Abstract
Objective
To examine the breadth of application and resulting outcomes in a university-based extracorporeal membrane oxygenation (ECMO) program directed by pediatric surgeons.
Summary Background Data
Several randomized control trials have supported the use of ECMO in neonates with respiratory failure. No comparable data exist for older children and young adults who may be afflicted with a variety of uncommon conditions. The indications for ECMO in these patients remain controversial.
Methods
Patient data were recorded prospectively and reported to the Extracorporeal Life Support Organization. These data were analyzed by indications and outcomes on all patients treated since the inception of the program.
Results
Two hundred sixteen patients were treated with 225 courses of ECMO. Neonates (188 [87%]) outnumbered 28 older patients (aged 6 weeks to 22 years). Overall, 174 patients survived (81%). Sixty-four of 65 (98.5%) neonates with meconium aspiration syndrome survived. ECMO support after heart (3), lung (2), heart-lung (1), and liver (1) transplant yielded a 57% survival to discharge. ECMO also resulted in survival of patients with uncommon conditions, including severe asthma (1), hydrocarbon aspiration (1/2), congestive heart failure due to a cerebral arteriovenous malformation (1), tracheal occlusion incurred during endoscopic stent manipulation (2), meningitis (1), and viral pneumonia (3/5).
Conclusions
ECMO can potentially eliminate mortality for meconium aspiration syndrome. Survival for other causes of respiratory failure in neonates and older children, while not as dramatic, still surpasses that anticipated with conventional therapy. Moreover, survival of transplant patients has been comparable to that achieved in other children.
Extracorporeal membrane oxygenation (ECMO) was developed conceptually and experimentally in the 1960s. Several teams pioneered partial support of children and infants, 1,2 including a report by Haller and White. 3 In 1975 Bartlett’s team at University of California–Irvine successfully treated an infant with meconium aspiration syndrome. 4 This report stimulated a surge of interest in the technique as a method to salvage newborns with pulmonary hypertension caused by a variety of disorders. During the 1980s there was a rapid expansion of the number of ECMO programs. 5–8 Several randomized controlled trials confirmed that ECMO improves survival when used in infants with respiratory failure 9–11 but not in adult patients. 12,13 Substantial data suggest that ECMO also improves outcome in neonates with congenital diaphragmatic hernia. 14 These reports and others have resulted in acceptance of ECMO as the treatment of choice for refractory respiratory failure in newborns with meconium aspiration syndrome, sepsis, persistent pulmonary hypertension of the newborn, and congenital diaphragmatic hernia.
ECMO has also been used to support children and adults with a broad range of less common causes of pulmonary failure, only a few of which are referenced. 15–21 Most reports of such use are limited to case reports or very small series. Other reported uses of ECMO include support for cardiovascular instability before or after repair of congenital heart disease 22–25 or for perioperative support for heart 26–28 or lung transplantation. 29–31 Patients receiving support for these conditions have usually been reported separately from neonatal populations and may have been treated by different teams within the same institution. Rare instances of intraoperative ECMO for noncardiac support have also been reported. 32,33
At the University of Florida, pediatric surgeons who as a consequence of their training and experience may be uniquely equipped to oversee such an effort direct a single ECMO program. This program oversees all extracorporeal life support activities at our institution, with the exception of prolonged bypass following complex congenital heart repairs. A small group of patients cannulated in the operating room who have survived the perioperative period and who have been converted to conventional ECMO are included in this report. This paper documents our experience using both veno-arterial (V-A) and veno-venous (V-V) ECMO for treating patients with a variety of illnesses resulting in refractory pulmonary hypertension or pulmonary or cardiac failure occurring between birth and adulthood, reflecting the age range seen in a pediatric surgery practice.
METHODS
A multidisciplinary team of surgeons, other critical care physicians, respiratory therapists, and nurses provide ECMO care in our institution for patients with reversible causes of pulmonary or cardiac failure. The service was organized in 1991. Care is provided at the bedside in the neonatal, pediatric, cardiac, or medical intensive care unit.
All infants less than 45 days of age at the time of ECMO were treated in the neonatal unit and are considered neonates for the purpose of data analysis. Ventilation for most neonates has been provided with a pressure-limited, time-cycled synchronized intermittent mandatory ventilator. Since 1992 we have emphasized permissive hypercapnia, with minimal, not maximal, ventilator settings for neonates, as previously described. 34 A variety of modern ventilation techniques are employed, including high-frequency oscillatory ventilation with ancillary measures that include nitric oxide and surfactant.
Indications for instituting ECMO support include inability to ventilate or oxygenate patients with safe ventilator settings. Neonates with an oxygenation index of greater than 40 for 4 hours are considered ECMO candidates in our hospital-based criteria, which were developed using a review of patients treated in the 1980s that suggested a high mortality in this group. 35 Independent of the oxygenation index, failure to provide satisfactory oxygenation, as judged by inadequate tissue perfusion, declining organ function, and rising serum lactic acid levels, is considered an indication for ECMO. Failure of ventilation in patients with adequate oxygenation is a less common problem, but ECMO is considered for patients with a Paco2 persistently higher than 85 mmHg.
At the inception of the program all patients were supported with V-A ECMO. Cannulation was performed at the bedside through an incision in the right neck with ligation of both the right carotid artery and right internal jugular vein. V-V ECMO using a single double-lumen cannula placed via cutdown was introduced in our unit in April 1994, with subsequent adoption in July 1998 of the percutaneous V-V method described by Reickert et al. 36 These three distinct periods represent the major changes in the technical performance of neonatal ECMO during the period of study. Larger children and adults cannot be supported with available small double-lumen cannulas and require simultaneous dual cannulation of the jugular and femoral veins. Cannulation for second runs of ECMO has been successful with both V-A and V-V techniques reusing vessels in the right neck, employing thrombectomy and dilation if required.
