Abstract
Objective
To define the role of surgical shunting for patients with poor hepatic reserve (Child’s class C) in the era of TIPS.
Summary Background Data
Most physicians prefer TIPS to surgical shunting for patients with poor hepatic reserve because of anticipated poor long-term survival.
Methods
Sixty-two patients of Child’s class C with bleeding varices not amenable to endoscopic sclerotherapy or banding were prospectively randomized to undergo TIPS or 8-mm prosthetic H-graft portacaval shunt (HGPCS) from 1993 to 1999. Resource consumption and survival after shunting were determined.
Results
Twenty-nine patients underwent TIPS and 33 underwent HGPCS. After HGPCS, survival at 3 years was favorable but not statistically superior. TIPS was more often associated with shunt stenoses/occlusions, recurrent hemorrhage, shunt revisions, and shunt failure. Long-term follow-up documented that after HGPCS, patients required fewer hospital and ICU days and fewer units of RBCs transfused. After HGPCS, cost of care was less, as was the median cost of care per day of survival.
Conclusions
For Child’s class C patients undergoing HGPCS or TIPS, long-term survival is similar, though favoring HGPCS. Similarly, measures of resource consumption and cost of care following hospital discharge favor HGPCS. HGPCS should be preferentially applied for acceptable patients without access to convenient capable post-shunt care or without definitive plans for imminent transplantation.
The deficiencies and inadequacies of the transjugular intrahepatic portasystemic shunt (TIPS) are increasingly well documented. Stent stenosis, occlusion, and foreshortening are common stent-related complications noted by all investigators familiar with TIPS. 1–4 As well, progression of hepatic dysfunction, often necessitating transplantation, is frequently noted after TIPS. 5–7 This deterioration in hepatic function is generally attributed to underlying cirrhosis and hepatic dysfunction rather than to diversion of nutrient portal blood flow. While the diversion of nutrient portal blood flow is an inevitable consequence of portasystemic shunting, it has been shown to be a particularly notable event after TIPS when compared to 8-mm prosthetic H-graft portacaval shunts (HGPCS). 8,9
Admittedly, TIPS is a commonly employed treatment option for patients with bleeding varices, portal hypertension, and cirrhosis and does provide partial portal decompression. TIPS has been shown by many to be effective in ameliorating portal hypertension and bleeding varices and is accepted as a bridge to imminent hepatic transplantation. 1,6,10–12 Despite the absence of randomized trials supporting its expanded use, TIPS has become a first-line option for many physicians treating bleeding varices due to cirrhosis and portal hypertension.
While patients with bleeding varices, portal hypertension, cirrhosis, and quantifiable objective measures of mild or moderate hepatic insufficiency (i.e., Child’s classes A and B) may be considered for surgical shunting, patients with advanced hepatic dysfunction (i.e., Child’s class C) generally undergo TIPS even when transplantation is not a possibility, for whatever reason. It seems TIPS is favored for these patients because it avoids an abdominal operation and a postoperative period perceived to carry increased attendant morbidity and mortality for patients with limited prospects for long-term survival. Efficacy equal to surgical shunting is presumed. Unfortunately, many patients undergoing TIPS will have poor access to subsequent healthcare, including TIPS follow-up, maintenance, and salvage. This is particularly true for unfunded patients or patients who live far from facilities with the resources and technology to monitor and therapeutically intervene for TIPS dysfunction.
This study was undertaken to review outcomes of patients with severe hepatic dysfunction (i.e., Child’s class C), varices, and cirrhosis undergoing TIPS or 8-mm prosthetic HGPCS. The intent was to compare periprocedural mortality, long-term survival, and resource allocation following TIPS or 8-mm prosthetic HGPCS. Specifically, using patients with advanced cirrhosis and severe hepatic dysfunction (i.e., Child’s class C) who participated in a randomized trial comparing TIPS versus 8-mm HGPCS, we sought to compare efficacy, complications, cost, and survival after each of these shunts. Our hypotheses in undertaking this study were that following TIPS, early survival would be better, long-term survival would be better, and resource allocation would be similar to that seen after 8-mm prosthetic HGPCS.
METHODS
The patients in this study were a subset of patients who were accrued into a protocol to compare TIPS to prosthetic 8-mm HGPCS in the treatment of variceal hemorrhage due to portal hypertension and cirrhosis. 13–15 This prospective randomized clinical trial began with institutional review board approval in 1993. All patients entered into this trial had bled from esophageal or gastric varices or portal gastropathy and had failed nonoperative therapy such as endoscopic sclerotherapy or banding. For all patients, portacaval shunting, whether obtained by TIPS or 8-mm HGPCS, was always undertaken as definitive therapy, never as a bridge to transplantation. For this reason, subsequent liver transplantation undertaken for progressive hepatic dysfunction was considered as an intervention to stave off death and was, therefore, identified prospectively as tantamount to death.
