Abstract
Objective
To determine the influence of route of nutrition on gut mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) expression and the effect of MAdCAM-1 blockade on gut-associated lymphoid tissue (GALT) lymphocyte populations and established respiratory antibacterial immunity.
Summary Background Data
Lymphocytes, sensitized to antigens in Peyer’s patches, migrate via mesenteric lymph nodes and home to intestinal lamina propria. MAdCAM-1 located on endothelial cells regulates this trafficking. Experimentally, parenteral nutrition (PN) decreases GALT cell mass and mucosal immunity when compared with enteral feeding.
Methods
In experiment 1, MAdCAM-1 expression was quantified in 32 mice after 4 days of feeding chow, a complex diet, intragastric (IG)-PN, or PN. In experiment 2, MAdCAM-1 was measured in 102 mice 0, 4, 8, 12, 24, 48, or 72 hours after starting PN and at 0, 4, 8, 12, 24, or 48 hours after reinstituting chow following 5 days of PN. In experiment 3, 56 mice received chow, PN, chow + MECA-367 (anti-MAdCAM-1 mAb), or chow + Isotype control Ab (IsoAb) for 5 days, followed by Peyer’s patches, lamina propria, and intraepithelial lymphocyte yield with respiratory and intestinal IgA levels. In experiment 4, 10 days after Pseudomonas immunization, mice received chow + MECA-367 or chow + IsoAb for 4 days followed by 1.2 × 108Pseudomonas intratracheally.
Results
Diet and route affect MAdCAM-1 expression (chow > complex diet > IG-PN > PN). Decreased MAdCAM-1 expression occurred within hours of starting PN in Peyer’s patches, but not mesenteric lymph nodes or the intestine, and recovered quickly with enteral refeeding. MAdCAM-1 blockade reduced all GALT populations. Blockade had little effect on IgA levels and partially impaired the late response of established respiratory immunity.
Conclusions
Enteral feeding affects MAdCAM-1 expression. Complete MAdCAM-1 blockade reduces GALT lymphocytes to PN levels, but the chow feeding stimulus preserves IgA and early antibacterial resistance, implying the existence of non-MAdCAM-1 mechanisms to preserve mucosal immunity.
Enteral feeding reduces the incidence of pneumonia in critically injured patients, implicating a defect in mucosal defenses. 1–3 Mucosal surfaces throughout the body are in constant contact with environmental antigens and are in a position to sample antigens for the immune system. One of the largest complexes of mucosal immune cells is in the gut-associated lymphoid tissue (GALT). Specialized antigen sampling M cells overlying the Peyer’s patches (PP) and associated fixed dendritic cells within PP process antigen and present it to circulating naïve T and B cells, which traffic through the PP. According to the common mucosal immune hypothesis, they then enter the thoracic duct and systemic circulation and home to various mucosal effector sites in the GI tract and the upper and lower respiratory tract. In these sites, the sensitized B and T cells protect against potential pathogens by producing a specific IgA against their antigens. 4–6
Several adhesion molecules, including mucosal addressin cellular adhesion molecule-1 (MAdCAM-1), L-selectin, α4β7 integrin, lymphocyte function-associated antigen-1 (LFA-1), and intercellular adhesion molecule-1 (ICAM-1), regulate trafficking of lymphocytes within the mucosal immune system. 7 MAdCAM-1 is considered the key molecule for this direction and is expressed on the surface of endothelial cells within the high endothelial venules of PP, mesenteric lymph nodes, and venules of the lamina propria. 8 MAdCAM-1 has also been observed on splenic sinusoidal cells and within the nasal-associated lymphoid tissue. 9,10 Experimentally, lack of enteral stimulation decreases GALT cell mass, reduces intestinal and respiratory tract IgA levels, and impairs established mucosal immunity to specific pathogens. 11–13 Because of these diffuse mucosal immune changes, we hypothesized that lack of enteral feeding decreased MAdCAM-1 expression in the GALT and other mucosal areas, leading to the reduction of lymphocyte number and impairment in mucosal immunity. These experiment quantify the effects of the route and type of nutrition on the magnitude and/or kinetics of MAdCAM-1 expression and the response of GALT cell populations to MAdCAM-1 blockade and examines the effect of MAdCAM-1 blockade on established immunity to Pseudomonas aeruginosa.
METHODS
Animals
All experimental protocols were approved by the Animal Care and Use Committee of the University of Tennessee, the University of Wisconsin–Madison, and the Middleton Veterans Administration Hospital, Madison. Male ICR (Institute of Cancer Research) mice were purchased from Harlan (Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility. The environment was controlled with regard to temperature and humidity, with a 12-hour light:dark cycle. Mice were fed ad libitum chow (RMH3200 Agway, Syracuse, NY) and water for 2 weeks before entry into this study protocol. During feeding protocols, mice were housed in metal metabolism cages with wire grid floors to eliminate coprophagia.
