Abstract
Objective
To determine if transfusion affected perioperative and long-term outcome in patients undergoing liver resection for metastatic colorectal cancer.
Summary Background Data
Blood transfusion produces host immunosuppression and has been postulated to result in adverse outcome for patients undergoing surgical resection of malignancies.
Methods
Blood transfusion records and clinical outcomes for 1,351 patients undergoing liver resection at a tertiary cancer referral center were analyzed.
Results
Blood transfusion was associated with adverse outcome after liver resection. The greatest effect was in the perioperative course, where transfusion was an independent predictor of operative mortality, complications, major complications, and length of hospital stay. This effect was dose-related. Patients receiving one or two units or more than two units had an operative mortality of 2.5% and 11.1%, respectively, compared to 1.2% for patients not requiring transfusions. Transfusion was also associated with adverse long-term survival by univariate analysis, but this factor was not significant on multivariate analysis. Even patients receiving only one or two units had a more adverse outcome.
Conclusions
Perioperative blood transfusion is a risk factor for poor outcome after liver resection. Blood conservation methods should be used to avoid transfusion, especially in patents currently requiring limited amounts of transfused blood products.
The development of blood transfusion and blood banking was no doubt one of the major advances in medicine in the 20th century, greatly improving the safety of surgical therapies and saving countless lives. An untoward effect of blood transfusions is immunosuppression, 1–4 which has been postulated to result in decreased tumor surveillance and detrimental outcome. Burrows and Tartter first proposed that blood transfusion may result in adverse outcome after cancer surgery in their report examining blood transfusion for patients undergoing resection of primary colorectal cancers. 5 Since then, similar studies have been performed for many tumor types, including colorectal cancer, 6–9 lung cancer, 10–14 breast cancer, 15–17 renal cell carcinoma, 18 gastric cancer, 19–23 and soft tissue sarcoma. 24 However, despite numerous published studies over the last three decades, the premise that transfusions may adversely effect outcome of cancer surgery remains controversial because almost as many studies exist that refute 9,14,16,17,21,22 as studies that support 6–8,11–13,15,18,24 this hypothesis. In fact, eight meta-analyses evaluating whether perioperative allogeneic transfusion is associated with adverse sequelae in patients with cancer are equally divided in conclusion. 25–32
For hepatic surgery, this subject is particularly relevant because of the high incidence of transfusion at most centers. 33–40 To date, data from the few published studies have both supported 41,42 and refuted 43,44 the notion that transfusion compromises outcome after liver resection. However, there have been many obstacles to definitive studies. First, liver resections were uncommon until recently, so most published series have relatively small sample size. Furthermore, the high incidence of blood transfusion in most published series resulted in only small numbers of nontransfused patients for comparison. Recent improvements in surgical techniques to limit blood loss and transfusion requirements, 36–40 combined with increasingly large experience with hepatic resections, have renewed interest in this subject. The current report examines the clinical outcome of over 1,300 patients undergoing hepatic resection for metastatic colorectal cancer, comparing the 749 transfused patients with the 602 patients not requiring a transfusion. The goal of this analysis was to analyze specifically the effects of transfusion. Of particular interest were the outcomes of patients who were transfused with limited amounts of blood products, since these are patients for whom allogeneic transfusion may be avoided using blood conservation or autologous transfusional techniques.
METHODS
All patients undergoing liver resection for metastatic colorectal cancer at the Memorial Sloan-Kettering Cancer Center (MSKCC) during the 15-year period spanning January 1986 and September 2001 were identified in the prospectively maintained Department of Surgery liver resection database. Patients undergoing only a diagnostic biopsy were excluded. In this period, hepatic resection with curative intent was performed in 1,351 patients as treatment of metastatic disease from a colorectal primary site. Criteria used to select patients for resection included medical fitness for major operation, absence of disseminated disease on preoperative imaging, and presence of adequate uninvolved residual hepatic parenchyma. Routine preoperative imaging studies included computed tomography of the abdomen and pelvis and chest x-ray. Intraoperative ultrasonography has been standard for the past 10 years 45 and selective laparoscopic survey for the past 5. 46
Patient, tumor, and operative information was obtained from patient records, and before analysis the database was stripped of all identifiers and coded by a study identification number to maintain patient confidentiality in agreement with our Institutional Review Board. Transfusion data were obtained from the computerized record system of the MSKCC blood bank. Data examined included demographics (age and gender), operative procedure, size and number of tumors, carcinoembryonic antigen level (CEA), nodal status, disease-free interval of the primary tumor, site of primary disease (colon vs. rectum), resection margin status at liver surgery, presence of bilobar or extrahepatic disease, intraoperative blood loss, length of operation, length of hospital stay (LOS), 60-day or in-hospital mortality, perioperative transfusion information, postoperative complications, and long-term survival. All factors that were not inherently dichotomous were analyzed as continuous variables.
Definitions
Nomenclature used to describe the extent of hepatic resection is that defined by Goldsmith and Woodburne. 47 An extended right hepatectomy refers to resection of Couinaud’s 48 segments 4 though 8; an extended left hepatectomy refers to resection of segments 2–5 and 8; a right lobectomy is resection of segments 5–8; a left lobectomy is resection of segments 2–4; a left lateral segmentectomy is resection of segments 2 and 3; a right anterior sectorectomy is resection of segments 5 and 8; a right posterior sectorectomy is resection of segments 6 and 7; a central hepatectomy is resection of segments 4, 5, and 8. For purposes of analysis, resections were also classified as less than a lobectomy, or lobectomy or more, according to these criteria.
Transfusion of any blood products (whole blood, packed cells, fresh-frozen plasma, or platelets) within 30 days of the operative procedure was considered a perioperative transfusion. Specific variables of interest include whether the patient had any units transfused (red blood cells, fresh-frozen plasma, or platelets), whether the patient received a red blood cell transfusion, if the patient received one or two units of red blood cells, and if the patient received an autologous blood transfusion only.
In an effort to standardize reporting, complications were graded on a 1-to-5 scale according to a previously published grading system. 49 In this classification system, grade 0 represents cases with no complications. Grade 1 complications are those requiring no intervention or minor interventions such as oral antibiotics, bowel rest, or basic monitoring. Grade 2 complications are those requiring moderate interventions such as intravenous medications (e.g., antibiotics or antiarrhythmics), TPN, prolonged tube feeding, or chest tube insertion. Grade 3 complications are those requiring hospital readmission, surgical intervention, or radiologic intervention. Grade 4 complications are those producing chronic disability, organ resection, or enteral diversion. Grade 5 complications result in death. Grades 1 and 2 are grouped as “minor” and grades 3 to 5 are considered “major” complications for the purpose of analysis.
Statistics
Survival was measured from two perspectives: postoperative and long-term. Postoperative mortality was defined as mortality within 60 days of surgery or an in-hospital death (two patients died shortly after day 60 but had never been discharged and were included in the postoperative mortality group). Long-term survival was defined as the survival of patients who were alive after the postoperative period. Thus, for postoperative mortality, we considered data from the first 60 days by censoring everyone still at risk at 60 days (n = 1,351). For long-term survival we considered only patients still at risk at 60 days (n = 1,261). These two time periods were incorporated into a Cox regression model, 50 which allowed for estimation and comparison of different hazard ratios for the two time periods. For each of the two time periods, Kaplan-Meier estimates of overall survival time in various groups were compared using the log-rank test. For continuous variables a Cox regression model was used. A significance level of 5% in the univariate setting was used as the criterion for including a variable in the multivariate modeling procedure. Stepwise regression was used to determine variables incorporated into the multivariate Cox regression model.