During ECMO, ventilator settings are manipulated to achieve mean airway pressures of less than 10 cm of water in neonates and less than 15 cm of water in older patients. The Fio2 is adjusted to 0.40 or less, and positive end-expiratory pressure is maintained at less than 10 cm of water. Continuous heparin infusion is used to keep activated clotting times between 190 and 220 seconds (typical). Echocardiograms are recorded frequently before and during ECMO to assess ventricular dimensions and wall motion. Cardiotropic support, nitric oxide, and other vasoactive medication may be employed based on the underlying pathology. Weaning from ECMO is based on clinical parameters. Cannulas may be left in position for 24 to 36 hours after discontinuation of ECMO in case of sudden decompensation. If the patient has been on V-A ECMO, no attempt is made to repair the carotid artery. CT scans or ultrasounds of the brain are obtained on all patients surviving ECMO before discharge.
Since the inception of our ECMO program, patient data have been recorded prospectively and reported to the Extracorporeal Life Support Organization (ELSO). This information stored in the ELSO database was requested for our center and compared with original data. Few errors were found, and these were corrected. For the purpose of this analysis ELSO data without identifiable patient identifiers were used. The University of Florida Institutional Review Board allows such use as an exempt protocol. Missing data are reported as such. Data related to indications and outcomes on all patients treated since the inception of the program are included. Statistical tests were performed using the SAS system for PC.
RESULTS
Two hundred sixteen patients were treated with 225 courses of ECMO. One hundred thirty-five (63%) were male and 81 (37%) were female. Neonates (188 [87%]) represent the majority of this experience and 28 patients (45 days to 22 years of age) make up the remainder. The most common diagnostic groups are listed in Table 1. Diagnoses recorded as “other” include asthma (1), hydrocarbon aspiration (2), tracheal occlusion during stent manipulation with inability to ventilate and pneumothorax (2), Potter syndrome (1), interstitial emphysema (1), near drowning (1), and congestive heart failure in a neonate due to an intracerebral arteriovenous malformation. Bacterial sepsis treated included one case of streptococcal meningitis. The three viral infections treated in neonates were influenza A (1), adenovirus (1), and cytomegalovirus (1).
Table 1. DIAGNOSES REQUIRING ECMO SUPPORT, WITH DIAGNOSIS-SPECIFIC SURVIVAL
Neonates ranged in birth weight from 1,880 g to 5,329 g, with a mean birth weight of 3,248 ± 617 g. Mean gestational age ranged from 32 to 42 weeks, with a mean of 38.8 ± 2.0 weeks. Apgar scores at 1 minute ranged from 0 to 9 (median 4), while 5-minute Apgars ranged from 0 to 10 (median 7). Delivery was accomplished vaginally in 86 (47%), by elective cesarean section in 46 (25%), and by emergent cesarean section in 50 (26%), with 2% of the data missing. Boys outnumbered girls 119 (63%) to 69. Forty-five (24%) of our neonatal patients were born at our hospital and the remaining 143 were transported to Shands Children’s Hospital specifically for evaluation and treatment by the ECMO team.
Our older patients ranged in age from 48 days of age to 23.4 years (median 5.8 years). There were 16 boys and 12 girls. In contrast to our neonatal program, only 3 of the 28 (11%) were transferred from other institutions for ECMO support. The remainder of these children were already receiving treatment in our hospital and were referred by colleagues within the medical center.
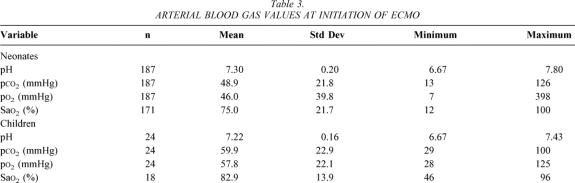
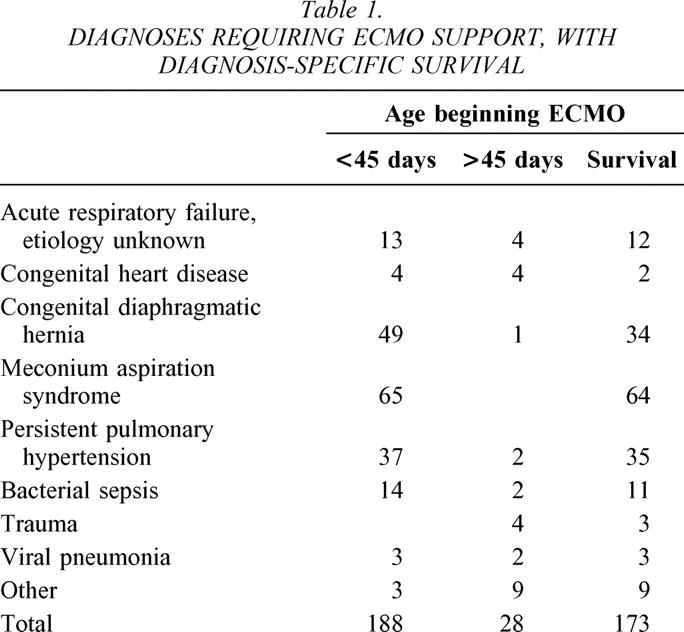
Neonates were treated primarily with conventional ventilators. At the time ECMO was instituted, 163 were on a conventional ventilator, 1 was on high-frequency oscillatory ventilation, 1 was on a jet ventilator, and 14 were being hand-ventilated with an Ambu-bag (data for 9 patients missing). Four other neonates had a previous trial of high-frequency oscillatory ventilation. Twenty-one of the older children were on conventional ventilators at the time ECMO commenced, while seven were ventilated using high-frequency oscillatory ventilation. Ventilator settings recorded both before and 24 hours after initiation of ECMO are listed in Table 2. Most patients were both moderately acidotic and hypoxemic at the onset of ECMO. The mean values and ranges for arterial pH, Pco2, and Po2 immediately before beginning ECMO are listed in Table 3, along with the same values 24 hours after initiating ECMO support.
