Abstract
Objective
To evaluate the preoperative relationships of hypertension and diabetes mellitus in severe obesity and the effects of gastric bypass (GBP)-induced weight loss.
Summary Background Data
Severe obesity is associated with multiple comorbidities, particularly hypertension and type 2 diabetes mellitus, that may affect life expectancy.
Methods
The database of patients who had undergone GBP by one general surgeon at a university hospital between September 1981 and January 2000 was queried as to weight, body mass index (BMI), pre- and postoperative diabetes, hypertension, and other comorbidities, including sleep apnea, hypoventilation, gastroesophageal reflux, degenerative joint disease, urinary incontinence, venous stasis, and pseudotumor cerebri.
Results
Of 1,025 patients treated, 15% had type 2 diabetes mellitus and 51% had hypertension. Of those with diabetes, 75% also had hypertension. There was a progressive increase in age between patients who had neither diabetes nor hypertension, either diabetes or hypertension, or both diabetes and hypertension. At 1 year after GBP (91% follow-up), patients lost 66 ± 18% excess weight (%EWL) or 35 ± 9% of their initial weight (%WL). Hypertension resolved in 69% and diabetes in 83%. Patients who resolved their hypertension or diabetes had greater %EWL and %WL than those who did not. African-American patients had a higher risk of hypertension than whites before GBP and were less likely to correct their hypertension after GBP. There was significant resolution of other obesity comorbidity problems. At 5 to 7 years after GBP (50% follow-up), %EWL was 59 ± 24 and %WL was 31 ± 13; resolution of hypertension was 66% and diabetes 86%.
Conclusions
These data suggest that type 2 diabetes mellitus and hypertension may be indirectly related to each other through the effects of obesity, but not directly as to cause and effect. The longer a person remains severely obese, the more likely he or she is to develop diabetes, hypertension, or both. GBP-induced weight loss is effective in correcting diabetes, hypertension, and other comorbidities but is related to the %EWL achieved. Severely obese African-Americans were more likely to have hypertension and respond less well to GBP surgery than whites. These data suggest that GBP surgery for severe obesity should be provided earlier to patients to prevent the development of diabetes and hypertension and their complications.
Previous studies have evaluated the efficacy of gastric bypass (GBP) surgery for the treatment of severe obesity on systemic hypertension 1,2 and type 2 diabetes mellitus 3–7 separately. This retrospective study was designed to evaluate the relationships of diabetes and hypertension with each other in a cohort of severely obese patients before and after GBP, as well as the effects of age, race, gender, and percent excess weight loss (%EWL), as well as the relationships to other obesity comorbidity problems.
METHODS
We queried our database of primary GBP procedures performed by one surgeon (H.J.S.) between September 1981 and January 1999 to determine the prevalence of diabetes and hypertension as well as other comorbidities in severely obese patients before GBP. Institutional review board approval was obtained for collecting the data in a secure database and reporting on its analyses. Major efforts were made to contact patients who had not returned for follow-up visits. Patients were considered eligible for surgery for obesity according to the 1991 NIH Consensus Conference guidelines 8 if their body mass index (BMI, kg/m2) was at least 35 kg/m2 and associated with obesity comorbidity, or at least 40 kg/m2 in the absence of comorbidity. Before 1991 all patients had met the NIH criteria. Because randomized, prospective, and selective trials found GBP to be significantly more effective than vertical banded gastroplasty, we exclusively performed GBP after 1985. 9,10 After 1991 “super-obese” patients with a BMI of at least 50 kg/m2 underwent a “long-limb GBP” with a 150-cm Roux limb. 11 Follow-up consisted of postoperative clinic visits with the operating surgeon.
The diagnosis of diabetes required an elevated fasting blood sugar (≥150 mg/dL) and a “diabetic diet” recommended by their primary care physician, oral hypoglycemic medications, or insulin treatment. The diagnosis of hypertension required a sitting blood pressure at the time of their initial visit of at least 150 mmHg systolic and/or at least 90 mmHg diastolic (using a wide blood-pressure cuff taken with an automatic sphygmomanometer) or use of antihypertensive medications. Age, race, gender, and obesity comorbidities were recorded. Data were evaluated at 1 to 2, 5 to 7, and 10 to 12 years after surgery and included kg weight lost, % weight lost (%WL), and %EWL, with excess weight defined as weight in kg above ideal body weight measured according to the Metropolitan Life Insurance Tables for medium frame. 12 Resolution of diabetes was defined as a fasting blood sugar of 120 mg/dL or less and absence of diabetic medications, while resolution of hypertension required no use of antihypertensive medications, a systolic blood pressure of 135 mmHg or less and a diastolic blood pressure of 85 mmHg or less. Data on mortality was assessed from the National Death Index.
Obesity hypoventilation was defined as a PaO2 of 55 torr or less and/or a PaCO2 of at least 65 mmHg, sleep apnea as a respiratory disturbance index of at least 10 hypopneic and/or apneic episodes per hour of sleep, and degenerative joint disease as self-reported complaints of pain in the weight-bearing joints. Gastroesophageal reflux was defined as “heartburn.” Pseudotumor cerebri was documented by an elevated cerebrospinal fluid pressure of at least 200 cm H2O with a normal cerebral MRI or CT scan except a dilated sella turcica. Urinary incontinence was defined as a history of difficulty controlling the urine.
Data are expressed as mean ± standard deviation. Frequency data were analyzed using the Fisher exact test; means were analyzed using ANOVA; logistic regression was used to investigate the relationship between diabetes and hypertension and age, race, gender, and initial BMI.
