Abstract
Five ethylene-insensitive loci (wei1–wei5) were identified by using a low-dose screen for “weak” ethylene-insensitive mutants. wei1, wei2, and wei3 seedlings showed hormone insensitivity only in roots, whereas wei4 and wei5 displayed insensitivity in both roots and hypocotyls. The genes corresponding to wei1, wei4, and wei5 were isolated using a positional cloning approach. The wei1 mutant harbored a recessive mutation in TIR1, which encodes a component of the SCF protein ubiquitin ligase involved in the auxin response. wei4, a dominant mutant, resulted from a mutation in the ethylene receptor ERS, whereas wei5, a semidominant mutant, was caused by a mutation in the EIN3-related transcription factor gene EIL1. The simultaneous loss of functional WEI5/EIL1 and EIN3 nearly completely abolished the ethylene response in etiolated seedlings, and adult plants were highly susceptible to infection by the necrotrophic fungal pathogen Botrytis cinerea. Moreover, wei5/eil1 ein3 double mutants were able to fully suppress constitutive signaling caused by ctr1, suggesting a synergistic interaction among these gene products. Unlike previously known root ethylene-insensitive mutants, wei2 and wei3 were not affected in their response to auxin and showed a normal response to gravity. Genetic mapping studies indicate that wei2 and wei3 correspond to previously unidentified ethylene pathway genes that may control cell-elongation processes functioning at the intersection of the ethylene and auxin response pathways.
The gaseous plant hormone ethylene plays an important role in diverse physiological and developmental processes ranging from seed germination to organ senescence and abscission (1, 2). After treatment with ethylene, etiolated seedlings undergo a dramatic morphological transformation called the triple response, which consists of inhibition of hypocotyl and root-cell elongation, radial expansion of cells in the hypocotyl, and exaggeration in the differential elongation rate of cells in the apical hook. This robust phenotype has been used extensively for genetic screens to identify Arabidopsis mutants affected in hormone biosynthesis, perception, and signal transduction pathways (3–11). The majority of signaling pathway mutants (ein2–ein4, etr1, etr2, ers2, ein5, and ein6) show ethylene insensitivity in all tissues of the plant and interfere with general ethylene signaling (3, 4, 8–10). However, other mutants (aux1, axr1, eir1, and hls1) are tissue-specific, and the mutations probably only disable a downstream branch of the pathway (7, 8, 11).
Ethylene is perceived by a family of receptors that are similar to bacterial two-component histidine kinases (9, 10, 12, 13). Genetic studies suggest that in the absence of ethylene, the receptors are active and positively regulate the activity of CTR1, a Raf-like serine/threonine kinase (5). In turn, the negative regulator CTR1 directly or indirectly inhibits the positive regulator EIN2, an integral membrane protein of unknown function (8, 14). EIN2 transmits the ethylene signal to the EIN3 family of DNA-binding transcription factors (15, 16). Although there are a total of six EIN3-like genes in Arabidopsis, only EIN3 has been shown to be required for the normal ethylene response, as inferred from the hormone insensitivity of the ein3 loss-of-function mutant (15). Transgenic studies suggest that the EIN3-like proteins EIL1 and EIL2 may also be involved in ethylene signal transduction (15). However, mutations in these genes have not been identified. EIN3 and possibly other members of the EIN3 family bind to a DNA element in the promoter of the ERF1 gene, an ethylene-responsive element binding protein-type transcription factor (16), and presumably activate its transcription. ERF1, in turn, binds to the GCC box in the promoters of a variety of ethylene target genes (16). Because no physical interactions between CTR1 and EIN2 or EIN2 and the EIN3-like proteins have been observed, yet unknown proteins are likely to bridge these steps.
Here we describe a screen to identify additional ethylene-signaling pathway components. By using low doses of the hormone, five weak ethylene-insensitive (wei) mutant loci were identified. Characterization of these mutants along with positional cloning of three wei genes has allowed the identification of several previously unknown ethylene pathway components. The fact that only one allele belonging to each complementation group was found indicates that this screen will provide a valuable approach to uncover additional genes participating in the response to ethylene.
Materials and Methods
Strains and Growth Conditions.
Arabidopsis thaliana accession Columbia-0 (Col-0) was the parental strain of the ethylmethanesulfonate- and En-I transposon-mutagenized populations used for the forward and reverse genetic screens, respectively. Landsberg erecta (Ler) was the parental strain of the En-I populations used for forward genetic screens. For each of the hormone treatments, surface-sterilized seeds were grown on Arabidopsis thaliana plates [1× Murashige and Skoog salts (GIBCO), pH 6.0/1% sucrose/0.8% agar] supplemented with 1-aminocyclopropane-1-carboxylic acid (ACC, 0, 0.1, 0.2, 0.3, 0.5, 1.0, or 10.0 μM) or 2,4-dichlorophenoxyacetic acid (0, 0.1, 1.0, or 10.0 μM) or treated with 10 ppm ethylene or with hydrocarbon-free air. Seeds were cold-treated for 3–4 days at 4°C and then placed at 24°C in darkness. Phenotypes were scored after ≈72 h. For propagation, seedlings from plates were transferred to MetroMix-200 (Scotts, Marysville, OH) and grown to maturity at 22°C under a 16-h light/8-h dark cycle.
