Abstract
The geminivirus protein AL1 initiates viral DNA replication, regulates its own expression, and induces plant gene transcription. To better understand how AL1 interacts with host proteins during these processes, we used yeast two-hybrid library screening and a baculovirus protein interaction system to identify plant proteins that interact with AL1. These studies identified a Ser/Thr kinase, a kinesin, and histone H3 as AL1 partners. The kinase is autophosphorylated and can phosphorylate common kinase substrates in vitro. The kinesin is phosphorylated in insect cells by a cyclin-dependent kinase. Immunostaining of Nicotiana benthamiana and Arabidopsis showed that kinase protein levels and subcellular location are regulated during plant development and geminivirus infection. By contrast, the kinesin is ubiquitous even though it is associated with the spindle apparatus in mitotic cells. Together, our results establish that AL1 interacts with host proteins involved in plant cell division and development. Possible functions of these host factors in healthy and geminivirus-infected plants are discussed.
INTRODUCTION
Geminiviruses are a group of DNA viruses that replicate their single-stranded genomes through a rolling-circle replication mechanism in nuclei of infected plant cells (Saunders et al., 1991; Stenger et al., 1991). They have small genomes, comprising one or two DNA components of <3 kb with limited coding capacity. Hence, they rely heavily on host factors to support their replication. However, because they infect terminally differentiated cells that have already exited the cell division cycle and no longer contain detectable levels of DNA replication enzymes (Coello et al., 1992; Nagar et al., 1995), geminiviruses first must induce their plant hosts to express the requisite replication machinery. As a consequence, geminiviruses are valuable tools for studying virus–host interactions, DNA replication, and cell cycle regulation in plants (Hanley-Bowdoin et al., 1999).
Geminiviruses are classified into four genera—mastreviruses, begomoviruses, curtoviruses, and topocuviruses—based on their genome organization, insect vector, and host range. Tomato golden mosaic virus (TGMV) and Cabbage leaf curl virus (CbLCV) are typical begomoviruses with bipartite genomes that are transmitted by whiteflies and infect dicotyledonous species. Both viruses encode a single protein, AL1 (also called AC1, C1, or Rep), that is essential for viral DNA replication (Elmer et al., 1988a). AL1 also acts as a transcriptional regulator to repress its own synthesis (Sunter et al., 1993; Eagle et al., 1994) and activates host gene expression (Nagar et al., 1995; Egelkrout et al., 2001).
During replication, AL1 specifically recognizes the viral origin (Fontes et al., 1994) and acts as an endonuclease and ligase to initiate and terminate rolling-circle replication (Laufs et al., 1995; Orozco and Hanley-Bowdoin, 1998). The AL1 protein has ATP-dependent topoisomerase activity (Pant et al., 2001) and has been proposed to function as a DNA helicase during replication (Gorbalenya and Koonin, 1993).
In addition to its catalytic and DNA binding activities, AL1 is involved in several protein interactions, including oligomerization, binding to the viral replication enhancer AL3, and interaction with the retinoblastoma host protein (pRb in animals and RBR1 in plants) (Xie et al., 1995; Settlage et al., 1996; Ach et al., 1997). With the exception of the topoisomerase activity, all of the functions ascribed to AL1 have been mapped to overlapping domains in the N-terminal half of the protein (Orozco et al., 1997; Orozco and Hanley-Bowdoin, 1998; Kong et al., 2000).
In plants, DNA replication and the corresponding machinery are restricted to meristems, developing leaves and roots, and the cambium. Unlike some geminiviruses (Esau, 1977; Horns and Jeske, 1991; Sanderfoot and Lazarowitz, 1996), TGMV and CbLCV are not confined to vascular tissue; instead, they are found in terminally differentiated cells throughout the leaf, stem, and root (Nagar et al., 1995; Qin and Petty, 2001). These tissues are unable to support DNA replication and must be reprogrammed to produce the nuclear replication enzymes early during infection (Hanley-Bowdoin et al., 1999).
Recent experiments showed that both TGMV and CbLCV infection activates transcription of the host gene encoding proliferating cell nuclear antigen (PCNA), the processivity factor of DNA polymerase δ, in mature leaves (Egelkrout et al., 2001; E.M. Egelkrout, L. Mairconti, R. Cella, D. Robertson, and L. Hanley-Bowdoin, unpublished data). PCNA also is expressed in differentiated cells of transgenic plants expressing TGMV AL1 (Nagar et al., 1995), establishing AL1 as sufficient for host induction. The ability of AL1 to activate PCNA expression is linked tightly to its capacity to interact with RBR1 (Kong et al., 2000). In quiescent animal cells, pRb interacts with members of the E2F transcription factor family to negatively regulate genes whose products are required for S-phase (Sidle et al., 1996). Mammalian DNA tumor viruses overcome this inhibition by binding to pRb and disrupting pRb/E2F complexes (Weinberg, 1995). In plants, the PCNA promoter also is under E2F negative control (Egelkrout et al., 2001), suggesting that AL1 uses an analogous mechanism to activate PCNA expression during geminivirus infection.
pRb is only one of several host proteins that are targeted by mammalian DNA tumor viruses to induce replication proteins in their hosts (Jansen-Durr, 1996). Adenovirus, papillomavirus, and polyomavirus proteins interact with signal transduction pathways and the cell cycle regulatory machinery through interactions with kinases such as the src family kinases (Courtneidge and Smith, 1983; Cheng et al., 1988) and the cyclin-dependent kinases (CDKs) (McIntyre et al., 1996) as well as CDK inhibitors (Mal et al., 1996; Funk et al., 1997). They recruit components of the DNA replication apparatus, including DNA polymerase α and RPA, to viral origins through protein interactions (Wang et al., 2000; Weisshart et al., 2000). Viral proteins also bind to a variety of host transcription factors ranging from basal factors such as TATA binding protein (Massimi et al., 1997), to coactivators such as p300/CBP (Eckner et al., 1994), to p53 (Werness et al., 1990), which plays a central role in regulating cell growth and death (Amundson et al., 1998). The interactions between geminiviruses and their plant hosts are likely to be equally complex, with AL1 participating in many different protein complexes. We addressed this hypothesis by using AL1 to screen an Arabidopsis cDNA library for interacting plant proteins.
RESULTS
Identification of Arabidopsis Proteins That Interact with AL1
Previous studies showed that AL1 binds to the plant pRb homolog RBR1 (Ach et al., 1997) and that this interaction is important for host induction (Kong et al., 2000). Given the multifunctional character of AL1, it is likely that it also contacts other host factors. To address this possibility, we used AL1 as bait in a two-hybrid screen of a Matchmaker cDNA library generated from 3-week-old Arabidopsis seedlings at a complexity of 3 × 106. For these experiments, the bait was the AL1 protein from CbLCV, a pathogen of Arabidopsis (Hill et al., 1998). We screened >1 × 108 independent transformants using both full-length CbLCV AL1 and a truncation corresponding to amino acids 1 to 178 fused to the Gal4 DNA binding domain. Our screens yielded three classes of cDNA fragments (Figure 1A).
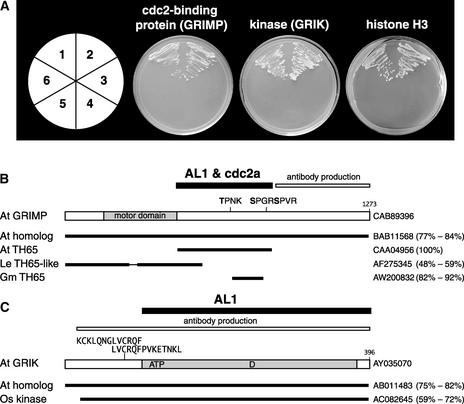
Figure 1.
Identification of Three Plant Proteins That Interact with AL1.
(A) Protein interactions in the yeast two-hybrid system. Arabidopsis cDNA sequences fused to the Gal4 activation domain (AD) vector isolated by library screening are shown. Center left, cdc2 binding protein (GRIMP473-866); center right, kinase (GRIK107-396); far right, histone (H358-136). The AD fusions were cotransformed with the various Gal4 DNA binding domain (BD) vectors into yeast strain AH109 and grown in the absence of Leu, Trp, His, and adenine. Section 1, AD-cDNA + BD-CbLCV AL1; section 2, AD-cDNA + BD-TGMV AL1; section 3, pGADT7 + pGBKT7; section 4, pGADT7 + BD-CbLCV AL1; section 5, pGADT7 + BD-TGMV AL1; section 6, AD-cDNA + pGBKT7.
(B) Diagram of GRIMP showing the motor domain (amino acids 108 to 369) and three consensus CDK recognition sites at positions 698, 841, and 845. The regions that interact with AL1 and Arabidopsis cdc2a and that were used for antibody production are indicated. Related sequences from Arabidopsis (At), tomato (Le), and soybean (Gm) are shown with their GenBank accession numbers at right. The percentage identity and homology are shown in parentheses.
(C) Diagram of GRIK showing the AL1-interacting region and the fragment used for antibody production. The ATP binding site (amino acids 114 to 137), the kinase active site D residue (amino acid 240), and the two overlapping calmodulin binding motifs (amino acids 73 to 85 and 80 to 93) are marked. Related sequences from Arabidopsis and rice (Os) are shown with their GenBank accession numbers at right. The percentage identity and homology are shown in parentheses.
The first class, which was isolated six times—four times with full-length AL1 and twice with AL11-178—was identical to a cDNA isolated previously in a screen using Arabidopsis cdc2a as bait (De Veylder et al., 1997). The second type was isolated two times using full-length AL1 and specified a protein kinase. The last class was isolated once using full-length AL1 and encoded a partial histone H3 cDNA. In all cases, yeast growth on selective synthetic dropout medium was dependent on the presence of both the AL1 bait and the Gal4 activation domain–Arabidopsis cDNA fusion. Experiments using full-length TGMV AL1 as bait were positive for all three Arabidopsis proteins (Figure 1A), demonstrating that the host interactions are conserved among geminivirus replication proteins.