Experiment 1: Effects of Route and Type of Nutrition on Total MAdCAM-1 Expression in Tissues
Thirty-two mice (6–8 weeks old) were randomized before cannulation to receive chow (n = 7), parenteral nutrition (PN; n = 8), intragastric (IG)-PN (n = 8), or a complex enteral diet (CED) (n = 9). Mice randomized to the chow and PN groups received internal jugular catheters and mice randomized to IG-PN and CED received a gastrostomy tube under anesthesia as previously detailed. 11–13 The technique does not induce physical or biochemical stress. 14
Catheterized mice were immediately connected to infusion pumps (Instech Laboratories, Plymouth Meeting, PA) and received 0.9% saline at 4 mL/d for 48 hours with ad libitum access to chow and water. On postoperative day 2, mice received their respective feeds. Chow-fed animals received 4 mL of 0.9% saline IV along with ad libitum chow and water throughout the study. The PN and IG-PN animals initially received 4 mL/d PN and were advanced to a goal rate of 10 mL/d by the third day of feeding. The PN solution contained 4.1% amino acids, 34.3% glucose (4878 kJ/L), electrolytes, and multivitamins with a nonprotein calorie to nitrogen ratio of 743 kJ/g nitrogen to meet the calculated nutritional requirements and provide 2,146 kJ/kg/d of nonprotein calories and 18.0 g protein/kg/d at goal rate. The CED mice received 6 mL/d of Isocal (Mead Johnson Co., Evansville, IN) with an increase in rate to a goal of 13 mL/d. Isocal contains 13.3% carbohydrate, 4.5% fat, and 3.4% protein (4,441 KJ/L) in addition to electrolytes and vitamins. The nonprotein calorie/nitrogen ratio of the CED was 709 kJ/g nitrogen. Thus, administered diets were almost isocaloric and isonitrogenous except for the chow mice.
After receiving their respective diets for 5 days, mice were killed for quantification of MAdCAM-1 expression in PP, mesenteric lymph nodes, small intestine, nasal-associated lymphoid tissue, nasal passages, bronchus, lung, and liver.
The binding mAb (MECA-367 rat IgG2aκ, the antimouse MAdCAM-1 mAb, PharMingen Inc., San Diego, CA) and the nonbinding mAb (R35–95, rat IgG2aκ isotypic Ab, PharMingen Inc) were radiolabeled with 125I and 131I, respectively, using the iodogen method as previously described in detail. 15–17
A mixture of 10 μg 125I-labeled MECA-367 was given with an appropriate amount of 131I-labeled nonbinding Ab (400,000–600,000 cpm) through the jugular vein catheter (total volume, 200 μL). 125I-labeled MECA-367 was injected to evaluate MAdCAM-1 expression on the endothelial cells, while 131I-labeled nonbinding mAb was given to eliminate the influence of nonspecific binding of MECA-367 to endothelial cells.
The mice were anesthetized with ketamine and acepromazine maleate and the radioisotopes administered after carotid cannulation as previously described in detail. 16,17 Animals were exsanguinated while perfused with bicarbonate-buffered saline through the jugular vein and carotid artery catheter after severing the inferior vena cava at the thoracic level as previously described. 16,17 The tissues were harvested for measurement of radioactivity.
The Cobra Automated Gamma Counting System (Packard Instrument, Meriden, CT) was used to count 125I (binding mAb) and 131I (nonbinding mAb) activities in each organ and in a 50-μL plasma sample as previously described. 15–17 The amount of radioactivity remaining in the tube and syringe used to mix and inject the mAbs was subtracted from the total calculated activity administered. Adhesion molecule expression was determined by subtracting the accumulated activity of the nonbinding mAb from that of the binding mAb, and expressed as ng of mAb tissue in the equation:
Adhesion molecule expression (ng mAb/tissue) = (125I cpm of tissue/125I cpm injected-131 I cpm of tissue/131I cpm injected) × (125I cpm/50 μL of serum/125I cpm injected)/(131I cpm/50 μL of serum/131I cpm injected) × total injected binding mAb (ng)
Experiment 2
Male mice (n = 65) were fed PN for 0, 4, 8, 12, 24, 48, or 72 hours, beginning 48 hours after venous cannulation. Subsequently, 37 animals who had received PN for 4 days were refed with chow diet and sacrificed at 0, 4, 8, 12, 24, and 48 hours after beginning refeeding. At each time point, MAdCAM-1 expression in PP was quantified using the techniques described in experiment 1.
Experiment 3: Effects of MAdCAM-1 Blockade on GALT Cell Yield
Pilot experiments performed to determine the amount of radiolabeled MAdCAM-1 necessary for saturation of cell binding sites revealed that a dose of 10 μg per mouse was adequate. Since the half-life of IgG2akappa monoclonal antibody is about 24 hours, we gave a loading dose of 20 μg anti-MAdCAM-1 antibody, followed by 10 μg per day. Fifty-six male mice (6–8 weeks old) were randomized to receive chow (n = 16), PN (n = 13), chow + MECA-367 (n = 15) or chow + isotypic antibody (n = 12). Their respective diets were started 2 days after jugular cannulation and isotypic Ab or saturating doses of MECA-367 were administered daily on days 3 to 6 of the experiment. All groups (except PN) had free access to water and chow throughout the study.
After 6 days of feeding (2 days of chow during the postsurgical recovery and 4 days of experimental infusion), the animals were weighed and anesthetized with a ketamine and acepromazine mixture. The thoracic and abdominal cavities were opened aseptically, and the animals were exsanguinated by cardiac puncture. The small intestine was removed from the duodenum to the terminal ileum and flushed with 10 mL calcium- and magnesium-free Hank’s balanced salt solution (HBSS) (Bio Whittaker, Walkersville, MD) to remove intestinal contents. The contents were collected and stored at −80°C for later analysis of mucosal IgA levels by ELISA (technique described later).
PP lymphocytes were isolated after excision from the serosal side of the intestine, as described in detail by Li et al. 11 The cell number was determined with a hemocytometer, and the viability of lymphocytes was determined by trypan blue exclusion. The number of PP retrieved from each animal was recorded.
Intraepithelial and lamina propria lymphocytes were isolated following the protocol as described in detail by Li et al. 11 The number and viability of lymphocytes were determined by trypan blue exclusion.