Through standard preoperative testing, patients were staged by assigning a Child’s class (A, B, or C). By protocol, each patient underwent pre-shunt color flow Doppler ultrasound imaging to document portal vein patency. If there were questions regarding the patency of the portal vein or the quality of the portal blood flow, visceral angiography with portal vein runoff was undertaken.
After portal vein patency was documented and it was confirmed that either TIPS or 8-mm prosthetic HGPCS could be undertaken, an informed consent was obtained and patients were randomized to receive either shunt. Patients were randomized to undergo TIPS or 8-mm HGPCS in pairs to allow for sequential analysis by pair differences. The investigators in the trial obtaining consent were blinded as to which shunt was next to be assigned. Patients were not randomized or considered for the trial only when the portal vein was thrombosed or chances of surviving shunting were thought to be hopeless because of ill health. Furthermore, patients were not randomized if they were felt not to be candidates for either TIPS or 8-mm prosthetic HGPCS for any particular reason. For example, patients with profound cardiorespiratory impairment that limited their candidacy for abdominal surgery may have been excluded from the protocol. As well, patients who had undergone multiple previous complex abdominal operations may not have been considered as candidates for the protocol because of their poor candidacy for abdominal surgery and, thereby, small-diameter prosthetic HGPCS.
Circumstances of shunting were defined as elective, urgent, or emergency. Elective shunts were those undertaken as scheduled procedures in hemodynamically stable patients. Urgent shunts were shunts undertaken within 24 hours of patient presentation. Emergency shunts were those undertaken within 8 hours of presentation.
Ascites was noted when present and was categorized as moderate (manageable by fluid restriction and diuretic therapy) or refractory to medical therapy.
Our technique in constructing an 8-mm prosthetic HGPCS has been described, 16 as has our technique used to construct TIPS. 13 Briefly, all TIPS procedures were undertaken under general anesthesia, and all but one TIPS were placed through a right internal jugular vein approach. Portal vein and hepatic vein pressures were measured before and after TIPS. Ultimately, a 10-mm by 68-mm Schneider Wallstent (Pfizer Inc., New York, NY) was placed. After appropriate positioning bridging the right branch of the portal vein and the right hepatic vein, the Wallstent was dilated under fluoroscopic guidance up to 8 to 10 mm or until an appropriate pressure gradient between the portal vein and hepatic vein was achieved. A portal vein/hepatic vein pressure gradient of no more than 10 mmHg was sought, although a lesser gradient was acceptable.
The 8-mm prosthetic HGPCS was constructed using external ring reinforced PTFE. The grafts were no longer than 3 cm from toe to toe and 1.5 cm from heel to heel, with bevels at 90 degrees to each other to allow for the orientation of the portal vein to the inferior vena cava. Portal pressures and inferior vena cava pressures were measured both before and after shunting. A decrease in the portal pressure of more than 10 mmHg, a decrease in the portal vein to inferior vena cava pressure gradient of more than 10 mmHg, a postshunt portal vein to inferior vena cava pressure gradient of less than 10 mmHg, and a thrill in the inferior vena cava cephalad to the shunt–cava anastomosis were all considered necessary elements in constructing a successful shunt. These findings with shunting predict long-term shunt patency. When necessary, a portion of the caudate lobe was excised to facilitate graft placement.
Before hospital discharge, shunt patency was documented. For the HGPCS, transfemoral cannulation of the shunts was undertaken on or near post-shunt day 4. TIPS function and patency were determined before discharge using color flow Doppler ultrasound study. Excessive flow turbulence in the stent, slow flow in the portal vein or the right branch of the portal vein, or extremely high focal shunt flow velocities led to transjugular cannulation of the TIPS with venography and pressure measurements. Evidence of stent stenosis, occlusions, or foreshortening led to stent dilation and the occasional need for placement of an additional stent to lengthen the previously placed stent or a larger stent to augment portal decompression. After discharge, patients with TIPS underwent color flow Doppler ultrasound evaluation at 6 weeks, 12 weeks, 6 months, and every 6 months thereafter. Again, studies compatible with stent stenosis or occlusion led to transjugular shunt assessment, with intervention as necessary. After discharge, patients with HGPCS underwent transfemoral cannulation of the shunts at 1, 3, 5, and 10 years.
Shunts were additionally studied with the onset of complications associated with cirrhosis, portal hypertension, or varices, including variceal re-hemorrhage, new or worsening ascites, or encephalopathy. For TIPS, the shunts were initially evaluated with color flow Doppler ultrasound study. The HGPCS were studied by transvenous cannulation of the shunts.