A GenBank search revealed that the first group of clones that interact with AL1 and cdc2a contains sequence from a gene that encodes a kinesin (Figure 1B), which we have designated GRIMP (Geminivirus Rep-Interacting Motor Protein). A full-length cDNA clone was generated from Arabidopsis seedling RNA by reverse transcriptase–mediated PCR using primers based on the Arabidopsis genomic sequence. Sequencing verified that the cDNA encodes a 1273–amino acid protein with a motor domain from positions 139 to 387. The original clone isolated from the yeast library screen contains amino acids 473 to 866 and includes three CDK phosphorylation consensus sequences. BLAST analysis identified GRIMP homologs in Arabidopsis and tomato (Mao et al., 2000) and a homologous partial cDNA in soybean.
A GenBank search identified the second cDNA as a fragment of Ser/Thr kinase (Figure 1C), which we have named GRIK (Geminivirus Rep-Interacting Kinase). Based on the Arabidopsis genomic sequence, we cloned the predicted full-length cDNA by reverse transcriptase–mediated PCR using gene-specific primers. However, the recent submission to GenBank of a cDNA sequence that was generated by rapid amplification of cDNA ends showed that our cDNA does not encode the first 11 amino acids of the protein. Both cDNA sequences revealed that the protein sequence predicted by GenBank also lacked three residues (VFK) at positions 263 to 265. Full-length GRIK contains 396 amino acids and two putative overlapping calmodulin binding motifs. The first motif from amino acids 73 to 85 is related to the 1-5-10 consensus, and the second motif from amino acids 80 to 93 is of the 1-8-14 type (Rhoads and Friedberg, 1997). The kinase domain is located between amino acids 108 and 369, with the ATP binding site at positions 114 to 137 and the active site containing the catalytic D residue from positions 235 to 247. AL1 binds to a region from positions 107 to 396 encompassing both motifs. BLAST analysis identified GRIK homologs in Arabidopsis and rice.
The third cDNA isolated in our AL1 two-hybrid screens encodes amino acids 58 to 136 of unprocessed histone H3 protein.
GRIK, GRIMP, and Histone H3 Interact with TGMV AL1 in Insect Cells
We used a baculovirus expression system to confirm the protein interactions detected by the two-hybrid assays. Insect cells were coinfected with a recombinant baculovirus corresponding to full-length TGMV AL1 and viruses for glutathione S-transferase (GST) fusion proteins of GRIK12C, GRIMP473-866, GRIMP, and histone H358-136, and protein com-plexes were isolated by glutathione affinity chromatography. Input (Figure 2A, lanes 1 to 5) and bound (lanes 6 to 10) fractions were resolved by SDS-PAGE and immunoblotted with anti-AL1 and anti-GST antibodies. AL1 copurified with GST-GRIK12C (lane7), GST-GRIMP473-866 (lane 8), GST-GRIMP (lane 9), and GST-H358-136 (lane 10). No AL1 protein was detected in the bound fraction from insect cells coexpressing AL1 and GST (lane 6), confirming the AL1 interactions with GRIK12C, GRIMP, and H3.
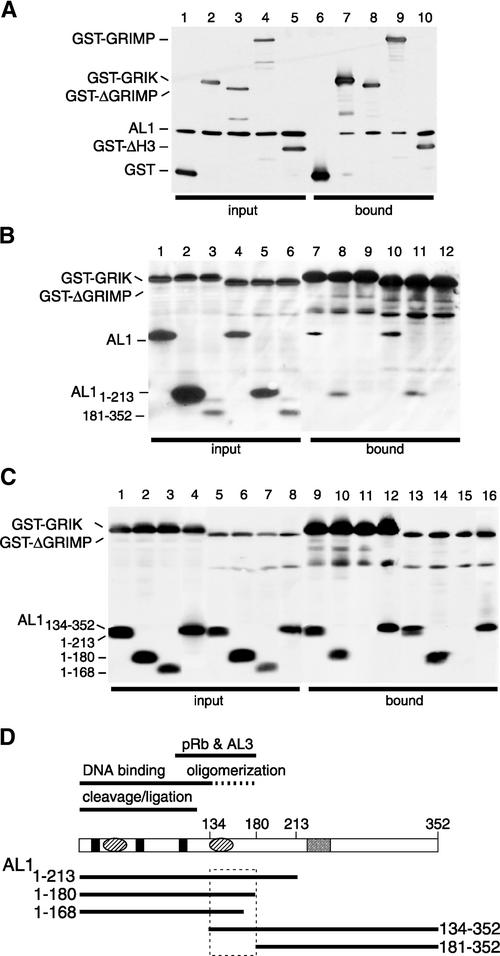
Figure 2.
AL1 Interacts with the Three Host Factors in Insect Cells.
Protein extracts from Sf9 cells coexpressing TGMV AL1 and GST fusion proteins were purified on glutathione-Sepharose resin. Total and bound proteins were resolved by SDS-PAGE and analyzed by immunoblotting using anti-AL1 and anti-GST antibodies. GST-GRIK includes amino acids 12 to 396 of GRIK. GST-GRIMP corresponds to full-length GRIMP. GST-ΔGRIMP contains amino acids 473 to 866 of GRIMP. GST-ΔH3 includes amino acids 58 to 136 of unprocessed histone H3.
(A) Verification of protein interactions in insect cells. Total (lanes 1 to 5) and bound (lanes 6 to 10) proteins are shown for assays containing full-length TGMV AL1 and GST (lanes 1 and 6), GST-GRIK (lanes 2 and 7), GST-ΔGRIMP (lanes 3 and 8), GST-GRIMP (lanes 4 and 9), or GST-ΔH3 (lanes 5 and 10).
(B) AL1 domains that interact with GRIK and GRIMP. Total (lanes 1 to 6) and bound (lanes 7 to 12) fractions from insect cells coexpressing TGMV AL1 (lanes 1, 4, 7, and 10) or the AL1 truncations AL11-213 (lanes 2, 5, 8, and 11) and AL1181-352 (lanes 3, 6, 9, and 12), with GST-GRIK (lanes 1 to 3 and 7 to 9) or GST-ΔGRIMP (lanes 4 to 6 and 10 to 12), are shown.
(C) Fine mapping of the AL1 domains that interact with GRIK and GRIMP. Total (lanes 1 to 8) and bound (lanes 9 to 16) fractions from insect cells coexpressing the TGMV truncations AL11-213 (lanes 1, 5, 9, and 13), AL11-180 (lanes 2, 6, 10, and 14), AL11-168 (lanes 3, 7, 11, and 15), and AL1134-352 (lanes 4, 8, 12, and 16), with GST-GRIK (lanes 1 to 4 and 9 to 12) or GST-GRIMP473-866 (lanes 5 to 8 and 13 to 16), are shown.
(D) The domain of AL1 that interacts with GRIK and GRIMP. Diagram of the AL1 protein showing the previously mapped domains for binding to pRb and AL3, oligomerization (dashed line), DNA binding (solid lines), and DNA cleavage/ligation activities. Three conserved DNA cleavage motifs (solid boxes), two predicted pairs of α-helices (ovals), and the ATP binding site (open box) are marked. Solid lines below the diagram show the positions of the AL1 truncations designated by their N- and C-terminal amino acids. The boxed region indicates the domain that interacts with GRIK and GRIMP.
TGMV AL1 truncations were used to determine what regions of the AL1 protein are responsible for interactions with GRIK and GRIMP. Infection experiments with various combinations of baculoviruses corresponding to AL11-213, AL1181-352, GST-GRIK12C, and GST-GRIMP473-866 were analyzed as described above. In Figure 2B, full-length AL1 (lanes 7 and 10) and AL11-213 (lanes 8 and 11) copurified with GST-GRIK12C and GST-GRIMP473-866. By contrast, no AL1181-352 (lanes 9 and 12) was detected in the bound fractions with either GST-GRIK12C or GST-GRIMP473-866, even upon long exposure of the immunoblot (data not shown).
In Figure 2C, AL11-180 (lanes 10 and 14) and AL1134-352 (lanes 12 and 16) as well AL11-213 (lanes 9 and 13) bound to GST-GRIK12C and GST-GRIMP473-866, whereas AL11-168 (lanes 11 and 15) failed to copurify with either GST fusion protein. Together, these results showed that the region of AL1 that interacts with both GRIK and GRIMP is between amino acids 134 and 180 (Figure 2D). This region is identical to the AL1 oligomerization domain and partially overlaps the pRb and AL3 binding domains (Kong et al., 2000; Orozco et al., 2000; Settlage et al., 2001).
GRIK and GRIMP Are Phosphorylated in Insect Cells
Sequence inspection identified several potential phosphor-ylation sites in GRIK and GRIMP. As a consequence, we asked if the proteins could be radiolabeled when 32P-orthophosphate was added to the culture medium of baculovirus-infected cells. In Figure 3A, 32P-labeled bands of the predicted sizes were detected for His-GRIK12C (lane 2), His-GRIMP473-866 (lane 3), and GST-GRIMP (lane 5). The identities of the GRIK and GRIMP phosphoproteins were verified by immunoblotting (data not shown). No radiolabeled band was seen for His-CAT (Figure 3A, lane 1), and only a weakly labeled band was observed for GST (lane 4).
Figure 3.
Phosphorylation of Recombinant GRIK and GRIMP in Insect Cells.
Sf9 cells infected with recombinant baculoviruses were labeled with 32P-orthophosphate. Radiolabeled proteins were resolved by SDS-PAGE. The positions of recombinant proteins are indicated at left. The migration and masses of protein markers are shown at right. The recombinant protein nomenclature is described for Figure 1. The various ΔGRIMP and GRIK fusions are nearly identical in size and migrate differently relative to each other depending on the tag. Their identities were verified using anti-GRIK and anti-GRIMP antibodies (data not shown).