The trachea was opened aseptically and clamped at the thoracic inlet with a vascular clip. Five hundred microliters cold HBSS was injected slowly into the tracheal lumen cephalic to the obstruction. The washing fluids draining from the nostrils were collected in microcentrifuge tubes and stored at −80°C for future analysis of IgA. Another 500 μL of cold HBSS was injected slowly through the tracheal lumen toward the alveoli. The alveolar washing fluids were collected and stored at −80°C for future analysis of IgA.
IgA was measured in intestinal, nasal, and alveolar washing fluids with a sandwich enzyme-linked immunosorbent assay (ELISA) using a polyclonal goat antimouse IgA (Sigma) to coat the plate, a purified mouse IgA (Sigma) as standard, and a horseradish peroxidase-conjugated goat antimouse IgA (Sigma).
Experiment 4: Effects of MAdCAM-1 Blockade on Respiratory Immunity
Eleven days before cannulation, 25 mice were immunized intranasally with P. aeruginosa antigen-containing liposomes as described below, and 19 mice received control liposomes containing no antigen. Two days after venous cannulation, immunized mice were randomized to the MECA-367 (n = 12) or IsoAb control group (n = 13). Beginning on postoperative day 2, each group was injected intravenously with 20 μg initially and then 10 μg daily of respective mAb. After 4 days, mice were anesthetized and challenged with 40 μL PBS containing 1.2 × 108 live bacteria intratracheally. Mice received ad libitum chow and water. Deaths were observed at 24, 48, and 72 hours.
Bacterial polysaccharide-containing liposomes were prepared by the detergent dialysis technique described by Abraham 18 and as described in our prior work. 12,13 Polysaccharide incorporation ranged from 30% to 70%. Calculation of immunization dose was based on the original lipid concentration (15 mg), because this produced consistent immunity. Each mouse was immunized intranasally with liposomes containing 160 μg lipid and 30 to 70 μg polysaccharide, or with liposomes (160 μg lipid) alone, as previously described. 12,13 Calculation of the liposome dose for immunization was based on the amount of lipid present.
P. aeruginosa was plated on trypticase soy agar with 5% sheep red blood cells 48 hours before the animals were inoculated and the plated P. aeruginosa was serially diluted and suspended to A595nm of 0.670 ± 0.010 after 24 hours, as previously described. 12,13
Statistics
Data are expressed as means ± SD and were analyzed using analysis of variance, followed by the Fisher protected least significant difference post hoc test or Student t test. The Fisher exact probability test was used for survival rate comparisons at 24, 48, and 72 hours after bacterial inoculation. Kaplan-Meier curves were used to analyze survival times.
RESULTS
Experiment 1
The pre-experiment body weights of all groups were similar (Table 1). CED, IG-PN, and PN mice lost more weight than chow mice, but there were no differences between these three groups. Chow-fed mice typically have 1.5 g of residual feces, while the GI tracts of IG-PN and IV-PN mice are empty. CED results in less than 0.5 g of feces.
Table 1. MICE BODY WEIGHT AND WEIGHT CHANGE
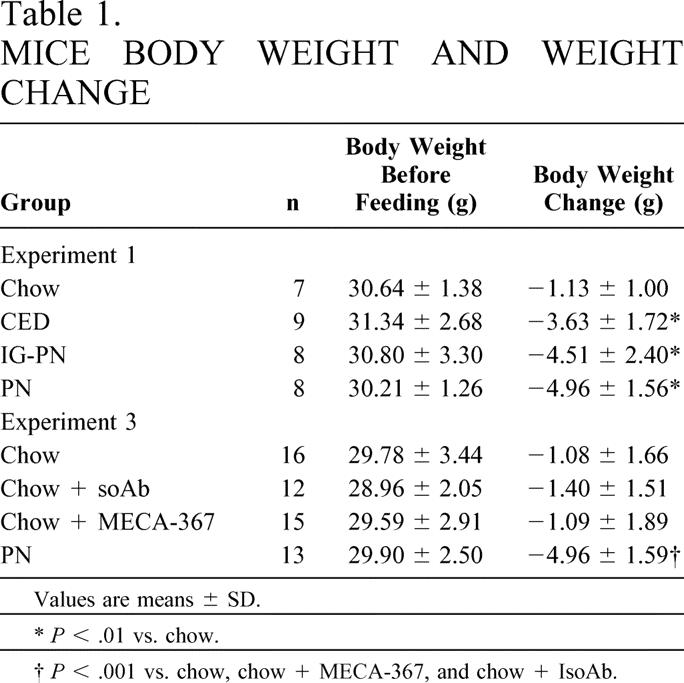
Values are means ± SD.
*P < .01 vs. chow.
†P < .001 vs. chow, chow + MECA-367, and chow + IsoAb.
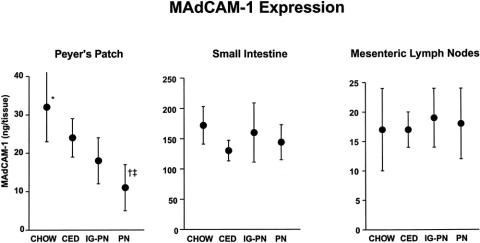
Total expression of MAdCAM-1 in PP was significantly lower in the PN group than in all enteral groups, with a significant decrease in the CED and IG-PN groups compared with chow mice (Fig. 1). There were no significant differences in MAdCAM-1 expression in the other GALT sites (i.e., small intestine or mesenteric lymph nodes) among the four diet groups.