Measures of resource consumption began at the time of shunting. Subsequent hospitalization and care necessitated by shunting, cirrhosis, portal hypertension, or varices were included in tabulations of resource consumption. Resource consumption was measured by determining units of packed red blood cells (RBCs) consumed, hospital days, ICU days, and hospital and professional fees charged. Cost of care per day of survival was determined by dividing cost of care for each patient by duration of survival or follow-up. This latter calculation was made to reflect the lesser cost of reduced survival.
All patients are being followed. Follow-up ranges from 3 to 9 years. Median follow-up after TIPS is 6 years and 1 month; after HGPCS it is 6 years and 2 months. Since patients were randomized in pairs, mean follow-up after TIPS or HGPCS is identical.
Shunt failure was defined prospectively as an inability to complete the shunt, irreversible shunt occlusion, major variceal re-hemorrhage, liver transplantation, or death.
Data are presented as mean ± standard deviation (SD) when appropriate. All data are stored in a file-based registry. Comparisons were undertaken utilizing True Epistat (Epistat Services, Richardson, TX). When chi-square testing was used, methods specific for prospective trials were used. Statistical significance was accepted with 95% confidence.
RESULTS
Sixty-two patients of Child’s class C with bleeding varices, portal hypertension, and cirrhosis underwent randomization and TIPS or 8-mm HGPCS per protocol. Thirty-three patients underwent 8-mm HGPCS and 29 patients underwent TIPS. The ages, gender, and causes of cirrhosis of the patients undergoing shunting are shown in Table 1. Ascites was noted preoperatively in 74% of the patients (84% of patients undergoing 8-mm HGPCS and 62% of patients undergoing TIPS). Ascites was refractory to medical therapy in 17 (95%) of the 18 patients with ascites undergoing TIPS and in 20 (71%) of the 28 patients with ascites undergoing 8-mm HGPCS. Encephalopathy was noted in 32% of the patients undergoing shunting (30% of patients undergoing 8-mm HGPCS and 34% of patients undergoing TIPS). Encephalopathy was dense in 40% of encephalopathic patients undergoing TIPS and in 60% of encephalopathic patients undergoing HGPCS.
Table 1. COMPARISON OF PATIENTS UNDERGOING TIPS OR 8-MM PROSTHETIC H-GRAFT PORTACAVAL SHUNTS
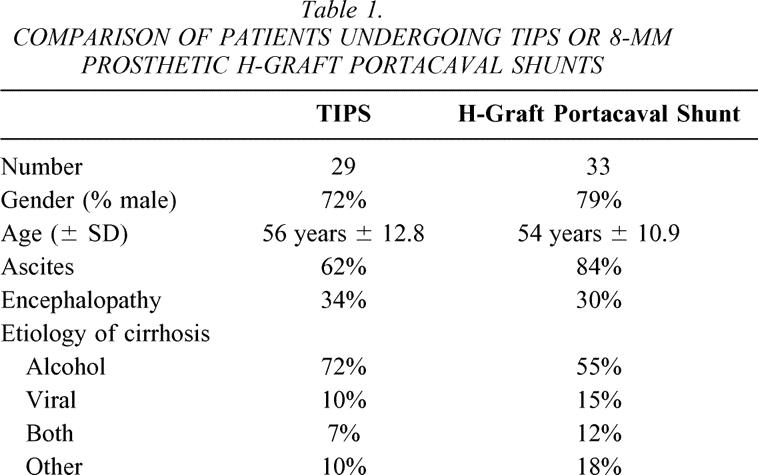
For patients undergoing TIPS, indications for shunting were bleeding esophageal varices refractory or not amenable to therapy in 41%, bleeding esophagogastric varices in 48%, or portal gastropathy in 10%. For patients undergoing HGPCS, indications for shunting were bleeding esophageal varices refractory or not amenable to therapy in 45%, bleeding esophagogastric varices in 42%, or portal gastropathy in 12%.
TIPS was undertaken electively in 19 (66%) patients, urgently in 5 (17%), and as emergencies in 5 (17%). HGPCS was undertaken electively in 23 (70%) patients, urgently in 2 (6%), and as emergencies in 8 (24%).
TIPS could not be completed in two patients. Otherwise, both shunts significantly reduced portal pressures and portal vein/inferior vena cava pressure gradients (Table 2). Portal pressures and portal vein/inferior vena cava pressure gradients were less after 8-mm HGPCS.