Overall complication rate and high-grade complications were secondary endpoints. The association of variables with complications was tested using the Fisher exact test for dichotomous co-variates and t test for continuous variables. Stepwise logistic regression was used for multivariate models, with variables significant on univariate analysis included in the modeling procedure.
RESULTS
Of the 1,351 patients, there were 776 deaths (43%), of which 50 (3.7% of total population) were postoperative mortalities. Median overall survival for the 1,351 patients was 42 months (95% CI 39–46;Fig. 1). Median follow-up for survivors was 35 months. The initial steep slope of the overall survival curve reflects the 50 postoperative mortalities. One-, 3-, and 5-year survival rates for the entire group were 88% (CI 86–90%), 56% (53–59%), and 36% (33–39%), respectively.
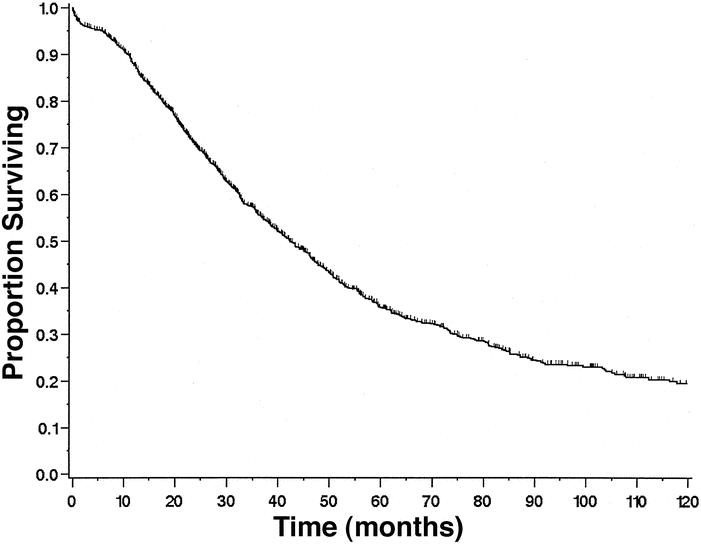
Figure 1. Overall survival of all 1,351 patients undergoing liver resection for metastatic colorectal cancer.
Transfusional Practice
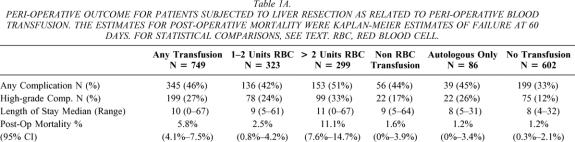
Fifty-five percent of the patients (n = 749) received some blood product transfusion (red blood cells, fresh-frozen plasma, or platelets), and 86 (6%) of the patients received autologous transfusion only. Forty-six percent of the patients (n = 622) received transfusion of red blood cells. Twenty-four percent (n = 323) received 1 or 2 units of red blood cells, 18% (n = 247) received 3 to 10 units, and 4% (n = 52) received more than 10 units; thus, 22% (n = 299) received more than 2 units.
Over time, there was a reduction in blood administration. In the early years (1986–1990) a large proportion (280/337 [83%]) of patients received some blood products. From 1991 to 1994 this proportion dropped to 54% (163/300), and in the last time period of 1995 to 2001, the percentage of patients receiving any transfusion dropped further to 43% (306/714).
Complications
Forty percent of patients experienced at least one surgical complication, and 274 (20%) patients experienced a high-grade complication (grade 3 or higher). Nontransfused patients had significantly fewer complications than those receiving blood products (33% vs. 46%, Fisher exact, P < .0001). The proportion of patients developing a complication was significantly less for patients receiving one or two units of red blood cells than for those receiving more than two units (42% vs. 51%, Fisher exact, P = .03) (Table 1). Patients receiving one or two units had significantly more complications than patients who did not receive any blood products (42% vs. 33%, Fisher exact, P = .03). The complication rate for patients transfused with blood products other than red blood cells was significantly higher than for patients receiving no transfusion (44% vs. 33%, Fisher exact, P = .02). Patients transfused with only autologous blood had complication rates similar to patients receiving one or two units of allogeneic red blood cells (Fisher exact, P = .52) and significantly more complications than patients who did not receive any transfusion (Fisher exact, P = .03). The same relationship was observed for patients with regards to development of major complications (grade 3 or greater) except in the case of transfusion only with blood products other than red blood cells, where there was not a significant difference in complication rates when compared with the no transfusion group (17% vs. 12%, Fisher exact, P = .15).
Table 1A. PERI-OPERATIVE OUTCOME FOR PATIENTS SUBJECTED TO LIVER RESECTION AS RELATED TO PERI-OPERATIVE BLOOD TRANSFUSION. The estimates for post-operative mortality were Kaplan-Meier estimates of failure at 60 days. For statistical comparisons, see text. RBC, red blood cell.
Table 1B. LONG-TERM OUTCOME FOR PATIENTS SUBJECTED TO LIVER RESECTION AS RELATED TO PERI-OPERATIVE BLOOD TRANSFUSION. Only the 1261 patients surviving the initial peri-operative period were considered.
Variables significant on univariate analysis for development of any complication were blood product transfusion, male gender, bilateral resection, lobe or more resection, increasing age, longer operation time, higher blood loss, and larger tumor size (data not shown). On multivariate analysis only blood product transfusion (odds ratio = 1.5;P = .0008), lobe or more resection (OR = 2.0;P < .0001), and male gender (OR = 1.4;P = .002) were independent predictors of any complication. Similarly, the variables significant on univariate analysis for development of high-grade complications were blood transfusion, bilateral resection, lobe or more resection, increased blood loss, and large tumor size (data not shown). On multivariate analysis only blood product transfusion (OR = 2.1;P < .0001) and lobe or more resection (OR = 2.4;P < .0001) were significant predictors of high-grade complications.
Length of Stay
Median LOS was 9 days (range 0–67). Comparing the LOS for patients grouped by amount transfused (see Table 1), patients receiving any transfusion (P < .01), patients receiving any red blood cell transfusion (P < .01), or patients receiving only autologous blood transfusion (P < .01) each had a longer LOS than nontransfused patients.
Perioperative and Long-Term Mortality
Comparison survival rates for the transfused patients are shown in Table 1 for perioperative morality and long-term survival. Patients receiving any transfusion (n = 749) had decreased survival (median survival 37 months, P = .0004) compared with patients who were not transfused (median survival 49 months, Fig. 2A). The initial slope of the “transfusion curve” is steep compared to that observed in the “no transfusion curve.” During the initial postoperative period (60 days), the hazard ratio for death was 4.2 (95% CI 2.0–9.0;P = .0002) for transfused patients compared with nontransfused patients. This increase in risk of perioperative mortality was directly related to the amount of blood product transfused. In fact, patients receiving more than two units of red blood cells had an 11% rate of mortality compared to 1.2% for patients not needing a transfusion.