Mutagenesis.
Ethylmethanesulfonate-mutagenized M2 families were obtained as described (4). Approximately 8,000 3-day-old M2 seedlings were screened for each of 10 independent M1 families. Sixty En-I transposon-tagged families were generated from single plants (gift of Andy Pereira, Center for Plant Breeding and Reproductive Research, Wageningen, The Netherlands) by two generations of self-fertilization of pools of ≈1,500 descendant plants. Approximately 8,000 seedlings from each of eight transposon families were initially screened for ethylene-response phenotypes.
Mutant Screen Using Low Concentrations of Hormone.
Mutagenized seeds were plated on Arabidopsis thaliana plates supplemented with 0.5 μM ACC at a density of 1,000–1,500 seeds per 150 × 15-mm plate, cold-treated, and incubated in the dark for 3 days at 24°C. Putative ethylene-insensitive seedlings were picked and grown in MetroMix-200 to maturity. In the next generation, plants were retested in 10.0 and 0.5 μM ACC, and those displaying weak ethylene insensitivity were kept and characterized. All wei mutants were backcrossed to the parental strain at least once before phenotypic analysis.
Genetic Mapping of the wei Mutations.
Mutants isolated in the Col-0 background (wei1, wei2, and wei4) were crossed to Ler, whereas mutants in the Ler background (wei3 and wei5-2) were crossed to Col-0. wei5-2 was mapped to the middle of chromosome 2 based on its cosegregation with the erecta mutation. wei1–wei4 were mapped by using simple sequence-length polymorphic (SSLP) markers (17) (see Results). Genomic DNAs were prepared from F3 seedlings as described (18).
PCR-Based Screen for eil1-1 (wei5-1).
To identify a second mutant allele of EIL1, a PCR-based screen was carried out by using pooled DNAs prepared from a population of 3,000 Arabidopsis insertion lines (ecotype Col-0) that contained ≈15,000 genome-insertion events of the maize transposable element En-I (19). Cosegregation of the transposon insertion and the ethylene-insensitive mutant phenotype in seedlings was confirmed by PCR analysis of 20 independent segregating descendents. The position of the transposon insertion within the EIL1 gene was determined by sequencing of the PCR product from the mutant.
Fungal Growth and Plant Inoculation.
A Botrytis cinerea isolate was obtained from cabbage and grown on potato dextrose agar (Difco) for 2 weeks at 24°C with a 12-h photoperiod before spore collection. Inoculation of Arabidopsis was performed on 4-week-old soil-grown plants by placing one 5-μl droplet of a suspension of 5 × 105 conidial spores per ml in 24 g⋅liter−1 potato dextrose broth (Difco) on each side of the midvein of each leaf. Four fully expanded leaves per plant were inoculated. Infected plants were incubated at 22–24°C with a 12-h photoperiod. High humidity was maintained by covering the plants with a clear plastic lid. The number of plants that were dead because the infection had spread to the central bud was scored 9 days after inoculation. The data were subjected to ANOVA, and P values were adjusted by using the Bonferroni method for multiple comparisons.
Results
Isolation of wei Mutants.
