Abstract
Objective
To describe a novel in vitro human tissue-based angiogenic model that can predict an individual tumor’s response to antiangiogenic drugs.
Summary Background Data
A number of in vitro and in vivo angiogenesis assays exist, but they do not provide potentially useful information for the treatment of an individual patient. Clonogenic assays have been used to evaluate the response of an individual’s tumor to antineoplastic agents, but these tumor fragments are cultured in an environment that does not lead to neovessel growth. The authors have previously demonstrated that human vein disks or human tumor xenograft fragments incorporated into a 0.3% fibrin-thrombin clot will develop angiogenic vessel growth from the cut edge of the vessel disk or xenograft fragment.
Methods
Fresh human tumor or normal tissue disks (2 × 1 mm) from fresh surgical specimens were incorporated into fibrin-thrombin clots overlain with nutrient medium containing either 20% fetal bovine serum alone or in combination with Epothilone B, a tubulin inhibitor with antiangiogenic properties. Tissue disks were visually assessed over time to determine the percentage of wells that developed an angiogenic response. Neovessel growth, density, and length were graded at intervals using a semiquantitative visual neovessel growth-rating scheme (angiogenic index, 0–16 scale) devised in the authors’ laboratory.
Results
Epothilone B treatment at doses of 10−6 mol/L and 10−8 mol/L decreased the number of wells that developed an invasive angiogenic response and limited the development of vessels that invaded the matrix. At these doses, Epothilone B also caused regression of vessels in wells that had been allowed to develop an angiogenic response. Treatment of tumors or normal tissues with Epothilone B at doses less than 10−8 mol/L was ineffective.
Conclusions
Epothilone B may be an effective antiangiogenic agent in a variety of tumor types. The authors speculate that this in vitro model might provide useful information to the clinician on the effect of specific antiangiogenic agents on individual tumors. This may be particularly useful in patients with tumors that, as a group, are unresponsive to treatment with antineoplastic agents.
A number of in vitro techniques have been developed to determine an individual tumor’s sensitivity to antineoplastics, hormones, and biologic response modifiers. Initially described by Salmon and Hamburger, the clonogenic or soft agar assay has been widely used to predict tumor chemosensitivity and chemoresistance. 1–3 Use of predictive assays such as hormone receptor assays and the evaluation of HER-2/neu have become common in the clinical management of patients with breast carcinomas. 4–9
A number of in vitro and in vivo models have been developed to assess the effect of a compound on the development of an angiogenic response. These predictive assays have had several significant drawbacks. First, many of these models do not use human tissue. 10–15 Conversely, if the angiogenic assay does use human tissue, it is either in the form of human umbilical vein endothelial cells (HUVECs) or disks of human veins or arteries embedded in a supportive matrix. 16–18 We have previously demonstrated that the angiogenic response from human tumor xenografts created in nude mice can also be assayed in a three-dimensional fibrin-thrombin clot angiogenesis model. 19 These human tumor/nude mouse xenografts have been successfully treated with drugs designed to attack the angiogenic response alone, the tumor cell, or both the angiogenic response and the tumor cell population. 19 However, the difficulty of routinely creating human tumor/mouse xenografts severely limits the utility of this technique for evaluating the sensitivity of an individual’s tumor-derived angiogenic response to a specific antiangiogenic therapy.
In an effort to devise an easy, reproducible assay that provides patient-specific, tumor-specific information on an antiangiogenic drug’s effects, we have developed an in vitro, three-dimensional fibrin-thrombin clot angiogenesis assay that allows the angiogenic responses of an individual tumor’s fragments to be evaluated over time. This assay allows tumors or normal tissue fragments to be treated with known inhibitors of angiogenesis over a wide range of clinically relevant concentrations.
We hypothesized that the preexisting (angiogenic) blood vessels contained within a tumor would rapidly invade into a fibrin-thrombin clot and would progressively grow and develop in a time-dependent fashion. We also hypothesized that effective antiangiogenic drugs would limit the invasion of angiogenic vessels into the fibrin-thrombin clot and would limit their subsequent development. In addition, we speculated that antiangiogenic agents may be either angiostatic or angiocidal to existing networks of angiogenic vessels that had developed over time in this assay.
METHODS
Tissue Preparation
To test these hypotheses, discarded portions of fresh human tumors or normal tissues were obtained anonymously with the approval of the Louisiana State University Health Sciences Center’s Institutional Review Board (New Orleans, LA). Tissues were obtained and transported to the laboratory in a saline-soaked gauze pad. Tumors were sliced into 1-mm-thick slices and 2-mm-diameter disks of fresh human tumor created using a sterile skin punch. Tumor disks were allocated to wells in a 96-well plate (Corning Inc., Corning, NY) in a pattern designed to minimize inclusion of one section of the tumor into a specific treatment group. All wells were preloaded with thrombin solution (0.05 IU in 1.0 μL/well) (Sigma Chemical Co., St. Louis, MO). Wells were allowed to evaporate to dryness before use.
The fibrin-thrombin clot was created using previously described methods. 20 Briefly, following placement of tissue disks in the bottom of thrombin-containing wells, the disks were covered with 100 μL of a clot-forming medium consisting of human fibrinogen (3 mg/mL) and ε-aminocaproic acid (5.0%) (Sigma) in HPVAM medium. HPVAM medium comprises Medium 199 and an antibiotic/antimycotic solution consisting of 100 U penicillin, 100 U streptomycin sulfate, and 0.25 μg amphotericin/mL (Gibco BRL, Gaithersburg, MD). This mixture was placed in a humidified incubator and allowed to clot at 37°C in a 6% CO2, 95% air environment. A nutrient medium (100 μL) containing the HPVAM supplemented with 20% bovine serum (Gibco BRL) was added to the tissue-containing clot. Drug-treated wells contained the nutrient medium supplemented with Epothilone B at appropriate concentrations. 20 Each well’s total volume was 200 μL.