Table 2. PRE-SHUNT AND POST-SHUNT PORTAL VEIN PRESSURES AND PORTAL VEIN–INFERIOR VENA CAVA PRESSURE GRADIENTS
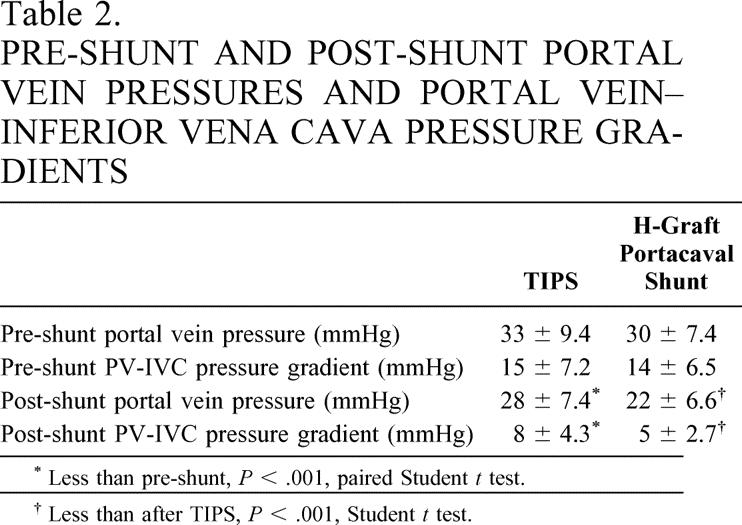
* Less than pre-shunt, P < .001, paired Student t test.
† Less than after TIPS, P < .001, Student t test.
Shunt thrombosis and occlusion occurred more frequently after TIPS (P < .01, chi-square), despite close follow-up and surveillance. After TIPS, 11 patients required interventions such as balloon angioplasty, thrombectomy, or placement of an additional stent. After TIPS, six patients underwent one intervention, three patients underwent two interventions, and two patients underwent four or more interventions. Irreversible shunt occlusion occurred with long-term follow-up in three patients after TIPS.
After 8-mm prosthetic HGPCS, two patients required interventions such as balloon thrombectomy and one patient required reoperation and reshunting for shunt occlusion. This reoperation occurred in the immediate postoperative period. All patients were discharged with patent shunts, and irreversible shunt occlusion did not occur thereafter.
Major re-hemorrhage recurred in six patients after TIPS and in four patients after 8-mm prosthetic HGPCS. After TIPS, shunt stenosis or occlusion was a precipitating factor in each occurrence of re-hemorrhage, which on each occasion was due to variceal bleeding. Alcohol recidivism was the major factor in two patients after HGPCS. Overall, patients undergoing HGPCS received fewer units of packed RBCs than patients undergoing TIPS (0.1 unit ± 1.4 vs. 1.3 units ± 2.7, P = .05, Student t test).
Ascites was not a notable problem after either shunt and generally improved when present preoperatively. By 30 days after shunting, ascites resolved or improved in 72% of patients with ascites before TIPS and in 90% of patients with ascites before 8-mm prosthetic HGPCS. With long-term follow-up, ascites was noted in two patients with ascites preceding TIPS and in two patients with ascites preceding 8-mm prosthetic HGPCS.
Seldom was new-onset encephalopathy noted after shunting. One patient developed new-onset encephalopathy 32 months after TIPS. Problematic encephalopathy persisted in three patients after TIPS and in three patients after HGPCS.
After TIPS, one patient underwent liver transplantation at 7 months after shunting. Follow-up ended for this patient at that point. After HGPCS, no patients underwent liver transplantation.
Six (21%) patients died within 30 days after undergoing TIPS and seven (21%) patients died within 30 days after undergoing HGPCS. Death was due to progressive liver failure, except for one patient suffering variceal re-hemorrhage after undergoing TIPS and two patients suffering multisystem organ failure after HGPCS.
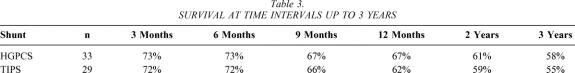
The last patients entered into this trial were entered 3 years ago; therefore, all patients are eligible for 3-year survival data. Survival at 3, 6, and 12 months and at 2 and 3 years after shunting is shown in Table 3. No significant differences between survival after TIPS or 8-mm prosthetic HGPCS at these time points were noted. Of patients dying after TIPS, death was due to progressive hepatic dysfunction, as well as variceal bleeding in three patients and unrelated causes in one patient (gastric cancer). Of patients dying after HGPCS, death was due to progressive hepatic dysfunction, as well as unrelated causes in five patients (carcinoid tumor, pneumonia, and ARDS). After HGPCS, no patients died of variceal bleeding.
Table 3. SURVIVAL AT TIME INTERVALS UP TO 3 YEARS
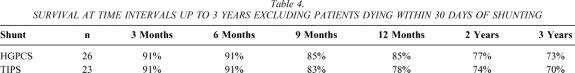
After TIPS, 23 patients were discharged for follow-up. After HGPCS, 28 patients were discharged for follow-up. Survival at time intervals up to 3 years after either shunt is noted for patients discharged after shunting in Table 4. Two patients died soon after discharge following HGPCS (i.e., within 30 days of shunting), as noted above.