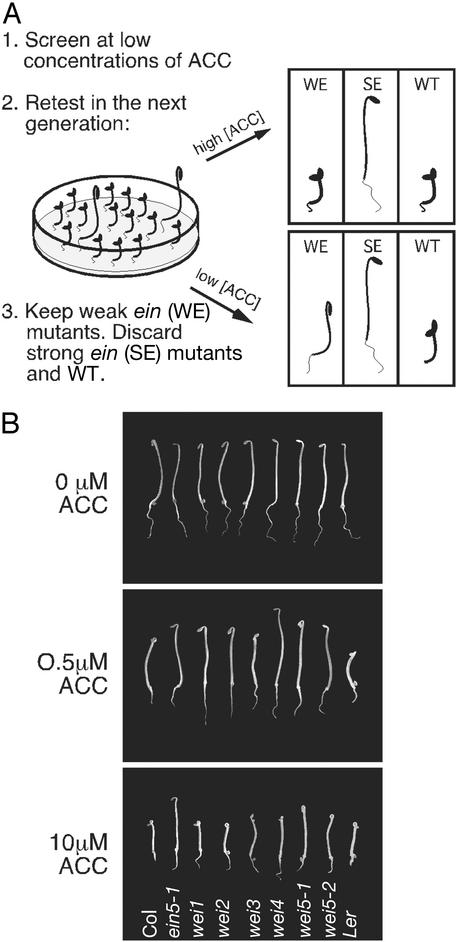
To select the appropriate concentration to screen for wei mutants, we analyzed the hormone response of 3-day-old etiolated seedlings grown in the presence of various concentrations of the ethylene precursor ACC (Fig. 1). Approximately 3,000 wild-type Arabidopsis seedlings were examined at each of seven different hormone concentrations (see Materials and Methods). At 0.5 μM ACC, the seedlings showed a uniform intermediate phenotype, and this concentration was chosen for further mutant screens. Progenies of the plants selected from primary mutant screens were retested using 0.5 and 10 μM ACC (Fig. 1A). Two phenotypic categories of mutants were selected by comparison with the weakest known ein mutant (ein5): those that showed strong ethylene insensitivity (more resistant to ethylene than ein5 seedlings) and those that showed weak insensitivity to the hormone (less resistant to ethylene than ein5 seedlings). This second group was referred to collectively as wei mutants. Five putative mutants that showed significant ethylene insensitivity at low concentrations of ACC and a near wild-type phenotype at higher concentrations of the hormone were selected for further characterization (Fig. 1B). Similar phenotypes were observed when ethylene gas was used instead of ACC (data not shown).
Figure 1.
Mutant screen using nonsaturating levels of ethylene. (A) Schematic representation of the screening strategy. Mutagenized Arabidopsis plants were screened at a low concentration of the ethylene precursor ACC. Plants that showed hormone insensitivity were selected and retested in the next generation. Only putative mutants that showed weak hormone insensitivity were characterized further. (B) Comparison of the phenotypes of wei mutants in different concentrations of ACC. Seedlings were grown in the dark for 3 days in the presence of the indicated concentrations of ACC and photographed.
Genetic and Phenotypic Characterization.
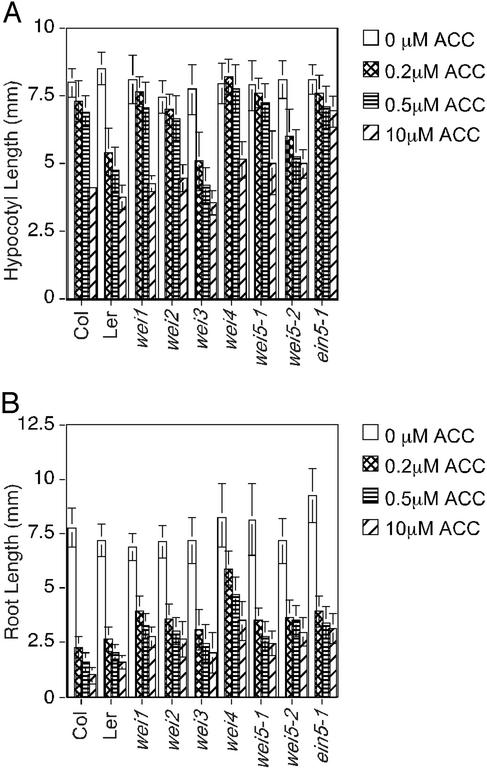
Genetic analysis of the progeny produced from backcrosses of these five mutants to wild type revealed that wei1 and wei2 are recessive mutations and wei3 and wei4 are dominant mutations, whereas wei5 is a semidominant mutation. The phenotypic responses of wild type, wei mutants, and ein5 to different concentrations of the ethylene precursor ACC were compared (Fig. 1B). In the absence of the hormone, the mutant plants were indistinguishable from wild type. Conversely, at both high and low concentrations of the hormone, the mutant seedlings showed a significant degree of ethylene insensitivity (Fig. 1B). To establish whether the ethylene-response defect of the mutants was more profound at lower rather than higher concentrations of the hormone, the responses were quantified. Importantly, in some of the wei mutants, hypocotyl and root tissues showed different degrees of ethylene sensitivity. Therefore, the effect of the ethylene treatment was assayed separately for roots and hypocotyls. At 10 μM ACC, wei4 and wei5 hypocotyls showed clear resistance to the hormone, although the degree of insensitivity of these mutants was significantly less than that observed in ein5. Conversely, the hypocotyl responses to ethylene found in wei1, wei2, and wei3 seedlings were indistinguishable from wild type. Interestingly, hormone insensitivity in the root response to ethylene was apparent in each of the wei mutants. The degree of root response varied among the mutants. wei4 roots showed a greater degree of insensitivity to ethylene than ein5 roots, whereas the root length of wei5 was similar to that of the ein5 mutant. The roots of wei1, wei2, and wei3 showed more response to the hormone but, nevertheless, when compared with wild type were clearly ethylene-insensitive (Fig. 1B, 2).