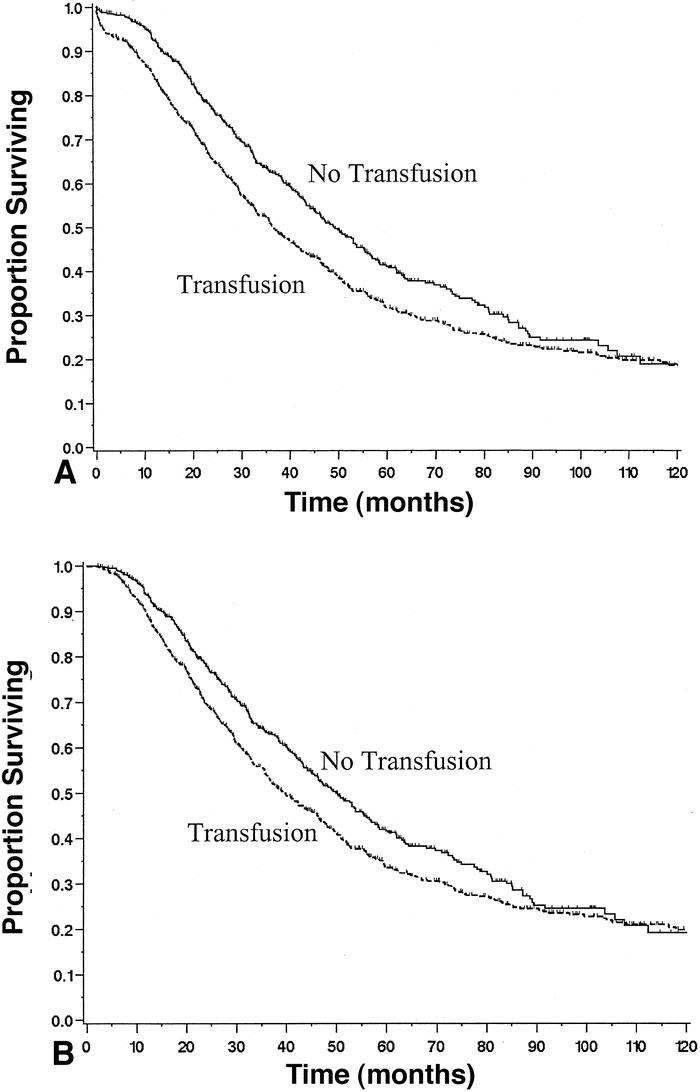
Figure 2. Survival of patients undergoing liver resection for metastatic colorectal cancer stratified by transfusion status. (A) Data for all 1,351 patients (P = .0001). (B) Data from the 1,261 patients who survived the initial operative period (P = .009).
For patients who survived at least 60 days (n = 1,261), the hazard ratio fell to 1.3 (95% CI 1.1–1.5;P = .0004) for transfusion-related deaths, and the curves are noticeably more parallel. The difference between the hazard ratios reported for the two separate portions of the transfusion curve was significant (P = .002). Thus, although the univariate risk of death associated with transfusion following liver resection for colorectal cancer appears significant, this risk falls tremendously after the 60-day postoperative period. Figure 2B shows the same data minus the postoperative fatalities, demonstrating a minor difference in slopes for transfused and nontransfused patients. At 5 years, the difference in survival for transfused and nontransfused patients was 8%. Thus, the greatest influence of transfusion is on the perioperative course.
Survival curves for all red blood cell transfusions stratified by number of units transfused (none, 1–2 units, 3–10 units, and >10 units) are illustrated in Figure 3. Figure 3A shows results for all patients, while Figure 3B shows results for patients who survived the perioperative period. The postoperative mortality (2.5%) and long-term survival (median 46 months) for patients receiving one or two units of red blood cells was not significantly different (P = .14, P = .40) from those not transfused (mortality 1.2%, median 50 months). However, the postoperative mortality rate and long-term survival for the one-or-two-unit group was significantly different (P < .0001 and P = .009) from those patients receiving more than two units of red blood cells (mortality 11.1%, median 34 months).
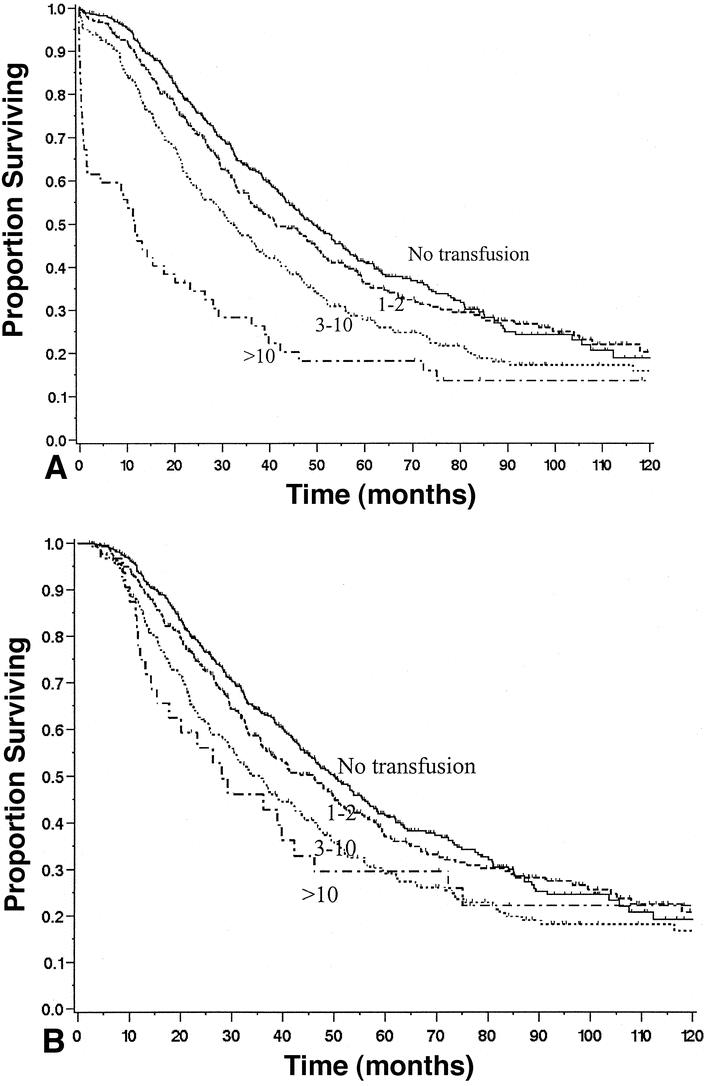
Figure 3. Survival of patients after liver resection for metastatic colorectal cancer stratified by amount of red blood cells (RBC) trans-fused. (A) Results for all 1,351 patients, grouped as: 1) no transfusion(n = 602), 2) 1 or 2 units transfused (n = 323), 3) 3 to 10 units transfused (n = 247), and 4) more than 10 units transfused (n = 52). P < .01 comparing all four curves (0, 1–2, 3–10, >10);P < .01 comparing only 3 to 10 and 10+. (B) Results for patients surviving beyond the perioperative period. P < .01 comparing all four curves (0, 1–2, 3–10, >10);P = .8 comparing only 3 to 10 and 10+.
Patients who were transfused only with fresh-frozen plasma or platelets (mortality 1.6%, median 45 months) had similar postoperative mortality and long-term survival rates (P = .72 and P = .63) to patients who were not transfused (mortality 1.2%, median 50 months). Patients who received an autologous transfusion only (mortality 1.2%, median 49 months) had similar postoperative mortality and long-term survival (P = .98, P = .66) to patients not receiving any transfusion (mortality 1.2%, median 50 months). This group also had similar postoperative mortality rates and survival (P = .35, P = .83) to those receiving one or two allogeneic units of red blood cells (mortality 3.0%, median 43 months) (Fig. 4).