(A) Phosphoproteins from cells infected with recombinant baculoviruses expressing His-CAT (lane1), His-GRIK (lane 2), His-ΔGRIMP (lane 3), GST (lane 4), and GST-GRIMP (lane 5) as indicated. The positions of GST and His-CAT are marked by dots at right of their respective lanes.
(B) A CDK inhibitor reduces GST-ΔGRIMP phosphorylation. Sf9 cells were infected with recombinant baculoviruses expressing GST-GRIK (lanes 1 to 3) or GST-ΔGRIMP (lanes 4 to 6) and labeled with 32P-orthophosphate in the presence of the CDK inhibitor olomoucine. The final concentrations of the inhibitor were 0 μM (lanes 1 and 4), 10 μM (lanes 2 and 5), and 20 μM (lanes 3 and 6), as indicated. The bottom panel shows an immunoblot using anti-GST antibody and 10% of the total protein analyzed by autoradiography.
GRIMP binds cdc2a and contains three putative CDK phosphorylation sites at positions 698, 841, and 845 (Figure 1B) (De Veylder et al., 1997). We used olomoucine, a specific CDK inhibitor (Vesely et al., 1994), to determine if GST-GRIMP473-866 is a CDK substrate in insect cells. 32P incorporation into GST-tagged GRIMP473-866 was reduced by olomoucine addition to the culture medium (Figure 3B, lanes 4 to 6). In contrast, there was no detectable effect of olomoucine on the phosphorylation of GST-GRIK12C (Figure 3B, lanes 1 to 3). Immunoblot analysis (Figure 3B) revealed that olomoucine had no effect on either GRIK (lanes 1 to 3) or GRIMP (lanes 4 to 6) protein accumulation in insect cells.
The reduction of GRIMP phosphorylation in the presence of 10 to 20 μM olomoucine was highly reproducible, but complete loss of labeling was seen only with 100 μM olomoucine (data not shown). Olomoucine inhibits CDK activity with a 50% inhibitory concentration of 7 μM, whereas other kinases require concentrations >25 μM for 50% inhibition (Vesely et al., 1994). In addition to the CDK sites, GRIMP473-866 contains multiple consensus phosphorylation sites for protein kinase C, and the residual labeling is likely to reflect incorporation at these sites. Together, these results showed that GRIK and GRIMP are phosphoproteins and suggested that GRIMP is a CDK substrate.
GRIK Is a Functional Protein Kinase
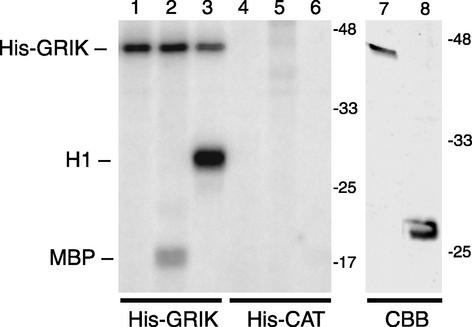
To determine if GRIK is a functional kinase, we purified His-GRIK12C and His-CAT by nickel-affinity chromatography from baculovirus-infected insect cells. Coomassie blue staining gave one major band of the predicted size for each fusion protein (Figure 4, lanes 7 and 8). The activities of the purified proteins were monitored by 32P labeling of the model kinase substrates, myelin basic protein and histone H1. Both substrates were phosphorylated by His-GRIK12C (Figure 4, lanes 2 and 3), with label incorporated into histone H1 more efficiently. No 32P incorporation was detected in parallel assays containing His-CAT (lanes 5 and 6), establishing that GRIK kinase activity was not attributable to a copurifying insect kinase. Interestingly, GRIK also displayed autophosphorylation activity independent of exogenous substrate (Figure 4, lanes 1 to 3).
Figure 4.
GRIK Acts as a Protein Kinase in Vitro.
His-GRIK (lanes 1 to 3) or His-CAT (lanes 4 to 6) was incubated in kinase reactions with no substrate (lanes 1 and 4), myelin basic protein (lanes 2 and 5 [MBP]), or histone H1 (lanes 3 and 6). The reactions were resolved by SDS-PAGE, and the 32P-labeled products marked at left were visualized by autoradiography. The right panel shows Coomassie blue staining (CBB) of the purified His-GRIK (lane 7; 0.6 μg) and His-CAT (lane 8; 1.8 μg) used in the kinase assays. The migration and masses of protein markers are shown at right. The recombinant protein nomenclature is as described for Figure 1.
We asked if AL1 or the AL1 partners AL3 and RBR1 (Settlage et al., 1996; Ach et al., 1997) are substrates of GRIK. For these assays, we used TGMV AL1, His-AL3, or His-RBR1 purified from insect cells as substrate (1-fold to 10-fold molar excess) in the kinase assay. None of the proteins was phosphorylated by GRIK in vitro (data not shown). In addition, AL1 had no detectable effect on GRIK activity in assays containing myelin basic protein or H1 substrate (data not shown). Sequence analysis showed that GRIK has two overlapping calmodulin binding motifs. However, we were unable to detect any impact of calcium (0.1 to 5 mM), calmodulin (40-fold molar excess), or EGTA (1 to 5 mM) on GRIK kinase activity in vitro (data not shown).
GRIMP and GRIK Display Different Expression Patterns in Plants
To gain insight into the functions of GRIMP and GRIK, we generated antibodies against His-tagged recombinant proteins produced in Escherichia coli and used them to study expression patterns in vivo. The GRIMP antiserum was raised against amino acids 857 to 1273 because sequence comparisons indicated that the C termini of GRIMP and its homolog are not related to any other proteins encoded by the Arabidopsis genome. Because of the strong degree of conservation between GRIMP and its homolog, it was not possible to identify a region that could be used to distinguish them.
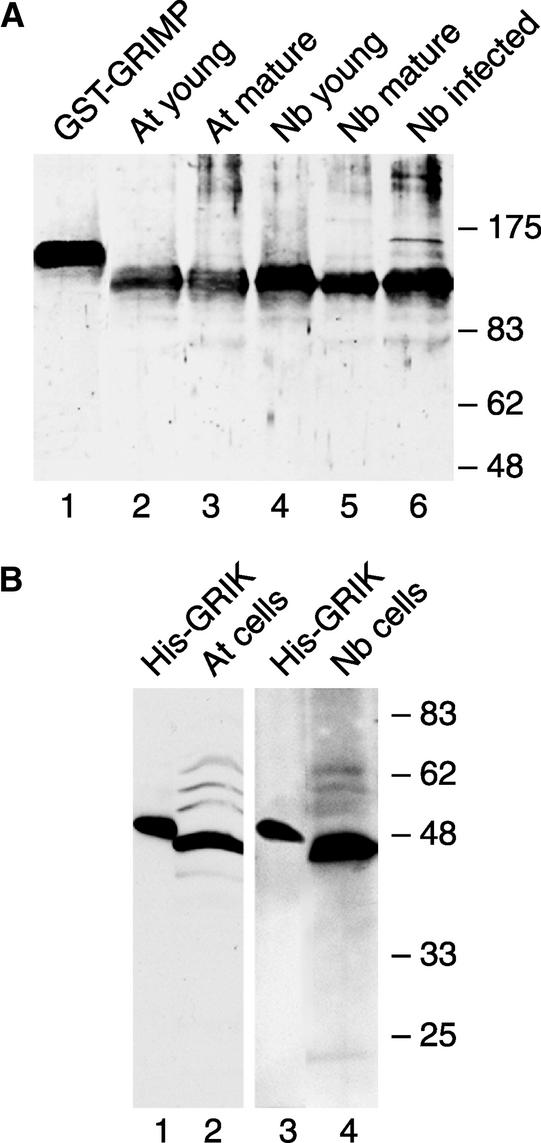
The polyclonal antiserum cross-reacted with a single band corresponding in size to recombinant GST-GRIMP on immunoblots (Figure 5A, lane 1). A single band of the predicted size (141 kD) for authentic GRIMP also was detected in total protein extracts from young and mature leaves of Arabidopsis (Figure 5A, lanes 2 and 3) and Nicotiana benthamiana (lanes 4 and 5). GRIMP levels were very similar in all tissues, indicating that its expression is not tissue specific or regulated developmentally. Similarly, TGMV infection had no apparent effect on GRIMP protein level in N. benthamiana (Figure 5A, lane 6). The slower migrating band seen in infected tissue was not observed reproducibly. Because the recombinant protein we used for antibody production did not contain the motor domain (Figure 1B), we did not expect to detect unrelated motor proteins. However, the predicted molecular mass for GRIMP and its homolog are very close, and we cannot exclude the possibility that the single band on the immunoblot corresponds to both GRIMP and its homolog.
Figure 5.
Immunodetection of GRIK and GRIMP in Plant Tissues and Cultured Cells.
(A) Total protein extracts from young leaves (lanes 2 and 4), mature leaves (lanes 3 and 5), or infected mature leaves (lane 6) of Arabidopsis (lanes 2 and 3) or N. benthamiana (lanes 4 to 6) were separated by SDS-PAGE and detected using anti-GRIMP antibody. Recombinant GST-GRIMP (lane 1) was used as a control.
(B) Total protein extracts from Arabidopsis (lane 2) or N. benthamiana (lane 4) cultured cells were resolved by SDS-PAGE and detected using anti-GRIK antibody. Recombinant His-GRIK (lanes 1 and 3) was used as a control.