Figure 2.
Quantification of the effects of ACC on hypocotyl (A) and root length (B) of the wei mutants. Wild-type and mutant seedlings (30–40 per treatment per genotype) were grown in the dark in the presence of the indicated concentrations of ACC for 3 days, photographed, and measured by using the program NIH IMAGE.
Overall, the differences in the sensitivity of the wei mutants to ethylene with respect to wild type were similar at both high and low concentrations of the hormone. The only exception was wei4 where the difference was greater at low ACC concentrations (Fig. 2). It should also be noted that the hormone-response phenotype of the wei3 mutant was highly variable both at high and low concentrations of ACC ranging from clear ethylene insensitivity to a near wild-type response. This variability was observed even when wei3 plants with clear ethylene-insensitive roots were selected and propagated for several generations. Although these results can be explained by low penetrance of the mutant phenotype, it is also possible that the phenotype observed may not be due to a single mutation but may reflect the quantitative interaction of several distinct loci.
Dose-response experiments also revealed that the hypocotyls of Ler plants were more sensitive to ethylene than those of Col (Fig. 2A). Interestingly, this effect was not found in the roots, where the hormone response was similar in both accessions (Fig. 2B).
Order of Action of Genes.
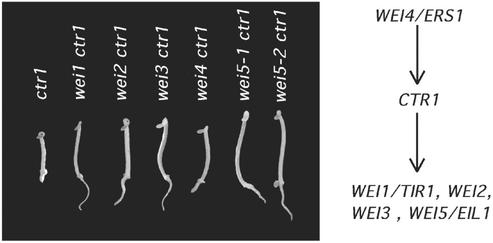
To position the wei mutants in the ethylene pathway, we examined the epistatic relationships between the wei mutants and ctr1. The morphology of ctr1 is similar to that of wild-type seedlings treated with saturating concentrations of ethylene (5). Double mutants were identified among F2 or F3 seedlings produced from crosses between each wei mutant and ctr1-1. In the case of wei1, wei2, wei3, and wei5, we identified several F2 seedlings that showed a constitutive wei ethylene phenotype in the absence of the hormone (Fig. 3). As anticipated for wei3, the number of seedlings showing a clear wei phenotype was less than that expected for a fully penetrant mutant. Putative double-mutant seedlings were retested in the next generation to confirm the initial phenotype. The results of the double-mutant studies indicated that wei1–wei3 and wei5 are epistatic to ctr1 in the ethylene-signaling pathway, and therefore these genes may act downstream of or at the same level as this RAF-like kinase.
Figure 3.
Epistasis analysis of the wei mutants. Double mutants were constructed between ctr1 and five wei mutants. Seedlings of the corresponding double mutants were grown in the dark for 3 days in the absence of exogenously applied ethylene or ACC and photographed.
When the phenotypes of the F2 seedlings from the cross between wei4 and ctr1-1 were analyzed, we were unable to identify any plants showing a constitutive wei ethylene phenotype in the absence of the hormone. These results suggested that ctr1 may be epistatic to wei4. To confirm this possibility, 10 ctr-like plants were crossed to wild type, and the phenotypes of F1 progeny from each cross were examined in the presence of a saturating level of hormone (10 μM ACC). All the F1 progeny produced from two of the crosses showed ethylene insensitivity (data not shown), implying that the genotype of the parental ctr-like plants was wei4 ctr1. These double mutants showed a phenotype indistinguishable from that of ctr1 both in seedlings and in adults. These results indicate that wei4 may act upstream of or at the same step as ctr1 in the ethylene-signaling pathway.
wei4 Is Allelic to ERS1.
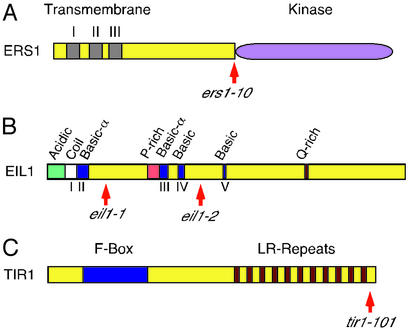
The wei4 mutation was mapped to the bottom of chromosome 2 between the SSLP markers BIO201 and nga168 in a region known to contain the ethylene receptor gene ERS1 (13, 20). Because all the dominant ethylene-insensitive mutants described to date belong to the ethylene receptor family and wei4 mapped to the same region of the chromosome 2 as ERS1, we tested whether wei4 was allelic to ERS1. Sequencing of ERS1 in the wei4 mutant plants identified a missense mutation that is predicted to produce a change of R320 to C. Interestingly, this amino acid is located between the hydrophobic amino end and the predicted kinase domain (Fig. 4A). Alignment of amino acid sequences for all the ethylene receptors showed that this arginine is conserved not only in the Arabidopsis ethylene receptor family but is also highly conserved in the members from other plant species (data not shown). Although several dominant mutations in other Arabidopsis ethylene receptors have been described, wei4 maps outside of the hydrophobic amino-end domain of ERS1, which forms the ethylene-binding pocket. We refer to this allele of ERS1 as ers1-10.