Drug Information
Epothilone B was a gift from Novartis Pharmaceuticals Corporation (East Hanover, NJ). This compound, a tubulin inhibitor, is in a variety of clinical phase 2 cancer trials, including trials for neuroendocrine tumors that traditionally are considered unresponsive to chemotherapy. 21 This agent, like other tubulin inhibitors such as paclitaxel, is thought to inhibit tumor growth by triggering cell cycle arrest in the G2/M phase and by inducing apoptosis. 22 Results from a phase 1 trial of this agent demonstrated that the mean (non-compartmental modeling) area under the curve was 94 ng*h/mL/mg, and the mean Cmax of this drug was 7.0 ng/mL/mg ± 2.5 (unpublished data, Novartis). Currently, this drug is being used clinically in doses of 2.5 mg/m2, administered as a 5-minute infusion once per week for 3 weeks. This is followed by a 1-week rest period. Concentrations of Epothilone B (molecular weight 507) used in our study ranged from 10−6 mol/L to 10−14 mol/L. Representative in vitro drug concentrations used in our study were 507 ng/mL (10−6 mol/L) and 5.07 ng/mL (10−8 mol/L). Thus, the Epothilone B concentrations used in our assay span a clinically achievable range of drug concentrations.
Treatment Schemes
Tumors were tested using two different assay methodologies. Eight tumors and four normal tissues were treated with either nutrient medium or drug-containing medium starting on the first day in culture, while three tumors and three normal tissues were allowed to develop an angiogenic response for 14 to 18 days. Subsequently, these tumors were treated with either control medium or drug-containing medium for 1 to 2 weeks.
Evaluation of Angiogenesis
Wells were assessed over time using an inverted phase microscope. Two different assay criteria were evaluated. The ability of the preformed blood vessels to invade into the fibrin-thrombin clot was assessed over time. The percentage of tumor implants that developed invasion (% I) was calculated using the formula “(# of wells exhibiting invasion/# of wells plated) × 100 = % I.” In addition, the degree of angiogenic response was assessed using a semiquantitative visual rating scale developed in our laboratory. Briefly, tissue disks were visually divided into four quadrants. Each quadrant was given a numeric score from 0 to 4 based on the neovessel’s length, density, and percentage of the quadrant’s circumference involved with the angiogenic response. Numeric results from the four quadrants were summed and expressed as an angiogenic index (AI, 0–16). These scores correlate well with more objective measures of vessel growth, such as vessel length or vessel surface area as determined by digital image analysis. 23 At the completion of the experiment, selected wells were fixed in 10% neutral buffered formalin for hematoxylin and eosin staining or for immunohistochemical evaluation for factor VIII (Fig. 1).
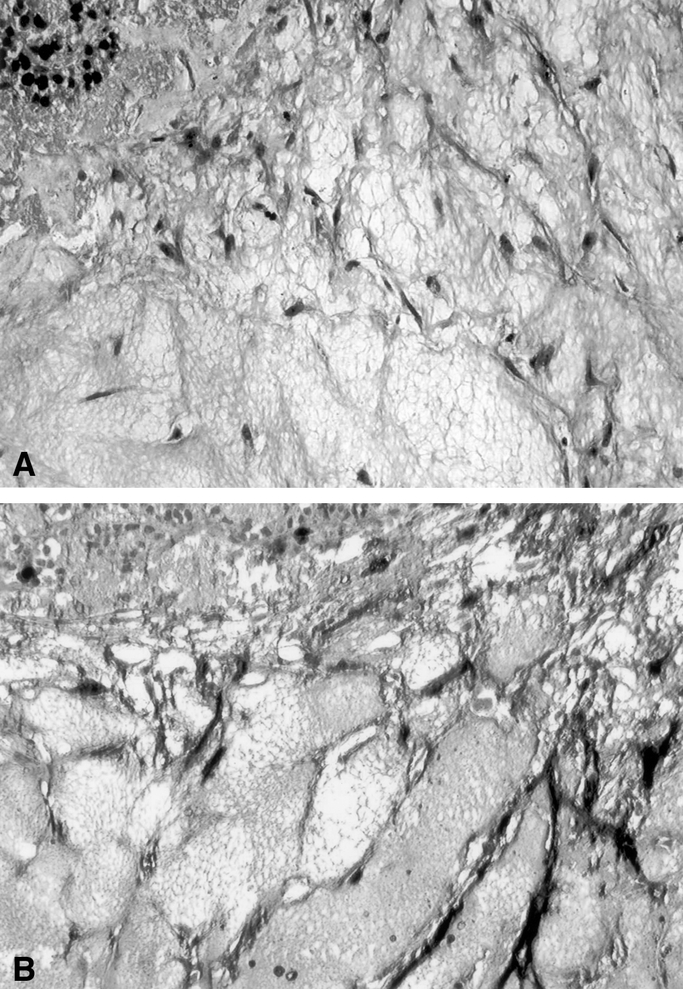
Figure 1. Photomicrograph of human parathyroid tissue following culture. (A) Hematoxylin and eosin stain (100×). (B) Immunohistochemical localization of factor VIII on the endothelial tubes invading the fibrin-thrombin clot matrix (100×).
