Abstract
The Arabidopsis phloem channel AKT3 is the founder of a subfamily of shaker-like plant potassium channels characterized by weak rectification, Ca2+ block, proton inhibition, and, as shown in this study, K+ sensitivity. In contrast to inward-rectifying, acid-activated K+ channels of the KAT1 family, extracellular acidification decreases AKT3 currents at the macroscopic and single-channel levels. Here, we show that two distinct sites within the outer mouth of the K+-conducting pore provide the molecular basis for the pH sensitivity of this phloem channel. After generation of mutant channels and functional expression in Xenopus oocytes, we identified the His residue His-228, which is proximal to the K+ selectivity filter (GYGD) and the distal Ser residue Ser-271, to be involved in proton susceptibility. Mutations of these sites, H228D and S271E, drastically reduced the H+ and K+ sensitivity of AKT3. Although in K+-free bath solutions outward K+ currents were abolished completely in wild-type AKT3, S271E as well as the AKT3-HDSE double mutant still mediated K+ efflux. We conclude that the pH- and K+-dependent properties of the AKT3 channel involve residues in the outer mouth of the pore. Both properties, H+ and K+ sensitivity, allow the fine-tuning of the phloem channel and thus seem to represent important elements in the control of membrane potential and sugar loading.
INTRODUCTION
The Arabidopsis genome encodes nine shaker-like potassium channels that share a common structure composed of six transmembrane domains (S1 to S6) and a pore region (P) located between S5 and S6 (Roelfsema and Hedrich, 1999; Zimmermann and Sentenac, 1999). Based on sequence similarity, these channels group into five subfamilies exhibiting different molecular and biophysical properties (Mäser et al., 2001). These five branches were named according to the first channel identified within each subfamily: KAT1, AKT1, AKT2/3, ATKC1, and SKOR.
Members of the KAT1 subfamily are voltage-dependent, acid-activated inward rectifiers, providing a molecular pathway for potassium uptake into guard cells (Schachtman et al., 1992; Hedrich et al., 1995; Müller-Röber et al., 1995; Nakamura et al., 1995; Pilot et al., 2001; Szyroki et al., 2001). The voltage-dependent gating of the Arabidopsis guard cell inward rectifier as well as the KAT1 α-subunit is potassium insensitive and thus independent of the reversal potential for potassium (EK) (Very et al., 1995; Brüggemann et al., 1999). Thus, in the membrane potential range positive to EK and negative to the activation potential, the inward rectifier will even mediate K+ release (Brüggemann et al., 1999).
The acid activation of this channel, as well as that of the potato guard cell channel KST1, has been shown to result from a positive shift of the half-maximal activation voltage upon extracellular acidification, which in turn increases the open probability of this channel type at a given membrane potential (Hoth and Hedrich, 1999a). Although structure–function studies identified two His residues in the S3-S4 linker and in the pore region to control the pH regulation in KST1 (Hoth et al., 1997), distinct molecular elements seem to regulate the proton-induced activation of KAT1 (Hoth and Hedrich, 1999a).
AKT2/3-like channels represent phloem-localized transporters, the pH sensitivity of which determines the redistribution of potassium, control of the membrane potential, sugar loading, and thus long-distance solute transport within the phloem network (Philippar et al., 1999; Deeken et al., 2000; Ache et al., 2001; Dennison et al., 2001). AKT2 and AKT3 are two proteins encoded by the same gene (At4 g22200); AKT3 (Ketchum and Slayman, 1996; Marten et al., 1999; Hoth et al., 2001) represents a truncated version of AKT2 (Cao et al., 1995; Lacombe et al., 2000b) characterized by a 15–amino acid shorter cytoplasmic N terminus. The presence of one or both of these proteins in planta has not yet been determined. Nevertheless, AKT2 and AKT3 have the same functional properties in Xenopus oocytes (Marten et al., 1999; Lacombe et al., 2000b).