RESULTS
Of 1,025 patients who had GBP, 70% had a standard GBP and 30% a long-limb GBP; 15% had diabetes and 51% hypertension (Table 1). The average age was 39 ± 10 years (range 12–69), 75% were white and 25% were African-American, and 78% were women. Of those patients with diabetes, 75% also had hypertension (P < .001). Of the patients with hypertension, 79% did not have diabetes. Of those with diabetes, 26% were “diet-controlled,” 35% required oral agents, and 39% required insulin. Hypertension was present in 66% of the diabetic patients who were “diet-controlled”, 74% of those on oral agents, and 79% of those taking insulin. Patients with diabetes were older than those without, and patients with hypertension were older than those without. Patients with both hypertension and diabetes were significantly older than those with either hypertension or diabetes alone (Table 2). There were no significant differences in BMI in patients without diabetes or hypertension, with hypertension but without diabetes, with diabetes but without hypertension, and with both hypertension and diabetes.
Table 1. FREQUENCY OF TYPE 2 DIABETES MELLITUS AND SYSTEMIC HYPERTENSION BEFORE GASTRIC BYPASS FOR SEVERE OBESITY
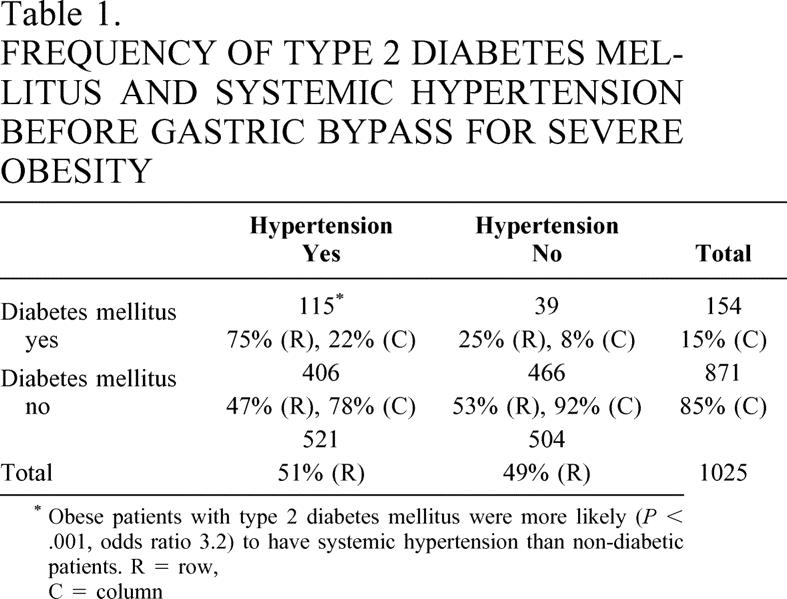
* Obese patients with type 2 diabetes mellitus were more likely (P < .001, odds ratio 3.2) to have systemic hypertension than non-diabetic patients. R = row, C = column
Table 2. AGE (YEARS) OF PATIENTS WITH DIABETES OR HYPERTENSION
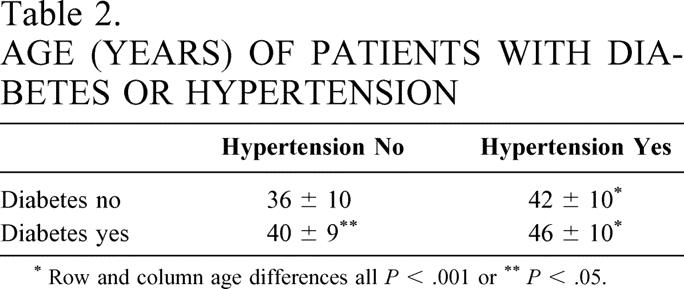
* Row and column age differences all P < .001 or
**P < .05.
African-American patients weighed more than whites (147 ± 31 vs. 138 ± 29 kg, BMI 54 ± 10 vs. 49 ± 10 kg/m2, P < .0001) and were more likely to have hypertension than whites (60% vs. 48%, P = .002). The frequency of diabetes was not different between ethnic groups. Men were also more likely to have hypertension independent of diabetes than women (64% vs. 47%, P < .0001). With logistic regression analysis, significant relationships existed for age, diabetes, gender, BMI, and race relevant to hypertension (P = .002), whereas for diabetes only hypertension and age were significant.
A number of other obesity comorbidities were seen, several of which have an interrelationship with blood pressure. These included obstructive sleep apnea syndrome in 27% (men 61% vs. women 18%, P < .0001), obesity hypoventilation syndrome 8% (men 18% vs. women 6%, P < .0001), pseudotumor cerebri 3% (women 3% vs. men 0.5%, P < .05), urinary incontinence 24% (women 30% vs. men 4%, P < .0001), and chronic venous stasis disease 5% (men 12% vs. women 3%, P < .0001). Gastroesophageal reflux and degenerative joint disease symptoms were observed in 32% and 67% of these patients, respectively. There was a greater risk of venous stasis, obesity hypoventilation, sleep apnea, and degenerative joint disease in patients with hypertension and a greater risk of venous stasis disease, sleep apnea, and degenerative joint disease in patients with diabetes.
There were nine deaths (0.9%) within 30 days of surgery, two from known pulmonary embolism, two from unknown causes, and five from postoperative septic complications. There were 74 late deaths, 15 within the first year of surgery. The majority of late deaths were from myocardial infarction, but other causes included arrhythmia, stroke, homicide, suicide, cancer, and motor vehicle accidents. One late death was from a complication of GBP (small bowel infarction from an incarcerated internal hernia).