RESULTS
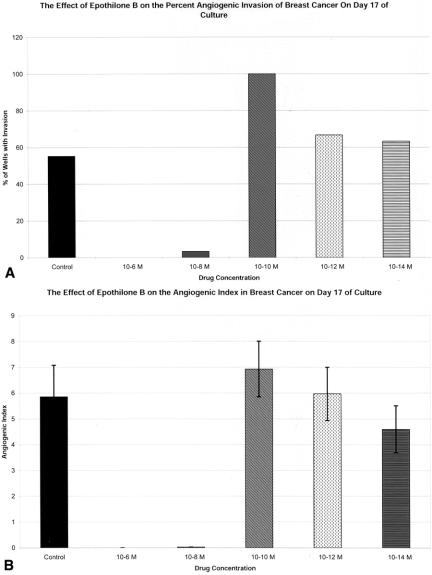
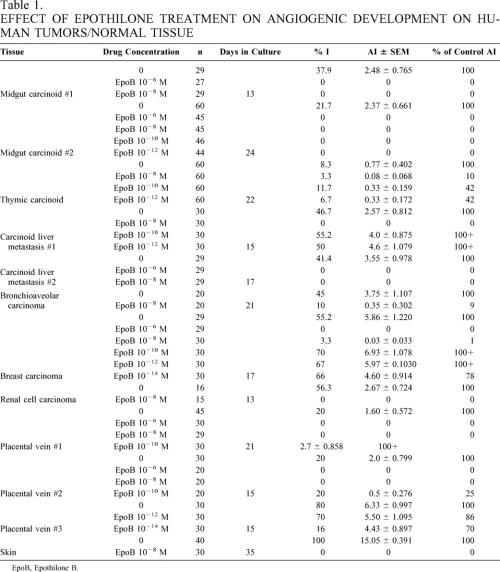
The effect of drug therapy on the angiogenic response was evaluated using two methods (Figs. 2–4). In the first set of experiments (Table 1), eight tumors and four normal tissues were exposed to drug from day 1 in culture. This set of experiments was designed to represent the effect of early or adjuvant Epothilone B treatment on the development of a tumor- or normal tissue-derived angiogenic response. The angiogenic response of the eight tumors treated in this method was highly sensitive to the higher doses of Epothilone B. All four of these tumors treated with Epothilone B at 10−6 mol/L demonstrated complete inhibition of angiogenic invasion into the clot. All eight tumors were treated with Epothilone B at 10−8 mol/L. At this dose, five of the eight tumors had complete inhibition of angiogenic vessel development (% I, AI), and the remaining three tumors had 90%, 91%, and 99% inhibition of angiogenic vessel development (AI). The angiogenic responses derived from the three normal tissues were also sensitive to Epothilone B at doses of 10−6 mol/L and 10−8 mol/L. In both tumors and normal tissues, the angiogenic response was not effectively inhibited by Epothilone B at doses less than 10−8 mol/L (see Fig. 2).
Figure 2. Effect of Epothilone B treatment at different doses on the percentage of wells that develop evidence of invasion (% I) (A) and the development of the angiogenic response (AI) (B). % I, % of wells demonstrating invasion of neovessels into the fibrin-thrombin clot ([# of wells with invasion/# of wells plated] × 100). AI ± SEM, mean angiogenic index ± SEM of wells. Data from wells that demonstrated invasion into the clot and those that did not invade the clot are included. Wells that did not demonstrate invasion are represented by a score of zero.
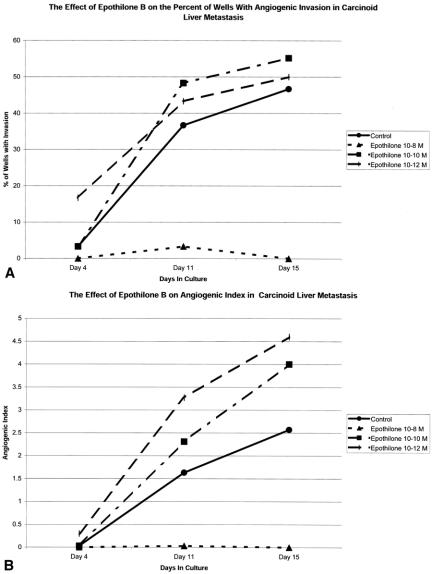
Figure 3. Time course for the development of an angiogenic response in a carcinoid liver metastasis in vitro. In this experiment, wells were exposed to Epothilone B with 10−8, 10−10, or 10−12 mol/L from day 1 in culture. Epothilone B treatment was effective at the 10−8 mol/L concentration but was ineffective at higher doses. % I, % of wells demonstrating invasion of neovessels into the fibrin-thrombin clot ([# of wells with invasion/# of wells plated] × 100). AI ± SEM, mean angiogenic index ± SEM of wells. Data from wells that demonstrated invasion into the clot and those that did not invade the clot are included. Wells that did not demonstrate invasion are represented by a score of zero.
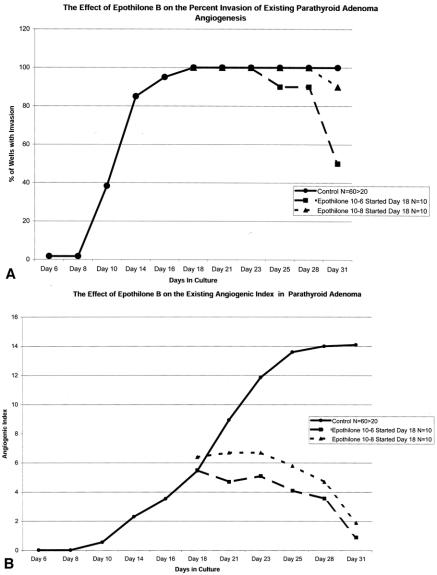
Figure 4. Regression of established neovessels following therapy with Epothilone B at 10−6 and 10−8 mol/L. Wells were allowed to grow until day 18, when therapy was initiated. Epothilone B treatment at these doses inhibited further neovessel development and caused a decrease in the percentage of wells that exhibited evidence of neovessel invasion (A) and decreased the mean AI in treated wells (B). % I, % of wells demonstrating invasion of neovessels into the fibrin-thrombin clot ([# of wells with invasion/# of wells plated] × 100). AI ± SEM, mean angiogenic index ± SEM of wells. Data from wells that demonstrated invasion into the clot and those that did not invade the clot are included. Wells that did not demonstrate invasion are represented by a score of zero.
