Abstract
Objective
To prospectively compare the Lower Extremity Grading System (LEGS)-derived “recommended treatment” to the actual treatment performed and to analyze LEGS intergrader scoring consistency by comparing blinded scoring results between physician graders.
Summary Background Data
Due to technical advances and the increased medical complexity of the aging population, the most appropriate treatment for chronic lower extremity ischemia—open surgery versus endovascular—is again in flux. In an attempt to standardize management, the LEGS score, based on the best available outcomes data, was devised by the physicians of an established vascular service.
Methods
From March to June 2002, all chronically ischemic lower extremities that met standard indications for revascularization were prospectively enrolled and independently graded with the LEGS score by an “endovascular surgeon” and an “open surgeon” for comparative analysis. The results were then blindly evaluated to determine whether the LEGS-derived “recommended treatment” agreed with the actual treatment rendered and to assess for intergrader consistency. Agreement was assessed using kappa statistical analysis.
Results
Of the 137 presenting limbs (mean patient age 66.4 yo; 43% claudication, 57% limb-threatening ischemia), 107 were treated (65% endovascular, 30% open surgery, 5% amputation), 16 were pending treatment, and 14 were not treated because of patient refusal (n = 13) or death (n = 1). The LEGS score predicted the actual or offered clinical treatment in 90% of cases. The LEGS score comparison between physician graders resulted in identical “recommended treatment” in 116 of 128 cases for a 90.6% agreement.
Conclusions
A reproducible scoring system to guide the treatment of patients with chronic lower extremity ischemia is possible. While systems like the LEGS score may have potential clinical application, their use as a treatment standardization tool for future prospective outcomes comparisons between open and endovascular surgery will be essential.
As the number of medically complex elderly patients increases, chronic lower extremity ischemia presenting as claudication or limb-threatening ischemia will continue to be an important clinical problem. At one time, the treatment of chronic limb ischemia was the exclusive domain of the surgeon, who assigned patients to medical therapy, angioplasty, or open surgery based on presentation. However, much has changed over the past decade. Multiple medical specialties, including general surgery, vascular surgery, cardiothoracic surgery, vascular medicine, cardiology, and interventional radiology, now independently treat a growing population of patients with symptoms of chronic lower extremity ischemia. A trend toward more invasive therapy has also emerged, supported by outcomes data, 1–3 driven by technical endovascular advances and motivated by economic incentives. As different specialists have entered the treatment realm, literature regarding the most appropriate use of angioplasty and surgery is often ignored. The care rendered has become as diverse as the treatment backgrounds of the providers rendering the care. Unfortunately, the management of chronic lower extremity ischemia has fallen into a state of flux.
Treatment standardization that can be applied across specialty lines is needed. Realizing this, we devised an objective scoring system designed to recommend the most appropriate invasive therapy, angioplasty, reconstructive surgery, or primary amputation, for the treatment of chronic lower extremity ischemia. The scoring system, developed using the best available outcomes data and our collective experience, takes into consideration a variety of clinical factors and theoretically directs care, neutralizing the specialty bias of the treating physician. The purpose of this study, therefore, was to prospectively assess the utility of this system as a reproducible scoring tool. Specifically, this was done by blindly comparing the “recommended treatment” as directed by the scoring system with the actual treatment performed by the treating physician and by analyzing intergrader scoring consistency by comparing blinded scoring results between physician graders with differing specialty backgrounds.