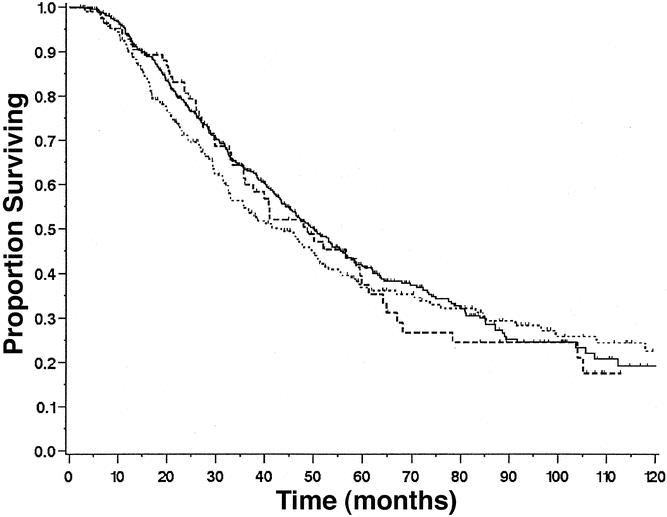
Figure 4. Survival of patients who did not receive transfusion (solid line, n = 602) compared to those who only received autologous blood products (long dashed line, n = 86) and those who had one or two units of allogeneic blood (short dashed line, n = 322). P = .98, no transfusion versus autologous;P = .13, autologous versus one- or two-unit allogeneic.
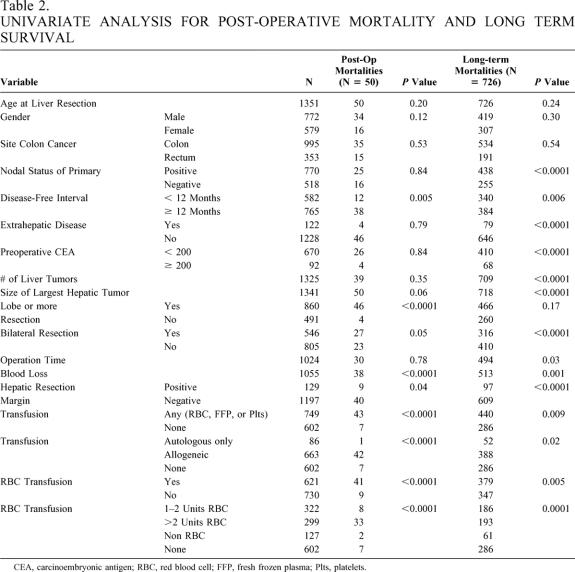
Table 2 shows the univariate analyses both for patients at risk of postoperative mortality (n = 1,351) as well as those in the long-term survival group (n = 1,261). Factors predictive of postoperative mortality were transfusion, bilateral resection, lobe or more resection, positive liver resection margin, short disease-free interval, and increased blood loss. Since increasing blood loss and administration of blood products are highly correlated, only transfusion was included in the multivariate model. The two significant predictors of postoperative mortality on multivariate analysis were blood transfusion (HR 3.7; 95% CI 1.7–8.4;P = .001) and lobe or more resection (HR 4.9; 95% CI 1.8–13.8;P = .003).
Table 2. UNIVARIATE ANALYSIS FOR POST-OPERATIVE MORTALITY AND LONG TERM SURVIVAL
CEA, carcinoembryonic antigen; RBC, red blood cell; FFP, fresh frozen plasma; Plts, platelets.
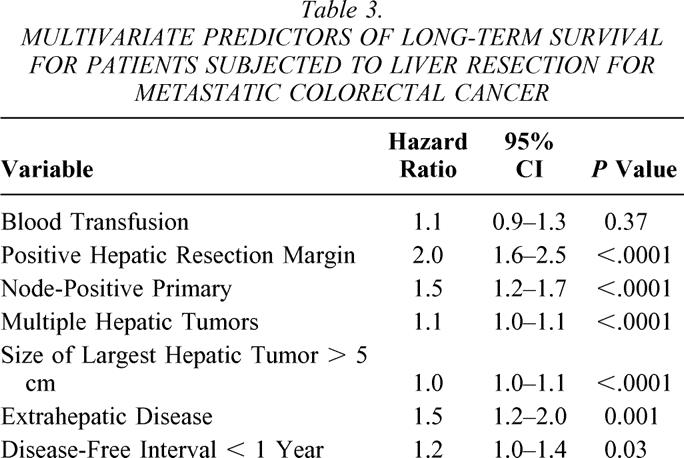
The factors predictive of long-term survival following liver resection for colorectal cancer metastases were identified in a previous analysis. 51 These are positive hepatic resection margin, node-positive primary, number of hepatic tumors greater than one, size of largest hepatic tumor greater than 5 cm, presence of extrahepatic disease, preoperative CEA greater than 200, and disease-free interval from time of colorectal resection of less than 1 year. To provide clinical relevance to the current analysis, these factors were included along with transfusion data in a multivariate regression model (Table 3). CEA was excluded from the multivariate analysis due to incomplete data accrual. Transfusion was not a significant predictor in this model (HR 1.1;P = .37). Similarly, when red blood cell transfusion (excluding autologous-only patients) was examined in the same multivariate model with the above-mentioned predictors, the variable was not significant in the model (HR 1.1;P = .20; data not shown).
Table 3. MULTIVARIATE PREDICTORS OF LONG-TERM SURVIVAL FOR PATIENTS SUBJECTED TO LIVER RESECTION FOR METASTATIC COLORECTAL CANCER
DISCUSSION
Numerous studies have examined the association of transfusion and adverse oncologic outcome for primary and secondary liver cancers. In hepatocellular carcinoma, most studies with small sample size such as those of Makuuchi et al. (13 transfused, 14 nontransfused patients), 52 Itasaka et al. (38 transfused, 33 nontransfused), 53 Matsumata et al. (54 transfused, 72 nontransfused), 54 or Kwon et al. (53 transfused, 55 nontransfused) 55 have been unable to identify a difference in recurrence or long-term survival. Three studies with moderate sample size have suggested an adverse effect for transfusions. Fujimoto et al. 56 examined 235 patients undergoing resection for hepatocellular carcinoma and found that by univariate analysis, the amount of blood products transfused in the perioperative period was a prognostic factor for poor long-term survival; however, only eight patients in this study were not transfused, and no rigorous multivariate analysis was performed. Asahara et al., in a report of 175 patients, 57 and Yamamoto et al., in a report of 252 patients, 58 found transfusion to be associated with decreased disease-free survival.
There are three studies in hepatic colorectal metastases that have focused on the effects of transfusion. Stephenson et al. reported the results of 59 patients undergoing liver resection at the National Cancer Institute. 41 By univariate analysis, patients who received 3- to 10-unit transfusions had better survival than those receiving more than 10 units. With the limited numbers of subjects, a rigorous multivariate analysis was not possible. Furthermore, since only five patients received no perioperative transfusions, a comparison with patients receiving transfusion was not possible. Younes et al. examined the influence of transfusions in 116 patients. 43 By univariate analysis, patients who received whole blood (P = .046) but not those who received packed red blood cells (P = .5) had a worse outcome than those not transfused. However, multivariate analysis did not find an influence of transfusion on outcome. Rosen et al. examined the clinical course of 280 patients treated at the Mayo Clinic. 44 By univariate analysis, the 81 patients who did not receive blood transfusion had a significantly better survival (P = .03). However, by multivariate analysis using primary tumor stage, method of initial detection, configuration of metastases, extrahepatic nodal status, extrahepatic disease, and transfusion as covariates, transfusion was not a predictor of outcome.