Figure 1. Total expression of MAdCAM-1 (measured as nanograms of monoclonal antibody in whole tissues) in the Peyer’s patches, small intestine, and mesenteric lymph nodes. Mice fed chow, complex enteral diet (CED), intragastric parenteral nutrition (IG-PN), and intravenous PN (PN) received respective diets for 4 days. *P < .03 vs. CED and IG-PN, †P < .05 vs. IG-PN, ‡P < .0005 vs. chow and CED.
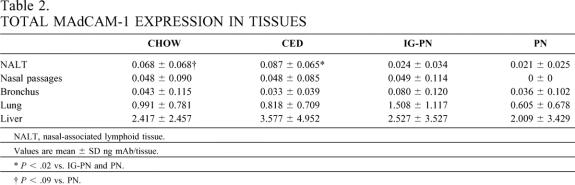
In extraintestinal sites, CED mice showed significantly higher MAdCAM-1 expression in the nasal-associated lymphoid tissue compared with IG-PN and PN groups (Table 2). Chow tended to maintain MAdCAM-1 levels in the nasal-associated lymphoid tissue when compared to PN (P = .09), with no difference compared to CED. No significant differences were observed in the nasal passages, bronchus, lung, or liver among the four groups.
Table 2. TOTAL MAdCAM-1 EXPRESSION IN TISSUES
NALT, nasal-associated lymphoid tissue.
Values are mean ± SD ng mAb/tissue.
*P < .02 vs. IG-PN and PN.
†P < .09 vs. PN.
Experiment 2
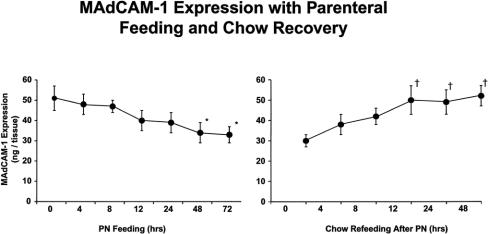
PP MAdCAM-1 expression dropped shortly after instituting PN, reaching statistical significance at 24 hours in this sample size and a nadir by 48 hours. Reinstitution of chow feeding lead to a rapid recovery of PP MAdCAM-1 expression, which reached normal levels and statistical significance 12 hours after refeeding (Fig. 2).
Figure 2. MAdCAM kinetics. MAdCAM-1 expression in Peyer’s patches dropped after starting PN from baseline and reached significance at 48 and 72 hours. Chow refeeding of animals fed PN for 5 days lead to rapid increases in MAdCAM-1 expression, which reached significance by 12 hours. *P < .05 vs. 0, 4, and 8 hours, †P < .02 vs. 0 hours.
Experiment 3
The pre-experiment weight of all groups were similar (see Table 1). PN mice lost more weight than all chow groups (P < .001), but there were no differences between the three chow-fed groups.
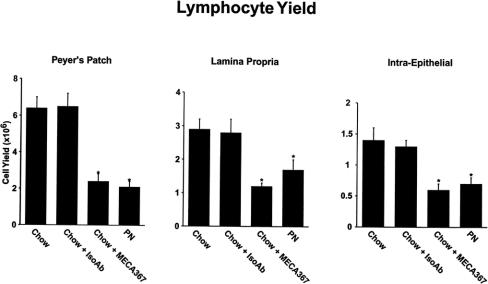
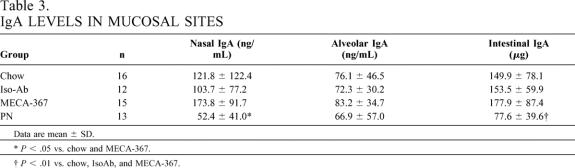
Total lymphocyte yield in PP, lamina propria, and intraepithelial sites were significantly lower in PN and chow + MECA-367 groups than in chow or chow + IsoAb groups (P < .05)(Fig. 3). There were no significant differences in cell numbers between MECA-367 and PN animals. IgA levels in nasal and intestinal wash fluids were significantly lower in PN animals than in chow groups (Table 3). There were no significant differences in the number of PP retrieved between groups (chow, 10.4 ± 2.1; chow + MECA-367, 9.5 ± 1.8; chow + IsoAb, 11.6 ± 1.8; PN, 10.6 ± 2.9).
Figure 3. GALT lymphocyte yield after MAdCAM-1 blockade. MECA-367 blockade of MAdCAM-1 significantly reduced Peyer’s patch, lamina propria, and intraepithelial lymphocytes to PN levels. *P < .02 vs. chow and IsoAb.
Table 3. IgA LEVELS IN MUCOSAL SITES
Data are mean ± SD.
*P < .05 vs. chow and MECA-367.
†P < .01 vs. chow, IsoAb, and MECA-367.
Experiment 4
Immunized animals in both the chow + MECA-367 group and the chow + IsoAb group had significantly lower mortality than unimmunized animals at 24 hours (Table 4). However, at 48 and 72 hours, mortality increased in the MECA-367 group to a level midway between the unimmunized animals and those receiving IsoAb. At both 48 and 72 hours, animals receiving IsoAb sustained a significantly lower mortality than unimmunized mice. When survival times were analyzed using Kaplan-Meier survival curves, survival times were significantly different between the unimmunized animals and the chow + MECA-367 group and the chow + IsoAb group. There were no differences in survival time between the MECA-367 and the IsoAb groups.
Table 4. MORTALITY FOLLOWING PSEUDOMONAS CHALLENGE

*P < .01 vs. unimmunized.