Table 4. SURVIVAL AT TIME INTERVALS UP TO 3 YEARS EXCLUDING PATIENTS DYING WITHIN 30 DAYS OF SHUNTING
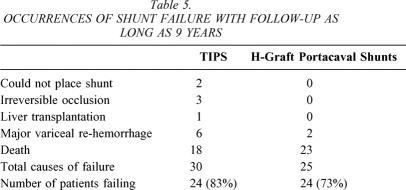
With follow-up as long as 9 years, shunt failure has occurred in 24 (83%) of 29 patients undergoing TIPS and in 24 (73%) of 33 patients undergoing HGPCS (Table 5). Unlike Table 3, which reported survival, and thereby mortality, data up to 3 years after shunting, Table 5 reports the number of patients who have died to date with follow-up up to 9 years for the first patients entered into this trial.
Table 5. OCCURRENCES OF SHUNT FAILURE WITH FOLLOW-UP AS LONG AS 9 YEARS
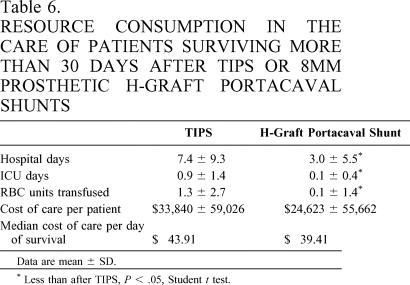
Following discharge after shunting, patients undergoing HGPCS required fewer hospital days and fewer ICU days (Table 6). As well, cost of care after HGPCS was less, though not statistically different, than the cost of care after TIPS (see Table 5). Similarly, the median cost of care per day of survival after shunting was less after 8-mm prosthetic HGPCS ($39.41 vs. $43.91).
Table 6. RESOURCE CONSUMPTION IN THE CARE OF PATIENTS SURVIVING MORE THAN 30 DAYS AFTER TIPS OR 8MM PROSTHETIC H-GRAFT PORTACAVAL SHUNTS
Data are mean ± SD.
* Less than after TIPS, P < .05, Student t test.
DISCUSSION
Many physicians caring for patients with varices, cirrhosis, and portal hypertension are enamored of TIPS because of its ability to provide portal decompression and relieve variceal bleeding. Furthermore, the shunt can be “ordered,” bespeaking its convenience and availability. Efficacy equivalent to surgical shunting is presumed, particularly for patients with poor hepatic reserve, as they are thought to do poorly with portal decompression and, consequently, have short life expectancy. While initially promoted as a bridge to imminent transplantation, TIPS has become widely employed despite a lack of comparative trials supporting its more general application. This study documents that when compared to a surgical shunt that also achieves partial portal decompression, TIPS offers no quantifiable benefits and is associated with higher resource consumption.
The patients undergoing TIPS or 8-mm prosthetic HGPCS in this trial were very similar. The patients were, on average, of similar ages and of a similar gender distribution. Of the patients undergoing TIPS, a few more had alcoholic cirrhosis and a few less had cirrhosis due to viral hepatitis or unknown causes. In general, ascites was more common in patients undergoing surgical shunting, but refractory ascites was relatively equally common in the patients undergoing TIPS or HGPCS. Encephalopathy was seen to the same extent in patients undergoing either shunt, though dense encephalopathy was relatively more common in patients undergoing HGPCS. In all, nothing of note discriminated the groups of patients undergoing either of the shunts.
Both shunts were undertaken under similar circumstances for similar causes and sites of variceal bleeding. Consistent with many reports, TIPS could not be placed in a small number of patients. In each, the liver was too hard to allow for bridging between the portal and hepatic veins.
Both shunts decompressed the portal system and brought the portal vein/inferior vena cava pressure gradient to normal or near-normal levels. The H-graft shunts provided better portal decompression, though decompression was always partial and never complete. As a result of portal decompression, and possibly more attentive care, ascites generally improved and was not much of a problem after either shunt. Surprisingly, given the magnitude of hepatic dysfunction in these patients, neither was new-onset encephalopathy. We cannot attest to improvements in Child’s class long term after shunting beyond generally noting that it occurred after each shunt with improvements in ascites and avoidance of variceal re-hemorrhage.
Shunt stenosis, occlusion, and thrombosis were more common after TIPS, undoubtedly reflecting its long course through the hepatic parenchyma. For those patients experiencing stenosis or occlusion, nearly half had recurring problems leading to further interventions. Irreversible shunt occlusions occurred in more than 10%, denoting a problem plaguing TIPS as definitive therapy for variceal bleeding.
Shunt occlusion and thrombosis were more common despite close follow-up, not a consequence of it. Less vigilant follow-up would have allowed the stenoses and occlusions to pass undetected, at least temporarily, but would have presaged other problems, such as variceal rebleeding or reaccumulation of ascites. The body of data about TIPS firmly establishes the need for close and long-term follow-up. The recurrences of major variceal bleeding in more than 20% of the patients in this trial undergoing TIPS document the consequences and magnitude of problems with stent narrowing and occlusion and underscore the need for long-term follow-up.