Figure 4.
Schematic representation of the ERS1 (A), EIL1 (B), and TIR1 (C) proteins. The approximate positions of the mutations found in wei4/ers1-10 (R to C), wei5-1/eil1-1 (transposon insertion), wei5-2/eil1-2 (frame shift), and wei1/tir1-101 (stop codon) are indicated.
wei5 Is Allelic to EIL1.
wei5 was mapped to the middle of chromosome 2 near the ERECTA gene. Because the EIN3-like gene, EIL1, is also closely linked to ERECTA (20), we inferred that wei5 might be allelic to EIL1. Sequencing of the EIL1 gene from wei5-2 plants revealed a 4-bp insertion at position 1,390 (GenBank accession no. AF004213) (Fig. 4B). Although the wei5-2 allele of EIL1 is in the Ler background, we also identified a Col-0 allele of EIL1 using a PCR-based reverse-genetics approach (see Materials and Methods). wei5-1 harbors an En-I transposon insertion at position 697 (Fig. 4B), and the phenotype of this mutant is similar to that of the wei5-2 allele in all of the aspects studied: resistance to ethylene, epistatic analysis, and genetic interaction with ein3-1 (see below). The transposon insertion in wei5-1 removes a significant part of both the putative DNA-binding domain and the dimerization domain (as predicted by similarity with the tobacco TEIL) (21). We renamed wei5-1 and wei5-2 as eil1-1 and eil1-2, respectively.
eil1 Genetically Interacts with ein3.
Previous studies suggest that the EIL genes may act in parallel with EIN3 (15, 16). By genetically combining ein3 with mutations in the other transcription factor family members, a further reduction of ethylene sensitivity may be evident. To test this hypothesis, we crossed the eil1 mutants to ein3-1. The F1 progeny of the crosses between eil1-1 or eil1-2 and ein3-1 showed a phenotype that was intermediate between that of the eil1 and ein3 homozygous mutants. eil1-1 ein3-1 and eil1-2 ein3-1 plants were identified in the F2 generation by PCR and DNA sequencing of candidate double-mutant plants. Quantification of the hypocotyl and root responses to ethylene in the eil1 ein3 double-mutant seedlings revealed that these plants were almost completely hormone-insensitive and indistinguishable from the ethylene-response null mutant ein2-5 (Table 1).
Table 1.
Quantification of the effects of ACC treatment on root and hypocotyl lengths in various genotypes
| Background | Root length, mm*
|
Relative root length, %† | Hypocotyl length, mm*
|
Relative hypocotyl length, %† | ||
|---|---|---|---|---|---|---|
| 0 mM ACC | 10 mM ACC | 0 mM ACC | 10 mM ACC | |||
| Col | 6.51 ± 0.85 | 1.88 ± 0.27 | 28.8 | 9.36 ± 1.13 | 4.72 ± 0.38 | 50.4 |
| Ler | 5.14 ± 1.23 | 2.14 ± 0.50 | 41.6 | 7.86 ± 1.25 | 3.56 ± 0.80 | 45.3 |
| eil1-1 | 5.42 ± 0.85 | 2.55 ± 0.38 | 47.0 | 9.20 ± 0.91 | 5.52 ± 0.49 | 60.0 |
| eil1-2 | 5.78 ± 1.38 | 3.09 ± 0.63 | 53.5 | 8.07 ± 1.44 | 4.99 ± 1.07 | 61.8 |
| ein3-1 | 6.14 ± 1.02 | 4.54 ± 0.98 | 73.9 | 8.72 ± 1.28 | 7.23 ± 0.83 | 82.9 |
| ein2-5 | 6.20 ± 0.88 | 5.83 ± 0.98 | 94.0 | 9.44 ± 1.33 | 8.99 ± 1.67 | 95.2 |
| eil1-1 ein3-1 | 5.79 ± 1.19 | 5.15 ± 0.88 | 88.9 | 8.36 ± 1.40 | 8.23 ± 1.20 | 98.4 |
| eil1-2 ein3-1 | 6.58 ± 1.90 | 6.61 ± 1.59 | 100.4 | 9.24 ± 1.60 | 8.89 ± 1.53 | 96.2 |
Fifty or more seedlings per treatment per genotype were measured.
Expressed as a ratio of organ length in the presence of 10 μM ACC over the organ length in the presence of 0 μM ACC (multiplied by 100).