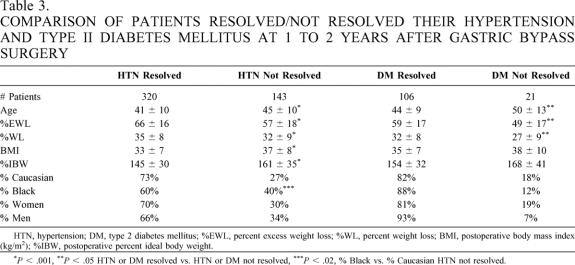
One to 2 years after GBP (91% follow-up), %EWL was 66 ± 18 and %WL was 35 ± 9. BMI decreased from 51 ± 10 to 33 ± 7 kg/m2 (P < .001), with a 50 ± 17-kg weight loss. Hypertension and diabetes resolved in 69% and 83%, respectively. Oral hypoglycemic medications were required in 4 (9%) but in only 1 of the 44 (2%) previously using them; 9 of 46 (20%) still required insulin. Patients who resolved their hypertension or diabetes had greater %EWL and were typically younger and white (Table 3). Overall, African-American patients had a smaller %EWL than whites (58 ± 16 vs. 69 ± 18, P < .0001). GBP resulted in significant improvements in a number of the other obesity comorbidity problems: urinary incontinence decreased from 23% to 1.7%, gastroesophageal reflux symptoms from 32% to 2.6%, venous stasis from 5% to 0.2%, pseudotumor cerebri from 3% to 0.2%, obesity hypoventilation from 9% to 0.2%, and degenerative joint disease symptoms 18%. Most patients no longer had symptoms of sleep apnea and were no longer using nasal CPAP; all tracheostomies were allowed to close.
Table 3. COMPARISON OF PATIENTS RESOLVED/NOT RESOLVED THEIR HYPERTENSION AND TYPE II DIABETES MELLITUS AT 1 TO 2 YEARS AFTER GASTRIC BYPASS SURGERY
HTN, hypertension; DM, type 2 diabetes mellitus; %EWL, percent excess weight loss; %WL, percent weight loss; BMI, postoperative body mass index (kg/m2); %IBW, postoperative percent ideal body weight.
*P < .001,
**P < .05 HTN or DM resolved vs. HTN or DM not resolved,
***P < .02, % Black vs. % Caucasian HTN not resolved.
Complications following surgery included anastomotic leaks (3%), severe wound infections (6%), minor wound infections (8%), marginal ulcers (9%; 5 [1%] required surgical correction), pulmonary embolism (1%), small bowel obstructions (4%), and anastomotic stenoses (15%, all but one treated with endoscopic dilatation, one required surgical revision).
At 5 to 7 years after GBP (50% [342/684] follow-up), average %EWL was 59 ± 24, %WL 31 ± 13, ideal body weight 153 ± 38%, and BMI 35 ± 9 kg/m2. Hypertension resolved in 66%, diabetes in 86% (in those available for assessment). None of the 21 diabetic patients who required an oral hypoglycemic agent before GBP was using them. Of the 21 patients requiring insulin before their GBP, only 3 were still using insulin and 3 a hypoglycemic agent. There was no difference in the average weight regain at 5 to 7 years between the patients whose hypertension remained controlled (13 ± 11 kg) compared to those who required medication for the treatment of hypertension (14 ± 11 kg).
At 10 to 12 years, follow-up decreased to 37% (135/361). %EWL was 52 ± 25, %WL 28 ± 13, and BMI had decreased from 50 ± 10 to 36 ± 9 kg/m2 (P < .001). Of the 18 patients with diabetes before surgery (7 had required insulin), 1 was using an oral hypoglycemic agent and none was using insulin. Of the patients with preoperative hypertension, 51% were no longer hypertensive.
