Abstract
Objective
To examine survival and toxicity by querying a single-institutional experience with adjuvant hepatic arterial infusional (HAI) chemotherapy.
Summary Background Data
Three randomized series in the literature have examined adjuvant HAI after complete resection of liver metastases. Only one of these trials showed an overall survival benefit at 2 years but not over the entire time period of the study. Previous studies in patients with unresectable disease were plagued by high rates of biliary toxicity.
Methods
A retrospective review of a prospectively maintained database was performed. Hepatic arterial pumps were placed in the standard fashion. Patients received floxuridine at doses previously demonstrated as safe in the literature. Standard statistical methods were used.
Results
Twenty-one of 92 patients underwent placement of hepatic arterial pumps at the time of liver resection. The HAI group was similar in all demographic measures to the non-HAI group, with the exception that the HAI patients were significantly younger. No differences were seen between the groups in either disease-free or overall survival, although a trend toward improved hepatic progression-free survival was noted. Significant biliary sclerosis developed in six patients in the HAI group, requiring chronic indwelling stents in four patients. One patient died of progressive liver failure associated with this toxicity.
Conclusions
Biliary toxicity is an important potential side effect of hepatic arterial chemotherapy. Although larger randomized studies and this one suggest significant improvements in hepatic recurrences, these have not reliably translated into overall survival benefit. This fact, in light of the potential toxicity, would argue for a larger confirmatory trial of HAI in the adjuvant setting, incorporating recent advances in systemic therapy and careful attention to hepatotoxicity.
Colorectal cancer affects approximately 130,000 patients per year and is the direct cause of death in 30,000. 1 The liver is the predominant site of metastases, and for many patients with recurrent disease this is the main determinant of survival. Liver metastases are synchronously present in 20% of patients undergoing primary resection, and they develop subsequently in an additional 40%. 2 Survival of patients with untreated liver metastases rarely exceeds 2 years, with a median of approximately 6 to 9 months. 3
Surgical resection remains the only modality of treatment for isolated hepatic metastases that is associated with a significant proportion of long-term survivors. Five-year survival following metastasectomy in patients with isolated liver metastases is approximately 30% to 40%, while 10-year survival rates range from 15% to 25%. 4–9 Median survival within these large experiences ranges from 23 to 42 months. Actual long-term survivors are well documented and are consistent with older autopsy studies 10 that demonstrated that a significant proportion of patients die with hepatic disease as the only evident site of disease. Moreover, retrospective reviews of patients with unresected “resectable” disease clearly document the absence of significant long-term survival.
A number of factors have been studied as potential predictors of recurrence following metastasectomy and include patient, primary tumor, and metastasis features. 4–7 Age, sex, primary tumor location, and the presence of bilateral metastases do not appear to be important independent predictors of recurrence or survival following metastasectomy. Primary tumor stage is a constant predictor of patient outcome, while the preoperative carcinoembryonic antigen (CEA) level, disease-free interval (time from primary to metastases), number of metastases, and size (of the largest metastasis) also appear to be important. Fong et al. 4 have developed a clinical risk score based on an experience with 1,001 patients undergoing hepatic resection that takes into account five variables that can be ascertained preoperatively: disease-free interval less than 12 months, size greater than 5 cm, more than one metastasis, preoperative CEA level greater than 200 ng/mL, and presence of a node-positive primary. In the group without any of these adverse prognostic factors (score = 0), the median survival was 74 months and the 5-year survival was 60%. Survival gradually diminished as the score increased, such that patients with all five of these adverse factors had a median survival of 22 months and an actuarial survival of 14%. There appeared to be a breakpoint beyond a score of 2 (possessing two adverse features) where 5-year survival greatly diminished from 40% to 20%. Even in the most adverse group, though (score = 5), long-term survival and median survival were much greater than would be expected from series of unresected, “resectable” disease.