Table 2. VENTILATOR SETTINGS AT INITIATION OF ECMO AND 24 HOURS LATER
Table 3. ARTERIAL BLOOD GAS VALUES AT INITIATION OF ECMO
At the time of ECMO, 164 neonates (87%) were on vasopressors or inotropic medication, while 35 (19%) were on intravenous vasodilators. Nitric oxide was used to support 83 neonates of 117 (71%) cared for since the drug became available at our institution in March 1996. Surfactant use was documented in 32 neonates of 137 (23%) treated after it became available in October 1994. Paralysis was used in 42 patients early in the series; the remainder were sedated but not paralyzed. Systemic acidemia was treated with sodium bicarbonate in 43 children and with THAM in 10. Support for older children included inotropic or vasopressor support in 19, intravenous vasodilators in 4, nitric oxide in 18 of 22 (82%) treated after the drug became available, and surfactant in 2.
Initial ECMO cannulation was V-A in the 45 patients treated before April 1994. Between April 1994 and July 1998 66 patients were treated. Thirty-eight of these were placed on V-V ECMO (58%) with 6 converted to V-A ECMO, which was used initially in 27 patients (41%). Since July 1998 105 patients have been treated, of whom 89 (85%) have been placed on V-V ECMO with 16 converted to V-A, and another 16 (15%) were treated with V-A ECMO from the outset. Interestingly, 6 (21%) of the older children were cannulated and supported using V-A ECMO and 3 of the 22 initially treated with V-V ECMO had to be converted to V-A ECMO. Thus, the proportion of older children on V-A ECMO is similar to our current practice in neonates.
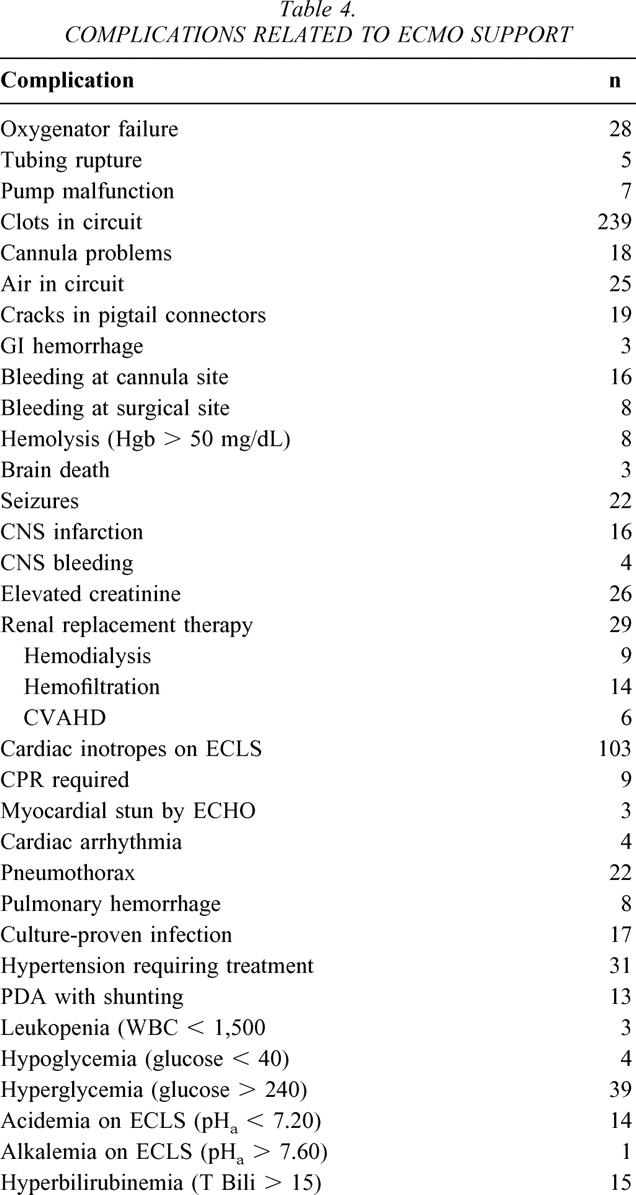
Adverse events occurred during 196 of the 225 courses of ECMO. Between 1 and 12 adverse events were noted for individual ECMO runs. The specific adverse events or complications are listed in Table 4. No patient deaths could be directly attributed to malfunction of the circuit, despite a number of equipment failures. While many adverse events were trivial or of only potential importance, the most serious were devastating, and included brain death (3), cerebral infarction (16), cerebral hemorrhage (4), and seizures (22). These 45 events occurred in 33 patients, representing 15% of the total population. Neurotoxicity was not confined to neonates but occurred with equal frequency in older patients. Taking the entire series as a whole, these complications were more common on V-A ECMO than on V-V ECMO (P < .01) in both neonates and children. The association has not held up recently, however. The incidence was 29.6% in 45 patients treated with V-A ECMO before April 1994. Between April 1994 and July 1998 the incidence was 29.6% in 27 patients treated with V-A ECMO and 3% in patients treated with V-V ECMO. Since July 1998 the incidence has been 6% in V-A ECMO, 10% in V-V ECMO, and 25% in patients converted from V-V to V-A ECMO (P = .10). Other complications of note included significant peripheral thrombotic problems in one of two neonates treated with epsilon-aminocaproic acid (Amicar), leading to this patient’s death.