The current study, with over 1,300 patients, only half of whom received perioperative transfusions, clearly demonstrates an adverse effect of such transfusions on outcome. The overall survival data from this study demonstrate that transfused patients are more likely to die in the immediate postoperative period. If the patients who fail in the first 60 days following resection are omitted from the analysis, transfused patients still fare worse than nontransfused patients on univariate analysis. However, when entered into a multivariate analysis with relevant cofactors, both transfusion of any blood product and red cell transfusion do not remain independent predictors of survival for this group of patients. This suggests that the major effect of transfusion is on perioperative outcome. Though there is still a perceptible influence on long-term survival, other tumor-related factors are more dominant determinants of long-term cancer outcome in these patients with stage IV cancer.
There is no doubt that transfusion has a significant effect on perioperative outcome as measured by perioperative mortality, complications, and LOS. An association between transfusion and postoperative complications has been shown both in preclinical models 59,60 and in clinical studies of other cancer types. 61–65 In a prospective analysis of 740 patients undergoing resection for colorectal cancer, 19% of 288 nontransfused and 31% of 452 transfused patients developed postoperative infectious complications (P < .001). 65 In a recent review of 254 consecutive elective liver resections (all diseases), Alfieri et al. found a significant association between administration of blood products and development of complications. 66 An earlier, small series of 43 patients with colorectal liver metastases also demonstrated a correlation between number of units of perioperative blood transfused and development of postoperative complications. 67 In the current study, transfusion was a prognostic factor for the development of complications in univariate and multivariate analysis. Transfusion predicted development of both minor and major complications. Transfused patients had twice as high a chance of developing major complications and four times the risk of perioperative death. Transfused patients also had a higher incidence of infectious complications (17% vs. 13%, P = .03).
One mechanistic basis for the adverse effects of transfusion has to be the altered immune function following administration of blood products, which has been recognized for over two decades. 68 Studies have demonstrated blood transfusions suppress host immunity via reductions in natural killer cell activity and cytotoxic T-cell function. There is also a reduction in NK cell number and a decrease in the T4 to T8 ratio. 1,2 In liver cancer patients, Kwon et al. 55 found that transfusions produced an increase in CD8 lymphocytes and diminished PHA responses. Many of these immunosuppressive effects are thought to be related to the number of leukocytes within the stored blood as well as to the length of blood storage. 4 These immunosuppressive effects of blood transfusion are being exploited in treatment of patients with autoimmune disease processes 3 and for organ transplant recipients. 68 In cancer patients undergoing major surgery, these immunosuppressive effects are detrimental and likely contribute to the adverse perioperative and long-term outcomes.
Examination of the influence of transfusion on clinical outcome in the current patient population is not purely academic, since 24% of patients in the current study received only one- or two-unit transfusions. In the current study, transfusing one or two units of allogeneic red blood cells was associated with significantly more all complications and high-grade complications. Certainly a large number, if not all, of these transfusions could have been avoided. In addition, intraoperative blood salvage, 69 leukocyte-depleted packed cells, 70,71 frozen washed red cells, 72 and perioperative administration of hematopoietic growth factors are potentially less immunogenic alternatives to allogeneic whole blood or packed red blood cells. Whether any of these will prove to be useful in the clinical situation is unknown and awaits prospective clinical evaluation. Interestingly, patients with one- or two-unit transfusions had no significant difference in short- or long-term survival than nontransfused patients. This likely is the result of recent improvements in perioperative support such that even major complications rarely result in mortality. Disappointing was the result that patients receiving only autologous blood transfusions were indistinguishable from patients receiving one or two units of allogeneic blood transfusion. Studies in other cancer types have suggested that use of autologous transfusion limits the requirement for allogeneic exposure and can reduce the incidence of complications. 39,63 Since hepatectomy is an immunosuppressive event that results in significant Kupffer cell and T-cell dysfunction, 73 small benefits produced by autologous transfusion may be undetectable with a sample size of only 86 patients so treated. Certainly, the current data should not be used to abandon autologous blood donation. Autologous blood can prevent other complications of allogeneic blood transfusion and preserve the allogeneic blood supply for other patients.
The current study presents further data implicating blood transfusions as influential on outcome after resections for cancer. These data emphasize the importance of avoiding unnecessary transfusions. In addition, these data would encourage consideration of less immunogenic alternatives to allogeneic whole blood or packed cells in the restoration of oxygen-carrying capacity in cancer patients.
Discussion
Dr. John S. Bolton (New Orleans, LA): I want to thank Dr. Fong for allowing me to see his manuscript and to discuss this paper, and to acknowledge the authors for a well-written, careful analysis of their large database. They have a long-standing interest in the relationship of blood transfusion to outcome after liver resection and also a long-standing interest in efforts to reduce blood loss through low CVP anesthetic technique and reducing allogeneic blood through the use of autologous blood. Their low utilization of blood transfusion is as good as any in the world, and a little better than most of us, myself included. However, lest we look at blood transfusion as an unmitigated evil, I would ask a rhetorical question: In the 22% of patients who require greater than 2 units of blood, and in particular those who required 10 units or more, would their outcome have been better without transfusion? I submit that it probably would not have been. While we all agree that blood loss is a bad thing, and that minimizing blood loss is desirable and a prerequisite to low morbidity, the next time I have an hypotensive patient with uncontrolled bleeding from the middle hepatic vein, I am not going to wring my hands about whether or not to give blood. In fact, I will do even less hand-wringing now that our colleagues at Memorial Sloan-Kettering have shown that blood transfusion is not a significant predictor of long-term survival on multivariate analysis. I have several questions for Dr. Fong. Number one, what was the MSKCC prognosis score for those who received greater than two units versus zero units of blood? Second, was there any difference in margin status for these two groups? And third, can he tell us more about the excess perioperative mortality in the greater-than-2-units transfusion group, and especially that 39% mortality in the greater-than-10-units group? What did those patients die of? How do we recognize these patients beforehand and manage them better before the major blood loss starts?
There is a lot more that I could ask about this marvelous paper, but I think I will stop here and allow other discussants to have their chance.
Dr. Michael A. Choti (Baltimore, MD): Congratulations on another excellent report from the Memorial group, Drs. Fong, Blumgart and coauthors, for all their contributions in the field of hepatic surgery. This report, as mentioned, evaluates the possible effect of blood and blood product transfusion on outcomes following liver resection for colorectal metastases. The question of the impact of the transfusion on outcome following major surgery, including liver resection in cancer patients, is a long-standing interest and something we have all been interested in, and, frankly, something we have all been kind of hoping that there is an impact, mostly because we are improving, our transfusion rates are going down, there certainly is an appealing theoretic mechanism regarding immunosuppression. So it is certainly something we are looking to find. I think perhaps this study may in many ways put some of these questions to rest. I think, though, in other areas it is still unclear what the answer is, this issue of short-term versus long-term outcomes, is it really an independent predictor? I think they did find that it is an independent predictor of short-term outcome, mortality, complication rate, and length of stay, but not in multivariate analysis on long-term outcomes. I have several questions.