Table 1. EFFECT OF EPOTHILONE TREATMENT ON ANGIOGENIC DEVELOPMENT ON HUMAN TUMORS/NORMAL TISSUE
EpoB, Epothilone B.
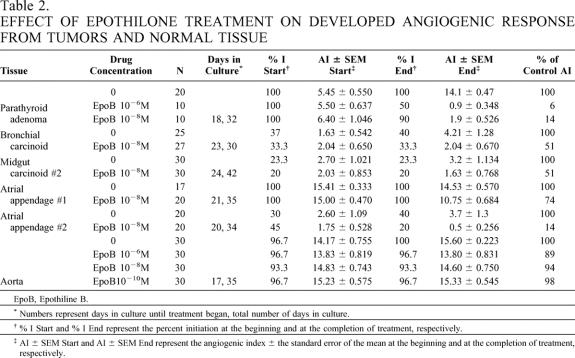
In the second set of experiments (Table 2), tumors or normal tissues were allowed to develop an angiogenic response. Wells were then randomly allocated to a “no treatment” group or treatment groups. This method was developed to more accurately determine the therapeutic response of developed, mature neovessel networks, such as those seen in widely metastatic tumors. Effective antiangiogenic agents should be able to act in an angiostatic or angiocidal manner in these mature neovessel networks. Three tumors were evaluated, including one tumor (midgut carcinoid #2) that was also evaluated by the early treatment method. Treatment of the parathyroid adenoma with Epothilone B at 10−6 mol/L and 10−8 mol/L caused regression of the vessels. The number of wells exhibiting visible angiogenesis decreased by 50% and 10% when treated with Epothilone B at concentrations of 10−6 mol/L and 10−8 mol/L, respectively. The AI of Epothilone B 10−6 mol/L-treated wells decreased by 94%, and the AI of Epothilone B 10−8 mol/L-treated wells decreased by 86%. Similar effects on AI were seen in the bronchial and midgut carcinoid #2. In both of these tumors, treatment with 10−8 mol/L Epothilone B decreased the AI by 50% (see Table 1).
Table 2. EFFECT OF EPOTHILONE TREATMENT ON DEVELOPED ANGIOGENIC RESPONSE FROM TUMORS AND NORMAL TISSUE
EpoB, Epothiline B.
* Numbers represent days in culture until treatment began, total number of days in culture.
† % I Start and % I End represent the percent initiation at the beginning and at the completion of treatment, respectively.
‡ AI ± SEM Start and AI ± SEM End represent the angiogenic index ± the standard error of the mean at the beginning and at the completion of treatment, respectively.
Three normal tissues (two atrial appendages and a segment of human aorta) were treated with Epothilone B after being allowed to develop an angiogenic response. Epothilone B 10−8 mol/L treatment decreased the AI by 36% and 86%, respectively, in atrial appendages #1 and #2. Epothilone B treatment at 10−6, 10−8, and 10−10 mol/L had little or no effect on the angiogenic response derived from fragments of human aorta (see Table 2). The lack of response of this aortic specimen to Epothilone is puzzling. In other experiments (data not shown) using aortic disks as targets for antiangiogenics, aortic tissue responded like other vein-derived neovessels.
DISCUSSION
The quest for in vitro assays that accurately predict the response of an individual tumor to antineoplastics, biologic response modifiers, hormones, and antiangiogenics has spanned several decades. Salmon and Hamburger in 1977 described a human tumor cloning assay in which small tumor fragments were dispersed in a soft agar matrix. 1,2 The tumor’s anchorage-independent growth allowed for proliferation of the malignant cells while specifically preventing the growth of anchorage-dependent “normal” or benign cells, including vessels and other supporting stromal cells. A number of investigators have used this methodology to predict the chemosensitivity and chemoresistance as well as the hormone sensitivity resistance in human tumor specimens. 3,24–28 A prospective trial of the human tumor cloning system as a means for selecting single-agent chemotherapeutics in patients with advanced cancer was undertaken by Von Hoff et al. 24 In this trial, 604 single-agent trials were performed in 470 patients. Each tumor was evaluated for drug sensitivity. Only 41% of the specimens submitted had adequate tumor growth and effectively grew in a soft agar system. In the 246 prospective drug in vitro/in vivo comparisons, there was a 60% true-positive and an 85% true-negative rate for predicting the response or lack of response of an individual tumor to the single chemotherapeutic agent.
Anchorage-independent cell growth has been considered a key concept for cell growth in the soft agar assay. Normal tissues and benign tumors were thought to be unlikely candidates for successful culturing in an anchorage-independent matrix. However, Von Hoff et al. demonstrated that benign human parathyroid tumors removed from patients with clinical hyperparathyroidism could be grown in soft agar culture. 29 Four patients with parathyroid hyperplasia and one with a parathyroid adenoma had their cells dispersed into single-cell suspensions of soft agar. These parathyroid cells grew into colonies and produced measurable levels of parathyroid hormone. These tissues remained viable for approximately 3 weeks. Von Hoff et al.’s study confirmed that malignancy was not necessary for colony formation in agar-based systems. Based on these observations, we tested a benign parathyroid adenoma in our assay. This tissue developed a robust angiogenic response over 18 to 32 days in culture. Subsequent treatment of this developed response with Epothilone B at 10−6 and 10−8 mol/L caused regression of neovessel formation. Effective destruction of an angiogenic response in a benign (functional) neuroendocrine tumor may cause clinical tumor regression, may limit peptide production, and may decrease the symptoms associated with these tumors.