METHODS
Grading System
From September 2001 through February 2002, we developed and agreed on a novel lower extremity ischemia scoring system to direct the most appropriate invasive therapy at presentation—endovascular intervention, open surgery, or primary amputation. For the purpose of the study the scoring system was then incorporated into the practice of our physician group, which consisted of six non-competing vascular surgeons and two vascular internists. Since 1998 all open and endovascular procedures have been performed by members of this group. Within the group the endovascular expertise is variable, with three surgeons having only open surgical interests and no endovascular privileges, three surgeons having both open surgical and endovascular privileges, one vascular internist with extensive endovascular experience but no open surgical privileges, and one vascular internist with neither open surgical or endovascular skills or privileges. Before the study, lower extremity cases referred to the group were distributed equitably to all attendings, and treatment was based on the individual attending’s clinical judgment. The use of endovascular surgery versus open surgery was often inconsistent and was subject to the bias of the treating physician. In a previously reported analysis during the 2-year period 1999 to 2000, the group invasively treated approximately 625 limbs for chronic lower extremity ischemia, 76% with open surgery and 24% with endovascular intervention. 4
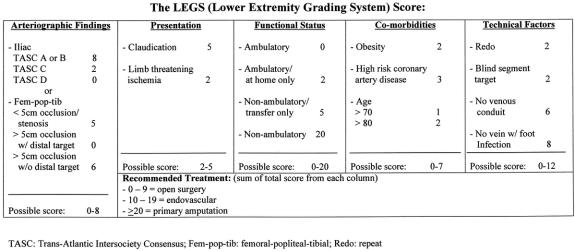
In constructing the system, five categories were established for the scoring of each limb: 1) the arteriographic anatomic findings, 2) the clinical presentation (claudication vs. limb-threatening ischemia), 3) the functional status of the patient, 4) medical comorbidities, and 5) other technical factors. The result was the Lower Extremity Grading System (LEGS) score (Fig. 1). In the system points are given to each of the five categories of clinical consideration, and the sum of these points results in a grade that recommends treatment (0–9 points = open surgery; 10–19 points = endovascular intervention; ≥20 points = primary amputation).
Figure 1. The LEGS score, used to recommend invasive treatment for patients with chronic lower extremity ischemia.
The first category in the scoring system, which considered the arteriographic findings, was divided into iliac occlusive disease and femoral-popliteal and tibial disease. A total of 8 points could be awarded in this category. Arteriographic anatomic findings were classified using the Trans-Atlantic Inter-Society Consensus (TASC) as a guide. 5 The TASC classification for aortoiliac occlusive disease (Table 1) 5 increases in anatomic involvement, and thus overall clinical severity, from A to D, with angioplasty being generally more acceptable for TASC A and B lesions and surgery for TASC C and D lesions. With femoro-popliteal-tibial arterial occlusive disease, the TASC classification was modified. By definition our scoring system defined a “distal target” for infrainguinal disease as any patent vessel (popliteal artery or any tibial artery) of more than 20 cm in length distal to a proximal occlusion (≥5 cm), regardless of runoff to the foot. A “blind segment” arterial target was defined as a patent arterial segment greater than 10 cm but less than 20 cm distal to a proximal occlusion (≥5 cm). Any named artery that reconstituted distal to a proximal occlusion for less than 10 cm or did not reconstitute at all was classified as “no distal target.”
Table 1. TRANSATLANTIC INTER-SOCIETY CONSENSUS (TASC) CLASSIFICATION FOR ILIAC ARTERIAL OCCLUSIVE DISEASE

CIA, common iliac artery; EIA, external iliac artery; CFA, common femoral artery.
Transatlantic Inter-Society Consensus Working Group. Outcomes assessment methodology in peripheral arterial disease. J Vasc Surg. 2000;31: S35–S43.
The second clinical category in the scoring system considered symptoms at presentation. These were categorized as claudication or limb-threatening ischemia. A total of 5 points could be awarded in this category. Claudication was defined as leg/foot pain with ambulation, relieved at rest, and associated with a diminished ankle/brachial index (<0.8 at rest or after exercise). Limb-threatening ischemia was defined as a limb with ischemic rest pain (ankle/brachial index < 0.5), ischemic ulcers (ulcers and an ankle/brachial index < 0.5), or ischemic foot gangrene.
The third category in the scoring system dealt with overall functional status. A total of 20 points could be given in this category. For the purpose of the scoring system, functional status was classified as “ambulatory” in patients who were fully functional but were experiencing symptoms of chronic lower extremity ischemia. Functional status was described as “ambulatory at home” in patients who were severely hampered by physical disabilities other than chronic lower extremity ischemia (e.g., severe arthritis, medical comorbidities, advanced age) but were able to ambulate without assistance at home and had chronic lower extremity ischemia. Functional status was defined as “non-ambulatory/transfer only” in patients with severe disabilities in addition to chronic lower extremity ischemia who required assistance with any ambulation or who were only able to bear weight to transfer. Functional status was defined as “non-ambulatory” in bedridden patients incapable of any ambulatory effort.