You emphasize the possible immunologic mechanism, and yet it is really only the short-term outcomes. So I would like to hear some kind of conceptual ideas about why immunologic mechanisms may have an impact on complication rate and mortality. Also, did you look specifically at infectious complications, which perhaps one may more associate with immunosuppression in the short-term postoperative period? Also, have you looked at the influence of transfusion comparing the types of resection, specifically benign disease? If we think that it perhaps is the cancer patient undergoing major liver resection, then I am sure the series of comparable resections for benign tumors may shed some light as to whether the cancer itself may have some impact in the short-term, and perhaps long-term, outcomes. You elaborated somewhat on the question of autologous transfusion. You mention this is being done more commonly and currently among your group. You found in this series only 6% of the cases had autologous blood transfusion. It is interesting that there were no differences between, as I read it, between allogeneic and autologous, in complication rate at least. So again, does this speak against the immunologic mechanism? Perhaps you can comment on that. And perhaps you could elaborate a little bit more on your indications for autologous transfusion, which patients, and what is your current practice, is this number going up? You comment on the rate of transfusion in recent years has gone down significantly, I think from 80% down to 43%. We and others have reported that there is a clear trend in improved outcome, both long-term and short-term outcomes, in recent years compared to, for example, a decade ago—perhaps for many reasons, some of which are not identified. And although you found transfusion to be an independent factor related to short-term outcome, are you really sure that it is not related to other factors of improved technique, anesthesia, postoperative care, and so forth, that really reflect this short-term impact? Did you find that mortality rate and complication rate have changed over time, for example in the first half versus second half of your series? Finally, you concluded that even still, I think, that blood transfusion has an impact on long-term outcome. I would like to think that perhaps this large series has finally put to bed the question that transfusion does not have an impact on long-term outcomes, oncologic outcomes, in patients undergoing liver resection for colorectal cancer. Perhaps you can comment on that.
Dr. Bryan Clary (Durham, NC): The authors in this report address the hypothesis that transfusions of blood products inherently lead to adverse short- and long-term outcomes following hepatic resection for colorectal metastasis and, as a means of addressing this paper, they perform a very large retrospective analysis of over 1,300 patients. It is difficult in this retrospective experience to determine that the transfusion itself and not the reason for that transfusion was the cause of the perioperative morbidity. Not included in the analysis are hemodynamic parameters such as the degree of intraoperative hypotension that led to the decision to transfuse the patient. As far as complexity of the operation, the authors grouped hemihepatectomies and extended hepatectomies together and compared them to patients undergoing lesser resections. As the degree of liver dysfunction and perioperative morbidity is likely greater with extended hepatectomies than hemihepatectomies, it is possible that if one approaches the complexity of the operation as extended versus other, that this effect may be negated. We as well do not know much about the preoperative status of these patients, and specifically their preoperative chemotherapy history, which may impact upon the blood count indices and also the function of the liver after large-volume resections, and as such may alter the perioperative outcome and the need for transfusion. They report in the manuscript and also in the presentation today that again over the successive three 5-year intervals of this 15-year experience that the transfusion rates decrease from 83% to 43%. As such, the transfusion group is weighted with individuals from the earlier time period. Experience and volume are generally felt to be associated with improved outcomes. In addition, one should note that over this time period, changes in potential adjuvant strategies such as hepatic arterial chemotherapy and new systemic agents, including CPT-11, were pioneered at Memorial. As was discussed yesterday, the authors of this study reported in 1999 that adjuvant hepatic arterial radiotherapy was associated with improved 2-year survival rates. In light of these data and my knowledge from having been at Memorial in the past 5 years, it is my impression that a large proportion of patients in the latter 5 years of the study likely received hepatic arterial pumps, whereas in the first 5 years of the study it was much less common. Despite these issues, the authors did not include analyses incorporating the era in which the patients were treated and, more specifically, the adjuvant therapies the patients may have received.
Given these thoughts, I have a few questions. Did the adverse perioperative outcomes associated with transfusion hold up through these different intervals of time where you reported the transfusion rates to be markedly different? Specifically, if you compare patients with similar adjuvant treatments, does there exist an effect of transfusion on long-term outcome? Second, do the authors have any data on the intraoperative hemodynamics or the specific indications for the transfusion that may explain the different perioperative mortality? In addition, was the effect of transfusion separate from postoperative liver dysfunction? I as well, in addition to Dr. Choti, have a question regarding autologous transfusion and your indication for that. Is this blood given back as a matter of routine even if there do not exist other indications for transfusion? If so, what percentage of these patients did not have other indications for transfusion? Lastly, if indeed transfusing only a limited number of patients is associated with perioperative morbidity, have you changed your indications for transfusion? Have you changed the manner in which perform the parenchymal transection? More specifically, what is your experience with some of these newer devices for parenchymal transection, including the ligature device and the tissue link device? And lastly, do your anesthesiologists, whose pay more attention to the immediate intraoperative consequences, share your conclusions?
Dr. Clinton E. Baisden (Temple, TX): A few years ago we looked at our elderly coronary bypass and/or valve replacement patients to see if we could determine why some of them had perioperative strokes. We looked at a lot of preoperative, intraoperative, and postoperative variables. In addition to advancing age, atherosclerosis of the ascending aorta, hyperlipidemia, and hypertension, we found that a hemoglobin less than 7 grams during surgery was significantly associated with stroke. The number of transfusions was not. So my question is, did you look at lowest hemoglobin during surgery to see if it was a predictor of bad outcome in your patients? Dr. James C. Thompson (Galveston, TX): Logicians caution us not to be confused by events that are true, true, and not related. In 1921, the coroner of the City of Philadelphia wrote the president of the University of Pennsylvania telling him that he was greatly concerned by the fact that nearly everyone they had autopsied had been receiving this new intravenous fluid therapy, and he was concerned lest that was the cause of death. The president of the university wrote back to the coroner that they would certainly look into that, and at the same time they wanted to look into beds, too.
Dr. Yuman Fong (New York, NY): I think it is important to comment on Dr. Thompson’s comments first, and that is because it may be that transfusion is a surrogate marker of something else that is producing the complications. So the correlation of transfusion with complications should not be interpreted as a direct cause and effect relationship; this paper is merely trying to point out this correlation. Also important is the fact that patients receiving very few units of blood transfusion nevertheless have higher complication rates. In response to Dr. Bolton’s question, here are increased infectious complications in those transfused. The more transfusions that are done, the higher rate of total complications and infectious complications. Therefore, one hypothesis that could be addressed in future studies is whether by reducing those transfusions we may be able to reduce the complications, and whether by using blood conservation techniques we can reduce complications and improve outcome. Therefore, these studies are meant to emphasize that we should do all we can to reduce blood transfusion and conserve blood in these patients. It is not so much a question as to whether we can avoid using blood in those patients that have major bleeding; for those patients, the blood transfusion may be life-saving. The blood bank and the blood transfusional practice is what allows us to do these big operations now. The question is, can we reduce some of the transfusions, particularly in those patients who have had only one or two units of blood? There are other new techniques and not-so-new techniques for blood conservation that have been around that we don’t use very much, including hemodilution, cell saver, and use of leukocyte-depleted blood, things that may change the amount of leukocytes infused or total blood infused. Those are the things that we should concentrate on in these days when the blood supply is in increasing short supply many times of the year, particularly now that the donors from Europe and donors who have traveled to Europe have been taken out of our blood donation pool.