Angiogenesis is a critical determinant of tumor growth and the development of metastasis. It is widely accepted that tumors cannot grow beyond a diameter of 2 mm without the development of an angiogenic response. A number of investigators have developed in vitro angiogenesis assays that combine some of the “gel” characteristics of the soft agar culture system but also provide a “scaffolding” for the development of anchorage-dependent endothelial tubes. We and others have used normal animal and human blood vessel fragments in a three-dimensional fibrin-thrombin clot, overlain with a nutrient tissue culture medium and supplemented with fetal bovine serum either alone or in combination with angiogenesis stimulators or inhibitors. 13,16–20 Over 1 to 2 weeks, these vascular explants begin to develop endothelial tubes from the cut vessel edge. Proliferative endothelial tubes are solid at the tip and vacuolized in the center and develop a lumen in their most mature, proximal location. 23 Developed vascular outgrowths also exhibit tight junctions and Weibel-Palade bodies, characteristic of their endothelial origin. They anastomose, branch, and express endothelial cell markers such as factor VIII, confirming their endothelial origin (see Fig. 1). Vascular explants have been used in a fibrin-thrombin clot assay to test experimental agents for their ability to inhibit the initiation or subsequent development of an angiogenic response. 20,30–32
Based on these concepts and our observations on the development of angiogenic vessels from the cut edge of a native vein or arterial specimen, we felt that the angiogenic vessels contained within a benign or malignant tumor would act as a source of endothelial cell growth, resulting in new vessel invasion and growth into a fibrin-thrombin clot matrix. Preliminary work from our laboratory using animal tissues obtained at necropsy demonstrated that tumor or normal tissue could develop an angiogenic response in this assay system. Gulec et al. used this system to test for the cytotoxic or angiocidal effect of a radiolabeled somatostatin analog, 111In-DTPA-JIC 2DL, in human tumor/nude mouse xenograft fragments. 19 In this study, the radiolabeled somatostatin analog destroyed tumor fragments whose cells expressed somatostatin receptor subtype 2 (sst 2) but had no effect on tumor fragments whose cells lacked this receptor. Conversely, angiogenic vessels from both sst 2-expressing and sst 2-nonexpressing tumors were destroyed by the treatment with this radiolabeled compound. This observation is consistent with the unique overexpression of sst 2 on human angiogenic vessels demonstrated by Watson et al. 18 The radiolabeled sst 2-preferring analog selectively attacked sst 2 receptors on cancer cells, angiogenic endothelial cells, or both. 19
Epothilones are a new class of antitumor compounds that have been isolated from Myxobacterium sporangium cellulosum. These compounds are naturally occurring cytotoxic macrolides that function as an antineoplastic by arresting cell division and by stabilizing cellular microtubule assemblies. Epothilones are part of a larger family of microtubule inhibitors that contain drugs such as paclitaxel (Taxol). These compounds stabilize the cytoskeleton and inhibit cell proliferation by stabilizing microtubules. Both paclitaxel and Epothilone compete for the same binding site. Unlike the paclitaxel members of this drug family, the epothilones appear to retain activity against multiple drug-resistant cell lines. 33 Paclitaxel and other members of this class of antineoplastics have been demonstrated to have significant inhibitory effects on angiogenesis, directly by effecting vascular endothelial cell proliferation and indirectly by decreasing VEGF production. 34–38 However, preliminary studies from our laboratory suggest that Epothilone B is significantly more potent than paclitaxel in its ability to inhibit angiogenesis (data not shown).
In our in vitro assay, we demonstrated that drug levels of 10−8 mol/L or greater produced a significant decrease in the angiogenic response in the majority of tissues studied, including carcinoids that are generally considered unresponsive to chemotherapy 21,39 (see Tables 1 and 2). These drug levels are within the range of blood levels seen in phase 1 trials of Epothilone B. In early phase 2 clinical trials of Epothilone B in patients with carcinoid tumors, both biochemical and clinical responses have been seen. These clinical trials remain in their infancy, so the number of patients who have exhibited a radiologic tumor response cannot yet be determined (personal communication, Lowell Anthony, MD, LSUHSC Department of Hematology and Oncology, New Orleans, LA). Based on the in vitro results from our laboratory, we predict that patients treated with Epothilone B will also experience radiologic tumor responses over time. In comparison, in a recent phase 2 trial of paclitaxel in patients with advanced neuroendocrine tumors, only 1 of 14 patients treated with this agent had a response, and the response was not durable. 39
CONCLUSIONS
We have developed a novel in vitro angiogenesis assay that allows individual tumors to be tested against a variety of antiangiogenic agents in clinically relevant concentrations. Epothilone B, a tubulin inhibitor, exhibits significant antiangiogenic effects in this assay at doses of 10−8 mol/L and greater. The ability to test an individual tumor against a wide range of antiangiogenic agents or against a single antiangiogenic compound over a wide range of clinically relevant doses may provide clinically useful information in the future. These concepts are under investigation in our laboratory.
Discussion
Dr. James C. Thompson (Galveston, TX): If you don’t die, and if you continue to go to meetings, generous colleagues may ask you to comment on work in areas on which your own competence, if not previously exposed, is at least recently untested. Now, I did attend, I believe at the Surgical Biology Club, the first public demonstration of an angiogenic agent, and I remember Judah Folkman’s showing us blood vessels creeping onto a rabbit’s cornea. I became familiar with the concept that these tumor-angiogenic factors were responsible for allowing circulating microfragments of tumor to gain a foothold on distant tissue and survive as metastases. I learned, to my own personal great interest, of the Jekyll-Hyde salutary role of VEGF in stimulating ingrowth of capillaries into ischemic myocardium.
The present study is directed towards measuring the antiangiogenic effects of a new agent from Novartis named Epothilone B. You will forgive me if I spend some time on that agent, because it is a fascinating thing. It is what happens when you are a new boy on the block. Now, knowing full well that the answers may be well known to the cognoscenti, I would like to ask the following.