DISCUSSION
Enteral feeding of trauma patients decreases the incidence of pneumonia compared with starvation or PN. 1–3 Our prior work showing an impaired mucosal immune system with a reduction in secretory IgA at mucosal sites provides a cogent hypothesis for this phenomenon. 4–6,11–13 The current work demonstrates that lack of enteral feeding rapidly reduces expression of the primary adhesion molecule, MAdCAM-1, responsible for directing cells into the PP, resulting in lymphocyte depletion, which recovers quickly with enteral feeding. The cause and effect is confirmed by reducing GALT cell populations to the level of PN-fed mice by administering MECA-367, an antibody that blocks all MAdCAM-1. This blockade reduces GALT cell populations, but IgA levels in the respiratory and GI tract are preserved with chow feeding. However, the effectiveness of respiratory defenses established against Pseudomonas, although probably weakened, is not reduced to the same degree as parenteral feeding, which completely destroys immunity. 13 These data continue to define an intriguing link between the gut and respiratory immunity discovered in our earlier work.
A specific subset of naïve lymphocytes destined to join the mucosal immune system is defined by a distinct adhesion molecule profile, L-selectin and α4β7 integrin, expressed on their cell surfaces. 19,20 The high endothelial venules of PP, the main inductive site of the GALT, express the ligand for these molecules, a MAdCAM-1 modified slightly by the addition of a carbohydrate molecule. Modified MAdCAM-1 interacts principally with L-selectin to attract the naïve lymphocytes into the PP for sensitization to processed antigen. 20 The adhesion molecule profile on lymphocytes changes after sensitization, with a decrease in L-selectin expression and an increase in α4β7 expression. 21 The altered profile more strongly interacts with the unmodified form of MAdCAM-1 expressed by endothelial cells of the intestinal lamina propria venules. The unmodified MAdCAM-1 has less affinity for L-selectin but greater affinity for α4β7, which excludes naïve lymphocytes. 21 MAdCAM-1 expressed within sites such as the nasal passages, mammary glands, and genitourinary system allows for sharing of immunologic protection generated within the gut according to the common mucosal immune hypothesis.
GALT cell populations drop and reach a nadir within 3 to 4 days with PN when no enteral stimulation is provided. This system cannot be studied by starvation in the mouse, since rapid malnutrition results in death within 72 hours. Use of PN prevents the progressive malnutrition that itself impairs immunity and allows isolated study of the effects of enteral stimulation on mucosal immunity. Animals fed parenterally rapidly lose mucosal immune competence as intestinal and respiratory IgA levels drop in association with rapid decreases (over 72 hours) of PP and lamina propria lymphocyte populations. 22 In the intestinal lamina propria, these changes reduce the Th2-type IgA-promoting cytokines, IL-4 and IL-10. 23 Functionally, established respiratory immunity to viral and bacterial pathogens is lost as antigen-specific IgA levels drop. 12,13 IgA normally prevents attachment of pathogens to mucosal surfaces; without attachment, the pathogens cannot infect the host. 24
Since the MECA-367 antibody recognizes and binds to both the modified and unmodified MAdCAM-1 molecule, 6,20 the dual radiolabeled antibody technique quantified expression of MAdCAM-1 at key sites of the mucosal immune system. This technique detects quantitatively small changes in expression of adhesion molecules better than immunohistochemistry and provides an advantage over mRNA quantification, since message is not always translated into functional expression of proteins. 15,25–27 Since the size of the PPs decrease, PPs were marked, in pilot experiments, with a suture before diet manipulation to be sure that all PPs were harvested and to eliminate the chow of spuriously low radioactivity and an undercalculation of MAdCAM-1 expression due to low PP retrieval. MAdCAM-1 expression was significantly higher in the chow-fed mice compared with the PN mice with respect to both total expression and on a per-PP basis (data not shown). In subsequent experiments PP number were counted to ensure there were no differences in the number of PPs harvested between groups.
In the four diet studies, chow feeding resulted in the highest MAdCAM-1 expression, followed by CED, IG-PN, and finally PN. As MAdCAM-1 expression decreases, we speculate that fewer naïve cells enter the PP, which leads to reduced lymphocyte cell numbers. In our kinetic study, lack of enteral feeding resulted in fairly rapid decreases in MAdCAM-1 expression (which reached significance at 24 hours in the sample size) that rapidly recovered within 12 hours with enteral refeeding. 28 MAdCAM-1 control of lymphocyte entry into PP was definitively established with the use of MECA-367 in saturating doses to block this ligand, which produced decreases in all lymphocyte numbers similar to PN-fed mice in PPs as well as in distant lamina propria and intraepithelial sites. Chow feeding with MECA-367 significantly reduced lymphocyte cell yield compared with animals administered control antibody injections within 4 days, proving the importance of MAdCAM-1 on lymphocyte populations in GALT in vivo.