Despite the relative success of surgical resection of hepatic metastases and the use of adjuvant systemic chemotherapeutic regimens, recurrence occurs in as many as 60% of patients following hepatectomy. 4,5,11 Half of these recurrences are confined to the liver. Systemic treatment of unresectable liver metastases with 5-fluorouracil-based chemotherapy has yielded disappointing response rates of 25% to 30%. 12 More recent studies using irinotecan or oxaliplatin have increased this rate to almost 40%. 13,14
In an attempt to improve these recurrence rates, three randomized trials were recently reported on the use of hepatic arterial infusion (HAI) of chemotherapy as an adjuvant to metastasectomy. HAI takes advantage of the fact that the liver derives most of its blood supply from the portal vein, whereas hepatic metastases are supplied predominantly by the hepatic arterial system. 15 Older prospective, randomized trials of HAI in patients with unresectable disease previously demonstrated partial response rates in the liver of 40% to 60% in those receiving HAI compared to only 10% to 20% in those undergoing systemic chemotherapy. 16–21 Kemeny et al. from Memorial Sloan-Kettering recently reported that HAI (in combination with systemic therapy) as an adjuvant following hepatic metastasectomy resulted in improved disease-free and overall survival rates at 2 years compared to systemic therapy alone. 22 The combined treatment group in this study (HAI plus systemic chemotherapy) had a higher rate of hospitalization for diarrhea, leukopenia, mucositis, and small bowel obstruction than patients receiving only systemic adjuvant therapy. In the combined group, 4 of 74 patients developed biliary sclerosis requiring biliary stents compared to 2 of 82 in the systemic therapy arm. Two subsequent randomized studies have failed to replicate these survival benefits. 23,24
Concerns regarding efficacy and potential toxicities have precluded HAI from being more widely adopted by the surgical and medical oncology communities. We present a retrospective review of a single-institutional experience with adjuvant use of HAI following hepatic metastasectomy.
METHODS
A prospectively maintained database of consecutive liver resections performed by two surgeons (P.C., B.C.) was queried for demographics, survival, and toxicity. Patients with less than 1 year of follow-up were excluded. All patients underwent preoperative abdominal/pelvic computed tomography scan and chest roentgenogram to rule out disseminated metastases. Anatomic liver resections were performed in almost all patients, with wedge resections reserved for situations where the adequacy of the liver remnant was thought to be in jeopardy. The decision to place a pump was left to the discretion of the treating surgeon. The method of pump insertion entails clearance of the common hepatic artery for 1 cm, followed by standard placement within the gastroduodenal artery after ligation of accessory vessels. Most patients underwent either transcutaneous arteriography or computed tomographic angiography preoperatively.
Treatment regimens varied according to oncologist. At Duke, most patients received a first cycle of FUDR at a dose of 0.1 mg/kg/d along with 20 mg dexamethasone. If this was well tolerated, subsequent cycles were increased to a dose of 0.2 mg/kg/d. It was recommended that hepatic enzyme levels were to be closely monitored, and dosages decreased or cycles terminated if these values significantly increased. Concomitant systemic therapy was delivered in approximately half of the patients receiving adjuvant hepatic arterial chemotherapy. All patients in the non-HAI group received adjuvant systemic chemotherapy; the regimens were varied.
Statistical analysis was performed using the Student t test for parametric variables and Kaplan-Meier analysis for survival. The statistical software Statistica (Statsoft, Tulsa, OK) was used to perform these analyses.
RESULTS
Demographics
Over a consecutive 5-year period, 92 patients underwent liver resection for colorectal metastases. Twenty-one patients underwent concomitant placement of a hepatic arterial pump; 10 were male and 11 female (38 were male and 33 female in the non-HAI group). As Table 1 reveals, the two groups did not differ significantly in mean disease-free interval, mean preoperative CEA level, mean size of the largest hepatic tumor, mean number of hepatic tumors, the percentage of patients with node-positive primary tumors, or the proportion of patients with a Blumgart-Fong clinical risk score of 2 or less. The HAI group was significantly younger, reflecting a selection bias in reserving a more aggressive approach to these younger individuals. Median follow-up was 29 months.