DISCUSSION
The data in this study are what one would predict: the longer an individual is severely obese, the more likely he or she is to develop hypertension and type 2 diabetes, and the combination of hypertension and diabetes is more frequent the older the obese patient. As in other studies, 13,14 African-American patients and men were at a greater risk for hypertension but not diabetes with severe obesity. As in our previous studies, severely obese men were more likely to suffer from obstructive sleep apnea syndrome, 16 obesity hypoventilation syndrome, 15,16 and venous stasis disease, 17 and women from urinary incontinence 18 and pseudotumor cerebri. 19
As in other studies, both hypertension and diabetes improved in the vast majority of patients at 1 to 2 years following GBP-induced weight loss. 1,2 The positive consequences of such weight loss cannot be minimized. One study noted a significant decrease in mortality in a group of severely obese diabetic patients having undergone GBP compared to a control group of morbidly obese patients who had not undergone surgery due to personal preference or refusal of insurance coverage. 6
The data in this study support previous studies documenting a marked reduction in other obesity comorbidity problems, including sleep apnea, 16,20,21 obesity hypoventilation, 16,22 venous stasis, 17 urinary incontinence, 18 pseudotumor cerebri, 19,23 and heartburn. 24,25 Although as little as a 5% to 10% reduction in weight may be very beneficial to obese patients, 26 patients in this study were more likely to resolve their metabolic and hypertensive abnormalities if they lost a greater percentage of their excess weight. Similar results were noted in the previous studies of the effects of GBP on systemic hypertension. 1,2
The largest prospective trial to date is the Swedish Obesity Subjects (SOS) study. Ten thousand Swedes have been entered in this study. One thousand of them have undergone bariatric surgery and are being compared to age-, sex-, weight-, and comorbidity-matched patients treated by conventional means. 27 Most operated patients in the SOS study underwent a gastroplasty or gastric banding procedure; 6% underwent GBP. Randomized, prospective as well as retrospective trials have shown that these operations produce significantly less weight loss than does GBP. 9,10,28–31 The SOS study also noted a significantly greater weight loss in their GBP patients. 32 The SOS found a significant improvement at 2 years in the quality of life and the use of sick leave and disability pension. 33,34 In a recent report hypertension resolved in only 42% of SOS patients, 35 in contrast to 70% in the present report and 65% to 75% of patients in other reports. 1,2 Diabetes resolved in only 47% of patients, in contrast to 84% in the current report and a similar percentage in other series. 3–7 Furthermore, the SOS found a complete relapse in hypertension in their surgical patients over 8 years, despite a maintained weight loss. 32 In a subsequent report from the SOS, there were still significant decreases in both systolic and diastolic blood pressure in the small (6%) subset of patients who had GBP and lost more weight than the patients who had gastroplasty or gastric banding. 36 The greater weight loss with GBP may be due to a significant decrease in the gastric hormone ghrelin, which increases food intake. 37 This increased weight loss would seem to provide a plausible explanation for the improved control of hypertension and diabetes with GBP. We have previously noted greater decreases in blood glucose and serum insulin levels following GBP compared to vertical banded gastroplasty, suggesting that gastrointestinal peptide secretion might be responsible for improved control of diabetes following GBP. 38 A similar hypothesis has been proposed by Pories et al. 5
Interpretation of our 5-to-7-year and 10-to-12-year data is complicated by the loss of patients to follow-up. In our prior report, with enormous effort, when we were able to achieve 80% follow-up at 5 years, the GBP patients had lost 60% EWL. 39 The East Carolina University group, with 95% follow-up, had a similar %EWL at 5 years, 50%EWL at 10 years, and 47% at 14 years after GBP. 3–7 However, in consideration of a 50% follow-up in the current study, the improvement in diabetes and hypertension appeared to hold at 5 to 7 years after surgery. Diabetes was still improved at 10 to 12 years, but there was a fall-off in the control of hypertension from 66% to 51%, with a further decrease in follow-up to 37%.
The data in our study suggest that the earlier severely obese patients have surgery, the less likely they are to develop hypertension and diabetes and presumably their associated complications. Peripheral neuropathy, nephropathy, and retinopathy are significant complications of prolonged diabetes. 40 As with diabetes, the longer that hypertension persists, the more permanent the accompanying functional and structural changes. The same is probably true for the other obesity comorbidities, such as venous stasis disease, obesity hypoventilation syndrome, and degenerative joint disease. The currently available medications for the treatment of obesity, such as sibutramine and orlistat, are associated with only a 10% weight loss in responding patients, 41,42 and sibutramine may be associated with statistically significant increases in blood pressure and pulse rate. 43 The data in this study suggest that current pharmacologic therapy would not be an adequate or sustainable method for the effective control of hypertension and diabetes in the majority of these severely obese patients. Bariatric surgery is the most effective management for the hypertensive, diabetic severely obese patient if remission of either of these diseases is sought.
Although there was a significant association between diabetes and hypertension, one fourth of the diabetic patients did not have hypertension, and 47% of the hypertensive patients were not diabetic. It has been proposed that the metabolic syndrome of central, or android, obesity leads to the development of hypertension through the effects of insulin-induced sodium reabsorption and increased catecholamine sensitivity. 44–50 However, it is possible that insulin resistance and hypertension simply coexist in centrally obese subjects without a specific causal relationship. In one study, hypertension in severely obese patients was not associated with hyperinsulinemia, and they had a decreased renal blood flow, glomerular filtration rate, and proteinuria. 51 A number of other studies have also questioned the relationship between insulin and hypertension. 52–57
As an alternative to the insulin hypothesis, we have postulated that increased intra-abdominal pressure, as seen in central obesity, is the cause of systemic hypertension through one, or a combination, of three mechanisms. 58 These include increased renal venous pressure leading to activation of the renin-angiotensin-aldosterone system, with resultant glomerular capillary injury and proteinuria; direct renal compression with activation of the renin-angiotensin-aldosterone system; and/or an increased intrathoracic pressure leading to a decreased venous return and cardiac output with further activation of the renin-angiotensin-aldosterone system. The net effect of these physiologic perturbations is sodium and water retention, increased blood pressure, and the development of proteinuria, as seen in severely obese patients. 51 In an acute porcine model of increased intra-abdominal pressure, intrathoracic pressure rises and cardiac output decreases, necessitating volume resuscitation, 59 with an increased plasma renin activity and serum aldosterone and an increased arterial pressure and systemic vascular resistance. 60 Renal vein constriction to 30 mmHg was associated with an increased plasma renin activity and aldosterone, decreased renal blood flow and glomerular filtration rate and proteinuria. 61 In a chronic canine model a progressive increase in intra-abdominal pressure to 25 mmHg with inflation of an intra-abdominal balloon at 5 weeks was associated with a marked increase in both systolic and diastolic pressure, which decreased following release of the intraperitoneal balloon. 62
In summary, this report has found that hypertension and diabetes are associated with age in severely obese patients, consistent with the conclusion that the longer a person is obese, the more likely he or she is to develop these complications of obesity, and that GBP is an effective treatment for most patients with hypertension or diabetes. African-American patients are more likely to be hypertensive than whites and are less likely to respond to obesity surgery-induced weight loss. The risk of major complications was not significantly increased in the diabetic patients. This study also suggests that diabetes and hypertension are merely co-conspirators in the metabolic syndrome of obesity and may be secondary to different mechanisms. Prior studies suggest that hypertension in the centrally obese may be a result of increased intra-abdominal pressure and activation of the renin-angiotensin-aldosterone system.