What is this stuff? Your manuscript calls it a macrolide. What exactly is that? Its molecular weight is only 507 Daltons, so it is a small molecule.
You say in your manuscript that it is a tubulin inhibitor that works by causing cell-cycle arrest and by inducing apoptosis. What is the mechanism for these actions? My colleagues have been interested in the genetic mechanisms for stimulating or inhibiting apoptosis for years. How does this agent work in the genetic cascade of intracellular signals?
As to this spiffy new assay that you have developed to measure angiogenesis, how easy is it to set up? What are its limitations?
How do normal and neoplastic tissues rank in order of angiogenic actions? For example, I was surprised by that parathyroid adenoma. It looked like it might be calling in blood vessels from all over. What does it mean? Why was the effect of Epothilone B so much stronger on atrial tissue than on aorta? I know you said you don’t know, but can you speculate?
Of all the tumors you have studied, only breast cancer is common; that is the only common tumor in your study. Have you tried your assay on cancer tissue from the lung, or ovary, or colon, or prostate? Finding growth suppression in these tumors would really get the attention of this audience.
Dr. C. Wright Pinson (Nashville, TN): Oncologists have long had a goal of predicting for a given patient the response to a given therapy. Examples pointed out today include clonogenic assays and the hormonal receptor assays. Today, we have heard described a novel in vitro angiogenic model. Normal blood vessel fragments or blood vessels in tumor fragments will develop and invade into the fibrin-thrombin clot progressively. Angiogenic drugs will limit and possibly force regression of this growth, termed respectively angiostatic and angiocidal effects. In particular we have heard about this Epothilone B that is a compound isolated from a myxobacterium and a supposed microtubular inhibitor from the same family as Taxol. In clinically relevant doses of 10−8 molar or greater, there is an antiangiogenic effect.
Eight tumors and four normal pieces of tissue were treated from day 1, demonstrating the angiostatic effects in all 12. Three tumors and three normal tissues were treated after 2 weeks of culture, demonstrating angiocidal effects in both, the one exception being the aortic tissues.
I have three lines of questioning for the authors.
First, I too would like to know why the aortic tissue did not respond like all the other blood vessels originating from vein, placental vein, atrial appendage, and tumors. You do not discuss this in your manuscript. Is there some difference in arterial angiogenesis from others?
Second, why was there an increase in angiogenesis in the doses less than 10 −8 molar in some of the specimens? You don’t discuss this biphasic response in your manuscript. And in particular, could this finding potentially make low-dose Epothilone B helpful, for example, in ischemic myocardium or ischemic peripheral tissue?
Finally, in your discussion you mention clinical trials are now in their infancy with Epothilone B, while this manuscript shows studies from exactly 10 tumor and 7 normal tissue specimens. Yet the conclusions stated in your abstract include, “This in vitro model may provide useful information to clinicians on the effect of specific angiogenic agents on an individual patient tumor. This may be particularly useful in patients with tumors that are as a group unresponsive to treatment with antineoplastic agents.” Given the limited data presented in this paper, don’t you think these are really interesting speculations for discussion, not really conclusions? And I also would suggest that perhaps the title should be altered as well.
I thank the Association and the authors for the opportunity to discuss this paper. It contains very innovative concepts that may help patients in a variety of ways.
Dr. Samuel W. Beenken (Birmingham, AL): This is an important, timely, and concise report on the utilization of an in vitro angiogenesis assay for assessing the sensitivity of an individual patient’s tumor-derived angiogenic response to a specific therapy. Unlike other in vitro assays, which measure proliferation and migration of isolated endothelial cells, this assay simulates more faithfully the microvascular sprouting and branching seen in vivo.
I was privileged to review the manuscript, which is well written and describes a technique which could be utilized in the clinical setting. As the authors indicate, this assay holds potential for being applied in two directions: first, to evaluate and screen new candidate antiangiogenesis agents for use in treating specific human tumor types; and, two, to provide predictive information regarding the efficacy of a specific antiangiogenesis drug against discrete tumor tissues from a specific individual.
I have three questions for the authors.
First, regarding the specificity of the assay, 20% fetal bovine serum in medium is added to the fibrin clot in which angiogenesis is evaluated. I am somewhat naive regarding the nutrient requirements necessary for tumor neogenesis and whether this varies between tumor types. Since fetal bovine serum is rich in fetal angiogenic growth factors, could it possibly confound the predictive value of the assay? In fact, I too was somewhat amazed by the brisk angiogenic response you got for all of the tissues tested, be they normal, adenoma, invasive cancer tissues.
Second, regarding clinical utilization of the assay, it measures angiogenesis, not tumor response. Can the authors describe what clinically related developmental steps are required for this assay before it can be used to determine “tailor-made” therapies for individual patients? In other words, how does one validate angiogenesis and its inhibition in this assay as being predictive of overall tumor response?
Third, regarding the action of Epothilone B, the drug inhibited angiogenesis in placental vein fragments and also caused regression of developing vessels from certain tumors. However, as has been mentioned by the other discussants, developing vessels from aorta fragments were largely unaffected by the drug even in high dose. Again, can the authors speculate on why aortic explant angiogenesis was not susceptible to this drug? Will an understanding of the mechanism of resistance provide a means for minimizing the toxicity of some antiangiogenesis drugs, particularly their effect on normal angiogenic processes?
Dr. Eugene A. Woltering (New Orleans, LA): Dr. Thompson, you asked what is Epothilone B, how does it work, and what are the genetics? I can’t comment on the genetics, but this drug stabilizes the microtubules and thus you can’t pull the DNA and the chromosomes apart at metaphase and you get a G0 to M1 arrest.