The fourth category in the scoring system considered comorbidities and awarded a total of 7 points. Comorbidities factored into the scoring system included obesity, coronary artery disease, and advanced age. A patient was considered “obese” if he or she met the generally accepted criteria for morbid obesity, a body mass index (weight in kilograms divided by the height in meters2) greater than 35. By definition, the patient was considered “high risk for coronary artery disease” if his or her cardiac risk score as derived by the Eagle Criteria was 3 or more. 6
The last category of the scoring system dealt with other technical factors that may alter treatment decisions, including “redo surgery,” blind segment arterial anatomy (previously defined), the lack of venous conduit, and the presence or absence of active foot infection. A total of 12 points were possible in this category. A patient requiring an additional operation on the same arterial segment was defined as needing a “redo” procedure. “No venous conduit” was defined as 1) a patient with previous bilateral greater saphenous vein stripping or harvesting and no other venous option; 2) no upper or lower extremity vein by venogram; or 3) no adequate vein by direct exploration. A patient was considered to have “no other venous option” when a patent greater or lesser saphenous vein in the contralateral extremity was present but with significant chronic limb ischemia in that extremity such that harvest would potentially result in a healing problem; when usable upper extremity vein was present but in a patient with impending end-stage renal disease; or when available vein was present but of insufficient length or quality to be used for bypass, as judged by the operating surgeon. “Foot infection” was defined as acute or chronic cellulitis in the presence of ischemia to include chronic osteomyelitis, severe lymphangitis, or groin lymphadenopathy. The presence of foot infection without adequate autogenous conduit increased the patient’s score, favoring endovascular treatment, because of the risk of infection with a prosthetic conduit.
Application of the LEGS Score
The LEGS score is applicable for limbs that present with chronic lower extremity ischemia or its immediate sequelae (e.g., forefoot gangrene). The implication before scoring is that the primary problem is chronic ischemia and that the problem will be corrected once the ischemia is alleviated. Limbs with incidental ischemia but with a more acute presenting problem such as gas gangrene or overwhelming foot sepsis should have the more acute problem treated before scoring. Feet that are not salvageable because of advanced ischemia, infection, or other systemic diseases are not applicable for LEGS scoring. However, if the foot sepsis or other problem can be stabilized to where the chronic ischemia becomes the primary problem, then LEGS scoring is applicable.
Conversely, legs with chronic lower extremity ischemia are graded only when, in the judgment of the treating physician, invasive intervention is clinically indicated. In general, intervention, whether open surgery or endovascular surgery, is reserved for patients with limb-threatening ischemia or severe recalcitrant claudication. Patients are not graded when they originally present to the clinic with claudication or mild ischemia amenable to medical therapy.
Each case presenting for LEGS scoring before grading is defined upfront as a bilateral or unilateral leg problem. For example, a patient with severe bilateral aortoiliac occlusive disease and well-compensated claudication who develops a unilateral ischemic ulcer with osteomyelitis and thus unilateral limb-threatening ischemia could be defined in one of two ways: 1) a bilateral leg problem with new onset of tissue loss, tipping the scale away from conservative medical management and toward invasive treatment of both legs, or 2) a unilateral leg problem with limb-threatening ischemia mandating intervention only on that affected leg, accepting the stable, well-compensated claudication in the contralateral limb. Since the grading system for aortoiliac disease depends on the TASC classification, where bilaterality affects the class, it is necessary to clinically define the goals of treatment as bilateral (scenario 1) or unilateral (scenario 2) upfront, since the LEGS-derived “recommended treatment” will potentially change.
Each ischemic limb or pair of limbs that present with multisegmental arterial disease are graded with a suprainguinal score and an infrainguinal score. Treatment then proceeds using the principle of repairing the proximal disease before the distal disease. If, in the judgment of the treating physician, surgical indications for intervention persist after correction of the proximal (suprainguinal) disease, the limb is then re-scored using the distal (infrainguinal) score, and a new recommended treatment is derived.
Study Methods
Before patient enrollment, the LEGS score concept and this proposed study were approved by the Investigational Review Committee of the Greenville Hospital System. From March 1, 2002, through June 1, 2002, all limbs with evidence of chronic lower extremity ischemia that met indications for invasive intervention, as judged by the treating physicians, were prospectively enrolled into this analysis of the LEGS scoring system. The analysis was divided into two parts. First, the scoring system was assessed to determine if the LEGS score-derived “recommended treatment” agreed with the actual treatment performed, which during the study period was directed by the clinical judgment of the treating physician. Second, the LEGS scoring system was assessed for intergrader consistency by comparing blinded individual scores of the same patient graded independently by two physicians with different specialty training backgrounds.