Discussion
Dr. John J. Gleysteen (Birmingham, AL): This has been an excellent presentation of retrospective collated data that really makes the point for gastric bypass surgery. We have morbid obesity on one hand and morbid obesity with complications on the other. I think you all may know that in many situations, in order to do this surgery you have to get insurance pre-approval in order to carry it out. So let me share a small vignette with you in order to ask a question. A few years ago, a 33-year-old Alabama farmer came to see me. He was 5-foot-9 tall; he weighed 426 pounds. That is a body mass index of about 63. He was married, with two children. He considered himself active; in fact, he had to be active to deal with all the farm machinery and tasks. He did not have hypertension. He did not have diabetes. And he probably did not have sleep apnea. He desired an operation. I wrote our Alabama Blue Cross agency. And perhaps I made an error, but I pointed out the chronicity and the magnitude of his morbid obesity, his absence of hypertension and diabetes, and said that we shouldn’t need to wait for these comorbidities to appear in order to justify this surgical venture. The response back was, yes, we should, Blue Cross would be happy to cover this man’s gastric bypass hospitalization after there was evidence of disease and complication as a result of his morbid obesity. Now, Dr. Sugerman, you make the point that these diseases clear after surgery and weight loss, and clear more readily when they are present only a short time. So what should be our attitude for these individuals that are massively obese before their diabetes or their hypertension or their sleep apnea have yet appeared?
Dr. J. Patrick O’leary (New Orleans, LA): I would like to rise to compliment Dr. Sugerman and to tell you that the last time I rose to comment on his paper, one of the authors’ names had been left off. I checked with Betsy Sugerman this morning and I am happy to know that she actually didn’t contribute to this paper, so her name was left off on purpose today, not just to make the home scene a little more difficult. Bariatric surgery has gone from rogue to vogue, and it has been in part because of the studies that Dr. Sugerman and the group at Richmond have accomplished. This study, however, leaves something to be desired, in my opinion. Firstly, the diagnosis of diabetes mellitus based on a history or based on whether or not some practitioner had placed the patient on a diet is problematic for me. For this study to have real merit you would have to know what the glucose tolerance or intolerance was. Therefore, an IV glucose tolerance test or a PO glucose tolerance test would have been appropriate. I wonder, Dr. Sugerman, did you have those studies done?
The second thing is that this is a hodgepodge of patients. And they have diabetes, but it may not be the same diabetes in each patient. It could be that they have a decrease in insulin, but it may also be insulin resistance. And insulin resistance has been shown in a publication in Science magazine to be in part related to what the intake is. And I think that the diabetes in seriously obese individuals is in part related to intake. So I would like to know if you looked at that as a part of your evaluation. We published an article in ’76 from the Forum that showed that malabsorptive also produced amelioration of the diabetes in patients, both PO and IV glucose tolerance testing in those patients.
Finally, Dr. Sugerman, the real question I have for you is, if diabetes improved, did hypertension improve? In other words, in that cohort of patients that you followed out to 5 to 7 years, was there a relationship in the improvement of the diabetes with the improvement in the hypertension?
Dr. Ward O. Griffen, Jr. (Frankfort, MI): Now that gastric restrictive operations have become nationally famous—as you know, the weatherman on The Today Show and the Brooklyn, New York, Congressman have both had gastric restrictive operations—I think we need to take bariatric surgery as a very serious undertaking. I would like to congratulate Dr. Sugerman and his coworkers on this paper. And even though I agree to some extent with what Dr. O’Leary said, I still think it is significant that it has been so successful. I compliment him also on his 91% follow-up for 1 to 2 years. It is unfortunate that his follow-up at 5 to 7 and 10 to 12 years was so low, particularly the 10 to 12 years, which was only about 35% as I recall, because that makes the data less meaningful. I am not surprised at the essential message of this study. Basically it says that the longer obesity exists, the more likely you are to have diabetes or hypertension and/or both, and the higher percentage of weight loss produced by the gastric restrictive operation, the more likely it is to ameliorate both comorbidities. This is brought out very well in the manuscript, which I received this morning to review. The obvious implications of these data are that the earlier the weight loss is achieved, the less likely the comorbidity entities will occur, and, therefore, we should consider doing this operation at an earlier time.
However, I have two concerns. One is that, as Dr. Sugerman and most serious bariatric surgeons are aware, there are many less-committed surgeons who enter the field primarily for its remunerative potential. By suggesting that we do earlier operations on patients with less obesity, are we perhaps increasing the clinical pool for these particular surgeons? Secondly, in view of the 1992 NIH study, which clearly established the levels of the BMI to be met for both patients with and without comorbidities and the suggestion that we do earlier operations on patients who perhaps don’t meet these standards, who is going to pay for it? That is sort of the same question that Dr. Gleysteen asked.