Dr. Bolton asked whether the rate of positive margins and the colorectal risk scores were equivalent in the patients not transfused and those transfused with very few units of blood. The answer is that the two groups looked equivalent. How do the patients die? In the patients who die after major transfusions, the eventual cause of death is usually multiorgan failure. But it is usually related to some infectious complication, either pneumonia or intra-abdominal abscess, that leads to the organ failure.
Dr. Choti asked about the immunologic mechanisms. These have been postulated for over 20 years. Nobody has clearly proven it. Investigators have done studies where they have measured immunologic parameters and shown that they are different in patients who got transfusion as opposed to those who did not get transfused. In our current study, there was no measurement of any immunologic parameter. Again, the infectious complications were different in the patients transfused and not transfused, but we cannot say for sure that immunologic derangements are what produced the difference.
Dr. Choti asked whether we have looked at transfusional practice in patients who had benign tumors resected and whether the outcomes are different. We have not yet done that analysis. That certainly is a very good suggestion that we will accept and hopefully show you some data in the future. In terms of autologous blood donation, it was disappointing to us that as hard as we have worked over the last 5 to 7 years to encourage people to donate blood, that we cannot show a difference in mortality depending on whether they donated blood or not. In this series, over 10% of the patients donated blood for themselves. What I showed was the 6% who only got back their own blood. It was not standard practice to give back the patient’s own blood just because the patient donated it ahead of time. We only gave it back according to strict transfusion criteria in terms of hemoglobin, hemodynamics. But when we look at the autologous blood issue, it is more than whether these patients do better clinically. We are also trying to preserve the blood supply. We are trying to increase the patient’s level of comfort about receiving blood transfusions. So I think there are other positives. I am hoping that if we have a larger series we may see some difference.
In terms of long-term outcome and chemotherapy, we have not directly looked at the chemotherapeutic backgrounds of these patients to stratify for which type of chemotherapy and how much chemotherapy. That certainly is a great recommendation by Dr. Clary, and we will go back and look at that.
Lastly, in terms of the conclusions. Again, we think that these data show that complications are higher in those patients who got blood transfusion. We don’t know whether it is a cause and effect. We certainly think that blood conservation makes sense. As Dr. Choti points out, these data do not support the supposition that long-term outcome in terms of cancer outcome is changed by transfusion, but that shouldn’t be very surprising. After a hepatectomy we know that the patient goes into an immunosuppressive state. We know that none of the NK cells in the liver, the Kupffer cells, work, for example. That has been shown in a number of animal models and in people. So to say that one or two units of extra blood transfusion is going to change the immunology such that the long-term cancer outcomes are different probably is asking too much. The cancer outcome is not different in these patients.
References
- 1.Gascon P, Zoumbos NC, Young NS. Immunological abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984; 100: 173–177. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan J, Sarnaik S, Gitlin J, et al. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984; 64: 308–310. [PubMed] [Google Scholar]
- 3.Peters WR, Fry RD, Fleshman JW, et al. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum. 1989; 32: 749–753. [DOI] [PubMed] [Google Scholar]
- 4.Ghio M, Contini P, Mazzei C, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: A possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999; 93: 1770–1777. [PubMed] [Google Scholar]
- 5.Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrence rate. Lancet. 1982; 2: 662. [DOI] [PubMed] [Google Scholar]
- 6.Foster RS, Costanza MC, Foster JC, et al. Adverse relationship between blood transfusions and survival after colectomy for colon cancer. Cancer. 1985; 55: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 7.Parrott NR, Lennard TWJ, Proud G, et al. Perioperative blood transfusion and recurrence of colorectal cancer. Br J Surg. 1986; 73: 505. [DOI] [PubMed] [Google Scholar]
- 8.Tartter PI. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg. 1992; 216: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiden PL, Bean MA, Schultz P. Perioperative blood transfusion does not increase the risk of colorectal cancer recurrence. Cancer. 1987; 60: 870–874. [DOI] [PubMed] [Google Scholar]
- 10.Tartter PI, Burrows L, Kirschner P. Perioperative blood transfusion adversely affects prognosis after resection of stage I (subset No) non-oat cell lung cancer. J Thorac Cardiovasc Surg. 1984; 88: 659–662. [PubMed] [Google Scholar]
- 11.Hyman NH, Foster RS, DeMeules JE, et al. Blood transfusions and survival after lung cancer resection. Am J Surg. 1985; 149: 502–507. [DOI] [PubMed] [Google Scholar]
- 12.Moores DWO, Piantadosi S, Mckneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989; 47: 346–351. [DOI] [PubMed] [Google Scholar]
- 13.Little AG, Wu HS, Ferguson MK, et al. Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg. 1990; 160: 630–633. [DOI] [PubMed] [Google Scholar]
- 14.Keller SM, Groshen S, Martini N, et al. Blood transfusion and lung cancer recurrence. Cancer. 1988; 62: 606–610. [DOI] [PubMed] [Google Scholar]
- 15.Nowak MM, Ponsky JL. Blood transfusion and disease-free survival in carcinoma of the breast. J Surg Oncol. 1984; 27: 124–130. [DOI] [PubMed] [Google Scholar]
- 16.Foster RS, Foster JC, Costanza MC. Blood transfusions and survival after surgery for breast cancer. Arch Surg. 1984; 119: 1138–1140. [DOI] [PubMed] [Google Scholar]
- 17.Voogt PJ, Vandevelde CJH, Brand A, et al. Perioperative blood transfusion and cancer prognosis: Different effects of blood transfusion on prognosis of colon and breast cancer patients. Cancer. 1987; 59: 836–843. [DOI] [PubMed] [Google Scholar]
- 18.Manyonda IT, Shaw DE, Foulkes A, et al. Renal cell carcinoma: blood transfusion and survival. Br Med J. 1986; 293: 537–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong Y, Karpeh M, Mayer K, et al. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg. 1994; 167: 256–260. [DOI] [PubMed] [Google Scholar]
- 20.Sugezawa A, Kaibara N, Sumi K, et al. Blood transfusion and the prognosis of patients with gastric cancer. J Surg Oncol. 1989; 42: 113–116. [DOI] [PubMed] [Google Scholar]
- 21.Kampschoer GHM, Maruyama K, Kinoshita T, et al. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989; 13: 637–643. [DOI] [PubMed] [Google Scholar]
- 22.Moriguchi S, Maehara Y, Akazawa K, et al. Lack of relationship between perioperative blood transfusion and survival time after curative resection for gastric cancer. Cancer. 1990; 66: 2331–2335. [DOI] [PubMed] [Google Scholar]
- 23.Kaneda M, Horimi T, Ninomiya M, et al. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion (Paris). 1987; 27: 375–377. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Seipp CA, White DE, et al. Perioperative blood transfusions are associated with increased rates of recurrence and decreased survival in patients with high-grade soft-tissue sarcomas of the extremities. J Clin Oncol. 1985; 3: 698–709. [DOI] [PubMed] [Google Scholar]