Table 4. COMPLICATIONS RELATED TO ECMO SUPPORT
Survival to discharge from the hospital was achieved for 175 (81%) patients. Survival was better for the 146 patients referred from other hospitals specifically for ECMO than for 70 inpatients (86% vs. 67%, P < .01). Neonates had a better survival (85% vs. 54%, P < .01) than older patients. Second courses of V-A ECMO were used in seven babies with congenital diaphragmatic hernia, three of whom survived (43%). Treatment of refractory RDS after heart (3), lung (2), heart-lung (1), and liver (1) transplant yielded a 57% survival to discharge. ECMO resulted in survival of patients with severe asthma (1), hydrocarbon aspiration (1/2), congestive heart failure due to a cerebral arteriovenous malformation (1), tracheal occlusion from malacia during stent manipulation (2), meningitis (1), and viral pneumonia (3/5).
DISCUSSION
Over the past three decades, the use of ECMO for support of children has changed dramatically. Improved results have encouraged most physicians to consider ECMO when confronted by a patient with life-threatening but potentially reversible hypoxemia. A significant investment was made by our institution and faculty to ensure the absence of a “learning curve.” This paper suggests that while quality of service for uncomplicated newborns with meconium aspiration syndrome was good from the outset, the expanded use of ECMO typical in a busy medical center illustrates several areas where progress has been achieved over the past decade, and identifies existing problems that require further improvement.
Our data support others 37 who have suggested that modern methods used in neonatal intensive care units have dramatically decreased the historically high mortality associated with meconium aspiration syndrome. With current use of nitric oxide, 38,39 “gentle” ventilation, 40 and surfactant replacement, 41 the need for ECMO in this population has diminished. 42–44 Those children requiring ECMO can generally be cared for with V-V ECMO with good results. The babies with meconium aspiration syndrome that we have found most difficult to care for are those with significant barotrauma sustained as a consequence of prolonged treatment at a non-ECMO center before referral for treatment. Unfortunately, the incidence of such delays has not significantly decreased. In fact, with the advent of NO and high-frequency oscillating ventilators, such occurrences are more frequent today than 10 years ago. 45,46 While in our experience such delay has not resulted in increased mortality in those patients successfully completing transfer for treatment, these infants are inevitably exposed to an increased risk of catastrophic complications, which may render transport difficult or impossible. 47 Therefore, we would suggest that optimal treatment for babies with meconium aspiration syndrome includes early use of surfactant, gentle ventilation with either conventional methods or low-pressure high-frequency oscillation, the use of nitric oxide for infants exhibiting profound hypoxemia, and early transfer of such infants to an ECMO center in case of abrupt decompensation.
Optimal treatment for the remainder of infants with respiratory distress is not as clearly defined. Significant improvement in the care of infants with congenital diaphragmatic hernia has been made with the realization of the critical importance of limiting barotrauma in these babies with hypoplastic lungs. Survival to discharge from the hospital has dramatically improved in our center 34 and in many others. 48–50 Nevertheless, these children may have persistent or recurrent pulmonary hypertension 51 and may require both prolonged and occasionally repeat ECMO runs, 52 exposing them to an increased hazard of complications. Neonates with viral infections or sepsis and those with persistent pulmonary hypertension of the newborn have fewer problems than babies with congenital diaphragmatic hernia, but a higher rate compared with infants with meconium aspiration syndrome. In all of these children, more effective methods to specifically treat the underlying disorder should improve the results of generally supportive measures such as ECMO. One common link in all of these patients is the increased need for cardiovascular support. V-A ECMO has been used preferentially for many of these conditions because it decompresses the right ventricle and provides direct support of mean arterial pressure and central perfusion.
Our results suggest that V-A support may incur an added risk of neural toxicity. This increased incidence of neurotoxicity was apparent in our series before but not after July 1998. Some authors have failed to show such a clear difference, 53 while others have confirmed our findings. 54,55 We do not have a plausible explanation for the recent decrease in these events noted in our own series. CT scans on all patients regardless of physical examination over this later period suggest that decreased detection of clinically silent events is not a factor. The fact that neural toxicity is seen in older children as well as neonates, that it occurs in all diagnoses, and that percutaneous access to the jugular vein has increased our complication rates compared with cutdowns suggest to us that damage to the carotid artery may be related to the complications. Others have suggested carotid repair to prevent long-term neurologic complications, but data presented to date have failed to demonstrate any short-term benefit. Techniques designed to protect desperately ill neonates and children from neurologic complications of long-term bypass would have a profound outcome on the results we are currently able to achieve.
ECMO support either preceding or following repair of congenital heart disease continues to be associated with a higher mortality rate than the use of ECMO for other disorders, both in our series as well as others. There has been a significant interest in early and more aggressive application of the technique in certain clinical situations, although this is still controversial. 56 Our own experience is too limited to clarify this particular issue.
The role of ECMO for patients who have exhibited hypoxemia after organ transplantation and immunosuppression has been controversial. Some authors have suggested that ECMO not be used in this setting. 57 Others have reported increasing success in its use after heart, 26–28 lung, 29–31 and bone marrow transplantation 58 and in patients receiving chemotherapy. 59 Our results in a small number of patients suggest that the use of ECMO in such patients who have hypoxemia due to viral infections or pulmonary hypertension results in survival identical to that of patients with similar problems who have not had an organ transplant.
Finally, there are many uncommon conditions that result in hypoxemia for which ECMO may be used. We have successfully used ECMO intraoperatively for support during bronchoscopic removal of tracheal stents twice, for the treatment of inhalation injury from a house fire, and for the treatment of hydrocarbon aspiration pneumonitis with success in one of two patients. To our knowledge, the successful use of ECMO for congestive heart failure, and hypoxemia during treatment of an intracranial arteriovenous malformation, with survival is unique. None of these conditions is common enough, nor are all combined common enough, to support clinical expertise required for a high-quality ECMO program. One of the benefits from such a commitment is the freedom ECMO provides to physicians and healthcare workers to provide the best supportive care possible for a broad range of babies and children suffering from the effects of hypoxemia.