Dr. Walter J. Pories (Greenville, NC): I am not surprised by the excellence of Dr. Sugerman’s paper. He can be counted on to bring us innovative ideas and always does. I was intrigued, however, by his finding that African-American patients had a higher risk of hypertension than Caucasians prior to the gastric bypass and were less likely to correct their hypertension after gastric bypass. At East Carolina University we have also observed major differences in the response to the gastric bypass between the races. African-Americans lose less weight after the operation than Caucasians. This ethnic difference in response to bariatric surgery led Dr. Hisham Barakat of our group to investigate the mobilization of fat from adipose stores as reflected by the activity of the hormone sensitive lipase, HSL. HSL, as you recall, catalyzes the rate-limiting step of lipolysis by hydrolyzing the stored triglycerides and diglycerides into fatty acid and glycerol. The differences were startling. HSL mass was approximately 35% lower in both subcutaneous and omental adipose tissue of African-Americans. In addition, basal lipolytic rates in African-Americans were 53% and 44% lower in the subcutaneous and omental fat, respectively, compared to Caucasian women, despite a lack of difference in cell size between the two groups, suggesting that the signaling pathway of HSL stimulation is more efficient in African-American women. In summary, there are major differences in both fat storage and mobilization. I congratulate you on your noting the differences among individuals and the recognition that we need to be aware of these variations as we select and advise our patients. My question is, have you noted any other differences in the outcomes of the gastric bypass between African-Americans and Caucasians and, if so, how has this affected your practice?
Dr. Kenneth G. Macdonald (Greenville, NC): Congratulations on a nicely and cleanly presented paper. We found almost identical results at East Carolina, where 82.9% of our patients became euglycemic after gastric bypass. Sort of a difference is what happened to the 17%, though, that did not. Of those, about one third were considered weight-loss failures. But the other two thirds of those that didn’t respond had similar weight loss than those that did. When we looked at these differences in who responded and who didn’t, we found that the group that did not become euglycemic were on an average of 7 years older and had had diabetes for 3 years longer. So we hypothesized then that with advancing age and duration of diabetes, there is some sort of structural or functional change in the islet cell which basically renders you much like a type 1 diabetic that can’t secrete the elevated insulin levels required. My question is, did you notice any independent correlation between age and duration of the diabetes, whether or not the diabetes resolved? Or was the amount of weight loss clearly the primary determining factor? Also, as 30% of your patients had a long limb gastric bypass, did your data show an increase in percent excess weight loss in these individuals and a corresponding increase in the resolution of their diabetes compared with a standard limb?
Dr. William C. Lineaweaver (Jackson, MS): A real number of these patients come to secondary plastic surgical operations 1 or 2 or 3 years out. And by the time they have had their arms and breasts and abdomens and thighs and various liposuctions done, the add-up of surgical specimens is sometimes another 5, 10, or even 15 kg. Has this group looked at those secondary surgeries and the overall impact of those surgeries on the final weight loss and the systemic illnesses that they studied?
Dr. Harvey J. Sugerman (Richmond, VA): In response to Dr. Gleysteen, and also to Dr. Pories and Dr. MacDonald, the concern is our data did not include the duration of diabetes prior to their gastric bypass. But as Dr. MacDonald pointed out, both he and Dr. Pories in their papers have shown that the longer you have diabetes, the less likely you are to respond to gastric bypass-induced weight loss. The other information that we didn’t have in our database, which I wish we had had, was the presence or absence of secondary diabetes-related complications such as neuropathy or retinopathy. What is really upsetting is getting a number of these patients with these secondary complications (we had one who had a toe amputation) where we think if we could have gotten these patients earlier, we could have prevented the development of these secondary complications of diabetes. We have also had the experience with insurance coverage that Dr. Gleysteen mentioned, although it appears that there is progressively more support for bariatric surgery from both insurance companies and primary care physicians.
I have to agree with Dr. O’Leary that our diagnosis of diabetes is certainly less than ideal, especially with the issue regarding dietary-managed patients. We would like to have had, but didn’t have over the last 25 years, hemoglobin A1c levels, and we certainly didn’t have glucose tolerance tests on a thousand patients. We did have that information in a selected, small group of patients when we presented our data to the Southern Surgical Association about 15 years ago looking specifically at the areas under the curve for insulin and glucose, comparing gastroplasty to gastric bypass. This was a large database looking at perhaps superficial aspects, but in our opinion very important aspects, of these patients. We also didn’t have information with regards to whether or not they were insulin-resistant. We had data regarding oral intake but didn’t analyze them for this report. We perhaps should have.
Were hypertension and diabetes correlated in terms of being improved together? The answer is yes. And again, it was related to percent excess weight loss. In both groups with diabetes and hypertension, the more you lost, the more likely you were to reverse both of those comorbidities.
Dr. Griffen, for one who has really been the pioneer for the development of gastric bypass, I have to take issue with the fact that Al Roker had a gastric restrictive operation. He had a gastric bypass, which is somewhat both restrictive and malabsorptive. We think there is a major difference between gastric banding, gastroplasty, and gastric bypass. So Carnie Wilson and Al Roker and the congressman who had a duodenal switch operation, these are certainly not gastroplasty operations.
I certainly agree with you that our follow-up at 10 to 12 years is very poor. And I wish it could be better. But we do back flips to get these patients back for follow-up. They are living all over the United States. The only thing that made me feel a little bit better was in the Swedish Obesity Trial, where you would think that they would have spectacular follow-up in Sweden, they also only had 47% follow-up in one of their reports on their operations in Sweden at 10 years. We try hard, but it is really, really difficult to follow patients long term.
