary tract drainage in chronic pancreatitis. Arch Surg. 1985; 120: 361–366. [DOI] [PubMed] [Google Scholar]
- 11.Heider BS, Meyer AA, et al. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999; 229: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criadoe-Destreano AA, Weiner TM, et al. Long-term results of percutaneous catheter drainage of pancreatic pseudocysts. Surg Gynecol Obstet. 1992; 175: 293–297. [PubMed] [Google Scholar]
- 13.Adams DB, Anderson MC. Percutaneous catheter drainage compared to internal drainage in the management of pancreatic pseudocysts. Ann Surg. 1992; 215: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spivak H, Galloway JR, et al. Management of pancreatic pseudocysts. American College of Surgeons. 1998; 168: 507–511. [DOI] [PubMed] [Google Scholar]
- 15.Lehman G. Chronic pancreatitis disease: role of endoscopy. Gastrointest Endosc. 1999; 49: S81–84.10049456 [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: William H. Nealon, MD, Department of Surgery, 301 University Boulevard, Galveston, TX 77555-0544.
E-mail: wnealon@utmb.edu
Accepted for publication December 2002.