Progressive hepatic dysfunction was an issue after both shunts. Thirty-day mortality was near 20% after shunting, almost always being a consequence of hepatic insufficiency. If hepatic insufficiency did not cause a given death, it certainly acted to limit recovery. In that way, pneumonia and respiratory problems carried a high morbidity. Late mortality was high. Overall, survival was just over 50% at 3 years. In an effort to discount the high 30-day mortality after shunting, survival was also determined for patients discharged to home after shunting. For these patients, survival was more than 70% at 3 years. This latter view of survival after shunting patients with limited hepatic reserve documents that long-term survival is not only possible but probable given periprocedural survival. Given similar 30-day survival rates after TIPS versus H-graft shunts, the high-risk nature of surgical shunting is discounted. Given the rather impressive survival at up to 3 years after shunting, the issues of high maintenance of TIPS become paramount.
Because of issues with stent stenosis and occlusion, resource consumption after TIPS was higher. After TIPS, more hospital and ICU days were consumed and more units of red blood cells were transfused, leading to higher costs of care. Though these measures were higher after TIPS, it can be argued that they were not meaningfully higher. Whether hospital and professional charges should be used to quantify care, as opposed to hospital and professional costs, is an unending and banal debate. Our intent was to compare relative measures of resource consumption and post-shunt care. The dollar figures attributed to the cost of care after each shunt fairly denote the care given these patients.
For patients with significant hepatic dysfunction (i.e., Child’s class C), varices, portal hypertension, and cirrhosis undergoing portal decompression, early mortality is high, though not excessive. Long-term survival is possible and probable for those surviving the shunting procedure and the early period thereafter. Given such survival, portacaval shunts should be durable and effective to avoid recurrences of variceal bleeding and consumption of resources necessary to forestall hepatic decompensation and death.
Given the results of shunting patients with significant hepatic dysfunction (i.e., Child’s class C), varices, portal hypertension, and cirrhosis in this trial, there is little support to recommend TIPS. TIPS is indicated for patients with significant hepatic dysfunction (i.e., Child’s class C), varices, portal hypertension, and cirrhosis waiting imminent transplantation and those with contraindications for an abdominal operation.
Discussion
Dr. J. Michael Henderson (Cleveland, OH): First I would like to say that Dr. Rosemurgy should be complimented on doing this study. This is the only published randomized controlled trial looking at surgical shunts versus TIPS to date. We are also conducting a similar study, but it is not published yet. The emphasis of this particular paper looking at resource consumption is important.
Dr. Rosemurgy for this presentation has analyzed his Child C patients, which represent half the patients entered into this randomized trial. He has shown you that the hospital mortality was the same for the two groups. That is testimony to his group’s surgical expertise and their experience in getting Child C patients through their operative procedure. You would anticipate the TIPS patients would have a lower hospital mortality. He has also shown you that at a median 6-year follow-up, there is an equivalent mortality in the TIPS and the operative shunt groups. Their background becomes important as he looks at resource consumption. I would like to ask a few questions related to that.
First, when you looked at resource consumption, how did you manage to track down all the patients? Did they all come back to you for follow-up? Or how many of them were seen at other locations?
In quantifying resources, you looked at the relatively easy part of hospital stay, ICU stay, blood transfusion requirement. Then you give us a dollar number: What is really in that dollar cost number? When we have looked at this we have broken down into hospital costs, the major components are hospital days, laboratory costs, and the radiology costs. The latter is particularly important in this trial.
The protocol that you used for following your two groups of patients surprised me in that you have mandatory recatheterizations for your surgical shunt group but not for your TIPS group. Your reintervention rate for your TIPS group at 30% is remarkably low. Although I don’t have the full data from our trial and I can’t really talk about it yet, ours is much higher than that. Radiology resource consumption becomes a major cost issue in our study. Going to the radiology suite is as expensive as going to the operating room. I would be interested in knowing the components for your hospital costs, particularly in terms of hospital stay, lab expenses, and radiology expenses.
In this cost analysis, did you include the components of “protocol” follow-up studies or just “event” follow-up interventions? This is an issue we struggle with in our ongoing study. Clearly, there are significant costs related to study protocol visits. Are those costs included?
This is an important study; Dr. Rosemurgy is to be commended. Resource consumption is one of the key outcomes in your trial.
Dr. David V. Feliciano (Atlanta, GA): Can you clarify your short- or long-term management of these grafts? Were these patients maintained on aspirin or any other antiplatelets? To what do you attribute your superior patency?
Dr. Layton F. Rikkers (Madison, WI): As each new therapy for variceal bleeding has come along, it has tended to dominate the field, especially if less invasive than its forebears, and then over time, after a number of randomized trials, it finds its place among the available alternatives.