CONCLUSIONS
ECMO has essentially eliminated mortality for meconium aspiration syndrome. Survival for RDS in other neonates and older children has improved dramatically from earlier reports. Survival rates for transplant patients with RDS are similar to those for other pediatric patients. ECMO is a life-saving clinical tool for treating a selected group of infants and children suffering from refractory RDS from a broad variety of etiologies.
Discussion
Dr. Samuel D. Smith (Little Rock, AR): I appreciate the opportunity to review the manuscript and to hear the excellent presentation. I have a few comments and about four questions to ask.
My comments would be that this is a large ECMO experience with neonates, but it is a fairly small pediatric series with only 28 patients, and you could see the difference in survival and the continued challenge of pediatric ECMO, with only 54% surviving versus 85% in the neonates.
The authors state that the in-house patients did worse than the ones who were transferred. Most of their pediatric patients were in-house, so once you mixed that in, it wouldn’t be surprising that the mortality would be higher on the patients from within their center.
They had a trend in the increased use of veno-venous or percutaneous cannulation method. And I think that is a tribute to their skill in doing ECMO, because it is lot harder to do veno-venous and support patients.
The information that I found interesting to me as a pediatric surgeon was the use of a second course of ECMO in seven patients who had diaphragmatic hernia. And while you may have commented on this in previous presentations, it obviously leads me to ask: How did you know to do it again? Why did you have to do it again? And you had a 40% survival after the second run. With that kind of survival, why didn’t the pediatric patients get a second run of ECMO if their survival was only 54% with a single run?
The problem with pediatric series in regards to ECMO support is that the indications for ECMO vary from institutions and within institutions as to whether to use alternative modes of ventilation before using any other sorts of therapy.
In your series, you make it clear if you have an oxygenation index for the neonates greater than 40 for 4 hours, that is an indication for ECMO. In your pediatric series it varied from inability to ventilate with CO2 retention, inability to oxygenate, and it looked like it was much more variable. I would appreciate any information you can provide in that regard.
Only 7 of the 21 older children were ventilated using some type of alternative mode of ventilation. In our institution the intensivists are into alternative modes of ventilation before they will consider ECMO. Why were only 7 of the 21 children ventilated using high-frequency oscillator or some other mode of alternative ventilation?
You mentioned in at least the manuscript that you had seven episodes of CPR. I didn’t really know whether those were before going on ECMO support or during ECMO support with some of the complications. I would be interested if you have any information on the patients that needed CPR. At least in my hands and in the other people in my institution’s hands, if you are doing CPR at the time you are having to put someone on ECMO, they are neurologically not going to do very well if they survive to come off ECMO.
Dr. J. Alex Haller, Jr. (Baltimore, MD): I also want to congratulate the authors on an excellent paper and thank them for letting me see the manuscript. I have just a few comments about the groups of patients that were managed with ECMO.
If there are any obstetricians in the audience I think they would immediately pick up the very high percentage of these patients who had meconium aspiration syndrome. I wanted to ask whether that was a reflection of referrals from other obstetrical services, or just what was going on. Modern obstetrical management can more or less prevent meconium aspiration syndrome by early aspiration of the trachea as the baby’s head is delivered, and yet this is a very sizable group in your series.
The second comment has to do with the use of ECMO for patients with overwhelming pulmonary sepsis. I think it is obvious, but it needs to be emphasized, that this is a particularly important technology. Because if these patients are ventilated, then you have the additional barotrauma from the ventilator that adds to their pulmonary infections, and that simply compounds the problem of pulmonary damage. So the use of ECMO—an artificial lung—clearly has improved the management of those patients by avoiding mechanical ventilation.
You did not mention anything about the use of the oscillator-type ventilation, particularly for some of the meconium aspiration patients. Do you use oscillation ventilation in your unit? Is this an adjunct, or do you use it preferentially in certain patients?
Finally, I noticed that you have gone from the use of the carotid artery for your perfusion to veno-venous perfusion. Is that because you are worried about sacrificing the carotid artery? What are the advantages of veno-venous circulation rather than veno-arterial?
Dr. Alden H. Harken (Denver, CO): Dr. Langham, I am very impressed with not only your presentation of this paper, but also this has to be the penultimate example of the marriage of bioengineering with complex patient physiology. To carry this off in a hospital is exceedingly difficult, and you and your group must be commended for that.
I am interested in generating a hypothesis out of your cutoff at 2 months. What happens to babies at 2 months that makes them respond differently to this major therapeutic insult? Lots of groups have noticed that little kids or babies really don’t get multiple organ failure or ARDS at the same incidence that adults do. Is that because immunologically or from an inflammatory standpoint these kids become adults at 2 months of age?
I was interested at the juxtaposition of the previous paper and the observation that the ventilator days were prolonged in the staged closure of the gastroschisis. Is that because with continued open environment of the child’s abdomen, there is a continued smoldering inflammation that provokes lung damage in the delayed closure of patients?
Ultimately, my question is, what is the etiological mechanism of ARDS? Most of us believe that this is a neutrophil-mediated phenomenon. If so, is there a difference, can you characterize the difference in the neutrophil of the under-2-month-old patient in their primed versus activated versus class and stage relative to superoxide production, beta-integrin expression, adhesion molecules, elastase production?
And then, ultimately, a question for both you and Dr. Haller. If indeed kids become, immunologically and from an inflammatory standpoint, adults at age 2 months, might a 3-month-old child better be treated in an adult hospital?
Dr. Ross M. Ungerleider (Portland, OR): It is clear that the neurological outcomes that are poor are the most devastating complication of the application of ECMO. And it seemed that the neurological outcome failures were most prevalent in the kids who were sickest at the time they were placed on ECMO, melactic acidosis was the greatest in those who had been delayed for ECMO over the longest period.