How easy is this assay? It is probably as easy as bacterial culture. Fibrin and thrombin are inexpensive and readily available; 96-well plates are also inexpensive. The only potential large expense here is the cost of the labor it takes to read the plates. One of the things that we are trying to do is to make this a robotic high-throughput assay so that we can do this without having to laboriously read each plate. But the assay is simple and is reproducible. We have graders that have ranged from medical students to MD PhDs, and they all have very consistent scores.
What other tissues have we studied? We are just now embarking on trying to expand the number of tissues. We now have studied rectal cancers, prostate cancers, and the more common cancers. Because of the unique practice that I have, we began doing mostly endocrine tissue, and more common cancers followed.
Dr. Pinson and all the other reviewers asked me probably the most fascinating biologic question, and that is, why, when every tissue we have ever studied is sensitive to this drug, is the aorta totally resistant? The easy way out would be to say that I don’t know, because I really don’t. Clearly, one of the things we have done in this assay is to freeze tissue in its native state and then take the tissue-matched specimens that have become angiogenic and look at differences in gene expression. We have now found two different genes that are uniquely upregulated when a normal vein fragment tissue becomes angiogenic. We are now going back and looking at whether those same genes are upregulated in aortic tissue. Clearly there must be morphological, histologic, or genetic differences in the arterially derived angiogenic vessels versus the venous-derived neovessels. It is interesting that most tumors derive their angiogenic response from the venous side, not from the arterial side, an observation consistent with our work.
Dr. Pinson also brought up a very interesting question, which is, why is there a biphasic dose-response curve? Why does one concentration of drug inhibit angiogenesis while a thousand-fold or ten thousand-fold less drug appears to stimulate angiogenesis? I don’t know exactly why that is in this particular drug. But I can tell you that it is a characteristic of many antiangiogenics. We started out in this assay many years ago looking at the antiproliferative effects of the somatostatin analog octreotide and reported a similar biphasic dose curve.
Do I think that you could use very low doses of Epothilone or some of these other antiangiogenics as angiogenesis stimulators? Yes, potentially. But I think there are many more potent angiogenic stimulators, like VEGF, FGF, that you could use for this purpose.
Dr. Beenken asked about the specificity of using 20% fetal bovine serum in our assay. In work that we didn’t present today, we also tested sheep serum, horse serum, human serum, and autologous serum in the placental vein model. FBS was chosen because it is inexpensive, readily available, and you can get 20- to 30-liter lots which you can screen and use for a long time so that the lot doesn’t change across tumors.
Dr. Beenken also asked about the clinical tumor responses versus angiogenesis and our prediction. And Dr. Pinson asked a similar question about our speculation for the future. I agree with Dr. Pinson that we are speculating in our conclusions, that this assay may be a good way to predict on an individual patient’s tumor what agents we can use and what clinical doses need to be used.
We recently received permission from our IRB to do a prospective trial where patients will have their tumor tested at the time of surgery. We will then predict what agent/dose will work. In addition to standard adjuvant chemotherapy, we will administer the antiangiogenic agent in a randomized fashion. This is going to be a very long study but one I think needs to be done.
References
- 1.Hamburger AW, Salmon SE. Primary bioassay of human myeloma stem cells. J Clin Invest. 1977; 60: 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977; 197: 461–463. [DOI] [PubMed] [Google Scholar]
- 3.Kern DH, Weisenthal LM. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990; 82: 582–588. [DOI] [PubMed] [Google Scholar]
- 4.Costa SD, Lange S, Klinga K, et al. Factors influencing the prognostic role of oestrogen and progesterone receptor levels in breast cancer: results of the analysis of 670 patients with 11 years of follow-up. Eur J Cancer. 2002; 38: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins RA, Tesdale AL, Prescott RJ, et al. Outcome after extended follow-up in a prospective study of operable breast cancer: key factors and a prognostic index. Br J Cancer. 2002; 87: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990; 50: 4332–4337. [PubMed] [Google Scholar]
- 7.Marx D, Schauer A, Reiche C, et al. c-erbB2 expression in correlation to other biological parameters of breast cancer. J Cancer Res Clin Oncol. 1990; 116: 15–20. [DOI] [PubMed] [Google Scholar]
- 8.Bacus SS, Bacus JW, Slamon DJ, et al. HER-2/neu oncogene expression and DNA ploidy analysis in breast cancer. Arch Pathol Lab Med. 1990; 114: 164–169. [PubMed] [Google Scholar]
- 9.Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989; 7: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 10.Patel PC, Woltering EA, Maltese WA, et al. Squamous cell carcinoma-induced angiogenesis: a demonstration using cultured cells in the chick chorioallantoic membrane model. Surg Forum. 1996; 47: 786–789. [Google Scholar]
- 11.Woltering EA, Barrie R, O’Dorisio TM, et al. Somatostatin analogs inhibit angiogenesis in the chick chorioallantoic membrane. J Surg Res. 1991; 50: 245–251. [DOI] [PubMed] [Google Scholar]