TIPS is presently undergoing this process, and Dr. Rosemurgy and his associates are to be congratulated for doing a rigorous randomized control trial that is difficult to accomplish in this population of patients. In fact, as Dr. Henderson pointed out, there is little data comparing an operative shunt to TIPS in the literature. To my knowledge, there is virtually no data looking specifically at the Child’s class C group of patients, who are the subjects of this report.
Due to the high associated operative mortality and morbidity rates of Child’s class C patients after shunt surgery, and the belief that such patients will not outlive the limited life expectancy of their TIPS, most gastroenterologists and surgeons have relegated these patients to interventional radiologists when endoscopic therapy has failed. Dr. Rosemurgy in his prospective trial is challenging the prevailing concept that TIPS is superior to an operative shunt in this group of patients.
I have a number of questions for you, Dr. Rosemurgy.
First, I would like to know how he classified his patients. Child’s classification can be ambiguous unless a number system is used to objectively classify patients. Additionally, there are a number of patients who are Child’s class A patients, but during resuscitation from a variceal hemorrhage develop ascites and hyperbilirubinemia, and can transiently appear as Child’s class C patients. Since the operative mortality rate for both the TIPS and shunt patients was approximately 20%, one third of the patients had preoperative encephalopathy, and over one half had medically intractable ascites, these patients likely had quite advanced chronic liver disease. I would appreciate your comments as to how firmly you feel that all of your patients were in Child’s class C.
Second, what were the real differences between the groups? Operative mortality and long-term survival rates were nearly identical between groups. There was a greater need for hospitalization among the TIPS patients, but there was no significant difference in overall costs. You stated that TIPS was a bit more expensive; however, when statistics are applied, there was no difference. The usefulness of statistics is to find out which of the differences that we do see are meaningful.
No patient in either of the groups developed new-onset encephalopathy, and ascites was well controlled in both groups. My conclusion would be that operative shunts were every bit as good as TIPS, but little evidence that they were superior to TIPS. I would appreciate your comments.
Finally, why do you consider late death or the need for transplantation as failure of therapy? Both of these treatments were directed towards preventing rebleeding, and you can’t blame either the shunt or the implanted TIPS for a return to alcoholism and eventual death from hepatic failure.
I greatly appreciate the opportunity to have read the manuscript and to hear your presentation today.
Dr. Jeffrey H. Fair (Chapel Hill, North Carolina): I would like to congratulate the authors for a very straightforward and important study. I haven’t read the manuscript; I had a few simple questions.
I was wondering if you had a chance to track people into transplant, those who did go on to transplant, and look at the complications of TIPS versus the portacaval shunt in those patients. I just wondered what your opinions on this versus a C-type mesocaval shunt in this patient population would be and if the partial decompression you thought was significant in terms of problems with encephalopathy afterwards, and comparing the cost also in the transplant phenomenon.
Also, I wondered if there were any stents in the TIPS groups that were covered stents, or was this all the old-type net?
Dr. Alexander S. Rosemurgy, ii (Tampa, FL): About the covered stent, I don’t think there is any compelling difference that this scenario is going to dramatically change with the advent of covered stents, although people are looking at them. But the preliminary data that is out is not particularly promising.
Transplant population. Thankfully, I guess, transplantation has been a relatively uncommon occurrence in our patients. Generally transplantation at times is not undertaken because of socioeconomic problems or some kind of self-destructive behavior. But the patients aren’t transplanted. So, oftentimes for these patients shunting is their definitive therapy.
Why is transplantation tantamount to death in our trial? Because prospectively we looked at shunting as definitive therapy, not as a bridge to transplantation. So for that reason, transplantation was determined as death, because transplantation was going to be undertaken only in patients that were going to otherwise die if they didn’t get transplanted. So for our trial, the follow-up stops at the time of transplantation.
Cost data is difficult. It is a very difficult thing to get our arms around charges versus costs versus whatever. At best, it is the measure of care that has been given, and I don’t give it more weight than that. There is a lot that is buried in cost data, and the radiology costs are considerable.
I don’t have a sense that there is an underlying story. There was no story behind the story looking at the numbers. The numbers are what the numbers are. We can certainly argue that hospital charges don’t equate to costs. I think that is a banal debate. And this is simply a measure. I am sure it cost less to care for these patients when they were charged. But, on the other hand, that is a debate that we all face in our hospitals all the time.
The patients were followed very closely. It is—I can’t say 100% follow-up. I think our follow-up in this trial is in the high 90s, 96%, 98%. Some of these patients aren’t just lost to follow-up; some of them were hiding. I have had a couple that were declared dead to avoid bills. I have tracked them down. It seems to me impossible that a significant number of patients—I don’t know, pick a number, 300, 400, 500 of these patients could be followed. That to me is absolutely inconceivable. People move, things happen, they go back north, et cetera, et cetera. Sometimes I have had calls from St. Louis and other places because an encephalopathic patient was found wandering in an airport with my card in their wallet. That may be my eventual claim to fame!