So the question is simply now, as we have entered an era where there are so many other modalities available for treating these babies before they get to ECMO, how do you make your decision to put a baby on ECMO and yet prevent them from being treated too long with all these other modalities, which would only increase their neurological risk?
Dr. Max R. Langham, Jr. (Gainesville, FL): Dr. Smith asked how we chose whether to do a second run on babies with diaphragmatic hernia. I don’t think that there is an absolute answer to that. Part of it is stubbornness. I have been fortunate to have a partner who has a passion for this work, David Kay, and sits at the bedside of these babies watching them.
Many times patients who have done well and come off of ECMO and who clearly have enough pulmonary horsepower to survive have some adverse event that tips them over. As you and other pediatric surgeons in the audience know, once you are on this type of slippery slope, things go pretty quick. The ability to recoup during the second run of ECMO has given some of these children, although obviously not all of them, a second chance—which begs the question of why don’t we use second runs of ECMO for the patients who are older.
I think that the biases in this study are that of an ECMO program that was designed by pediatric surgeons who are interested in congenital diaphragmatic hernia. That is why we have an ECMO program at Florida, and that is why we have kept it up within our division.
We perhaps could be more aggressive with older children. And I think as we get more comfortable with some of these processes and as our techniques improve and the complications drop, we may in fact find older patients who would benefit from a second run. But we would be looking for a patient who had recovered and who had the pulmonary horsepower, had the ability, to do okay, but who somehow got on a slippery slope and ended up back in trouble again.
What are the indications for ECMO in older patients, and do we use the OI less and less commonly in newborns? I think the indications for newborns and older patients are blending together. A patient who cannot be maintained in aerobic metabolism with adequate perfusion of their vital organs is somebody who needs ECMO.
If you have a patient who is in renal failure, or who has a rising lactic acid, or who has a declining mean arterial pressure, that cannot be supported with inotropic support, who is on a ventilator with nitric oxide and so forth who cannot maintain oxygen delivery to his mitochondria, that patient is well served going on ECMO. And that becomes a clinical judgment.
Alternate modes of ventilation instead of ECMO; we have oscillatory ventilators. They are used primarily—and this goes to one of Dr. Haller’s questions—primarily in newborns who are premature. Those patients who have bronchopleural fistulae can be very nicely handled with oscillating ventilators.
Older patients in the PICU are probably not quite as good candidates, in our experience, as newborns. In some of these patients we have used inverse ratio ventilation, or jet ventilation—which I personally hate—and a number of other modalities. But quite frankly, when you are good at ECMO, you end up doing more ECMO.
Finally, Dr. Smith asked about CPR going on to ECMO. Most of the patients who required CPR were patients who were going on to ECMO. A couple of them required CPR on ECMO. Those patients arrested during circuit changes. When a problem arises and the circuit goes to hell and you have to clamp somebody out who has no lungs, you have to get the circuit fixed quickly, but you also have to be ready to pump on the chest while you are getting the circuit going again.
Dr. Haller talked about a high incidence of meconium aspiration syndrome, and that is absolutely true. One of the great resources we have at the University of Florida is the superb transport services set up by Jim Talbert in the early ‘70s after he arrived in Gainesville. We have not had an in-house meconium aspiration syndrome who needed ECMO, Dr. Haller, that I can recall. We take patients from south Georgia and north Florida, central Florida, and sometimes from south Florida.
Sepsis, I think, is a major issue. I think that if ECMO could work well for sepsis, that we would be much busier with ECMO. I think that there are some underlying problems both with the pulmonary morbidity of previous ventilatory treatment but also problems with treating specific viruses and some very aggressive bacteria.
Finally, why do we push veno-venous ECMO? There have been several authors that looked at cerebral hemodynamics during veno-arterial ECMO. While a majority of patients survive with no neurological complications, it has been clearly documented that cerebral blood flow is altered by ligation of carotid artery. In our experience, complications are higher with V-A ECMO; therefore, we feel that if veno-venous ECMO will work, why not use it?
Dr. Harken asks, as usual, stimulating questions. What is the pathophysiology of older patients that differ from the babies? My take on it is that the disease processes are different. Diaphragmatic hernias who are older don’t need ECMO. Folks don’t get meconium aspiration syndrome when they are 2 months old, and persistent pulmonary hypertension of a newborn does not occur outside 2 months of age. So I think it is the underlying disease process that is different.
Your tongue-in-cheek question about whether the patient should be treated in an adult hospital hits close to home, since we are a general hospital at Shands. We have a children’s hospital within a hospital. But we do have, I think, some advantages, particularly in some of the older patients and have resources that many children’s hospitals may not have.
Finally, Dr. Ungerleider points out a major problem in ECMO today. Patients are frequently treated with nitric oxide, surfactant, high-frequency oscillation, and the kitchen sink, and only when their lungs are completely shredded and they are grasping at straws do they get sent for ECMO. And those patients in fact do have more neurological problems. I think that systematic organization with early application of ECMO in those patients most likely to benefit would largely reduce the neurological complications we have presented.