Why lack of enteral feeding decreases MAdCAM-1 expression within PP and not other “downstream” GALT sites is not clear. A modified form of MAdCAM-1 is expressed only in the PP, and alterations in the PP environment (such as the cytokine milieu) with PN feeding may result in altered gene expression and a lack of transport of the modified molecule to the cell surface. 20,21 The unmodified form in peripheral sites may not be so affected. Although we have not measured cytokine changes in isolated PP, parenteral feeding results in a reduction of IL-4 levels within small intestinal homogenates. 23 IL-4 increases the affinity of lymphocytes to high endothelial venules of PP in in vitro models. 29 Interestingly, MAdCAM-1 expression after PN was depressed in nasal-associated lymphoid tissue, while CED and chow maintained higher levels. This is consistent with our ELISPOT work, which demonstrated a impaired cellular proliferation of IgA- and IgM-producing cells in nasal tissues of PN-fed mice compared to CED and chow mice in response to acute infection with the APR/8 influenza virus. 30
To examine the functional effects of the MAdCAM-1 molecule changes, we tested the effects of this in our model of established antibacterial respiratory immunity. This immunization technique with P. aeruginosa provides a specific immune response, results in established immunity within 10 days, and reduces mortality to subsequent intratracheal administration of live Pseudomonas from 80% to 100% in unimmunized mice to 10% to 20% in immunized mice. 13,18 We previously showed that 5 days of parenteral feeding destroys this immunity and increases mortality to the level of unimmunized controls. 13 Animals given MECA-367 blockade or nonspecific antibody for 4 days sustained no mortality at 24 hours, a significant improvement relative to the unimmunized mice. However, MECA-367 produced a progressive increase in mortality at 48 and 72 hours so that there was nearly a doubling of the mortality in this group compared with control antibody-treated mice. Mortality with blockade was still better than unimmunized animals, suggesting a partial loss of established immunity against the bacteria rather than the complete loss that occurs with PN. To reach statistical significance between these experimental groups would have required approximately 40 animals per group; therefore, we chose not to perform more experiments. However, we concluded that although anti-MAdCAM-1 therapy impaired immunity in immunized chow-fed mice, it did not impair it to the extent of animals receiving no enteral stimulation over a 4-day period of blockade.
There are several reasons why blockade may not have completely eliminated immunity against challenge. First, animals given the blocking antibody continued to receive chow and generated an enteral response to digest the food. Neuropeptides, such as gastrin-releasing peptide, gastrin, and cholecystokinin, are released in response to enteral feeding and are known to support mucosal immunity. 31–33 Administration of the gastrin-releasing peptide analog bombesin completely eliminates the impairment in antipseudomonal and antiviral defects in immunity induced by parenteral feeding. 34,35 MECA-367 should exert little if any effect on this neuropeptide release, which could keep cells upregulated and stimulated within the lamina propria, perhaps by maintaining a normal cytokine milieu. Recently we demonstrated that bombesin protection did not work by upregulating MAdCAM-1 expression. 36 Second, lack of enteral feeding is known to impair transport of IgA from the basal surface of epithelial cells to the luminal surface. 37 Chow-fed mice might keep this transport system intact so that even if less IgA were produced in the lamina propria, a more effective transport could tip the balance in favor of improved mucosal defenses. Third, another adhesion molecule, peripheral node adressin (PNAd), is expressed on endothelial cells within the inductive and effector sites in the murine nasal-associated lymphoid tissue. 10 While alterations in MAdCAM-1 expression or MAdCAM-1 blockade may decrease the number of lymphocytes committed for IgA production in the intestinal mucosa, there may be no effect on PNAd expression within the nasal-associated lymphoid tissue, potentially preserving IgA responses within the respiratory tract. 10 Finally, chow feeding might produce increased levels of IL-4 or IL-10—two important IgA-stimulating cytokines—in peripheral tissues to compensate for the GALT cell reduction induced by MECA-367. Each of these potential mechanisms warrants further investigation.
This work offers novel evidence partially explaining the effect of enteral feeding or lack of enteral stimulation on GALT during PN feeding. Rapid alterations of MAdCAM-1 expression within the main site of GALT entry with no enteral stimulation decrease the number of naïve lymphocytes entering the mucosal immune system. While functional effects of MAdCAM-1 blockade within the respiratory system are muted in chow-fed mice compared with animals receiving no enteral stimulation, there was still a dramatic effect on GALT cell numbers in our in vivo model. The limited functional respiratory defect raises interesting questions of the role of IgA transport, cytokine production, and expression of other adhesion molecules in the mucosal immune system associated with lack of enteral stimulation.
Discussion
Dr. R. Neal Garrison (Louisville, KY): The elegant and complex work just presented by Dr. Kudsk provides an important piece to the puzzle of our understanding of gut immunity. This is important because it is increasingly clear that the intestinal immunologic function is pivotal in the sequence of systemic inflammatory response that follows surgery in traumatic injury.
As Dr. Kudsk has shown, the GALT tissue and the expression of MAdCAM-1 closely coincide with enteral feeding. However, MAdCAM-1 blockade does not eliminate mucosal immunity, and protection against an infectious challenge is preserved in spite of that blockade.
I was intrigued in reading the discussion of this paper to learn that MAdCAM-1 expression is heaviest on microvascular venular endothelial cells in Peyer’s patches and the lamina propria. In thinking about blood vessels and food, two of my favorite topics, I remembered what all our mothers knew long ago: We couldn’t swim after lunch because all our blood ran to our stomachs, and we would suffer from belly cramps.
These two thoughts led to my one question: Could the explanation for these findings, that the root of feeding rather than the composition of the feeds, be simply a matter of augmented blood flow to the intestinal tract and the subsequent associated GALT tissue in response to exposure of nutrients to the gut mucosa?
Dr. David N. Herndon (Galveston, TX): I too would like to congratulate Dr. Kudsk for his fascinating and amazingly technically challenging studies that build very well on his seminal work in this area in the past. I too am perplexed by the paradox of this study, and I would like Dr. Kudsk to speculate a bit more on the mechanism whereby nasal and alveolar IgA is still elevated in chow-fed animals, mice, in which anti-MAdCAM monoclonal antibodies successfully decreased intestinal lymphocyte trafficking, just the opposite of what we would have expected. Why do these animals survive the Pseudomonas challenge longer than unimmunized mice? Decreased numbers of lymphocytes to recognize stimuli and produce signals is the opposite of what would be expected to create this effect.