Because GRIK amino acids 12 to 84 are outside of the kinase domain and are the least conserved compared with its homolog, we initially attempted to express this polypeptide in E. coli but were unsuccessful. As an alternative, we used recombinant His-GRIK12-396 for antibody production (Figure 1C). The resulting antiserum cross-reacted efficiently with recombinant His-GRIK12-396 on immunoblots (Figure 5B, lanes 1 and 3). By contrast, we detected only faint signals corresponding to GRIK in protein extracts from young and geminivirus-infected mature tissues and were unable to visualize GRIK in uninfected mature tissues of Arabidopsis or N. benthamiana (data not shown). However, a strong signal of the correct size (44 kD) for GRIK was seen in protein extracts from Arabidopsis (Figure 5B, lane 2) and N. benthamiana (lane 4) cultured cells. Only one major band was detected in both species, indicating that the antiserum does not cross-react with protein kinases in general. As in the case of GRIMP, we cannot exclude the possibility that the single band corresponds to both GRIK and its homolog. However, our results suggested that GRIMP is expressed constitutively, whereas GRIK levels are regulated differentially during development and geminivirus infection.
GRIMP Is Associated with Mitosis
The motor domain of GRIMP displays homology with C-terminal kinesins, many of which are involved in mitosis (Goldstein and Philp, 1999). Hence, we asked if GRIMP is in the nucleus and associated with mitotic chromosomes. The distribution of GRIMP protein was examined by immunolocalization using purified anti-GRIMP antibody and a secondary antibody conjugated to horseradish peroxidase. Because of the similar immunoblotting data in Figure 5 for Arabidopsis and N. benthamiana, we performed the analysis in the latter species, which is technically more amenable to immunolocalization studies because of larger cell size.
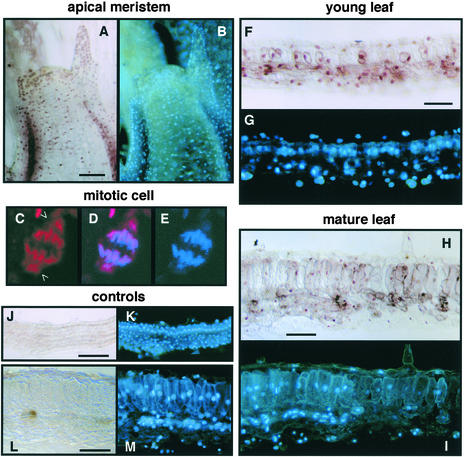
Consistent with the immunoblot data, GRIMP was detected in meristematic (Figure 6A), young leaf (Figure 6F), and mature leaf (Figure 6H) tissues. The peroxidase signal primarily coincided with 4′,6-diamidino-2-phenylindole (DAPI) staining in all three tissues (Figures 6B, 6G, and 6I). Faint background staining was seen across the entire section of control samples treated with preimmune rabbit IgG, but there was no evidence of nuclear staining in these samples (Figures 6J and 6L). Together, these results demonstrated that GRIMP is a nuclear protein.
Figure 6.
Immunolocalization of GRIMP.
N. benthamiana tissue sections were analyzed using anti-GRIMP IgG ([A] to [I]) or preimmune IgG ([J] to [M]) followed by anti-rabbit secondary antibody and peroxidase staining ([A], [B], and [F] to [M]) or Texas red–conjugated secondary antibody ([C] to [E]). All sections also were stained with DAPI. Bars = 50 μm.
(A) and (B) Meristem showing GRIMP (A) and DAPI (B) staining.
(C) to (E) Confocal image of a mitotic cell treated with anti-GRIMP IgG and a Texas red–labeled secondary antibody (C), DAPI staining (E), and the merged image (D). Open arrowheads show GRIMP staining associated with the spindle poles.
(F) and (G) Young leaf showing GRIMP (F) and DAPI (G) staining.
(H) and (I) Mature leaf showing GRIMP (H) and DAPI (I) staining.
(J) and (K) Young leaf treated with preimmune IgG showing no peroxidase staining (J) but stained with DAPI (K).
(L) and (M) Mature leaf treated with preimmune IgG showing no peroxidase staining (L) but stained with DAPI (M).
High-resolution analysis of meristematic cells using a Texas red–labeled secondary antibody showed that GRIMP colocalizes with mitotic figures (Figure 6C). Interestingly, the GRIMP signal extended beyond the DAPI signal (Figures 6D and 6E), indicating that it is located at the spindle poles as well as on segregating chromosomes. These data suggested that GRIMP is a mitotic kinesin. However, its constitutive expression pattern indicates that GRIMP function is not restricted to mitosis.
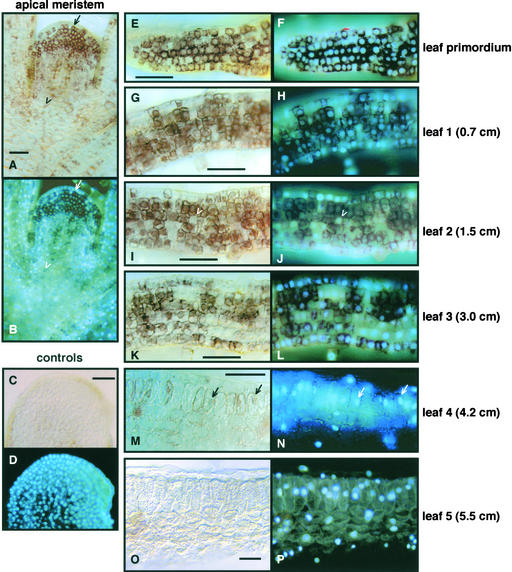
GRIK Moves from the Cytoplasm to the Nucleus before Disappearing during Leaf Development
Previous studies showed that immunolocalization can be more effective than immunoblotting in detecting proteins expressed at low levels in plant tissues (Nagar et al., 1995). Hence, we examined the distribution of GRIK in N. benthamiana tissue sections (Figure 7). GRIK was abundant in N. benthamiana meristem (Figure 7A), leaf primordium (Figure 7E), and leaves 1 to 3 (Figures 7G, 7I, and 7K). By leaf 4 (Figure 7M), which was ∼30% expanded and the first leaf to show mature palisade cell morphology, only a few cells were positive for GRIK. By leaf 5 (Figure 7O), which is <50% of mature size, GRIK was no longer detected. There was no GRIK signal in control hybridizations using preimmune serum to probe meristematic sections (Figure 7C), establishing the specificity of the GRIK signal. Similar results were seen in Arabidopsis, with GRIK staining strongest in young tissues and undetectable in mature rosette leaves (data not shown).
Figure 7.
Developmental Expression of GRIK.
N. benthamiana sections were analyzed using anti-GRIK IgG ([A], [B], and [E] to [P]) or preimmune IgG ([C] and [D]). Peroxidase-conjugated secondary antibody was used, followed by peroxidase and DAPI staining. The leaves are numbered from top to bottom of the plant, and their lengths are indicated at right. Bars = 50 μm.
(A) and (B) Meristem showing GRIK (A) and DAPI (B) staining. The arrow indicates a cell with GRIK in the cytosol, and the open arrowhead marks a cell with GRIK in the nucleus.
(C) and (D) Meristem treated with preimmune IgG showing no peroxidase staining (C) but stained with DAPI (D).
(E) and (F) Leaf primordium showing GRIK (E) and DAPI (F) staining.
(G) and (H) First leaf showing GRIK (G) and DAPI (H) staining.
(I) and (J) Second leaf showing GRIK (I) and DAPI (J) staining. The open arrowheads designate a cell negative for GRIK surrounded by cells containing high levels of the kinase.
(K) and (L) Third leaf showing GRIK (K) and DAPI (L) staining.
(M) and (N) Fourth leaf showing GRIK (M) and DAPI (N) staining. The arrows identify cells with nuclear GRIK staining.
(O) and (P) Fifth leaf showing GRIK (O) and DAPI (P) staining.
Comparison of the GRIK signal with DAPI staining revealed that its subcellular location changed during development. In N. benthamiana, GRIK was cytoplasmic in meristematic cells (Figures 7A and 7B) and leaf primordia cells (Figures 7E and 7F), began moving to the nucleus in leaf 1 (Figures 7G and 7H), and was primarily in the nucleus by leaf 3 (Figures 7K and 7L). Differential partitioning of GRIK also was seen in meristem sections with only those cells nearest the apex showing cytoplasmic localization and distal cells displaying nuclear localization (Figures 7A and 7B). GRIK also moved from the cytoplasm to the nucleus in Arabidopsis as leaf tissue matured (data not shown).
The GRIK protein signal was patchy, and it was common to see cells lacking GRIK antigen surrounded by cells staining positive for the protein independent of subcellular location (Figure 7I). Although the patchiness became more pronounced as tissue matured, it was readily apparent at all stages of development. Patchy staining is a common feature of proteins expressed at discrete stages of the cell cycle (Fobert et al., 1994). However, GRIK was found in both the cytoplasm and the nuclei of cultured cells in log phase (data not shown), suggesting that its subcellular localization is not regulated during the cell cycle.
GRIK Expression Is Induced during Geminivirus Infection
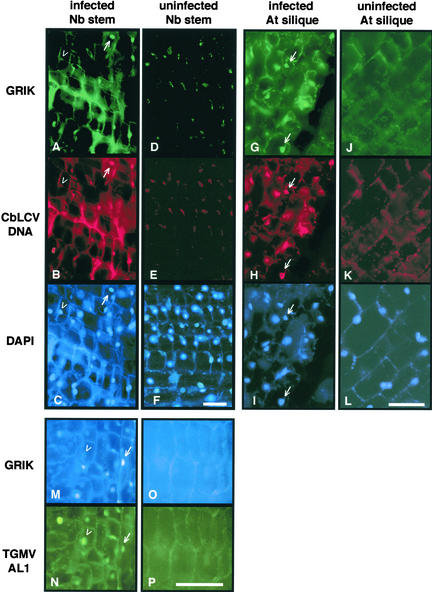
The GRIK expression pattern resembles that of another host protein, PCNA, which also is expressed in cultured cells and young tissue but not in mature tissue. During geminivirus infection, PCNA expression is induced in mature cells (Nagar et al., 1995). We asked if GRIK protein also accumulates during infection. Mature plant tissues were fixed and analyzed using anti-GRIK antibody in combination with anti-TGMV AL1 antibody or a fluorescent CbLCV DNA probe. The Texas red oligonucleotide was designed to bind specifically to the plus strand of the CbLCV AL1 gene; as such, it recognized both double- and single-stranded forms of viral DNA but not viral mRNA. To minimize autofluorescence, N. benthamiana stem and Arabidopsis silique tissues were used for these experiments. However, autofluorescence still was apparent in the cortical regions of cells with all of the probes (Figure 8).