References
- 1.Rashkind WJ, Freeman A, Klein D, et al. Evaluation of a disposable plastic, low volume, pumpless oxygenator as a lung substitute. J Pediatr. 1965; 66: 94–102. [DOI] [PubMed] [Google Scholar]
- 2.Dorson W Jr, Meyer B, Baker E, et al. Response of distressed infants to partial bypass lung assist. Trans ASAIO. 1970; 16: 345. [PubMed] [Google Scholar]
- 3.White JJ, Andrews HG, Rinsemberg H, et al. Prolonged respiratory support in newborn infants with a membrane oxygenator. Surgery. 1971; 70: 288–296. [PubMed] [Google Scholar]
- 4.Bartlett RH, Gassaniga AB, Jefferies R, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans ASAIO. 1976; 22: 80–88. [PubMed] [Google Scholar]
- 5.Krummel TM, Greenfield LJ, Kirkpatrick BV, et al. Clinical use of an extracorporeal membrane oxygenator in neonatal pulmonary failure. J Pediatr Surg. 1982; 17: 525–531. [DOI] [PubMed] [Google Scholar]
- 6.Hardesty RL, Griffith BP, Debski RF, et al. Extracorporeal membrane oxygenation: successful treatment of persistent fetal circulation following repair of congenital diaphragmatic hernia. J Thorac Cardiovasc Surg. 1981; 81: 556–563. [PubMed] [Google Scholar]
- 7.Short BL, Pearson GD. Neonatal extracorporeal membrane oxygenation: ar review. J Intens Care Med. 1986; 1: 47–54. [Google Scholar]
- 8.Loe W, Grave E, Ochsner J, et al. Extracorporeal membrane oxygenation for newborn respiratory failure. J Pediatr Surg. 1985;. 20: 684–688. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985; 4: 479–487. [PubMed] [Google Scholar]
- 10.O’Rourke PP, Krone R, Vacanti J, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989; 84: 957–963. [PubMed] [Google Scholar]
- 11.UK Collaborative ECMO Trial Group. UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996; 348: 75–82. [PubMed] [Google Scholar]
- 12.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe respiratory failure. JAMA. 1979; 242: 2193–2196. [DOI] [PubMed] [Google Scholar]
- 13.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Resp Crit Care Med. 1994; 149: 295–305. [DOI] [PubMed] [Google Scholar]
- 14.The Congenital Diaphragmatic Hernia Study Group. Does extracorporeal membrane oxygenation improve survival in neonates with congenital diaphragmatic hernia? J Pediatr Surg. 1999; 34: 720–724. [DOI] [PubMed] [Google Scholar]
- 15.Alpard SK, Zwishchenberger JB. Extracorporeal membrane oxygenation for severe respiratory failure. Chest Surg Clin North Am. 2002; 12: 355–378. [DOI] [PubMed] [Google Scholar]
- 16.Seo T, Ando H, Watanabe Y. Acute respiratory failure associated with intrathoracic masses in neonates. J Pediatr Surg. 1999; 34: 1633–1637. [DOI] [PubMed] [Google Scholar]
- 17.Sreenan CD, Osiovich H. Neonatal pertussis requiring extracorporeal membrane oxygenation. Pediatr Surg Int. 2001; 17: 201–203. [DOI] [PubMed] [Google Scholar]
- 18.O’Toole G, Peek G, Jaffe W, et al. Extracorporeal membrane oxygenation in the treatment of inhalation injuries. Burns. 1998; 24: 562–565. [DOI] [PubMed] [Google Scholar]
- 19.Masiakos PT, Islam S, Doody DP, et al. Extracorporeal membrane oxygenation for nonneonatal acute respiratory failure. Arch Surg. 1999; 134: 375–379. [DOI] [PubMed] [Google Scholar]
- 20.Goldman AP, Kerr SJ, Butt W, et al. Extracorporeal support for intractable cardiorespiratory failure due to meningococcal disease. Lancet. 1997; 349: 466–469. [DOI] [PubMed] [Google Scholar]
- 21.Linden V, Palmer K, Reinhard J, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000; 26: 1630–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aharon AS, Drinkwater DC Jr, Churchwell KB, et al. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Ann Thorac Surg. 2001; 72: 2095–2101. [DOI] [PubMed] [Google Scholar]
- 23.Mehta U, Laks H, Sadeghi A, et al. Extracorporeal membrane oxygenation for cardiac support in pediatric patients. Am Surg. 2000; 66: 879–886. [PubMed] [Google Scholar]
- 24.Magovern GJ Jr, Simpson KA. Extracorporeal membrane oxygenation for adult cardiac support: the Allegheny experience. Ann Thorac Surg. 1999; 68: 655–661. [DOI] [PubMed] [Google Scholar]
- 25.Kulik TJ, Moler FW, Palmisano JM, et al. Outcome-associated factors in pediatric patients treated with extracorporeal membrane oxygenator after cardiac surgery. Circulation. 1996; 94: II63–68. [PubMed] [Google Scholar]
- 26.Hopper AO, Pageau J, Job L, et al. Extracorporeal membrane oxygenation for perioperative support in neonatal and pediatric cardiac transplantation. Artif Organs. 1999; 23: 1006–1009. [DOI] [PubMed] [Google Scholar]
- 27.Ishino K, Weng Y, Alexi-Meskishvili V, et al. Extracorporeal membrane oxygenation as a bridge to cardiac transplantation in children. Artif Organs. 1996; 20: 728–732. [PubMed] [Google Scholar]
- 28.Adamson RM, Dembitsky WP, Daily PO, et al. Immediate cardiac allograft failure. ECMO versus total artificial heart support. ASAIO J. 1996; 42: 314–316. [PubMed] [Google Scholar]
- 29.Zenati M, Pham SM, Keenan RJ, et al. Extracorporeal membrane oxygenation for lung transplant recipients with primary severe donor lung dysfunction. Transplant Int. 1996; 9: 227–230. [DOI] [PubMed] [Google Scholar]
- 30.Koutlas TC, Bridges ND, Gaynor JW, et al. Pediatric lung transplantation—are there surgical contraindications. Transplantation. 1997; 63: 269–274. [DOI] [PubMed] [Google Scholar]
- 31.Chan CY, Chen YS, Ko W, et al. Extracorporeal membrane oxygenation support for single lung transplantation in a patient with primary pulmonary hypertension. J Heart Lung Transplant. 1998; 17: 325–327. [PubMed] [Google Scholar]