Second, does this also mean that the effect of TPN decreasing respiratory immunity is independent of its effect on decreasing gut-associated lymphoid tissue, as previously hypothesized? Is it perhaps more a physical effect, an effect on neuropeptides released by the GI tract or an effect on cytokine levels?
The immunization of Pseudomonas in this study is done intranasally. Are independent lymphoid tracking mechanisms present in nasal-associated lymphoid tissue than those associated with gut lymphoid tissue; can you simply indicate a different trafficking mechanism in nasal-associated lymphoid tissue?
Dr. George M. Watkins (Tampa, FL): I think it was an excellent paper. I would like the authors, if the authors did it, to tell us in their various regimes what the bacteriological counts were when they counted the lymphoids, the liver, the lungs, and the spleen again. Just as a comment: although we never published it, just presented it at one of the local American College meetings at a residents’ competition, we looked at colchicine once, and bacterial translocation, and we felt that the colchicine would probably block some of the translocation because colchicine reduces the vascularization transport across cells.
We found that actually very significant increases—and we did not know why and we didn’t do the experiment anymore—the colchicine increased markedly the bacterial counts in all these places that I mentioned. And I am wondering if the use of colchicine—I won’t be doing it anymore since I am long retired, but if you should look at one of these groups maybe thinking about colchicine and see if it doesn’t have a different effect than we thought it had.
Dr. Timothy L. Pruett (Charlottesville, VA): What you described is basically a starvation model for change and MAdCAM-1. Is there any information available with animal models? We usually see people who are stressed, either burn or infectious models in terms of that. And second, your feeding has been with enteral nutrition. Can you at all speculate on the role of the immunostimulatory fatty acids as a potential way in which the augmentation of gut immunity occurs?
Dr. Kenneth A. Kudsk (Madison, WI): I would like to thank all of the discussants for their insightful comments.
Dr. Garrison asked about blood flow. Certainly blood flow is an important issue in this since it carries the cells throughout the body. After eating a meal, there is a tremendous increase in blood flow and lymphocyte delivery to the lamina propria. Whether this affects initial education of cells and delivery to Peyer’s patches, I don’t know. I don’t believe that it would all be due to blood flow, since if we give parenterally fed animals either bombesin or glutamine, which are not known to increase blood flow to the gut tremendously, we see an upregulation of immunity in the gut-associated lymphoid tissue.
Dr. Herndon asked about the mechanisms to preserve defenses and why they might be. Last week I got some data and was surprised to find that animals given MECA-367 had very high IL-4 levels. Compared to chow-fed animals or those with isotypic antibody, IL-4 levels were almost twice as high as the chow-fed animals and almost four times as high as the animals fed parenterally.
Two things affect IgA production: the number of cells and the presence of this IL-4 and IL-10. I didn’t include these data in the manuscript because we are still doing the samples for IL-10. But clearly there is a cytokine change. This really reinforces a new body of literature coming out in the basic sciences which suggest a communication between the mucosa and the underlying GALT cells which keep them vigilant. Maybe they do this by affecting cytokines in the GALT, or by releasing cytokines themselves since the mucosa is known to be a cytokine producer.
Dr. Watkins mentioned the bacteriologic cultures. We don’t get mesenteric lymph nodes for cultures. I believe bacterial translocation is an epiphenomenon which reflects a breakdown in gut-associated lymphoid tissue. It has been well documented that parenteral feeding causes an overgrowth of aerobic bacteria and an increase in bacterial translocation. But no one has ever been able to relate those findings to clinical outcome. The colchicine is an interesting concept. Perhaps we could talk afterwards, because I didn’t understand its effects. If it impairs neutrophils, then I would be inclined to believe that it would hurt animals in our model, particularly in stress.
Dr. Pruett commented that this is a starvation model of Peyer’s patches. That is absolutely true. We are not studying some bad effect of TPN, as some people interpret our data. If we starve these animals and don’t provide parenteral feeding, they die within 3 days from starvation. Parenteral feeding allows the study of this mucosal immune system, which is 50% of our total immunity, and how, by taking food via the gastrointestinal tract, it keeps this system upregulated.
Stress does play a role, but not in the way you think. At this point I do have some stress data but it is not a downregulation, at least in the short term. Stress produces an upregulation in IgA production. But I don’t want to talk about that anymore at this point since it is preliminary.
Finally, I have done nothing with fatty acids, but I think it is an interesting field. I have a Ph.D. joining me in January who is a specialist in that area, so I may be able to share insights in another year.
References
- 1.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992; 215: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, McCroskey BL, Haddis T, et al. Total enteral nutrition vs total parenteral nutrition after major torso injury: Attenuation of hepatic protein reprioritization. Surgery. 1988; 104: 199–207. [PubMed] [Google Scholar]
- 3.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma. 1986; 26: 874–879. [DOI] [PubMed] [Google Scholar]
- 4.Tomasi TB Jr. Mechanisms of immune regulation at mucosal surfaces. Rev Infect Dis. 1983; 5: S784–S792. [DOI] [PubMed] [Google Scholar]
- 5.McGhee JR, Mestecky J, Dertzbaugh MT, et al. The mucosal immune system: From fundamental concepts to vaccine development. Vaccine. 1992; 10: 75–88. [DOI] [PubMed] [Google Scholar]
- 6.Butcher EC. Lymphocyte homing to mucosal effector sites. In: Ogra PL, Lamm ME, McGhee JR, et al., eds. Handbook of Mucosal Immunology. San Diego: Academic Press, Inc; 1994:507–522.