To compare “recommended treatment” to actual treatment, scores were calculated and recorded by a single individual (C.A.K.) not involved in the direct treatment of any of the patients and confirmed by at least one other author not involved in the care of the patient. The treatment of the patient, therefore, was blinded in every possible case from the scoring process. Actual treatment then proceeded based on the clinical judgment of the attending physician. The “recommended treatment” and actual treatment were then recorded and compared.
Intergrader consistency was assessed by comparing the calculated scores and “recommended treatments” of all limbs as graded by two groups of physician graders. In every case, each limb was scored by an individual who in his practice performs primarily endovascular surgery and then by an individual who in his practice performs nearly all open surgery. These two scores, calculated by physicians with theoretically opposite treatment biases, were blindly recorded and compared for agreement of total score and for agreement of “recommended treatment.”
Inclusion/Exclusion Criteria
All patients presenting with symptoms of chronic lower extremity ischemia were considered for prospective enrollment. Patients not meeting the clinical criteria as stated in the applications of the LEGS score section (see above) were excluded from enrollment. All ischemic limbs that presented with failed previous vascular reconstructions that were not previously enrolled in the study were excluded. Patients with acute ischemia (manifested by acute neurologic changes requiring emergent or urgent intervention) were excluded from enrollment. For the purpose of this study, microscopic atheroembolism (blue-toe syndrome) to the lower extremity was considered acute ischemia and thus was excluded.
Patients could be enrolled only if at presentation they were candidates for both endovascular therapy and surgical intervention. If the patient had medical or surgical issues that precluded one or the other treatment modalities, he or she was excluded from analysis. While rare, an example of this might be a patient with severe chronic renal failure who, in the judgment of the attending physician, would develop dialysis dependency because of contrast nephropathy after endovascular therapy.
Statistical Analysis
Statistical significance, where appropriate, was determined by the one-sample t test. The extent of agreement was determined using the kappa statistic. The kappa statistic is defined as a measure of agreement “beyond chance.” The interpretation of kappa was based on the classification proposed by Fleiss (1981) where values of 0.75 or greater are considered excellent agreement, 0.4 to 0.7 fair to good agreement, and 0 to 0.4 poor agreement.
RESULTS
During the 3-month study period, 87 patients with 137 chronically ischemic limbs were enrolled for analysis. The patients (white, n = 71 [81.6%]; African-American, n = 16 [18.4%]; diabetes present, n = 41 [47.1%]) had a mean age of 66.4 ± 11.8 years (range 43–90 years) and presented with limb-threatening ischemia (n = 78 [57%]) more frequently than claudication (n = 59 [43%]).
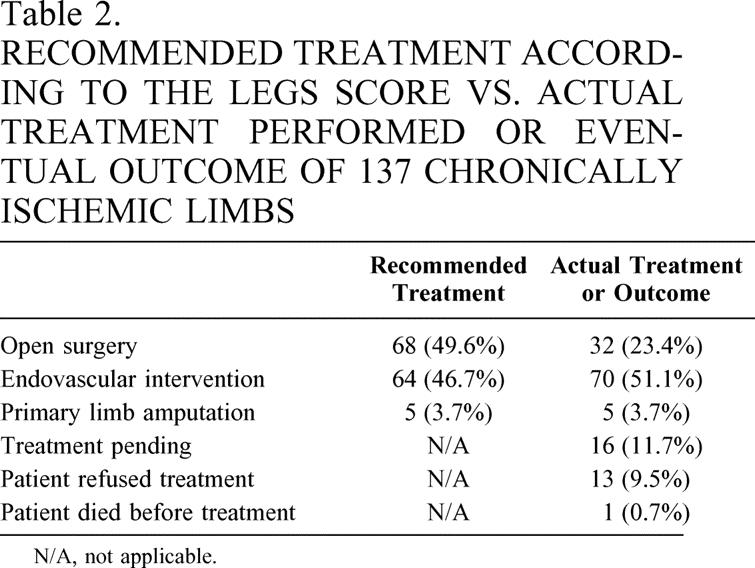
The recommended treatment as determined by the LEGS score and the outcomes that occurred on all 137 limbs are shown in Table 2. To summarize, at the end of the study period 107 limbs had been treated (65% by endovascular surgery; 30% by open bypass/endarterectomy; 5% by primary amputation), 1 patient (1 limb) died before treatment, 16 were pending treatment, and 13 refused treatment. Interestingly, 12 of these last 13 patients underwent arteriography with hopes of being candidates for angioplasty/stent, only to refuse treatment when open surgery was offered as the only option.