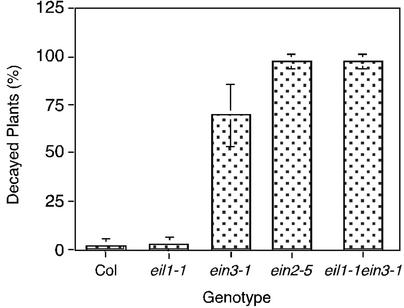
To address the requirement of eil1 and ein3 at later stages of plant development, we constructed a triple mutant: ctr1-1 eil1-2 ein3-1. Remarkably, when grown in soil this mutant was phenotypically indistinguishable from wild-type plants (data not shown), suggesting that the effect of ctr1-1 mutation was masked completely by the eil1-2 ein3-1 double knockout. To further investigate the role of EIL1 and EIN3 in mature plants, we examined the response of several ethylene mutants to a necrotrophic fungal pathogen B. cinerea. B. cinerea is known to be able to kill the ethylene-insensitive mutant ein2-5, whereas wild-type plants are largely resistant to this pathogen (22). Interestingly, eil1-1 mutant plants were also resistant to B. cinerea infection, unlike ein3-1, which showed an intermediate level of susceptibility between that of wild type and ein2-5 (Fig. 5). However, the eil1-1 ein3-1 double-mutant plants proved to be as susceptible to the pathogen as the strong ethylene-insensitive mutant ein2-5 (Fig. 5). Statistical analysis confirmed that the difference in average number of decayed plants between ein3 and ein3 eil1 or ein2-5 plants was significant (P < 0.015).
Figure 5.
Pathogen susceptibility of ethylene mutants. Rosettes of 4-week-old adult plants of Col, eil1-1, ein3-1, ein2-5, and eil1-1 ein3-1 were infected with the spore suspensions of B. cinerea. The percentage of dead plants was scored 9 days after inoculation. The data represent averages with standard deviations of three independent experiments performed with 12 or more plants per genotype.
wei1 Shows Altered Auxin Response.
Several mutations that interfere with auxin transport or sensitivity have also been found to affect the response to ethylene (8, 11). In these mutants, the abnormal ethylene response was restricted mainly to the root tissues, suggesting that the ethylene-induced inhibition of root-cell elongation may have depended on proper auxin biosynthesis, transport, or signaling processes. Because wei1–wei3 showed root-specific ethylene insensitivity, we also tested their response to exogenously applied auxin. Although wei1 seedlings displayed clear auxin insensitivity (Fig. 6), wei2 and wei3 phenotypes were indistinguishable from that of the wild type (data not shown). In addition, wei2 and wei3 also showed a normal gravitropic response (data not shown), further suggesting that wei2 and wei3 are root-specific ethylene-signaling components that do not affect the general auxin response.
Figure 6.
Quantification of the effects of 2,4-dichlorophenoxyacetic acid on the hypocotyls (A) and roots (B) of the wei mutants. Wild-type and mutant seedlings (30–40 per treatment per genotype) were grown in the dark for 3 days in the presence of the indicated concentrations of 2,4-dichlorophenoxyacetic acid, photographed, and measured by using the program NIH IMAGE.
wei1 Is a New Allele of tir1.
Because of the weak phenotype of wei1, F3 mapping populations from a cross of the mutant to Ler were used for genetic mapping of this mutation. wei1 was positioned on the bottom of chromosome 3, 8 centimorgans south of the nga6 marker. Interestingly, an auxin-insensitive mutant tir1 has been mapped previously to this region (23). Because wei1 is insensitive not only to ethylene but also to auxin, wei1 and tir1 might be alleles of the same gene. We therefore compared the phenotype of wei1 and tir1-1 in response to ethylene (ACC). Although both mutants showed ethylene insensitivity in the root, wei1 was clearly more hormone-insensitive than tir1-1 (data not shown). Similarly, the auxin insensitivity of wei1 was stronger than that of tir1-1 (Fig. 6). Genetic complementation analysis indicated that wei1 and tir1-1 were allelic (data not shown). Sequencing of the TIR1 gene in the wei1 mutant identified a G-to-A transition at nucleotide 2,898 (GenBank accession no. AF005047), changing W574 to a stop codon (Fig. 4C). The resulting TIR1 protein is predicted to lack 21 carboxyl-terminal amino acids. We named the wei1 allele of this gene tir1-101.
wei2 and wei3 Define New Ethylene-Insensitive Loci.
The wei2 and wei3 mutants were mapped by using SSLP markers to the top of chromosome 5, close to the EIN2 locus. Genetic complementation testing indicated that wei2 and wei3 were not alleles of the EIN2 gene. The wei2 mutant was further mapped to a region between the SSLP markers nga225 and nga249. Similarly, SSLP mapping placed wei3 to a region between the markers ca72 and nga106. Because wei2 and wei3 are distantly located from each other on the chromosome 5 and no other previously known ethylene-related mutants/genes have been mapped to these regions, we conclude that wei2 and wei3 represent two previously uncharacterized ethylene-insensitive loci.