- 32.Morneault L, Johnston A, Perreault T. Management of acute airway obstruction using extracorporeal membrane oxygenation. ASAIO J. 1996; 42: 321–323. [PubMed] [Google Scholar]
- 33.Connolly KM, McGuirt WF Jr. Elective extracorporeal membrane oxygenation: an improved perioperative technique in the treatment of tracheal obstruction. Ann Otol Rhinol Laryngol. 2001; 110: 205–209. [DOI] [PubMed] [Google Scholar]
- 34.Kays DW, Langham Jr. MR, Ledbetter DJ, et al. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg. 1999;230:340–351. [DOI] [PMC free article] [PubMed]
- 35.Langham MR Jr, Longmate JA, Elliott MJ, et al. ECMO criteria: an unsolved problem. Pediatr Res. 1992; 31: 208A. [Google Scholar]
- 36.Reickert CA, Schreiner RJ, Bartlett RH, et al. Percutaneous access for venovenous extracorporeal life support in neonates. J Pediatr Surg. 1998; 33: 365–369. [DOI] [PubMed] [Google Scholar]
- 37.Gross I. Recent advances in respiratory care of the term neonate. Ann NY Acad Sci. 2000; 900: 151–158. [DOI] [PubMed] [Google Scholar]
- 38.Cornfield DN, Maynard RC, deRegnier RA, et al. Randomized, controlled trial of low-dose inhaled nitric oxide in the treatment of term and near-term infants with respiratory failure and pulmonary hypertension. Pediatrics. 1999; 104: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 39.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2001;(4):CD000399. [DOI] [PubMed]
- 40.Wung JT, James LS, Kilchevsky E, et al. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985; 76: 488–494. [PubMed] [Google Scholar]
- 41.Marks SD, Nicholl RM. The reduction in the need for ECMO by using surfactant in meconium aspiration syndrome. J Pediatr. 1999; 135: 267–268. [DOI] [PubMed] [Google Scholar]
- 42.Roy BJ, Rycus P, Conrad SA, et al. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics. 2000; 106: 1334–1338. [DOI] [PubMed] [Google Scholar]
- 43.Hintz SR, Suttner DM, Sheehan AM, et al. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics. 2000; 106: 1339–1343. [DOI] [PubMed] [Google Scholar]
- 44.Kennaugh JM, Kinsella JP, Abman SH, et al. Impact of new treatments for neonatal pulmonary hypertension on extracorporeal membrane oxygenation use and outcome. J Perinatol. 1997; 17: 366–369. [PubMed] [Google Scholar]
- 45.Wilson JM, Bower LK, Thompson JE, et al. ECMO in evolution: the impact of changing patient demographics and alternative therapies on ECMO. J Pediatr Surg. 1996; 31: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 46.Gill BS, Neville HL, Khan AM, et al. Delayed institution of extracorporeal membrane oxygenation is associated with increased mortality rate and prolonged hospital stay. J Pediatr Surg. 2002; 37: 7–10. [DOI] [PubMed] [Google Scholar]
- 47.Wilson BJ, Heiman HS, Butler TJ, et al. A 16-year neonatal/pediatric extracorporeal membrane oxygenation transport experience. Pediatrics. 2002; 109: 189–193. [DOI] [PubMed] [Google Scholar]
- 48.Boloker J, Bateman DA, Wung JT, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 49.Muratore CS, Kharasch V, Lund DP, et al. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg. 2001; 36: 133–140. [DOI] [PubMed] [Google Scholar]
- 50.Weber TR, Kountzman B, Dillon PA, et al. Improved survival in congenital diaphragmatic hernia with evolving therapeutic strategies. Arch Surg. 1998; 133: 498–502. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz IP, Bernbaum JC, Rychik J, et al. Pulmonary hypertension in children following extracorporeal membrane oxygenation therapy and repair of congenital diaphragmatic hernia. J Perinatol. 1999; 19: 220–226. [DOI] [PubMed] [Google Scholar]
- 52.Dela-Cruz TV, Stewart DL, Robinson TW, et al. The use of a second course of extracorporeal membrane oxygenation in neonatal patients. ASAIO J. 1996; 42: 230–232. [PubMed] [Google Scholar]
- 53.Knight GR, Dudell GG, Evans ML, et al. A comparison of venovenous and venoarterial extracorporeal membrane oxygenation in the treatment of neonatal respiratory failure. Crit Care Med. 1996; 24: 1678–1683. [DOI] [PubMed] [Google Scholar]
- 54.Dimmitt RA, Moss RL, Rhine WD, et al. Venoarterial versus venovenous extracorporeal membrane oxygenation in congenital diaphragmatic hernia: the Extracorporeal Life Support Organization Registry, 1990–1999. J Pediatr Surg. 2001; 36: 1199–1204. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatr Surg Int. 1999; 15: 78–84. [DOI] [PubMed] [Google Scholar]
- 56.Langley SM, Sheppard SV, Tsang VT, et al. When is extracorporeal life support worthwhile following repair of congenital heart disease in children? Eur J Cardiothorac Surg. 1998; 13: 520–525. [DOI] [PubMed] [Google Scholar]
- 57.Macha M, Griffith BP, Keenan R, et al. ECMO support for adult patients with acute respiratory failure. ASAIO J. 1996; 42: M841–844. [DOI] [PubMed] [Google Scholar]
- 58.Leahey AM, Bunin NJ, Schears GJ, et al. Successful use of extracorporeal membrane oxygenation (ECMO) during EMT for SCID. Bone Marrow Transplant. 1998; 21: 839–840. [DOI] [PubMed] [Google Scholar]
- 59.Linden V, Karlen J, Olsson M, et al. Successful extracorporeal membrane oxygenation in four children with malignant disease and severe Pneumocystis carinii pneumonia. Med Pediatr Oncol. 1999; 32: 25–31. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Max R. Langham, Jr., MD, Division of Pediatric Surgery, Box J-100286 JHMHC, Gainesville, FL 32610–0286.
E-mail: langham@surgery.ulf.edu
Accepted for publication December 2002.