Table 2. RECOMMENDED TREATMENT ACCORDING TO THE LEGS SCORE VS. ACTUAL TREATMENT PERFORMED OR EVENTUAL OUTCOME OF 137 CHRONICALLY ISCHEMIC LIMBS
N/A, not applicable.
Recommended Treatment Versus Received Treatment
In 17 limbs (1 ischemic limb in a patient who died before a decision about treatment was made and 16 limbs where treatment was pending), the disposition of management was indeterminate. Because of the indeterminate nature of disposition in these 17 limbs, they were excluded from the analysis. Thus, in 107 cases, treatment was performed, and in 13 cases treatment was clearly offered but refused. Therefore, 120 limbs were available for comparison of LEGS score “recommended treatment” with actual or offered treatment. Of the 120 limbs, “recommended treatments” agreed with actual treatment in 108 limbs, for a percentage agreement of 90.0%. This resulted in an overall kappa of 0.81 (95% confidence interval 0.71–0.91).
Intergrader Consistency
Of the 137 limbs independently graded by an endovascular interventionalist and then by an open surgeon, “pull-back” arterial blood pressures were performed across suspicious angiographic atherosclerotic lesions in the aortoiliac vessels of nine limbs to assess the hemodynamic effect of the questionably significant lesions. This information was available only to the “endovascular” surgical graders, who factored these data into their grades, and not to the “open” surgical graders. Thus, these nine limbs were excluded from analysis. Of the remaining 128 limbs, the average limb score determined by the “endovascular interventionalist” was 9.43 ± 4.80 (median 10; range 2–23) and the average score determined by the “open surgeon” was 9.26 ± 4.81 (median 10; range 2–23). There was no statistical difference in total scoring between the two graders (P = .28). Overall agreement in the “recommended treatment” between graders occurred in 116 of 128 limbs for a percentage agreement of 90.6%. This resulted in an overall kappa of 0.82 (95% confidence interval 0.73–0.92).
DISCUSSION
This study represents an effort by a group of diverse vascular specialists to devise a scoring system that will direct the treatment of chronic lower extremity ischemia based on a series of clinical considerations and objective physical findings. This effort resulted in the LEGS score. Analysis showed that the LEGS score prospectively predicted the eventual treatment rendered in 90% of ischemic legs scored. In addition, there was minimal variability in eventual “recommended treatment” among graders when using the score. Kappa statistics confirmed excellent agreement for both parameters studied, treatment and intergrader consistency. We believe this study is important because it shows that the surgical treatment of chronic lower extremity ischemia can be objectively standardized. While the LEGS score in its current form could be used, as it is in our practice, to direct care, it should not be considered an authoritative source on the treatment of leg ischemia at this time. Although the scoring system was devised assimilating current available outcomes data, it still contains the group’s biases, right and wrong, and will result, if followed, in a management plan that would tend to mimic treatment seen in our practice. What is apparent, however, is that with modification this scoring system could be a very useful tool to direct the clinical management of lower extremity ischemia, neutralizing the training background of the treating physician. While the prospect of this is encouraging, much refinement is needed.
In its current form, the score’s present value is in its potential ability to standardize treatment. Why is standardization important? There are very few well-constructed randomized studies comparing angioplasty with bypass surgery for the treatment of leg ischemia. Indeed, most reports are case series or prospective studies that contain severely flawed selection bias. 7,8 While randomization of patients using appropriate selection and reporting criteria 8,9 continues to be the purest form of standardization, it is often difficult to do and fails to build on accepted results that have been accumulated to date. For example, do we really need to randomize a middle-aged man with an isolated common iliac artery stenosis and claudication to angioplasty versus surgery when we already know a great deal about the expected outcomes of this treatment? Clearly the LEGS score acknowledges a basic acceptance of the conventional results we have learned, including which procedures have the lowest mortality, the best reconstruction patency rates, and the highest limb salvage rates. Its application then allows us to build on these accepted outcomes and to study new ones.