Discussion
In this article, we describe the results of a genetic screen that was based on nonsaturating concentrations of the ethylene precursor ACC. Five loci (wei1–wei5) were identified that are required for a full response to ethylene in Arabidopsis. wei1, wei4, and wei5 were shown to be allelic to TIR1, ERS1, and EIL1, respectively, whereas wei2 and wei3 represent previously undiscovered ethylene-response loci. Positional cloning of wei4 revealed that the dominant phenotype was due to a mutation in one of the ethylene receptor genes (ERS1). Ethylene perception is carried out by a family of five membrane-bound proteins with sequence similarity to the two-component histidine kinases (reviewed in ref. 24). The wei4/ers1-10 mutation represents the first recovered for ERS1 in a mutant screen. Interestingly, only strong alleles of ers1 are obtained by creating substitution mutations in the wild-type ERS1 gene and reintroducing these transgenes into wild-type plants (13). The identification of “native” weak alleles of ers1 may provide important insights into gene function. The ethylmethanesulfonate-induced ers1-10 mutation creates a wei plant with a predominantly root-specific phenotype, possibly providing a clue as to its normal developmental context in the plant response to ethylene.
A number of receptor mutants that confer dominant ethylene insensitivity have been identified (3, 9, 10). Each of the mutations was found to affect the amino-terminal transmembrane part of the receptor protein. This region is predicted to form a hydrophobic pocket suitable for ethylene binding via copper-dependent coordination of the ethylene molecule (25–27). Although ethylene is thought to trigger a conformational change in the amino terminus that then is transmitted to the carboxyl part of the receptor, direct experimental evidence to support this model is still lacking. Remarkably, the missense mutation in wei4/ers1-10 maps in the region between the hydrophobic amino end and the putative histidine kinase domain. Thus, this mutation may interfere with the transmission of the signal within the receptor molecule by uncoupling the input from the output domains of the protein.
EIL1 is one of six EIN3-like genes in Arabidopsis (15, 20). Although several ein3 alleles have been identified using the triple-response assay, none of these mutants conferred complete ethylene insensitivity. These results suggest the involvement of one or more of the EIL genes in the ethylene response (15). This possibility is supported by previous studies demonstrating that overexpression of EIL1 and EIL2 was able to complement the ein3 mutant phenotype (15). Despite this evidence, direct involvement of the EILs in ethylene signaling had not been demonstrated conclusively. The isolation of wei5/eil1 not only confirms that EIL1 is a component of the ethylene-signaling cascade but also allows us to examine the contributions of the remaining EIN3 family members to the ethylene response. Interestingly, eil1 ein3 double-mutant seedlings show no morphological response to ethylene, indicating that EIL2–EIL5 genes may not contribute to the ethylene response at this stage of development. Moreover, in adult plants the effect of these two mutations on the plant resistance to the necrotrophic fungus B. cinerea is synergistic. Finally, phenotypic analysis of the soil-grown adult ctr1-1 eil1-2 ein3-1 triple-mutant plants suggests that the EIL1/EIN3 deficiency is able to mask the constitutive ethylene response of ctr1-1. Taken together, these results support the notion of the importance of EIL1 and EIN3 for plant sensitivity to the hormone ethylene throughout the plant-life cycle.
Among the ethylene-mediated responses, the growth inhibition of hypocotyl and root cells is the most apparent. Initially identified for their abnormal response to the plant hormone auxin (11), the aux1, axr1–axr3, and eir1 mutants are also affected in the ethylene response specifically in root tissues (8, 11). These findings suggest that some ethylene responses may be mediated by the interaction between these two hormones (8). The absence of response to ethylene in these multihormone-resistant mutants suggests that ethylene may regulate cell elongation, in part, by modulating the concentration and/or sensitivity to auxin. In agreement with this suggestion, we found that plants with mutations in TIR1, a gene that participates in a ubiquitin-mediated degradation of general components of the auxin response (23, 28), are also altered in their response to ethylene. The near wild-type levels of ethylene sensitivity and the localized effects of these mutations suggest that other TIR1-like genes present in the Arabidopsis genome (20) might also participate in the response to ethylene/auxin.