Figure 8.
GRIK Induction in Geminivirus-Infected Plants.
Sections of N. benthamiana stem (left two columns) or Arabidopsis silique tissues (right two columns) were fixed and analyzed using anti-GRIK IgG. In the same experiments, Texas red–labeled CbLCV DNA probe ([A] to [L]) or mouse anti-TGMV AL1 antibody ([M] to [P]) was used to detect the viruses. In (A) to (L), Alexa 488–conjugated goat anti-rabbit secondary antibody and DAPI were used to show specific proteins and nuclei, respectively. In (M) to (P), Cascade blue–conjugated goat anti-rabbit secondary antibody and Alexa 488–conjugated goat anti-mouse secondary antibody were used to show GRIK and TGMV AL1, respectively. The mock-inoculated controls ([D], [E], [F], [J], [K], [L], [O], and [P]) were exposed 15 times longer than infected tissues to show cell structure. Bars = 50 μm.
(A) to (C) CbLCV-infected N. benthamiana stem showing GRIK staining (A), CbLCV hybridization (B), and DAPI staining (C). The arrows mark an infected cell positive for GRIK, and the open arrowheads identify a nucleus with no detectable GRIK.
(D) to (F) Uninfected N. benthamiana stem tissue showing no GRIK staining (D) or CbLCV hybridization (E) but stained with DAPI (F).
(G) to (I) CbLCV-infected Arabidopsis silique showing GRIK staining (G), CbLCV hybridization (H), and DAPI staining (I). The arrows mark infected cells positive for GRIK.
(J) to (L) Uninfected Arabidopsis silique tissue showing no GRIK staining (J) or CbLCV hybridization (K) but stained with DAPI (L).
(M) and (N) TGMV-infected N. benthamiana stem stained for GRIK (M) and AL1 (N). The arrows show a nucleus that contains GRIK, and the open arrowheads mark a nucleus without detectable GRIK. Both nuclei are positive for AL1.
(O) and (P) Uninfected N. benthamiana stem showing no GRIK (O) or AL1 (P) staining.
As in mature leaves (Figure 7O), no GRIK protein was detected in uninfected sections from N. benthamiana (Figures 8D and 8O) and Arabidopsis (Figure 8J). By contrast, GRIK was detected in nuclei of equivalent geminivirus-infected tissues (Figures 8A, 8G, and 8M). This was true for N. benthamiana infected with either CbLCV (Figure 8A) or TGMV (Figure 8M) and for Arabidopsis infected with CbLCV (Figure 8G), indicating that GRIK accumulation is not limited to a specific geminivirus or host plant.
There was a strict relationship between the presence of GRIK and geminivirus infection. Only cells infected with virus as determined by hybridization to the CbLCV-specific DNA probe (Figures 8A, 8B, 8G, and 8H) or immunostaining of TGMV AL1 protein (Figures 8M and 8N) contained detectable GRIK. There were many examples of DAPI-stained nuclei that were not infected and that lacked GRIK (Figures 8A to 8C, 8G, and 8H), indicating that kinase induction is cell autonomous with geminivirus infection. All cells positive for CbLCV DNA (Figures 8B and 8H) also contained detectable levels of GRIK (Figures 8A and 8G), but not all cells positive for AL1 protein were positive for the kinase (Figures 8M and 8N). In all infected cells, GRIK was seen only in the nucleus, where AL1 also localized.
DISCUSSION
Many small DNA viruses replicate their genomes in nuclei of infected cells. Because of their limited coding capacities, these viruses depend on host replication enzymes to amplify their genomes. As a consequence, these viruses must induce the synthesis of host DNA replication machinery in quiescent cells, which have exited the cell division cycle and cannot support DNA replication. In animals, DNA tumor viruses induce the cell division cycle by encoding viral proteins that interact with cell cycle regulators. These viral proteins target pRb (Dyson et al., 1989), p53 (Werness et al., 1990), and many other components of the transcription apparatus, the cell cycle regulatory network, and signal transduction pathways (Jansen-Durr, 1996). Many of these alterations are achieved by direct protein–protein interactions. Previous research showed that geminiviruses induce PCNA transcription (Nagar et al., 1995), most likely by AL1 binding to RBR1 and disrupting E2F/RBR1 regulation (Kong et al., 2000; Egelkrout et al., 2001). In this work, we identified three new partners of AL1: a kinase, a kinesin, and histone H3. Our results established that analogous to the tumor antigen proteins of mammalian DNA viruses, AL1 interacts with diverse host factors that affect a broad range of viral and host functions.
When we initiated our studies, we anticipated that AL1 would interact with plant proteins associated with DNA replication, transcription, and the G1/S transition of the cell cycle. Of the three proteins we identified, only the interaction with histone H3 can be rationalized based on known functions of AL1. As a consequence, we suspect that even though we screened >108 individual library clones, or 30 times the complexity of the Arabidopsis cDNA library, this was not sufficient to identify many of the proteins that interact with AL1. This idea is consistent with the fact that our screen did not yield RBR1, which is known to interact with AL1 in yeast two-hybrid assays (Kong et al., 2000). This discrepancy may reflect an overestimation of the total number of colonies screened, which was based on the number of colonies that grew on complete medium. There is evidence that the AL1 protein is detrimental to yeast and impairs growth, as demonstrated by the slower growth of AL1 transformants versus colonies carrying the equivalent empty vector on complete medium. The viability of the AL1 transformants may decrease even more on selective medium. In addition, cDNAs corresponding to proteins that are expressed only in mature tissues may have been underrepresented in the library from 3-week-old Arabidopsis seedlings. This possibility is supported by the observation that the three AL1 partners we identified are all expressed in young tissues and that RBR1, which we did not isolate, is abundant in differentiated cells and almost undetectable in cycling cells (Huntley et al., 1998).
The interaction between histone H3 and AL1 is consistent with its role in viral replication and transcription. The double-stranded form of geminivirus DNA assembles with nucleosomes (Pilartz and Jeske, 1992) that could block access to the overlapping viral origin of replication and the bidirectional promoters (Hanley-Bowdoin et al., 1999). This obstacle may be overcome by recruitment of AL1 to its binding site in the 5′ intergenic region (Fontes et al., 1992) followed by interaction with H3 to alter or displace nucleosomes and allow access of the replication and transcription machinery. Replication of the SV40 minichromosome is facilitated by neutralization of histone charge (Alexiadis et al., 1997) and remodeling of minichromosome structure through the interactions of large T antigen with histones H1 and H3 (Ramsperger and Stahl, 1995). AL1 also interacts with GRIMP, a protein that includes a kinesin domain, a central region that binds to cdc2a, and a C-terminal domain that is unique. Even though the motor domains of GRIMP and its homolog are in their N termini, phylogenetic analysis based on the motor domain showed that they belong to the C-terminal kinesin subfamily (Reddy and Day, 2001). Members of the C-terminal subfamily localize to the spindle and modulate microtubule dynamics during mitosis (Goldstein and Philp, 1999).
GRIMP also localizes to the spindle apparatus and condensed chromosomes in mitotic cells, suggesting that it acts during cell division. A cell cycle role for GRIMP also is supported by its interaction with cdc2a and its CDK-dependent phosphorylation in insect cells. However, because GRIMP is present in mature cells, it also must be involved in other processes that are independent of cell cycle status. Alternatively, because our antibody most likely does not distinguish GRIMP and its homolog, it is possible that one of the proteins is in mitotic cells and the other performs an unrelated function in mature cells.
The consequences of AL1–GRIMP interactions with respect to geminivirus infection are not known, but it is interesting that AL1 and cdc2a bind to the same region of GRIMP. One possibility is that when it is bound to AL1, GRIMP cannot be bound or phosphorylated by the CDK, which in turn prevents its association with the spindle apparatus and mitosis. It was not feasible to address this hypothesis in our GRIMP phosphorylation assays because only a fraction of GRIMP was associated with AL1 when they were coexpressed in insect cells. Geminivirus-infected cells enter S-phase and support both viral and chromosomal DNA replication (S. Nagar, L. Hanley-Bowdoin, and D. Robertson, unpublished data) but do not proceed through mitosis (Nagar et al., 1995). Instead, infected cells contain condensed chromatin typical of early prophase (Bass et al., 2000). Arrest at this stage may be attributable to AL1–GRIMP interactions.
The third AL1 partner is a protein kinase with two overlapping calmodulin binding motifs in its N terminus. GRIK and its homolog constitute a distinct family (4.2.7) of the “cytoplasmic protein kinases unrelated to receptor kinases” (see http://plantsp.sdsc.edu). To date, no functions or substrates have been ascribed to GRIK or its homolog. We were unable to detect any effect of calcium or calmodulin on GRIK kinase activity in vitro. Even though Coomassie blue staining of GRIK gave primarily one band, we cannot exclude the possibility that GRIK purified from insect cells was bound to insect calmodulin. However, this is unlikely because EGTA also had no effect on activity. It is possible that the missing 11 amino acids at the GRIK N terminus are required for calmodulin and/or calcium to affect function.