Table 1. PATIENT CHARACTERISTICS
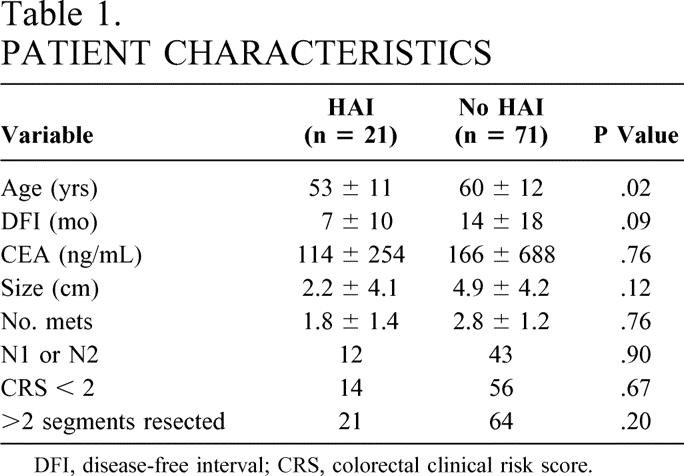
DFI, disease-free interval; CRS, colorectal clinical risk score.
Survival
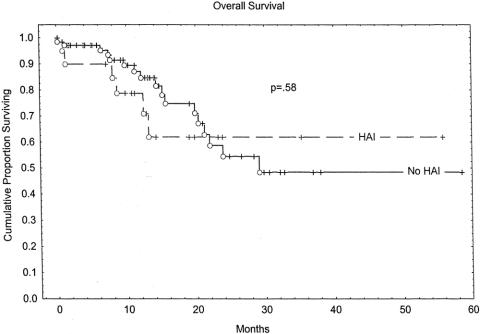
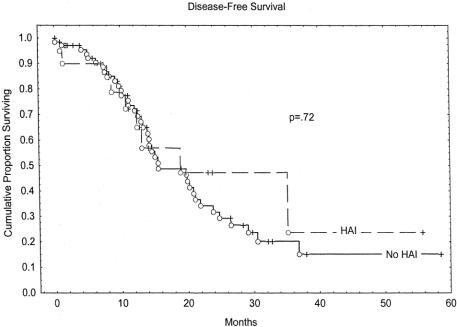
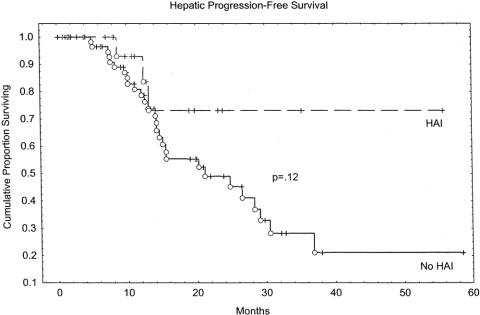
Overall survival rates at 2 years were 61% in the HAI group and 53% in the non-HAI group (P = .58). Kaplan-Meier overall survival estimates are depicted in Figure 1. The group receiving HAI had an early survival disadvantage, with the curves intersecting at approximately 22 months. Because of the relatively short median follow-up of this study, survival differences over longer time periods cannot be excluded. Disease-free survival is estimated in the Kaplan-Meier curves in Figure 2. No significant difference was seen between the HAI group and the non-HAI group. Hepatic recurrence-free survival is depicted in Figure 3. The HAI group trended toward increased hepatic-free survival; however, this trend must be viewed with caution as few patients are represented by the curve beyond 2 years.
Figure 1. Overall survival.
Figure 2. Disease-free survival.
Figure 3. Hepatic progression-free survival.
Toxicity
Perioperative complications in the non-HAI group included two dehiscences, two wound infections, one cerebrovascular accident, one perihepatic abscess, and two perioperative deaths. Two patients in the HAI group died postoperatively of overwhelming sepsis and liver failure respectively. One pump was removed in the early postoperative setting to rule it out as the infectious source in a septic patient. One additional patient subsequently declined HAI therapy after pump insertion, such that four patients who underwent pump placement ultimately did not receive that therapy.