I don’t think that we are going to be doing operations in patients with criteria less than the NIH Consensus Conference recommended in 1991 unless the patient is going to come to some surgeon with cash in pocket. And we certainly won’t accept that patient. The NIH actually wanted to do a study in diabetics between BMIs of 30 and 35 kg/m2. The only minor issue was, they weren’t willing to pay for it. And I asked them, “Well, how are we going to get the study? Because the NIH Consensus did not support bariatric surgery for a BMI less than 35, so that insurance will not pay for it.” So we couldn’t do that trial. Actually, I think there are very few patients who would fit into that category.
Dr. Pories, I think the HSL data with regards to African-American patients are certainly interesting. We did not find any other significant correlations with any other comorbidities. Of interest, when we presented to this Association 2 years ago our lap-band data, a gastroplasty-type procedure, African-American patients lost only 11% of their excess weight. So although the weight loss after gastric bypass wasn’t as good as the Caucasians, it is clearly much better than a purely gastric restrictive operation such as a vertical banded gastroplasty or a lap-band.
And finally, no, we did not look at the effects of plastic surgery on these patients in terms of the various comorbidities that we discussed today.
References
- 1.Foley EF, Benotti PN, Borlase BC, Hollingshead J, Blackburn GL. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg. 1992; 163: 294–297. [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Ruddy ME, Duff AE, et al. The effect of gastric bypass surgery on hypertension in morbidly obese patients. Ann Intern Med. 1994; 154: 193–200. [PubMed] [Google Scholar]
- 3.Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg. 1992; 215: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992; 55: 582S–585S. [DOI] [PubMed] [Google Scholar]
- 5.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995; 222: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald KG Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997; 1: 213–220. [DOI] [PubMed] [Google Scholar]
- 7.Hickey MS, Pories WJ, MacDonald KG Jr, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998; 227: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIH Conference. Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Consensus Development Conference, 30 March to 1 April 1992. Ann Intern Med. 1993; 119: 764–770. [PubMed] [Google Scholar]
- 9.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987; 205: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989; 157: 93–100. [DOI] [PubMed] [Google Scholar]
- 11.Brolin RE, Kenler HA, Gorman JH, et al. Long-limb gastric bypass in the superobese. A prospective randomized study. Ann Surg. 1992; 215: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metropolitan Life Insurance Co. New weight standards for men and women. Statistical Bulletin of the Metropolitan Life Insurance Company. 1959; 40: 1–4. [Google Scholar]
- 13.Burt VL, Culter JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995; 26: 60–69. [DOI] [PubMed] [Google Scholar]
- 14.Cooper RS, Liao Y, Rotimi C. Is hypertension more severe among U.S. blacks, or is severe hypertension more common? Ann Epidemiol. 1996; 6: 173–180. [DOI] [PubMed] [Google Scholar]
- 15.Sugerman HJ, Fairman RP, Baron PL, et al. Gastric surgery for respiratory insufficiency of obesity. Chest. 1986; 89: 81–86. [DOI] [PubMed] [Google Scholar]
- 16.Sugerman HJ, Fairman RP, Sood RK, et al. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr. 1992; 55: 597S–601S. [DOI] [PubMed] [Google Scholar]
- 17.Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks/benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001; 234: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bump RC, Sugerman HJ, Fantl JA, et al. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992; 167: 392–399. [DOI] [PubMed] [Google Scholar]
- 19.Sugerman HJ, Felton WL III, Sismanis A, et al. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999; 229: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charuzi I, Ovnat A, Peiser J, et al. The effect of surgical weight reduction on sleep quality in obesity-related sleep apnea syndrome. Surgery. 1985; 97: 535–538. [PubMed] [Google Scholar]
- 21.Charuzi I, Lavie P, Peiser J, et al. Bariatric surgery in morbidly obese sleep-apnea patients: short- and long-term follow-up. Am J Clin Nutr. 1992; 55: 594S–596S. [DOI] [PubMed] [Google Scholar]
- 22.Sugerman HJ, Baron PL, Fairman RP, et al. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg. 1988; 207: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugerman HJ, Felton WL, Sismanis A, et al. Effects of surgically induced weight loss on pseudotumor cerebri in morbid obesity. Neurology. 1995; 45: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 24.Smith SC, Edwards CB, Goodman GN. Symptomatic and clinical improvement in morbidly obese patients with gastroesophageal reflux disease following Roux-en-Y gastric bypass. Obes Surg. 1997; 7: 479–484. [DOI] [PubMed] [Google Scholar]
- 25.Jones KB Jr. Roux-en-Y gastric bypass: an effective antireflux procedure in the less than morbidly obese. Obes Surg. 1998; 8: 35–38. [DOI] [PubMed] [Google Scholar]
- 26.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995; 3: 211S–216S. [DOI] [PubMed] [Google Scholar]
- 27.Sjostrom L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992; 16: 465–479. [PubMed] [Google Scholar]
- 28.Maclean LD, Rhode BM, Sampalis J, et al. Results of the surgical treatment of obesity. Am J Surg. 1993; 165: 155–162. [DOI] [PubMed] [Google Scholar]