To this end, are there other important outcomes measures pertaining to this subject that need to be examined? Increasingly, respected authoritative leaders in the treatment of lower extremity ischemia have exposed several cracks in the foundation of our accepted surgical outcomes data pertaining to lower extremity revascularization. 10,11 Long felt to be above critical reproach, the infrainguinal venous surgical bypass, which achieves limb salvage and patency rates of 90% and 85% respectively at 3 to 5 years, 12 has suddenly became the focus of criticism as it pertains to functional outcomes. Using their own data, the vascular group at the University of Oregon, a group who has reported arguably the best conventional lower extremity bypass results in the world, noted that fewer than 15% of patients achieved the ideal results of an uncomplicated surgery, prompt wound healing, relief of distal ischemia, maintenance of independent living status, resumption of ambulation, and no need for surgical reintervention. They concluded that this ideal outcome was distinctly infrequent and suggested that the future will demand more than the conventional knowledge of graft or angioplasty patency rates and limb salvage expectations to decide the most appropriate treatment for limb ischemia. 11,13 Perhaps it is appropriate to perform a femoral artery angioplasty for a newly discovered ischemic ulcer and accept a 45% 1-year patency rate if it results in low morbidity, prompt healing of the ulcer, maintenance of ambulation, independent living status, and a return to baseline for the patient.
Outcomes data need to be redefined, adding among other things independent living status and maintenance of ambulation to the conventional accepted measures of graft patency and limb salvage. In this regard, we really do not know if angioplasty or surgery is superior. Much work needs to be done. A standardization tool for selection of therapy will be essential as other new outcome endpoints are defined and examined. Clearly a scoring system such as the LEGS score can provide a backdrop for new outcomes research on chronic lower extremity ischemia.
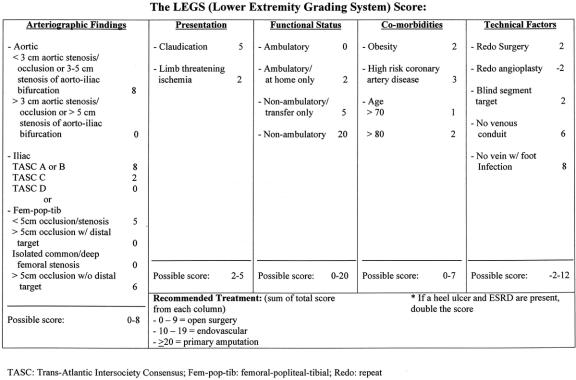
We have learned a great deal while working with the LEGS score. Restricted by the design of this study, individuals used the scoring system independently with essentially no group discussion of any particular case. We believe that this scoring system will be of even greater value when accompanied by group discussion of borderline cases. It is also paramount to stress that this current scoring system pertains only to cases with surgical indications for intervention; it cannot be applied to all patients who present to the clinic for a first-time consultation. The application of the LEGS score was found to be relatively straightforward. However, there were several clinical problems that arose that were not addressed in the original scoring system. For instance, disease isolated to the aorta was not specifically considered. In addition, isolated disease confined to the common femoral artery and its immediate branches, the profunda and superficial femoral arteries, was classified as being amenable only to surgery, with no angioplasty option available. This was an exclusion criterion that precluded patients from prospective entry into the study. “Redo” surgery, which implies failed surgery, favored (+2 points) angioplasty. There was, however, no consequence or penalty to favor surgery for cases of failed angioplasty (“redo” angioplasty). Finally, the difficult problem of chronic heel ulcers, which are occasionally seen in patients with end-stage renal disease, was not addressed. A modified LEGS score (Fig. 2) was therefore produced, adding provisions to deal with all of these specific clinical situations. Patient enrollment using this new scoring scale has proceeded at our institution, looking now at various outcomes measures after intervention.
Figure 2. A modification of the LEGS score, currently used at our institution.
In conclusion, this study shows that a reproducible scoring system to direct and standardize the treatment of chronic lower extremity ischemia is possible. As a standardization method the LEGS score can be a potentially valuable outcomes research tool for the comparison of endovascular and open surgery. With appropriate modification, such a scoring system can also be adapted in the future to direct the best clinical treatment of chronic lower extremity ischemia by practitioners of various training backgrounds and treatment biases.
Discussion
Dr. David B. Adams (Charleston, SC): I am a great admirer of Dr. Taylor’s surgical leadership in the upstate of South Carolina, and I have been impressed how he has exerted a positive force in developing a system that has brought the very best in multidisciplinary vascular care to his community. The work presented today represents a small fraction of Dr. Taylor’s long-standing interest in vascular registries, outcomes analysis, and innovative therapies.
Although I am not a practicing vascular surgeon, my participation in a multidisciplinary GI group speaks to some of the major issues of this report, and other issues out of my area of expertise were discussed with one of my vascular colleagues, Tom Brothers. So let me share with you some concerns that we have with this report.