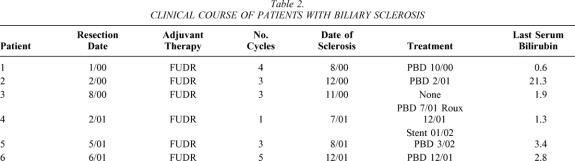
However, delayed complications in the HAI group were common. Pump-related problems included pump malfunction in four patients, three of whom required replacement in the operating room, one late pump pocket infection in one patient requiring removal, and one pump flipped within the pocket requiring operative repositioning. A significantly higher rate of biliary sclerosis was noted in the HAI group compared to the non-HAI group. Six of the 17 patients receiving HAI therapy (35%) developed persistently elevated serum bilirubin and alkaline phosphatase levels indicative of biliary sclerosis. Five of these patients underwent cholangiography, documenting a pattern of stricturing consistent with this diagnosis. Four of these five patients were managed with chronic indwelling stents. Table 2 demonstrates the clinical courses of the six patients with biliary sclerosis. One patient (patient 4) had a pattern of sclerosis involving a dominant hilar stricture that was repaired via intrahepatic Roux-en-Y hepaticojejunostomy, after which he did well. Three of these six patients received concomitant systemic chemotherapy as part of their adjuvant therapy, two with 5-FU/LV and one with CPT-11. Chemotherapy treatment plans were available in five of the six patients for review. In these five patients, the dose of FUDR given did not exceed 0.2 mg/kg/d, nor were the liver function tests abnormal before the last cycle of chemotherapy. One patient died of cirrhosis and subsequent liver failure associated with HAI therapy.
Table 2. CLINICAL COURSE OF PATIENTS WITH BILIARY SCLEROSIS
DISCUSSION
Three randomized studies have examined HAI in an adjuvant setting after complete resection of all hepatic metastases. The first of these, by the German Cooperative Study on Liver Metastases, randomized 226 patients to either resection alone or to resection followed by hepatic arterial 5-FU and leucovorin. 24 They found no significant differences in either disease-free or overall survival between the groups. The treated group trended toward more extrahepatic failures. This study has been criticized for the fact that the regimen used did not contain FUDR, which has a higher intrahepatic response rate.
The Memorial-Sloan Kettering study by Kemeny et al. randomly assigned 156 patients to HAI with FUDR and dexamethasone plus intravenous fluorouracil with or without leucovorin or to 6 weeks of systemic chemotherapy alone (same reagents as in HAI arm). 22 Exclusion criteria included the presence of extrahepatic disease, prior liver resection or irradiation, concurrent or recent cancer, or finding of metastasis to the portal lymph node at operation. Powered to address 2-year endpoints, this study revealed significant increases in hepatic recurrence-free survival, relapse-free survival, and overall survival at 2 years. Median survival was 72.2 months in the combined therapy group and 59.3 months in the monotherapy group. Overall survival between the two group was not significantly different (P = .21) by log-rank analysis of Kaplan-Meier estimates. The groups trended toward significance in progression-free survival, suggesting that some distant disease may arise from established hepatic metastases. However, if this metastasis sequence were common, the progression-free curves would be significantly different. Thus, HAI in this series could not overcome the predominant problem in these patients: systemic micrometastases present at the time of liver resection.
Finally, the Intergroup trial randomized 75 patients to either resection alone or to HAI with FUDR along with systemic continuous-infusion fluorouracil. 23 This study had originally planned accrual of 109 patients, but many were found ineligible after randomization. Accrual of patients for the study took 9 years. Significant differences in recurrence-free survival and hepatic recurrence-free survival were noted between the two groups, but no significant difference was noted in overall survival. The authors made the argument that the study was powered to reveal differences in recurrence-free but not overall survival. However, the overall Kaplan-Meier survival curves in this study are almost superimposable, with P = .6 at 48 months. Although our study is clearly underpowered to make meaningful conclusions about survival, the survival curves are not dissimilar from those of the Intergroup trial. The larger study from Memorial Sloan-Kettering (with the more active agent FUDR) would suggest that more patients and longer follow-up may demonstrate a benefit to this form of adjuvant therapy.