We were unable to show that AL1 is a substrate of GRIK or has any apparent effect on kinase activity in vitro using model substrates. The fact that AL1 is not phosphorylated by GRIK is consistent with the failure to radiolabel AL1 protein produced in plant or insect cells grown in the presence of 32P-orthophosphate (data not shown). Hence, a likely scenario is that AL1 recruits substrates for GRIK in infected cells. In insect cells, expression of AL1 reproducibly increased the 32P labeling of an endogenous 45-kD protein of unknown identity (data not shown). Even though this enhancement was independent of recombinant GRIK expression, it demonstrated that AL1 can influence the phosphorylation status of other proteins.
GRIK expression is regulated developmentally, with the protein level high in young tissue and not detectable in mature tissue. The spotted expression pattern of GRIK in young leaves and cycling cells suggested that its level also is regulated during the cell cycle. The induction of GRIK in mature cells during geminivirus infection may be by a mechanism similar to PCNA induction, which is dependent on E2F binding sites in its promoter (Egelkrout et al., 2001). However, there are no obvious E2F binding sites in the promoter region of the Arabidopsis GRIK gene or its homolog, suggesting that a different mechanism is involved. This idea is consistent with the presence of AL1-positive cells lacking GRIK. Because AL1 is expressed very early during infection and can be detected before viral DNA (S. Nagar, L. Hanley-Bowdoin, and D. Robertson, unpublished data), our data suggest that there is a time lag between the onset of viral infection and the induction of GRIK.
GRIK activity also is likely to be regulated by its location, which shifts from the cytosol to the nucleus during leaf development. The fact that GRIK is induced during infection and localizes to the nucleus of infected cells suggests that its primary targets are nuclear. If this is correct, GRIK protein may accumulate as an inactive enzyme in the cytosol of meristematic and leaf primordial cells, and then it moves to the nucleus in the first leaf, in which it is active. Phosphorylation of GRIK may affect its activity and subcellular location in plants. As with GRIMP, it is necessary to consider the possibility that GRIK and its homolog are located in different cellular compartments and that our expression pattern is a composite of the two proteins. The disappearance of GRIK at the time when mature leaf cell morphology is established strongly suggests that it plays a role in leaf maturation.
We demonstrated that AL1 binds to GRIK, GRIMP, and histone H3 in both yeast and insect cells but did not confirm the interactions in plant cells because of technical limitations attributable to difficulty in extracting AL1 under native conditions. However, several lines of evidence strongly suggested that AL1 interacts with the three host factors. First, not all of the putative AL1 partners that were identified by the two-hybrid screen could be verified in insect cells (data not shown). Second, domain-mapping experiments using truncated AL1 proteins established that GRIK and GRIMP interact with AL1 amino acids 134 to 180, indicating that their interactions with AL1 are highly specific. Third, all three host proteins and AL1 localize to the nucleus, thereby providing the opportunity for interaction. This observation is particularly striking for GRIK, which changes subcellular location from the cytoplasm to the nucleus during development but is found exclusively in nuclei of infected cells. One possibility is that GRIK moves into or is tethered in the nucleus because of its interaction with AL1.
AL1 interacts with GRIK and GRIMP through the same domain (amino acids 134 to 180). This region also contains the AL1 oligomerization domain (Orozco et al., 2000) and part of the RBR1 and AL3 binding domains (Kong et al., 2000; Settlage et al., 2001). The region includes a pair of conserved α-helices that are essential for RBR1 binding (Kong et al., 2000; G. Arguello-Astorga, L.-J. Kong, L. Lopez, and L. Hanley-Bowdoin, unpublished data) and are likely to provide contacts for other AL1 partners. The overlapping character of the protein interaction domains suggests that AL1 occurs in a variety of complexes that serve different functions, possibly at different times during the infection process. This idea is further supported by previous experiments suggesting that AL1 forms different oligomers with different activities (Orozco et al., 2000) and that oligomerization is a prerequisite for RBR1 binding (Kong et al., 2000). Oligomerization also may influence the formation of AL1/GRIK and AL1/GRIMP complexes.
We isolated three host proteins that interact with AL1. The interaction between AL1 and histone H3 may have direct consequences for geminivirus replication and transcription, whereas the interactions with GRIMP and GRIK are more likely to be involved in the establishment and maintenance of a cellular environment that is favorable for geminivirus infection. AL1–GRIMP interactions may prevent infected cells from undergoing mitosis. GRIK is an abundant nuclear protein in plant cells during the developmental transition from cell division to expansion. Based on these interactions, a possible scenario is that AL1–host protein interactions do not induce mature plant cells to reenter the cell division cycle; instead, they trigger the establishment of an endoreduplication cycle. Three lines of evidence support this idea. First, geminivirus infection alters the nuclear morphology and volume of mature cells to resemble endocycling cells in the absence of mitosis (Bass et al., 2000). Second, AL1 also binds to and inactivates RBR1 (Kong et al., 2000). Functional pRb is necessary to block endoreduplication in animal cells (Niculescu et al., 1998). Third, endoreduplication is a common event late in leaf development (Joubes and Chevalier, 2000), and as such, it may be induced readily in mature tissues by geminivirus infection. This hypothesis will be tested in future experiments that examine the effect of mutations in GRIMP, GRIK, and their homologs on plant development and geminivirus infection.
METHODS
Plasmids and Viruses
Yeast Two-Hybrid Clones
Plasmids pAS2-1, pGBKT7, and pGADT7 were from Clontech (Palo Alto, CA). pNSB736 containing the Gal4 DNA binding domain–Tomato golden mosaic virus (TGMV) AL1 fusion was described previously (Kong et al., 2000). A Cabbage leaf curl virus (CbLCV) AL1 fragment was amplified from CbLCV.A/pBS (Abouzid et al., 1992) using primers 5′-CCTAAATAagatctACAAggatccCACGAAACCCTA-3′ and 5′-GAGTTGAAACGGAGGAGCCC-3′ (uppercase letters are the CbLCV sequence, and lowercase letters are restriction site sequences). The PCR product was cut with BglII and NcoI and recloned into CbLCV.A/pBS to introduce a BamHI site. The BamHI fragment from this plasmid was cloned into the BamHI site of pAS2-1 to give plasmid pNSB909. The bait plasmid (pNSB981) with the full-length CbLCV AL1 gene was constructed by inserting the BamHI-PstI fragment of pNSB909 into pGBKT7. The bait plasmid (pNSB986) containing CbLCV AL1 amino acids 1 to 178 was constructed by inserting the NcoI fragment of pNSB981 into pGBKT7 also digested with NcoI.
cDNA Clones
Total RNA isolated from 2-week-old seedlings of Arabidopsis thaliana ecotype Columbia using a RNA mini kit (Qiagen, Valencia, CA) was used as a template in reverse transcriptase–mediated (RT)–PCR to obtain full-length cDNAs. A RT-PCR product corresponding to the 5′ end of the GRIK coding region was generated using primers 5′-CAggatccATCATGAGTTGCTTCGGGTGT-3′ and 5′-TATGAGCAT-GAAGGTACATGAG-3′ (uppercase letters indicate the Arabidopsis sequence, and lowercase letters are restriction site sequences). The product was digested with BamHI and NaeI and cloned into the same sites of the original GRIK library clone (At3) to give pNSB1016 encoding GRIK12C.
The SalI fragment of the original GRIMP library clone (At331) was cloned into pBS-KS(+) to give plasmid pNSB1035. A RT-PCR fragment corresponding to the 3′ end of the GRIMP coding sequence was generated with primers 5′-CACTAGCAGGGGTAGCAG-3′ and 5′-GGTTCctcgagTTACTCCAGTTCACTAACAAGG-3′, digested with NcoI and XhoI, and inserted into pNSB1035 to give pNSB1041. A RT-PCR fragment corresponding to the 5′ end of the GRIMP open reading frame was generated with primers 5′-CTGGatcgatCATGG-CCGATCAGAGAAGTAAAAC-3′ and 5′-CATTTGCAGCTTCTGTTCCTG-3′, digested with ClaI and AccI, and cloned into the same sites of pNSB1041 to give pNSB1042 specifying full-length GRIMP.
Baculovirus Transfer Vectors
The His-GRIK12C transfer vector pNSB1017 was obtained by in-sertion of a trimmed BamHI-EcoRI fragment of pNSB1016 into the repaired NdeI and EcoRI sites of pNSB910, a baculovirus transfer vector with a His tag sequence. The glutathione S-transferase (GST)–H358-136 transfer vector pNSB1049 was generated by cloning the SalI-DraI fragment of the original H3 library plasmid (At121) into pNSB314 (Orozco et al., 1997) digested with SalI and PvuII. The GST-GRIMP transfer vector pNSB1047 was made by cloning the SmaI-XhoI fragment of pNSB1042 into pNSB314 digested with SmaI and SalI. A BsiHKAI-XhoI fragment from the original GRIMP library plasmid (At331) was cloned into the SalI and PstI sites of pNSB314 to give the GST-GRIMP473-866 transfer vector pNSB1046.
Escherichia coli Expression Vectors
The His-GRIK12C expression vector pNSB1066 was generated by inserting a trimmed AccI-PvuII fragment of pNSB1017 into a trimmed BamHI site of pET16b (Novagen, Madison, WI). The fragment resulting from pNSB1041 digested with NcoI, trimmed, and then digested by XhoI was inserted into repaired NdeI and XhoI sites of pET-16b to give the His-GRIMP857-1273 expression vector pNSB1064.
Yeast Two-Hybrid Screen
A Gal4-based two-hybrid system was used as described by the manufacturer (Clontech). The Arabidopsis Matchmaker cDNA library (Clontech) and corresponding bait plasmids were used to cotransform yeast strain AH109 (MATa, trp1-90, leu2-3, 112, ura3-52, his3-200, gal4Δ, gal80Δ, LYS2::GAL1UAS-GAL1TATA-HIS3, GAL2UAS-GAL2TATA-ADE2, URA3::MEL1TATA-lacZ). Transformants were plated onto synthetic dropout medium with α-gal lacking Trp, Leu, His, and adenine. Plasmid DNA was recovered from blue yeast colonies, transformed into E. coli strain DH5α, recovered, and retransformed into yeast strain AH109 with control plasmids. β-Galactosidase activity was monitored using a colony-lift filter assay. Clones without autoactivation and positive in filter assays were selected for DNA sequencing and analyzed using BLAST against the Arabidopsis genomic database.