- 29.Howard L, Malone M, Michalek A, et al. Gastric bypass and vertical banded gastroplasty: a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995; 5: 55–60. [DOI] [PubMed] [Google Scholar]
- 30.Brolin RL, Robertson LB, Kenler HA, et al. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994; 220: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996; 171: 74–79. [DOI] [PubMed] [Google Scholar]
- 32.Sjostrom CD, Peltonen M, Sjostrom L. Blood pressure and pulse pressure during long-term weight loss in the obese: the Swedish Obese Subjects (SOS) intervention study. Obes Res. 2001; 9: 188–195. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS): an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998; 22: 113–126. [DOI] [PubMed] [Google Scholar]
- 34.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 1999; 23: 619–624. [DOI] [PubMed] [Google Scholar]
- 35.Karasson K, Lindroos AK, Stenlof K, et al. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000; 160: 1797–1802. [DOI] [PubMed] [Google Scholar]
- 36.Sjostrom CD, Peltonen M, Wedel H, et al. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000; 36: 20–25. [DOI] [PubMed] [Google Scholar]
- 37.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002; 346: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 38.Kellum JM, Kuemmerle JF, O’Dorisio TM, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990; 211: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992; 55: 560S–566S. [DOI] [PubMed] [Google Scholar]
- 40.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. UK Prospective Diabetes Study Group (UKPDS). Lancet. 1998; 352: 837–853. [PubMed] [Google Scholar]
- 41.Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res. 1999; 7: 189–198. [DOI] [PubMed] [Google Scholar]
- 42.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999; 281: 117–141. [DOI] [PubMed] [Google Scholar]
- 43.McMahon GF, Fujioka K, Singh BN, et al. Efficacy and safety of sibutramine in obese white and African-American patients with hypertension: a 1-year double-blind, placebo-controlled, multicenter trial. Arch Intern Med. 2000; 160: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 44.Landsberg L. Pathophysiology of obesity-related hypertension: role of insulin and the sympathetic nervous system. J Cardiovasc Pharmacol. 1994; 23 (Suppl 1): S1–S8. [PubMed] [Google Scholar]
- 45.Reisin E. Sodium and obesity in the pathogenesis of hypertension. Am J Hypertension. 1990; 3: 164–167. [DOI] [PubMed] [Google Scholar]
- 46.Modan M, Halkin H, Almay S, et al. Hyperinsulinemia: a link between hypertension, obesity and glucose intolerance. J Clin Invest. 1985; 75: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gans ROB, v.d. Toorn L, Bilo HJG, et al. Renal and cardiovascular effects of exogenous insulin in healthy volunteers. Clin Sci. 1991; 80: 219–225. [DOI] [PubMed] [Google Scholar]
- 48.Hsueh WA, Buchanan TA. Obesity and hypertension. Endocr Metab Clin North Am. 1994; 23: 405–427. [PubMed] [Google Scholar]
- 49.Cowley AW. Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr. 1997; 65: S587–S593. [DOI] [PubMed] [Google Scholar]
- 50.Rocchini AP. Insulin resistance, obesity and hypertension. J Nutr. 1995; 125: S1715–S1717. [DOI] [PubMed] [Google Scholar]
- 51.Scaglione R, Ganguzza A, Parrinello G, et al. Central obesity and hypertension: pathophysiologic role of renal haemodynamics and function. Int J Obesity Metab Disord. 1995; 19: 403–409. [PubMed] [Google Scholar]
- 52.Brands MW, Hall JE, Van Vliet BN, et al. Obesity and hypertension: roles of hyperinsulinemia, sympathetic nervous system and intrarenal mechanisms. J Nutr. 1995; 125: 1725S–1731S. [DOI] [PubMed] [Google Scholar]
- 53.Hall JE, Brands MW, Zappe DH, et al. Insulin resistance, hyperinsulinemia, and hypertension: causes, consequences or merely correlations? Proc Soc Exp Biol Med. 1995; 208: 317–329. [DOI] [PubMed] [Google Scholar]
- 54.Cigolini M, Seidell JC, Charzewska J, et al. Fasting serum insulin in relation to fat distribution, serum lipid profile, and blood pressure in European women: The European Fat Distribution Study. Metabolism. 1991; 40: 781–787. [DOI] [PubMed] [Google Scholar]
- 55.Saad MF, Knowler WC, Pettitt DJ, et al. Insulin and hypertension, Relationship to obesity and glucose intolerance in Pima Indians. Diabetes. 1990; 39: 1430–1435. [PubMed] [Google Scholar]
- 56.Briffeuil P, Huynh-Thu T, Kolanowski J. Reappraisal of the role of insulin on sodium handling by the kidney: effect of intrarenal insulin infusion in the dog. Eur J Clin Invest. 1992; 22: 523–528. [DOI] [PubMed] [Google Scholar]
- 57.Brands MW, Mizelle HL, Gaillard CA, et al. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens. 1991; 4: 164–168. [DOI] [PubMed] [Google Scholar]
- 58.Sugerman HJ, Windsor ACJ, Bessos MK, et al. Abdominal pressure, sagittal abdominal diameter and obesity co-morbidity. J Intern Med. 1997; 241: 71–79. [DOI] [PubMed] [Google Scholar]
- 59.Ridings PC, Bloomfield GL, Blocher CR, et al. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma. 1995; 39: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 60.Bloomfield GL, Blocher CR, Fakhry IF, et al. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997; 42: 997–1005. [DOI] [PubMed] [Google Scholar]
- 61.Doty JM, Saggi BH, Blocher CR, et al. The effect of increased renal venous pressure on renal function. J Trauma. 1999; 47: 1000–1003. [DOI] [PubMed] [Google Scholar]
- 62.Bloomfield GL, Sugerman HJ, Blocher CR, et al. Chronically increased intra-abdominal pressure produces systemic hypertension in dogs. Int J Obes Relat Metabol Disord. 2000; 24: 819–824. [DOI] [PubMed] [Google Scholar]
Footnotes
Correspondence: Harvey J. Sugerman, MD, Box 980519 MCV Station, Richmond, VA 23298-0519.
E-mail: hsugerma@hsc.vcu.edu
Accepted for publication December 2002.