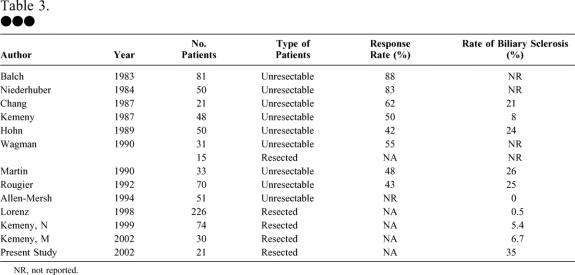
Biliary toxicity with HAI is a major concern, given the devastating consequences (Table 3). Many reports of palliative HAI do not specifically mention rates of biliary sclerosis. However, those that have meticulously documented biliary sclerosis rates have published rates of 20% to 30%. 17–19,21 These high rates have been treated in different ways. The Northern California Oncology group realized that early dose reduction from 0.3 mg/kg/d to 0.2 mg/kg/d markedly decreased the percentage of patients requiring biliary instrumentation. 18 Others have added dexamethasone to the intra-arterial treatment regimen, 25 which led to a reduced need for dose reduction and a trend toward lower serum bilirubin levels.
Table 3. •••
NR, not reported.
In the three randomized studies of adjuvant HAI, morbidity was significantly increased in comparison to the systemic or observation arms. In terms of perioperative complications, the German group had two pump infections, three malperfusion/technical complications, and a 7.5% perioperative mortality rate in 107 patients. The Memorial Sloan-Kettering study reported three small bowel obstructions, three wound infections, three pleural effusions, six pump infections, and a perioperative mortality rate of 2.7% in 74 patients. The Intergroup study described two pleural effusions, one bile leak, two biliary fistulas, one wound infection, one hemorrhage, and a perioperative mortality rate of 2.8% in 35 evaluable patients. These are comparable to the perioperative complication rates in our study. Thus, hepatic resection with adjuvant HAI appears to be safe perioperatively, with an acceptable incidence of complications.
Late complications were also documented in each of these randomized trials. The German group did not use intra-arterial FUDR and thus had only one case of biliary toxicity. 24 The Memorial Sloan-Kettering study provided HAI with FUDR at a dose of 0.25 mg/kg/d along with 20 mg dexamethasone. The biliary sclerosis rate was 6%; moreover, only 26% of the patients received more than 50% of the planned dose of FUDR due to hepatic enzyme elevation. 22 The Intergroup study administered FUDR to the treatment group at a dose of 0.1 mg/kg/d, which was increased to 0.2 mg/kg/d if no toxicity occurred. On this regimen, only two (6.7%) patients required instrumentation for biliary sclerosis. 23 In our study, biliary sclerosis occurred in 35% of the patients receiving HAI. Our patients received various regimens of the drug, but almost all received 0.2 mg/kg/d FUDR or less, plus or minus dexamethasone.
Our early experience with this form of therapy highlights some of its potential disadvantages, as defined by persistent hyperbilirubinemia and an increase in alkaline phosphatase and, in five of six patients, cholangiographic evidence of significant biliary toxicity. This occurred despite low- to moderate-dose FUDR regimens containing dexamethasone. In addition, as careful evaluation of the available literature demonstrates, long-term survival advantage for these patients is absent. Therefore, we recommend that use of HAI following complete metastasectomy for colorectal cancer be undertaken extremely cautiously and preferentially in the context of larger, randomized multi-institutional trials designed to confirm or deny the purported survival benefits reported by the Memorial Sloan-Kettering group. Education of participating medical oncologists and appropriate attention to detail with respect to dose reduction in the setting of hepatotoxicity will be critical in terms of patient safety.
CONCLUSIONS
Biliary toxicity is an important potential side effect of hepatic arterial chemotherapy. Although larger randomized studies and ours suggest significant improvements in hepatic recurrences, these have not reliably translated into overall survival benefit. This fact, in light of the potential toxicity, would argue for a larger confirmatory trial of HAI in the adjuvant setting, incorporating recent advances in systemic therapy and careful attention to hepatotoxicity and the need for dose modification.