Protein Expression and Analysis in Insect Cells
Recombinant baculoviruses expressing TGMV AL1, AL11-168, AL11-180, AL11-212, AL1134-352, AL1181-352, GST, and His-CAT were described previously (Settlage et al., 1996; Orozco et al., 2000). The generation of recombinant baculoviruses from transfer vectors and protein interaction assay was described previously (Orozco et al., 1997). For in vivo 32P labeling, 4 × 106 Spodoptera frugiperda Sf9 cells were infected with recombinant baculoviruses. At 24 h after infection, the medium was replaced by phosphate-free medium, and 32P-orthophosphate was added 2 h later to a final concentration of 25 μCi/mL. For assays with the cyclin-dependent kinase inhibitor, olomoucine (Promega, Madison, WI) was added at the indicated concentrations with the phosphate-free medium. Cells were harvested 24 h later and washed with PBS. Total protein was extracted and analyzed by SDS-PAGE. Phosphorylated proteins were visualized by phosphorimaging.
To purify His-tagged proteins, infected Sf9 cells were harvested, washed twice with PBS, and resuspended in 1 mL of lysis buffer (50 mM Na2HPO4, pH 8, 300 mM NaCl, 0.5 mM EDTA, 1% Nonidet P-40, 5 mM β-mercaptoethanol, and 20 mM imidazole with a protease inhibitor cocktail [Settlage et al., 1996]) for every 107 cells. The cells were mixed for 30 min at 4°C followed by centrifugation at 4000g for 5 min. One-tenth volume of nickel–nitrilotriacetic acid agarose magnetic agarose beads (Qiagen) was added to the supernatant, mixed for 1 h at 4°C, and centrifuged to remove the supernatant. The beads were washed three times with lysis buffer and eluted with elution buffer (lysis buffer with 250 mM imidazole and 30% glycerol).
The in vitro kinase assay was performed in 50 μL of kinase reaction buffer (50 mM Tris-HCl, pH 7.5, 1 mM DTT, 10 mM MgCl2, 1 μg of bovine histone H1 or bovine myelin basic protein [Life Technologies, Rockville, MD], 10 μM ATP, and 5 μCi of γ-32P-ATP) with 50 ng of purified His-GRIK12C or His-CAT. The reaction was stopped after a 20-min incubation at 37°C by adding one-half volume of 3 × SDS loading buffer, boiled for 3 min, and resolved by SDS-PAGE. The protein gel was dried and exposed to x-ray film overnight.
Antibodies
Monoclonal and polyclonal antibodies for AL1 and GST were described previously (Orozco et al., 1997). The 6xHis monoclonal antibody was from Clontech. His-GRIK12C and His-GRIMP473-866 were produced in E. coli using the pET expression system (Novagen, Madison, WI) and purified on nickel–nitrilotriacetic acid agarose magnetic agarose beads using 6 M guanidine denaturing conditions as suggested by the manufacturer (Qiagen). Rabbit antisera were generated against the purified proteins by Cocalico Biologicals (Reamstown, PA). Total IgG was purified using protein A–Sepharose CL-4B (Amersham, Piscataway, NJ).
Plant Infection, Protein Extraction, and Immunoblotting
Arabidopsis ecotype Columbia was grown at 20°C under an 8-/16-h light/dark cycle. Nicotiana benthamiana was grown at 25°C under a 13-/11-h light/dark cycle. Arabidopsis plants at the six-leaf stage were infected in the apex by syringe inoculation with Agrobacterium tumefaciens carrying pNSB1090 and pNSB1091, which contain partial tandem copies of CbLCV A and B DNA, respectively (E.M. Egelkrout, L. Mairconti, R. Cella, D. Robertson, and L. Hanley-Bowdoin, unpublished data). N. benthamiana plants at the eight-leaf stage were agroinoculated as described previously (Egelkrout et al., 2001) using pMON337 and pMON393 carrying partial tandem copies of TGMV A and B DNA, respectively (Elmer et al., 1988b). The N. benthamiana cell line was described previously (Eagle et al., 1994), whereas the Arabidopsis cell line was a gift from Iris Meier (Department of Plant Biology, Ohio State University, Columbus).
Plant tissues or cultured cells were ground in liquid nitrogen, resuspended in SDS gel loading buffer, boiled for 6 min, and centrifuged at 5000g for 10 min. Supernatants were diluted with PBS to quantify protein by Bradford (1976) assays. Total protein (100 μg) was analyzed by SDS-PAGE followed by immunoblotting with the corresponding antibody at 0.8 μg IgG/mL using the ECL Plus Western kit (Amersham). Young tissue was from leaves of 2-week-old plants, and mature tissue was from fully expanded leaves. For infected mature tissue, fully expanded leaves displaying systematic symptoms were selected.
Protein Immunolocalization and in Situ DNA Hybridization
Healthy and systemically infected plant tissue was fixed and sectioned as described previously (Nagar et al., 1995). AL1, GRIK, and GRIMP were immunolocalized using a mouse monoclonal antibody against TGMV AL1 or purified IgG from rabbit antiserum (80 μg IgG/mL) against GRIK or GRIMP. The secondary antibodies were conjugated to Alexa 488, Cascade blue (Molecular Probes, Eugene, OR), or horseradish peroxidase (Vector Laboratories, Burlingame, CA). When required, the Vectastain Elite ABC horseradish peroxidase kit (Vector Laboratories) was used to visualize the protein signals.
A Texas red–labeled oligonucleotide, 5′-CGTGCTGTGCAATCC-AGGAGAGGGGAGCAG-3′ (Genset, Paris, France), that specifically detected CbLCV A DNA, was used for in situ analysis as described previously (Bass et al., 2000). Images were collected using a Nikon Optiphot 800 microscope (Tokyo, Japan) as described previously (Nagar et al., 1995) or a Leica TCS SP confocal microscope (Wetzlar, Germany) with ×40 or ×63 PL APO objectives.
Upon request, all novel material described in this article will be made available in a timely manner for noncommercial purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Accession Numbers
The accession numbers for the sequences mentioned in this article are as follows: Arabidopsis GRIMP (AY100691) and its kinesin homolog (BAB11568); GRIMP TH65 homologs in Arabidopsis (CAA04956), tomato (AF275345), and soybean (AW200832); Arabidopsis GRIK (AY035070); GRIK Ser/Thr protein kinase homologs in Arabidopsis (AB011483) and rice (AC082645).
Acknowledgments
We thank Sharon Settlage (North Carolina State University [NCSU] Biochemistry) and Steven Spiker (NCSU Genetics) for helpful discussions and careful reading of the manuscript. We are indebted to Niki Robertson (NCSU Botany) for insight regarding the immunolocalization experiments and for providing microscope access. We thank Iris Meier (Department of Plant Biology, Ohio State University) for the Arabidopsis cell line. This research was supported by grants MCB-9809953 and MCB-0110536 from the National Science Foundation.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003681.