First, we should note that this scoring system was developed by a cohesive group of physicians and surgeons who carry a preexisting consensus into the system. The question I ask is whether LEGS can be applied in a different hospital, different state, and different country.
A second issue relates to the high number of exclusion criteria utilized. Aren’t you excluding an important group of patients when you exclude those with prior reconstruction, acute ischemia, and the blue-toe syndrome?
My third query concerns the 30 patients with treatment pending, those who refused treatment, and then the one who died. Would their inclusion in your treatment strategies have changed the results? Could you comment more about the indeterminate group?
My fourth question relates to a treatment pathway not taken; that is, nonintervention. In outcomes analysis we examine quality-of-life issues. Sometimes we should consider quality of death, since all of our treatments are ultimately palliative. Should a treatment strategy consider that some patients would prefer to end their life nonambulatory but with all their limbs present?
The LEGS score selects the worst patients for endovascular therapy. The New York Times Sunday Magazine has a frequent ad featuring a nattily dressed, robust man beaming after his intravascular intervention. He must have a low LEGS score, yet he is the poster child for endovascular treatment. The question I ask is, why not? Why not switch your strategy and do open surgery for high-risk patients and endovascular therapy for low-risk patients?
Most of the issues I raised are thoughtfully addressed in the manuscript, which I am grateful to have had the chance to review, and I am eager to give Dr. Taylor a chance to clarify these points on the podium, as well as to welcome him to this Association.
Dr. J. David Richardson (Louisville, KY): I also had the opportunity to read the manuscript and certainly commend it to our membership. This paper does represent an attempt that I think is certainly laudable, which is to create a standardized scoring system that would allow comparisons of outcomes to be made on patients that are stratified to a certain treatment group, whether it is open, endovascular, or amputation alone, based on this LEGS score. And I think the LEGS thing is acute.
In the manuscript, Dr. Taylor and his group note that this reflects the reality of their practice in which a group of vascular surgeons have agreed to see patients, to score them and monitor their outcomes. His vascular group, I think, includes surgeons with what do strike me to be world-class catheter-based skills that are often not available to many of us in our respective practices around the country.
In the LEGS system that Dr. Taylor presented, patients with a high score were recommended for endovascular treatment. In our practice in Louisville, patients with a low score would in fact be the ones that almost certainly would be treated by radiologists or cardiologists or other interventionalists who by and large in our community are not surgeons, while surgeons often end up seeing the end-stage patient with the only option being a high-risk revascularization or an amputation. So how representative do you think your practice is in a world where cardiologists and radiologists tend to “cherry pick” those patients who would have low scores?
And I guess the second question then, Dr. Taylor, is, did you actually prospectively use this to treat patients, or were you just sort of monitoring it as you treated patients as you ordinarily would?
I would note in my practice there are patients on whom I have done a distal bypass who would have certainly had a very high score in this system. For example, I can think of octogenarians in which we did distal bypasses to allow them to be able to pivot and turn on their preserved limb, which kept them out of a nursing home and allowed a family member to take care of them. If endovascular treatment is not available, I would presume that you would still go ahead and treat that patient or offer them an open operation, as many of us have heretofore done, assuming the conduit is available.
I do think this scoring system is a good first step by the authors who, as noted, have excellent reliability. I guess the question I would have, then, is, what is the next step, and where are you going to take it from there?
Dr. Thomas H. Schwarcz (Lexington, KY): I think that this, as Dr. Richardson says, is a very important step to start trying to make valid comparisons between open and endovascular therapy. However, my impression looking at this scale is it is very biased towards endovascular surgery, and obviously that may be the bias of your group for management of that patient population.
I think you clearly prove that given a certain set of criteria and arteriograms, that the physicians or surgeons will all agree on what the score should be. That does not necessarily mean that treatment is what should be done for each patient. We all use our clinical judgment, so to speak, to do what is right for each patient individually. This is not something you can computerize. However, I think this approach is an important first step.
I think next you would have to look at the outcomes of applying this system to see if the treatments from endovascular and open therapy do actually turn out to be equally effective; or is one better, and then modify the system. Do you have plans to do that in the future?
Dr. Spence M. Taylor (Greenville, SC): I appreciate all the discussants’ comments, and I agree with everything that has been said.