Discussion
Dr. Leslie H. Blumgart (New York, NY): Thank you very much for your invitation to discuss your paper and for letting me see the manuscript. While I share your recommendation and conclusion that further studies are necessary to confirm or refute the controlled studies done at Memorial and elsewhere, I have considerable reservations regarding your communication.
It is very important to note, and as you acknowledge, that this is a retrospective analysis (quite uncontrolled) of a very small number of patients with a short follow-up. Indeed, there are only 21 patients in your group selected for hepatic arterial infusion, and of these only 17 actually were treated. What were the criteria for selecting these patients? Perhaps you could help us. As judged by the clinical risk score described by Dr. Fong and myself which you quote in the manuscript, the groups were similar. However, 14 of the 21 patients, or 66%, had a clinical risk score less than 2. Were you selecting young patients with low risk for HAI?
In this context, you make no mention of patients with a higher risk or with positive margins. As you know, hepatic arterial infusion may be more effective in this situation as compared to systemic chemotherapy. Did you have no positive margins and no patients with multiple tumors who were subjected to infusion? Or were the numbers too small to allow assessment?
You show no difference in hepatic progression-free survival despite the fact that the survival curves appear very different. The corresponding data for our study at 5 years are now available. Hepatic progression-free survival data obtained from Dr. Kemeny just last week is 73% for hepatic arterial infusion versus 41% for systemic treatment alone (P = .001). Could your conclusion in respect of hepatic progression-free survival change with time and with more cases?
You show a very high rate of complications with biliary sclerosis, 29% of 21 patients. It is actually more if you take the 17 you actually treated. Recent data at MSKCC reveal a 4% sclerosis rate in 90 patients. Why do you have such a high rate of sclerosis? Is it a lack of vigilance in reducing dosage early and at the first sign of biliary toxicity?
Finally, you mention the important point that while HAI may very well control hepatic disease, the problem of extrahepatic disease remains. In this context, we are now engaged in a phase 2 study of FUDAR and dexamethasone given by arterial infusion combined with irinotecan given systemically. This may be much more effective in controlling extrahepatic disease. Further studies conducted in a prospective randomized manner and followed for a prolonged time are needed.
Two final small points: Please change the title “Metastasectomy.” It is hepatic resection for metastases. The second point refers to the patient on whom you were able to operate and reconstruct the biliary tree. We have had one such case and that turned out to be a technical error resulting in an iatrogenic stricture and had nothing to do with chemotherapy. Is there a chance that your case was in fact an iatrogenic problem?
Dr. Kelly M. McMasters (Louisville, KY): Congratulations to the authors for a nicely presented study. I too share your concern about the potential toxicity and complications associated with hepatic artery chemotherapy in the adjuvant setting.
If you look at your data from an intention-to-treat standpoint, how many patients were scheduled for hepatic artery pump placement who did not get a pump placed? It seems that maybe the hepatic artery chemotherapy group might have had less extensive liver resections and better performance status.
As you know, we have also been performing a multicenter phase II study of hepatic artery chemotherapy with radiofrequency ablation of liver metastases, which is moving forward slowly. Do you think there is any difference between resection and radiofrequency ablation in terms of toxicity with hepatic artery chemotherapy? Does the extent of liver resection affect the rate of complications, including biliary sclerosis? And do you think that adjuvant hepatic artery chemotherapy is feasible at this point in the multi-institutional setting?
Dr. William C. Chapman (St. Louis, MO): I too rise to congratulate the authors for bringing our attention to an important and I believe unresolved treatment problem; namely, what role should there be for adjuvant therapy following liver resection for metastatic colorectal cancer.
I would share some of the previous concerns that perhaps this is an underpowered study to draw any firm conclusions. The authors suggest that further multicenter trials should be conducted. I would be interested in their view on how realistic it will be to put such multicenter trials together. Dr. Kemeny was the sponsor of an intergroup study in the mid ’90s that was enrolling patients with unresectable colorectal metastases. That trial was slated to accrue over 600 patients. The trial was only able to accrue a little over 300 patients before it was closed for inadequate accrual. I think this is an area where treatment accrual is very difficult, because these patients’ treatment is heavily influenced by their medical oncologist, and general medical oncologists are reluctant to use hepatic arterial infusion therapy. So I would be interested in your views on how such a multicenter trial might actually be put together.