References
- Abouzid, A.M., Hiebert, E., and Strandberg, J.O. (1992). Cloning, identification, and partial sequencing of the genomic components of a geminivirus infecting the Brassicaceae. Phytopathology 82, 1070. [Google Scholar]
- Ach, R.A., Durfee, T., Miller, A.B., Taranto, P., Hanley-Bowdoin, L., Zambriski, P.C., and Gruissem, W. (1997). RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol. Cell. Biol. 17, 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiadis, V., Halmer, L., and Gruss, C. (1997). Influence of core histone acetylation on SV40 minichromosome replication in vitro. Chromosoma 105, 324–331. [DOI] [PubMed] [Google Scholar]
- Amundson, S.A., Myers, T.G., and Fornace, A.J. (1998). Roles for p53 in growth arrest and apoptosis: Putting on the brakes after genotoxic stress. Oncogene 17, 3287–3299. [DOI] [PubMed] [Google Scholar]
- Bass, H.W., Nagar, S., Hanley-Bowdoin, L., and Robertson, D. (2000). Chromosome condensation induced by geminivirus infection of mature plant cells. J. Cell Sci. 113, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Cheng, S.H., Harvey, R., Espino, P.C., Semba, K., Yamamoto, T., Toyoshima, K., and Smith, A.E. (1988). Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 7, 3845–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coello, P., Rodriguez, R., Garcia, E., and Vazquez-Ramos, J.M. (1992). A DNA polymerase from maize axes: Its purification and possible role. Plant Mol. Biol. 20, 1159–1168. [DOI] [PubMed] [Google Scholar]
- Courtneidge, S.A., and Smith, A.E. (1983). Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature 303, 435–439. [DOI] [PubMed] [Google Scholar]
- De Veylder, L., Segers, G., Glab, N., Van Montagu, M., and Inze, D. (1997). Identification of proteins interacting with the Arabidopsis Cdc2aAt protein. J. Exp. Bot. 48, 2113–2114. [Google Scholar]
- Dyson, N., Buchkovich, K., Whyte, P., and Harlow, E. (1989). The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243, 934–937. [DOI] [PubMed] [Google Scholar]
- Eagle, P.A., Orozco, B.M., and Hanley-Bowdoin, L. (1994). A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 6, 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner, R., Ewen, M.E., Newsome, D., Gerdes, M., Decaprio, J.A., Lawrence, J.B., and Livingston, D.M. (1994). Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8, 869–884. [DOI] [PubMed] [Google Scholar]
- Egelkrout, E.M., Robertson, D., and Hanley-Bowdoin, L. (2001). Proliferating cell nuclear antigen transcription is repressed through an E2F consensus element and activated by geminivirus infection in mature leaves. Plant Cell 13, 1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, J.S., Brand, L., Sunter, G., Gardiner, W.E., Bisaro, D.M., and Rogers, S.G. (1988. a). Genetic analysis of tomato golden mosaic virus. II. Requirement for the product of the highly conserved AL1 coding sequence for replication. Nucleic Acids Res. 16, 7043–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, J.S., Sunter, G., Gardiner, W.E., Brand, L., Browning, C.K., Bisaro, D.M., and Rogers, S.G. (1988. b). Agrobacterium-mediated inoculation of plants with tomato golden mosaic virus DNAs. Plant Mol. Biol. 10, 225–234. [DOI] [PubMed] [Google Scholar]
- Esau, K. (1977). Virus-like particles in the nuclei of phloem cells in spinach leaves infected with the curly top virus. J. Ultrastruct. Res. 61, 78–88. [DOI] [PubMed] [Google Scholar]
- Fobert, P.R., Coen, E.S., Murphy, G.J.P., and Doonan, J.H. (1994). Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J. 13, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes, E.P.B., Eagle, P.A., Sipe, P.A., Luckow, V.A., and Hanley-Bowdoin, L. (1994). Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 269, 8459–8465. [PubMed] [Google Scholar]
- Fontes, E.P.B., Luckow, V.A., and Hanley-Bowdoin, L. (1992). A geminivirus replication protein is a sequence-specific DNA binding protein. Plant Cell 4, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, J.O., Waga, S., Harry, J.B., Espling, E., Stillman, B., and Galloway, D.A. (1997). Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11, 2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, L.S., and Philp, A.V. (1999). The road less traveled: Emerging principles of kinesin motor utilization. Annu. Rev. Cell Dev. Biol. 15, 141–183. [DOI] [PubMed] [Google Scholar]
- Gorbalenya, A.E., and Koonin, E.V. (1993). Helicase: Amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 3, 419–429. [Google Scholar]
- Hanley-Bowdoin, L., Settlage, S.B., Orozco, B.M., Nagar, S., and Robertson, D. (1999). Geminiviruses: Models for plant DNA replication, transcription and cell cycle regulation. Crit. Rev. Plant Sci. 18, 71–106. [PubMed] [Google Scholar]
- Hill, J.E., Strandberg, J.O., Hiebert, E., and Lazarowitz, S.G. (1998). Asymmetric infectivity of pseudorecombinants of cabbage leaf curl virus and squash leaf curl virus: Implications for bipartite geminivirus evolution and movement. Virology 250, 283–292. [DOI] [PubMed] [Google Scholar]
- Horns, T., and Jeske, H. (1991). Localization of abutilon mosaic virus (AbMV) DNA within leaf tissue by in situ hybridization. Virology 181, 580–588. [DOI] [PubMed] [Google Scholar]
- Huntley, R., et al. (1998). The maize retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (CycD) proteins. Plant Mol. Biol. 37, 155–169. [DOI] [PubMed] [Google Scholar]
- Jansen-Durr, P. (1996). How viral oncogenes make the cell cycle. Trends Genet. 12, 270–275. [DOI] [PubMed] [Google Scholar]
- Joubes, J., and Chevalier, C. (2000). Endoreduplication in higher plants. Plant Mol. Biol. 43, 735–745. [DOI] [PubMed] [Google Scholar]
- Kong, L.J., Orozco, B.M., Roe, J.L., Nagar, S., Ou, S., Feiler, H.S., Durfee, T., Miller, A.B., Gruissem, W., Robertson, D., and Hanley-Bowdoin, L. (2000). A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19, 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs, J., Traut, W., Heyraud, F., Matzeit, V., Rogers, S.G., Schell, J., and Gronenborn, B. (1995). In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl. Acad. Sci. USA 92, 3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal, A., Poon, R.Y.C., Howe, P.H., Toyoshima, H., Hunter, T., and Harter, M.L. (1996). Inactivation of p27Kip1 by the viral E1A oncoprotein in TGFb-treated cells. Nature 380, 262–265. [DOI] [PubMed] [Google Scholar]
- Mao, L., Begum, D., Chuang, H.W., Budiman, M.A., Szymkowiak, E.J., Irish, E.E., and Wing, R.A. (2000). JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 406, 910–913. [DOI] [PubMed] [Google Scholar]
- Massimi, P., Pim, D., and Banks, L. (1997). Human papillomavirus type 16 E7 binds to the conserved carboxy-terminal region of the TATA box binding protein and this contributes to E7 transforming activity. J. Gen. Virol. 78, 2607–2613. [DOI] [PubMed] [Google Scholar]
- McIntyre, M.C., Ruesch, M.N., and Laimins, L.A. (1996). Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology 215, 73–82. [DOI] [PubMed] [Google Scholar]
- Nagar, S., Pedersen, T.J., Carrick, K., Hanley-Bowdoin, L., and Robertson, D. (1995). A geminivirus induces expression of a host DNA synthesis protein in terminally differentiated plant cells. Plant Cell 7, 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu, A.B., Chen, X.B., Smeets, M., Hengst, L., Prives, C., and Reed, S.I. (1998). Effects of p21(Cip1/Waf1) at both the G(1)/S and the G(2)/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco, B.M., and Hanley-Bowdoin, L. (1998). Conserved sequence and structural motifs contribute to the DNA binding and cleavage activities of a geminivirus replication protein. J. Biol. Chem. 273, 24448–24456. [DOI] [PubMed] [Google Scholar]
- Orozco, B.M., Kong, L.J., Batts, L.A., Elledge, S., and Hanley-Bowdoin, L. (2000). The multifunctional character of a geminivirus replication protein is reflected by its complex oligomerization properties. J. Biol. Chem. 275, 6114–6122. [DOI] [PubMed] [Google Scholar]
- Orozco, B.M., Miller, A.B., Settlage, S.B., and Hanley-Bowdoin, L. (1997). Functional domains of a geminivirus replication protein. J. Biol. Chem. 272, 9840–9846. [DOI] [PubMed] [Google Scholar]
- Pant, V., Gupta, D., Choudhury, N.R., Malathi, V.G., Varma, A., and Mukherjee, S.K. (2001). Molecular characterization of the Rep protein of the blackgram isolate of Indian mungbean yellow mosaic virus. J. Gen. Virol. 82, 2559–2567. [DOI] [PubMed] [Google Scholar]
- Pilartz, M., and Jeske, H. (1992). Abutilon mosaic geminivirus double-stranded DNA is packed into minichromosomes. Virology 189, 800–802. [DOI] [PubMed] [Google Scholar]
- Qin, Y., and Petty, I.T. (2001). Genetic analysis of bipartite geminivirus tissue tropism. Virology 291, 311–323. [DOI] [PubMed] [Google Scholar]
- Ramsperger, U., and Stahl, H. (1995). Unwinding of chromatin by the SV40 large T antigen DNA helicase. EMBO J. 14, 3215–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S., and Day, I.S. (2001). Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genomics 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads, A.R., and Friedberg, F. (1997). Sequence motifs for calmodulin recognition. FASEB J. 11, 331–340. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., and Lazarowitz, S.G. (1996). Getting it together in plant virus movement: Cooperative interactions between bipartite geminivirus movement proteins. Trends Cell Biol. 6, 353–358. [DOI] [PubMed] [Google Scholar]
- Saunders, K., Lucy, A., and Stanley, J. (1991). DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 19, 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settlage, S.B., Miller, A.B., Gruissem, W., and Hanley-Bowdoin, L. (2001). Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology 279, 570–576. [DOI] [PubMed] [Google Scholar]
- Settlage, S.B., Miller, B., and Hanley-Bowdoin, L. (1996). Interactions between geminivirus replication proteins. J. Virol. 70, 6790–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidle, A., Palaty, C., Dirks, P., Wiggan, O., Kiess, M., Gill, R.M., Wong, A.K., and Hamel, P.A. (1996). Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit. Rev. Biochem. Mol. Biol. 31, 237–271. [DOI] [PubMed] [Google Scholar]
- Stenger, D.C., Revington, G.N., Stevenson, M.C., and Bisaro, D.M. (1991). Replicational release of geminivirus genomes from tandemly repeated copies: Evidence for rolling-circle replication of a plant viral DNA. Proc. Natl. Acad. Sci. USA 88, 8029–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter, G., Hartitz, M.D., and Bisaro, D.M. (1993). Tomato golden mosaic virus leftward gene expression: Autoregulation of geminivirus replication protein. Virology 195, 275–280. [DOI] [PubMed] [Google Scholar]
- Vesely, J., Havlicek, L., Strnad, M., Blow, J.J., Donelladeana, A., Pinna, L., Letham, D.S., Kato, J., Detivaud, L., Leclerc, S., and Meijer, L. (1994). Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem. 224, 771–786. [DOI] [PubMed] [Google Scholar]
- Wang, M., Park, J.S., Ishiai, M., Hurwitz, J., and Lee, S.H. (2000). Species specificity of human RPA in simian virus 40 DNA replication lies in T-antigen-dependent RNA primer synthesis. Nucleic Acids Res. 28, 4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, R.A. (1995). The retinoblastoma protein and cell cycle control. Cell 81, 323–330. [DOI] [PubMed] [Google Scholar]
- Weisshart, K., Forster, H., Kremmer, E., Schlott, B., Grosse, F., and Nasheuer, H.P. (2000). Protein-protein interactions of the primase subunits p58 and p48 with simian virus 40 T antigen are required for efficient primer synthesis in a cell-free system. J. Biol. Chem. 275, 17328–17337. [DOI] [PubMed] [Google Scholar]
- Werness, B.A., Levine, A.J., and Howley, P.M. (1990). Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248, 76–79. [DOI] [PubMed] [Google Scholar]
- Xie, Q., Suarez-Lopez, P., and Gutierrez, C. (1995). Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant DNA virus: Requirement for efficient viral DNA replication. EMBO J. 14, 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]