It was interesting to look at the Medline and see how many papers about TIPS are written as opposed to papers on surgical shunting. The last time I looked there were something like, and I forget the exact time period, but the numbers were about 1,100 papers written about TIPS and there were about 20 papers written about surgical shunting. I fear that this is a dying field of sorts.
And I don’t want this to appear to you that this is an emotional pursuit on my part. It is not, although I can’t help ultimately sounding somewhat emotional. Because I must say I truly don’t understand how a therapy can become so well accepted and so embraced by our medical community, and I use that in the broadest of sense, without any compelling data that would support its application in any general sense.
In terms of the patients with A becoming a C, becoming an A, it is really an A but it might be a B, that is a real difficult thing to get my hands around. And I think that this question has been asked so many times now that I am going to have to look at the data and analyze the data to see if an A that becomes a C is really an A. Personally, I think if they are a C when you shunt them, they are a C. And if they get ascites and they get hyperbilirubinemia with resuscitation, it is because they don’t have much hepatic reserve and they are a C.
The patients in this trial, I think, were the dregs of a surgical practice. Eighty percent had ascites and 30% of the patients had encephalopathy at the time of shunting. My recollection of caring for these patients is that that is the way they were. This wasn’t new-onset encephalopathy that was 24 hours old; these patients had not been doing well.
Why do an H-graft portacaval shunt at this time, why not do a TIPS? I think Dr. Rikkers said it best, with the presumption that these patients were going to have short life expectancies, that the long-term wear and tear of the TIPS is not going to become an issue. But if patients do survive the periprocedural period, long-term survival is not only possible, it is probable and should be expected.
Long-term TIPS. The problems with TIPS are going to become more problematic. The problems with stenosis, occlusion, and so on aren’t going to go away, they are just going to get worse.
References
- 1.Zemel G, et al. Percutaneous transjugular portosystemic shunt. JAMA. 1991; 266: 390–393. [PubMed] [Google Scholar]
- 2.Conn H. Transjugular intrahepatic portal-systemic shunts: the state of the art. Hepatology. 1993; 17: 148–158. [PubMed] [Google Scholar]
- 3.Rossle M, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994; 330: 165–171. [DOI] [PubMed] [Google Scholar]
- 4.Kuhlman CG, et al. Use of balloon-expandable stents in transjugular intrahepatic portosystemic shunts in cases of Wallstent endoprosthesis technical failure and revision of shunt stenosis. J Vasc Interv Radiol. 2002; 13: 405–408. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Freedman AM, Luketic VA, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997; 112: 889–898. [DOI] [PubMed] [Google Scholar]
- 6.Barton RE, Rosch J, Saxon RR, et al. TIPS. Short- and long-term results: A survey of 1750 patients. Semin Intervent Radiol. 1995; 12: 364–367. [Google Scholar]
- 7.LaBerge JM, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995; 108: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 8.Rosemurgy AS, et al. Differential effects on portal and effective hepatic blood flow. A comparison between transjugular intrahepatic portasystemic shunt and small-diameter H-graft portacaval shunt. Ann Surg. 1997; 225: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zervos EE, Goode SE, Rosemurgy AS. Small-diameter H-graft portacaval shunt reduces portal flow yet maintains effective hepatic blood flow. Am Surg. 1998; 64: 71–76. [PubMed] [Google Scholar]
- 10.Wroblewski T, et al. TIPS: a therapy to prevent variceal rebleeding in patients listed for liver transplantation. Transplant Proc. 2002; 34: 635–637. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology. 1996; 111: 138–146. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Ann Intern Med. 1997; 126: 849–857. [DOI] [PubMed] [Google Scholar]
- 13.Rosemurgy AS, et al. A prospective trial of transjugular intrahepatic portasystemic stent shunts versus small-diameter prosthetic H-graft portacaval shunts in the treatment of bleeding varices. Ann Surg. 1996; 224: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zervos EE, Rosemurgy AS. Small-diameter portocaval shunt vs. transjugular intrahepatic portosystemic shunt for portal hypertension. Adv Surg. 1997; 31: 105–125. [PubMed] [Google Scholar]
- 15.Rosemurgy AS, et al. Transjugular intrahepatic portosystemic shunt vs. small-diameter prosthetic H-graft portacaval shunt: extended follow-up of an expanded randomized prospective trial. J Gastrointest Surg. 2000; 4: 589–597. [DOI] [PubMed] [Google Scholar]
- 16.Rosemurgy A. Small-diameter interposition shunt. Mastery of Surgery. 1998; 3: 1301–1307. [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Alexander S. Rosemurgy, MD, Tampa General Hospital, 1 Davis Blvd., Box 1289, Room F-145, Tampa, FL 33601.
E-mail: arosemur@hsc.usf.edu
Accepted for publication December 2002.