The other question that has been touched upon is whether or not the dose of hepatic artery infusional therapy was perhaps too high. So I would wonder if these patients had unrecognized toxicity when having their chemotherapy administered by a medical oncologist who perhaps only infrequently used pump chemotherapy.
I have three specific questions for the authors:
Number 1, what selection criteria were used to decide if pump placement should be utilized at the time of liver resection? Was this decision made or influenced by the medical oncologist? It appears that both high-risk and low-risk patients were included in the group that had pump placement.
Secondly, what regimen or surveillance scheme was in place for following patients who had pumps? You mentioned that the liver tests were normal prior to the last dose of pump chemotherapy. What about their prior doses? Did you look to see if patients did or did not appropriately have dose reductions and whether they had toxicity on that basis?
Finally, have you continued to place pumps in your high-risk patients? If so, what surveillance mechanisms have you put in place while they are on therapy?
Dr. John M. Daly (Philadelphia, PA): The evolution of adjuvant therapy for patients undergoing hepatic resection for colorectal metastatic disease has a lot to do with staging. The previously held dictum of 25% to 30% 5-year survival has improved over time. Your own information shows predicted survival curves from maybe 50% to 60%, acknowledging the fact that much of this is predicted. I wondered if you would comment on that.
The second issue is if you look at Nancy Kemeny’s published randomized trial, the post-hoc analysis showed that the group with one metastasis and the group with greater than four didn’t seem to benefit by the use of regional infusion along with systemic treatment. It was really that intermediate group of those with two to four metastatic foci that benefited the most. I wondered if you really have enough power to understand that group, because that was the group that seemed to influence overall survival differences in that previous study.
Lastly, this issue of biliary sclerosis is really vexing. If you are achieving a 29 to 30-some percent biliary sclerosis rate, it becomes unacceptable, because these patients clearly develop the symptoms of itching and other problems which occur with the biliary sclerosis. I wonder if you would comment upon anything you have done to try to reduce that rate further, having followed some of the stated protocols for doing this.
Dr. Mark W. Onaitis (Durham, NC): Addressing Dr. Blumgart’s questions, we certainly appreciate the small numbers of patients and the retrospective nature of the study. The study wasn’t actually meant to be a study of survival or hepatic regression-free survival but was instead a cautionary look at this experience as we have experienced such a high rate of biliary sclerosis.
Regarding the high rate of biliary sclerosis, our first thought was that the medical oncologists were not following these patients up appropriately. We used a standard definition of biliary sclerosis: patients with persistent hyperbilirubinemia as well as patients with prolonged elevations in alkaline phosphatase requiring intervention.
Was it an operative problem? We thought maybe it was an operative problem, but we only dissected within 1 cm of the gastroduodenal artery during pump insertion, and we minimized portal dissection using intraparenchymal portal pedicle control when possible.
As far as extrahepatic disease goes, only two patients in our series received CPT-11 in addition to hepatic arterial chemotherapy. Four others received 5-FU and the rest received hepatic arterial infusion alone. And I know there is a phase 2 trial at Memorial to address this.
Five out of the six patients experiencing biliary sclerosis had normal alkaline phosphatase levels prior to the last cycle. Two thirds of the patients had elevated bilirubins and needed a dose reduction during prior treatment cycles. I think this is pretty much in parallel with the other studies that have been presented in the literature.
Concerning survival, Dr. Blumgart quoted 73% hepatic-free survival rate versus 41% in the monotherapy group. Only time and more experience will tell if this will hold up in our series.
One note is the NSABP trial, which proposes using 0.3 mg/kg per day, which is a relatively high dose of hepatic arterial chemotherapy, is going to be coming out in Texas, and that trial will be interesting.