- 12.Woltering EA, Watson JC, Alperin-Lea RC, et al. Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs. 1997; 15: 77–86. [DOI] [PubMed] [Google Scholar]
- 13.Nicosia R, Ottinetti A. Growth of sulfoxide-1 in serum-free matrix culture of rat aorta: a quantitative assay of angiogenesis in vitro. Lab Invest. 1990; 63: 115–22. [PubMed] [Google Scholar]
- 14.Gimbrone M, Ramzi R, Leapman S, et al. Tumor growth and neovascularization: an experimental model using rabbit cornea. J Natl Cancer Inst. 1974; 52: 413–426. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay D, Nagy JA, Manseau EJ, et al. Vascular permeability factor/vascular endothelial growth factor-mediated signaling in mouse mesentery vascular endothelium. Cancer Res. 1998; 58: 1278–1284. [PubMed] [Google Scholar]
- 16.Brown K, Maynes S, Bezos A, et al. A novel in vitro assay for human angiogenesis. Lab Invest. 1996; 75: 539–555. [PubMed] [Google Scholar]
- 17.Parish CR, Freeman C, Brown KJ, et al. Identification of oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999; 59: 3433–3441. [PubMed] [Google Scholar]
- 18.Watson JC, Balster DA, Gebhardt BM, et al. Growing vascular endothelial cells express somatostatin subtype 2 receptors. Br J Cancer. 2001; 85: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulec SA, Drouant GJ, Fuselier J, et al. Antitumor and antiangiogenic effects of somatostatin receptor targeted in situ radiation with 111In-DTPA-JIC 2DL. J Surg Res. 2001; 97: 131–137. [DOI] [PubMed] [Google Scholar]
- 20.Jung SP, Siegrist B, Wade MR, et al. Inhibition of human angiogenesis with heparin and hydrocortisone. Angiogenesis. 2001; 4: 175–186. [DOI] [PubMed] [Google Scholar]
- 21.Kvols LK, Reubi JC. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Acta Oncol. 1993; 32: 197–201. [DOI] [PubMed] [Google Scholar]
- 22.Hood KA, West LM, Rouwe B, et al. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule-stabilizing activity. Cancer Res. 2002; 62: 3356–3360. [PubMed] [Google Scholar]
- 23.Watson JC, Redmann JG, Meyers MO, et al. Breast cancer increases initiation of angiogenesis without accelerating neovessel growth rate. Surgery. 1997; 122: 509–514. [DOI] [PubMed] [Google Scholar]
- 24.Von Hoff DD, Clark GM, Stogdill BJ, et al. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983; 43: 1926–1931. [PubMed] [Google Scholar]
- 25.Pommier RF, Woltering EA, Milo G, et al. Synergistic cytotoxicity between dimethyl sulfoxide and antineoplastics against ovarian cancer in vitro. Surg Forum. 1987; 38: 475–477. [DOI] [PubMed] [Google Scholar]
- 26.Pommier RF, Woltering EA, Milo G, et al. Cytotoxicity of dimethyl sulfoxide and antineoplastic combinations against human tumors. Am J Surg. 1988; 155: 672–676. [DOI] [PubMed] [Google Scholar]
- 27.Pommier RF, Woltering EA, Fletcher WS, et al. Synergistic cytotoxicity of dimethyl sulfoxide-antineoplastic combinations against P388 leukemia in CD-F1 mice. Surg Forum. 1988; 39: 471–473. [Google Scholar]
- 28.Pommier RF, Woltering EA, Milo G, et al. Synergistic cytotoxicity of combinations of dimethyl sulfoxide and antineoplastic agents against P388 leukemia in CD-F1 mice. Anticancer Drugs. 1993; 3: 635–639. [DOI] [PubMed] [Google Scholar]
- 29.Bradley EC, Reichert CM, Brennan MF, et al. Direct cloning of human parathyroid hyperplasia cells in soft agar culture. Cancer Res. 1980; 40: 3694–3696. [PubMed] [Google Scholar]
- 30.Kruger EA, Duray PH, Tsokos MG, et al. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun. 2000; 268: 183–191. [DOI] [PubMed] [Google Scholar]
- 31.Meyers MO, Gagliardi AR, Flattman GJ, et al. Suramin analogs inhibit human angiogenesis in vitro. J Surg Res. 2000; 91: 130–134. [DOI] [PubMed] [Google Scholar]
- 32.Jung SP, Siegrist B, Hornick CA, et al. Effect of human recombinant endostatin protein on human angiogenesis. Angiogenesis. 2002; 5: 111–118. [DOI] [PubMed] [Google Scholar]
- 33.Chou TC, Zhang XG, Harris CR, et al. Desoxyepothilone B is curative against human tumor xenografts that are refractory to paclitaxel. Proc Natl Acad Sci USA. 1998; 95: 15798–15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau DH, Xue L, Young LJ, et al. Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm. 1999; 14: 31–36. [DOI] [PubMed] [Google Scholar]
- 35.Sindermann JR, Skaletz-Rorowski A, Bartels A, et al. Paclitaxel and cyclosporine A show supra-additive antiproliferative effects on smooth muscle cells by activation of protein kinase C. Basic Res Cardiol. 2002; 97: 125–131. [DOI] [PubMed] [Google Scholar]
- 36.Celletti FL, Waugh JM, Amabile PG, et al. Inhibition of vascular endothelial growth factor-mediated neointima progression with angiostatin or paclitaxel. J Vasc Interv Radiol. 2002; 13: 703–707. [DOI] [PubMed] [Google Scholar]
- 37.Taraboletti G, Micheletti G, Rieppi M, et al. Antiangiogenic and antitumor activity of IDN 5390, a new taxanes derivative. Clin Cancer Res. 2002; 8: 1182–1188. [PubMed] [Google Scholar]
- 38.Xu G, Pan J, Martin C, et al. Angiogenesis inhibition in the in vivo antineoplastic effect of manumycin and paclitaxel against anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2001; 86: 1769–1777. [DOI] [PubMed] [Google Scholar]
- 39.Ansell SM, Pitot HC, Burch PA, et al. A phase II study of high-dose paclitaxel in patients with advanced neuroendocrine tumors. Cancer. 2001; 91: 1543–1548. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Supported in part by Novartis Pharmaceutical Corporation, East Hanover, New Jersey.
Correspondence: Eugene A. Woltering, MD, FACS, Section of Surgical Endocrinology, LSUHSC Department of Surgery, 1542 Tulane Avenue, New Orleans, LA 70112.
E-mail: ewolte@lsuhsc.edu
Accepted for publication December 2002.