If a functional auxin response is required for ethylene-mediated inhibition of root growth, then downstream components in the ethylene pathway may be found that function at intersection with the auxin biosynthesis, transport, or signaling pathways. In support of this idea, we previously identified two genetically downstream, tissue-specific ethylene-response mutants with alterations in auxin-mediated processes (4, 7, 8). The ethylene-insensitive root mutant eir1 (8), an auxin efflux carrier (29–32), and the apical hookless mutant hls1 (4, 7), a putative acetyltransferase (7), have been found to affect auxin transport in roots and auxin response gene expression in apical hooks, respectively. The two mutants described here, wei2 and wei3, also show wei phenotypes that are restricted almost exclusively to the root tissues. As mentioned earlier, selective ethylene insensitivity of roots is often a characteristic of the mutants also affected in the response to auxin. wei2 and wei3, however, show normal sensitivity to exogenously applied auxin, as well as a normal gravitropic response. The fact that these two downstream mutants are specifically affected in their response to ethylene may indicate that they function at steps connecting the general ethylene-response pathway, represented by the cascade from ETR1 to EIN3 (27), to the process of auxin-mediated growth. We anticipate that cloning of the genes affected in wei2 and wei3 may provide further understanding of how ethylene and auxin coordinate their biosynthesis, transport, and signaling pathways to precisely regulate uniform and differential cell-elongation processes.
Acknowledgments
We thank A. Hamilton and members of the Ecker Laboratory for critical reading of the manuscript and A. Pereira for the En-I transposon lines. J.M.A. was supported by a Spanish Ministerio de Educacion y Ciencia postdoctoral fellowship. A.N.S. was a recipient of Department of Energy Predoctoral Fellowship ER20162-A0003. R.S. was a recipient of Human Frontier Science Program Organization and Spanish Ministerio de Educacion y Ciencia postdoctoral fellowships. S.F. was a recipient of an Institute Pasteur–Fondazione Cenci Bolognetti fellowship. This work was supported by The Giovanni Armenise–Harvard Foundation, National Institutes of Health Grant GM48707 (to F.M.A.), and National Science Foundation Grant MCB-0213154, and Department of Energy Grant DE-FG03-00 ER15113 (to J.R.E.).
Abbreviations
- wei
weak ethylene-insensitive
- Col
Columbia
- Ler
Landsberg erecta
- ACC
1-aminocyclopropane-1-carboxylic acid
- SSLP
simple sequence-length polymorphic
References
- 1.Abeles F, Morgan P, Saltveit M. Ethylene in Plant Biology. San Diego: Academic; 1992. [Google Scholar]
- 2.Johnson P R, Ecker J R. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Bleecker A, Estelle M, Somerville C, Kende H. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 4.Guzman P, Ecker J. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieber J J, Rothenberg M, Roman G, Feldmann K A, Ecker J R. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 6.Ecker J R. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- 7.Lehman A, Black R, Ecker J. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 8.Roman G, Lubarsky B, Kieber J, Rothenberg M, Ecker J. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua J, Sakai H, Nourizadeh S, Chen Q H G, Bleecker A B, Ecker J R, Meyerowitz E M. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai H, Hua J, Chen Q G, Chang C, Medrano L J, Bleecker A B, Meyerowitz E M. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estelle M, Klee H J. In: Arabidopsis. Meyerovitz E M, Somerville C R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 555–578. [Google Scholar]
- 12.Chang C, Kwok S F, Bleecker A B, Meyerowitz E M. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 13.Hua J, Chang C, Sun Q, Meyerowitz E M. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- 14.Alonso J M, Hirayama T, Roman G, Nourizadeh S, Ecker J R. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 15.Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker J. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 16.Solano R, Stepanova A, Chao Q, Ecker J R. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell C, Ecker J. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 18.Doyle J J, Doyle J L. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 19.Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, Saedler H, Weisshaar B. Proc Natl Acad Sci USA. 1998;95:12432–12437. doi: 10.1073/pnas.95.21.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arabidopsis Genome Initiative. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 21.Kosugi S, Ohashi Y. Nucleic Acids Res. 2000;28:960–967. doi: 10.1093/nar/28.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomma B P, Eggermont K, Tierens K F, Broekaert W F. Plant Physiol. 1999;121:1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruegger M, Dewey E, Gray W M, Hobbie L, Turner J, Estelle M. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Shockey J A. Curr Opin Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez F I, Esch J J, Hall A E, Binder B M, Schaller G E, Bleecker A B. Science. 1999;283:996–998. doi: 10.1126/science.283.5404.996. [DOI] [PubMed] [Google Scholar]
- 26.Schaller G, Bleecker A. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- 27.Wang K L C, Li H, Ecker J R. Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Pozo J C, Estelle M. Proc Natl Acad Sci USA. 1999;96:15342–15347. doi: 10.1073/pnas.96.26.15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luschnig C, Gaxiola R A, Grisafi P, Fink G R. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utsuno K, Shikanai T, Yamada P, Hashimoto T. Plant Cell Physiol. 1998;39:1111–1118. doi: 10.1093/oxfordjournals.pcp.a029310. [DOI] [PubMed] [Google Scholar]
- 31.Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson P H. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]