Regarding which criteria we are using for pump placement now, we were very much more enthusiastic about pump therapy in the past, which is reflected in our data with a large number of patients with a colorectal score of less or equal to 2 being included. Since our examination of this data, we have really changed to trying to put these pumps in patients with a colorectal risk score of 4 or 5, patients that are high risk for recurrence. Only 4 patients of the 21 had multiple tumors in this group, so that small number really precludes looking at them as compared to the rest of the group.
Asking whether future trials in this area are realistic, the ones I mentioned are ongoing. But it is our hope there will be greater enthusiasm for future trials since the Memorial data has suggested a survival benefit. And only more time and more patients will allow us to see if that is really the case or not.
Finally, Dr. Daly’s comments about the actuarial survival. Only time will tell if our actuarial survival is as good as the Kaplan-Meier curves show. Generally, in other studies that we have done, they haven’t been.
The intermediate groups that have two or four tumors, certainly the number of patients with that intermediate number of tumors in our study was a small number. And the lack of power really precludes us from commenting on this.
We agree that biliary sclerosis is a vexing problem. In our study, we found no reason why the patients who developed it would have it compared to the other patients. We think it is something that needs to be monitored closely in future trials.
References
- 1.Landis SH, Murray T, Bolden S, et al. Cancer statistics. CA Cancer Clin. 1999; 49: 8–31. [DOI] [PubMed] [Google Scholar]
- 2.Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. Cancer. 1969; 23: 198–202. [DOI] [PubMed] [Google Scholar]
- 3.Wood CB, Gillis CR, Blumgart LH. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976; 2: 285–288. [PubMed] [Google Scholar]
- 4.Fong Y, Fortner JG, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann Surg. 1999; 230: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele J, Stangl R, Atendorf-Hofman A, et al. Resection of colorectal liver metastases. World J Surg. 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 6.Minigawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000; 231: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambiru S, Miyazaki M, Isono T, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999; 42: 632–639. [DOI] [PubMed] [Google Scholar]
- 8.Jamison R, Donohue J, Nagorney D, et al. Hepatic resection for metastatic colorectal cancer results in cure for some. Arch Surg. 1997; 132: 505–511. [DOI] [PubMed] [Google Scholar]
- 9.Gayowski T, Iwatsuki S, Madariaga J, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994; 116: 703–711. [PMC free article] [PubMed] [Google Scholar]
- 10.Welch J, Donaldson G. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg. 1978; 189: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordlinger B, Quilichini M-A, Parc M, et al. Hepatic resection for colorectal liver metastases. Ann Surg. 1987; 205: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson III AB. Therapy for advanced colorectal cancer. Semin Oncol. 1998;25:2–11. [PubMed]
- 13.Andre T, Bensmaine MA, Louvet C, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999; 17: 3560–3568. [DOI] [PubMed] [Google Scholar]
- 14.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000; 343: 905–914. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman NB. The blood supply of experimental liver metastases. Surgery. 1974; 75: 589–596. [PubMed] [Google Scholar]
- 16.Allen-Mersh TG, Earlem S, Fordy C, et al. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994; 344: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 17.Chang AE, Schneider PD, Sugarbaker PH, et al. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987; 206: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with coloreactal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989; 7: 1646–1654. [DOI] [PubMed] [Google Scholar]
- 19.Martin JK, O’Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs. systemic fluorouracil for hepatic metastases from colorectal cancer: a randomized trial. Arch Surg. 1990; 125: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 20.Kemeny N, Daly J, Reichman B, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma—a randomized trial. Ann Intern Med. 1987; 107: 459–465. [DOI] [PubMed] [Google Scholar]
- 21.Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992; 10: 1112–1118. [DOI] [PubMed] [Google Scholar]
- 22.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999; 341: 2039–2048. [DOI] [PubMed] [Google Scholar]
- 23.Kemeny N, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an Intergroup study. J Clin Oncol. 2002; 20: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz M, Muller H, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. Ann Surg. 1998; 228: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemeny N, Seiter K, Niedzwiecki D, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992; 69: 327–334. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Bryan Clary, MD, Chief of Hepatobiliary Surgery, Duke University Medical Center, Box 3247, Durham, NC 27710.
E-mail: clary001@mc.duke.edu
Accepted for publication December 2002.