- 7.Connor EM, Eppihimer MJ, Morise Z, et al. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999; 65: 349–355. [DOI] [PubMed] [Google Scholar]
- 8.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Path. 1997; 151: 97–110. [PMC free article] [PubMed] [Google Scholar]
- 9.Kraal G, Schornagel K, Streeter PR, et al. Expression of the mucosal vascular adressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Path. 1995; 147: 763–771. [PMC free article] [PubMed] [Google Scholar]
- 10.Csencsits KL, Jutila MA, Pasual DW. Nasal-associated lymphoid tissue: Phenotypic and functional evidence for the primary role of peripheral node adressin in naïve lymphocyte adhesion to high endothelial venules in a mucosal site. J Immunol. 1999; 163: 1382–1389. [PubMed] [Google Scholar]
- 11.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995; 39: 44–52. [DOI] [PubMed] [Google Scholar]
- 12.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996; 223: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999; 229: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. JPEN. 1983; 7: 582–586. [DOI] [PubMed] [Google Scholar]
- 15.Panes J, Perry MA, Anderson DC, et al. Regional differences in constitutive and induced ICAM-1 expression vivo. Am J Physiol. 1995; 269: H1955–H1964. [DOI] [PubMed] [Google Scholar]
- 16.Fukatsu K, Lundberg A, Hanna K, et al. Route of nutrition influences intercellular adhesion molecule-1 expression, neutrophil accumulation in intestine. Arch Surg, 1999:134. [DOI] [PubMed]
- 17.Fukatsu K, Lundberg A, Hanna MK, et al. Increased expression of intestinal P-selectin and pulmonary E-selectin during intravenous total parenteral nutrition. Arch Surg, 2000:135. [DOI] [PubMed]
- 18.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992; 10: 461–468. [DOI] [PubMed] [Google Scholar]
- 19.Berlin C, Berg EL, Briskin M, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993; 47: 185–195. [DOI] [PubMed] [Google Scholar]
- 20.Berg EL, McEvoy LM, Berlin C, et al. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993; 366: 695–698. [DOI] [PubMed] [Google Scholar]
- 21.Rott LS, Briskin MJ, Andrew DP, et al. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. J Immunol. 1996; 156: 3727–3736. [PubMed] [Google Scholar]
- 22.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997; 132: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediated intestinal cytokines. Ann Surg. 1999; 229: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mestecky J, Abraham R, Ogra PL. Common mucosal immune system and strategies for the development of vaccines effective at the mucosal surfaces. In: Ogra PL, Lamm ME, McGhee JR, et al., eds. Handbook of Mucosal Immunology. New York: Academic Press Inc; 1994: 357–372.
- 25.Eppihimer MJ, Russell J, Langley R, et al. Differential expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) in murine tissue. Microcirculation. 1998; 5: 179–188. [PubMed] [Google Scholar]
- 26.Henninger DW, Panes J, Eppihimer MJ, et al. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997; 158: 1825–1832. [PubMed] [Google Scholar]
- 27.Eppihimer MJ, Wolitzky B, Anderson DC, et al. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996; 79: 560–569. [DOI] [PubMed] [Google Scholar]
- 28.Zarzaur B, Fukatsu K, Johnson C, et al. A temporal study of diet-induced changes In Peyer patch MAdCAM-1 expression. Surg Forum. 2001; 52: 194–196. [Google Scholar]
- 29.Chin Y-H, Cai J-P, Xu X-M. Transforming growth factor-β1 and IL-4 regulate the adhesiveness of Peyer’s patch high endothelial venule cells for lymphocytes. J Immunol. 1992; 148: 1106–1112. [PubMed] [Google Scholar]
- 30.Kudsk K. Route of nutrition influences generation of antibody-forming Cells (AFCs), initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003 (in press). [DOI] [PMC free article] [PubMed]
- 31.Pascual DW, Stanisz AM, Bost KL. Functional aspects of the peptidergic circuit in mucosal immunity. In: Ogta PL, Lamm ME, McGhee JR, et al., eds. Handbook of Mucosal Immunology. New York: Academic Press Inc; 1994: 203–216.
- 32.Alverdy J, Stera E, Poticha S, et al. Cholecystokinin modulates mucosal immunoglobulin A function. Surgery. 1997; 122: 386–392. [DOI] [PubMed] [Google Scholar]
- 33.Hanna MK, Zarzaur BL, Fukatsu K, et al. Individual neuropeptides regulate gut-associated lymphoid tissue integrity, intestinal IgA levels, and respiratory antibacterial immunity. JPEN. 2000; 24: 261–269. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Kudsk KA, Hamidian M, et al. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in IV-fed mice. Arch Surg. 1995; 130: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 35.Janu PG, Kudsk KA, Li J, et al. Effect of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg. 1997; 132: 89–93. [DOI] [PubMed] [Google Scholar]
- 36.Zarzaur B, Ikeda S, Johnson C, et al. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN. 2002:26. [DOI] [PubMed]
- 37.Renegar KB, Kudsk KA, DeWitt RC, et al. Impairment of mucosal immunity by parenteral nutrition: Depressed nasotracheal influenza-specific secretory IgA levels and transport of parenterally fed mice. Ann Surg. 2001; 233: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Support by NIH grant #5 R01 GM53439.
Experiment 2 previously published (in part) in Surgical Forum 2001;L11:194–196, and pilot data of experiment 1 published in Surgical Forum 2000;L1:211–214.
Correspondence: Kenneth A. Kudsk, MD, 600 Highland Avenue, H4/736, Madison, WI 53792-7375. No reprints available.
E-mail: kudsk@surgery.wisc.edu
Accepted for publication December 2002.