Until we have long-term follow-up, this scoring system should not be judged as right or wrong. I have no idea whether the suggested treatment should be considered the right way or the wrong way to treat patients. It simply mimics the way our practice treats patients. While we believe we treat patients correctly, we acknowledge that we have a biased approach toward endovascular therapy.
This scale took 6 months to devise, with partners agreeing and disagreeing as to what they think is right or wrong. The only point is that can be derived by this study is that an objective scoring system, as Dr. Richardson pointed out, is possible to construct.
At this point we have enrolled over 400 limbs, and we are continuing to prospectively follow these patients, accumulating outcomes data. We have 6-month data now on the group that we have just presented today, looking at not just limb salvage and reconstruction patency, but clinical outcomes of ambulation and maintenance of independent living status—things that really count. We should learn a great deal about the LEGS score once we analyze these data.
This was a prospective study. The exclusion criteria were established upfront and the study ran its course. Interestingly, 12 of the 13 patients that refused surgery had arteriograms, thinking that they were going to be angioplasty candidates for their claudication, only to be told that open surgery was their only option. Open surgery was then refused, as these patients opted to live with their claudication. Most of the 16 patients listed in the study as indeterminate treatment have still not been treated for one reason or another.
We agree that the LEGS score favors endovascular therapy for the high-risk patient. But if you look at the scale as well, it also favors endovascular therapy for the best-risk patient too. These are patients with soft traditional indications for open surgery. The score does not affect that core group of patients that we typically advise to have surgery.
The scoring system acknowledges we are treating more patients invasively. If you look at specific patient examples, the score favors endovascular therapy for both ends of the spectrum, the best-risk and the highest-risk patients. I think the applicability of the LEGS score in its current form will only be determined once the outcomes data we are prospectively gathering can be analyzed.
References
- 1.Feinglass J, McCarthy WJ, Slavensky R, et al. Functional status and walking ability after lower extremity bypass grafting or angioplasty after intermittent claudication: Results from a prospective outcomes study. J Vasc Surg. 2000; 31: 93–103. [DOI] [PubMed] [Google Scholar]
- 2.Currie IC, Lamont PM, Baird RN, et al. Treatment of intermittent claudication: The impact on quality of life. Eur J Vasc Endovasc Surg. 1995; 10: 356–361. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons GW, Burgess AM, Guadagnoli E, et al. Return to well-being and function after infrainguinal revascularization. J Vasc Surg. 1995; 21: 35–44. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan TM, Taylor SM, Blackhurst DW, et al. Has endovascular surgery reduced the number of open vascular operations performed by an established surgical practice? J Vasc Surg. 2002; 36: 514–519. [DOI] [PubMed] [Google Scholar]
- 5.Transatlantic Inter-Society Consensus (TASC) Working Group. Treatment of intermittent claudication. J Vasc Surg. 2000; 31: S98–S104. [PubMed] [Google Scholar]
- 6.Eagle KA, Surger DE, Brewster DC, et al. Dipyridamole-thallium scanning in patients undergoing vascular surgery: Optimizing preoperative evaluation of cardiac risk. JAMA. 1987; 257: 2185–2189. [PubMed] [Google Scholar]
- 7.Transatlantic Inter-Society Consensus (TASC) Working Group. Outcomes assessment methodology in peripheral arterial disease. J Vasc Surg. 2000; 31: S35–S43. [PubMed] [Google Scholar]
- 8.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997; 26: 517–538. [DOI] [PubMed] [Google Scholar]
- 9.Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg. 1986; 4: 80–94. [PubMed] [Google Scholar]
- 10.Abou-Zamzam AM, Lee RW, Moneta GL, et al. Functional outcome after infrainguinal bypass for limb salvage. J Vasc Surg. 1997; 25: 287–295. [DOI] [PubMed] [Google Scholar]
- 11.Nicoloff AD, Taylor LM, McLafferty RB, et al. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998; 27: 256–266. [DOI] [PubMed] [Google Scholar]
- 12.Transatlantic Inter-Society Consensus (TASC) Working Group. Treatment of critical limb ischemia. J Vasc Surg. 2000; 31: S217–S226. [PubMed] [Google Scholar]
- 13.Gibbons GW, Burgess AM, Guadognoli E, et al. Return to well-being and function after infrainguinal revascularization. J Vasc Surg. 1995; 21: 35–45. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Spence M. Taylor, MD, Academic Department of Surgery, Greenville Hospital System, 701 Grove Road, Greenville, SC 29605.
E-mail: staylor2@ghs.org
Accepted for publication December 2002.