- 25.Francis DM. Relationship between blood transfusion and tumour behaviour. Br J Surg. 1991; 78: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 26.Vamvakas EC. Transfusion-associated cancer recurrence and postoperative infection: meta-analysis of randomized, controlled clinical trials. Transfusion (Paris). 1996; 36: 175–186. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995; 82: 582–587. [DOI] [PubMed] [Google Scholar]
- 28.McAlister FA, Clark HD, Wells PS, et al. Perioperative allogeneic blood transfusion does not cause adverse sequelae in patients with cancer: a meta-analysis of unconfounded studies. Br J Surg. 1998; 85: 171–178. [DOI] [PubMed] [Google Scholar]
- 29.Vamvakas E, Moore SB. Perioperative blood transfusion and colorectal cancer recurrence: a qualitative statistical overview and meta-analysis. Transfusion (Paris). 1993; 33: 754–765. [DOI] [PubMed] [Google Scholar]
- 30.Vamvakas EC. Perioperative blood transfusion and cancer recurrence: meta-analysis for explanation. Transfusion (Paris). 1995; 35: 760–768. [DOI] [PubMed] [Google Scholar]
- 31.Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1993; 80: 427–432. [DOI] [PubMed] [Google Scholar]
- 32.Duffy G, Neal KR. Differences in post-operative infection rates between patients receiving autologous and allogeneic blood transfusion: a meta-analysis of published randomized and nonrandomized studies. Transfus Med. 1996; 6: 325–328. [DOI] [PubMed] [Google Scholar]
- 33.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 34.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 35.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997; 15: 938–946. [DOI] [PubMed] [Google Scholar]
- 36.Belghiti J, Noun R, Zante E, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996; 224: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997; 226: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998; 187: 620–625. [DOI] [PubMed] [Google Scholar]
- 39.Chan AC, Blumgart LH, Wuest DL, et al. Use of preoperative autologous blood donation in liver resections for colorectal metastases. Am J Surg. 1998; 175: 461–465. [DOI] [PubMed] [Google Scholar]
- 40.Johnson LB, Plotkin JS, Kuo PC. Reduced transfusion requirements during major hepatic resection with use of intraoperative isovolemic hemodilution. Am J Surg. 1998; 176: 608–611. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson KR, Steinberg SM, Hughes KS, et al. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988; 208: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doci R, Gennari L, Bignami P, et al. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995; 82: 377–381. [DOI] [PubMed] [Google Scholar]
- 43.Younes RN, Rogatko A, Brennan MF. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991; 214: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992; 216: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarnagin WR, Bach AM, Winston CB, et al. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg. 2001; 192: 577–583. [DOI] [PubMed] [Google Scholar]
- 46.Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001; 91: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 47.Goldsmith N, Woodburne R. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957; 105: 310–318. [PubMed] [Google Scholar]
- 48.Couinaud C. Etudes anatomique et chirurgales. Paris: Mason, 1954.
- 49.Martin RC, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg 2002; 235: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Therneau TM, Grambsch PM. Modeling Survival Data. New York: Springer, 2001.
- 51.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makuuchi M, Takayama T, Gunven P, et al. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989; 13: 644–648. [DOI] [PubMed] [Google Scholar]
- 53.Itasaka H, Yamamoto K, Taketomi A, et al. Influence of blood transfusion on postoperative long-term liver function in patients with hepatocellular carcinoma. Hepato-Gastroenterology. 1995; 42: 465–468. [PubMed] [Google Scholar]
- 54.Matsumata T, Ikeda Y, Hayashi H, et al. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993; 72: 1866–1871. [DOI] [PubMed] [Google Scholar]
- 55.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001; 91: 771–778. [PubMed] [Google Scholar]
- 56.Fujimoto J, Okamoto E, Yamanaka N, et al. Adverse effect of perioperative blood transfusions on survival after hepatic resection for hepatocellular carcinoma. Hepato-Gastroenterology. 1997; 44: 1390–1396. [PubMed] [Google Scholar]
- 57.Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999; 23: 676–680. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994; 115: 303–309. [PubMed] [Google Scholar]
- 59.Tadros T, Wobbes T, Hendriks T. Blood transfusion impairs the healing of experimental intestinal anastomoses. Ann Surg. 1992; 215: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tadros T, Wobbes T, Hendriks T. Opposite effects of interleukin-2 on normal and transfusion-suppressed healing of experimental intestinal anastomoses. Ann Surg. 1993; 218: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998; 97: 562–568. [DOI] [PubMed] [Google Scholar]
- 62.Vamvakas EC, Carven JH. Allogeneic blood transfusion, hospital charges, and length of hospitalization: a study of 487 consecutive patients undergoing colorectal cancer resection. Arch Pathol Lab Med. 1998; 122: 145–151. [PubMed] [Google Scholar]
- 63.Bellantone R, Sitges-Serra A, Bossola M, et al. Transfusion timing and postoperative septic complications after gastric cancer surgery: a retrospective study of 179 consecutive patients. Arch Surg. 1998; 133: 988–992. [DOI] [PubMed] [Google Scholar]
- 64.Kinoshita Y, Udagawa H, Tsutsumi K, et al. Usefulness of autologous blood transfusion for avoiding allogenic transfusion and infectious complications after esophageal cancer resection. Surgery. 2000; 127: 185–192. [DOI] [PubMed] [Google Scholar]
- 65.Mynster T, Christensen IJ, Moesgaard F, et al. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000; 87: 1553–1562. [DOI] [PubMed] [Google Scholar]
- 66.Alfieri S, Carriero C, Caprino P, et al. Avoiding early postoperative complications in liver surgery. A multivariate analysis of 254 patients consecutively observed. Digestive Liver Dis. 2001; 33: 341–346. [DOI] [PubMed] [Google Scholar]
- 67.Cole DJ, Ferguson CM. Complications of hepatic resection for colorectal carcinoma metastasis. Am Surg. 1992; 58: 88–91. [PubMed] [Google Scholar]
- 68.Opelz G, Terasaki PI. Prolongation effect of blood transfusions on kidney graft survival. Transplantation. 1976; 22: 380–383. [DOI] [PubMed] [Google Scholar]
- 69.Adoute BG. Autotransfusion. Using Your Own Blood. New York: Springer-Verlag, 1991.
- 70.Meryman HT, Hornblower M. The preparation of red cells depleted of leukocytes: review and evaluation. Transfusion (Paris). 1986; 26: 101–106. [DOI] [PubMed] [Google Scholar]
- 71.Snyder EL. Prevention of HLA alloimmunization: role of leukocyte depletion and UV-B irradiation. Yale J Biol Med. 1990; 63: 419–427. [PMC free article] [PubMed] [Google Scholar]
- 72.Huggins CE, Russell PS, Winn HJ, et al. Frozen blood in transplant patients: hepatitis and HL-A isosensitization. Transplant Proc. 1973; 5: 809–812. [PubMed] [Google Scholar]
- 73.Karpoff HM, Tung C, Ng B, et al. Interferon gamma protects against hepatic tumor growth in rats by increasing Kupffer cell tumoricidal activity. Hepatology. 1996; 24: 374–379. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Supported in part by grants T32 CA 09501 (D.A.K.), RO1CA76416, RO1CA72632, and RO1CA61524 (Y.F.) from the National Institutes of Health and grant MBC-99366 (Y.F.) from the American Cancer Society.
Correspondence: Yuman Fong, MD, Memorial Sloan-Kettering Cancer Center, Department of Surgery, 1275 York Ave., New York, NY 10021.
E-mail: fongy@mskcc.org
Accepted for publication December 2002.