Members of the AKT2/3 subfamily (Cao et al., 1995), such as AKT2/3 and ZMK2, exhibit weak rectification properties only, allowing the uptake of potassium ions at membrane potentials negative and potassium release positive to EK (Marten et al., 1999; Philippar et al., 1999; Bauer et al., 2000; Lacombe et al., 2000b; Dreyer et al., 2001). Furthermore, AKT2/3-like channels are inhibited by extracellular protons. The proton-mediated decrease in macroscopic currents of AKT2/3 channels results from a reduction in single-channel conductance (Marten et al., 1999) rather than a decrease in the number of active channels (Lacombe et al., 2000a).
In a previous study, in which we characterized a chimera between members of the KAT1 and AKT2/3 families, we were able to demonstrate that the pore region contains all of the structural elements for rectification, susceptibility toward extracellular Ca2+, and regulation by extracellular protons (Hoth et al., 2001). The interaction of AKT2/3 with H+ represents a feature that distinguishes this channel type from the K+ uptake channels but that is shared with the Arabidopsis outward rectifiers SKOR and GORK (Lacombe et al., 2000a).
Gating of the latter has been shown to be sensitive to extracellular potassium. Decreasing extracellular potassium concentrations shift the half-maximal activation potential of SKOR and GORK towards negative membrane potentials, whereas complete removal of potassium renders these channels nonactive (Gaymard et al., 1998; Ache et al., 2000). This behavior and modulation of K+ susceptibility by H+ is well known for animal potassium channels of the shaker family (Schönherr and Heinemann, 1996; Jäger et al., 1998; Jäger and Grissmer, 2001).
In this report, we have investigated the molecular determinants of extracellular proton and potassium sensitivity in AKT3. Using site-directed mutagenesis in combination with heterologous expression in Xenopus oocytes, we provide evidence that the pH and potassium sensitivity of AKT3 depends on two distinct positions, His-228 and Ser-271, within the outer mouth of the pore region. Although the single mutants S271E and H228D exhibit a pronounced decrease in pH sensitivity, any mutant exhibiting changes at Ser-271, including the double mutant HDSE, lacks susceptibility to extracellular potassium. This finding indicates that H+ and K+ seem to compete for binding sites at the extracellular face of the channel pore.
RESULTS
Based on the analysis of chimeric channels between the proton-activated KST1 and the proton-blocked AKT3, we recently showed that the pore region harbors the AKT2/3-specific H+ sensor (Hoth et al., 2001). This finding is in agreement with a pore block of AKT3 channels by extracellular protons. Mutation of a Met (Met-260) highly conserved in the narrow pore of members of the AKT2/3 family, however, did not affect pH sensitivity. To explore the molecular basis for the peculiar pH sensitivity of this phloem K+ channel, we focused on residues in the outer mouth of the AKT3 pore. As a result of their pKa in the physiological range, His residues as well as charged amino acids have been implicated in mediating pH sensitivity in a number of potassium channels (Guy and Durell, 1995; Jäger and Grissmer, 2001).
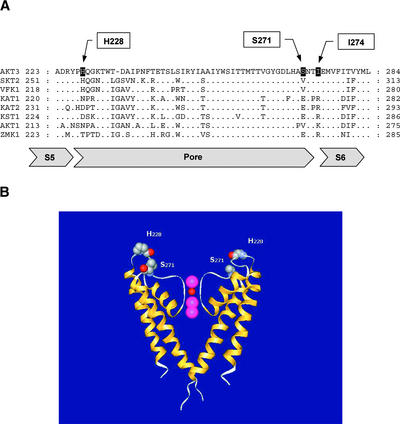
Comparing the extended pore region of different shaker-like plant potassium channels (Figure 1A) shows that the AKT3-like channels differ at three conspicuous positions with respect to members of the KAT1 and AKT1 subfamily. A conserved HQG motif in the S5-P linker is characteristic of AKT2/3 family members (Ehrhardt et al., 1997; Ache et al., 2001). In addition, the uncharged residues Ser-271 and Ile-274 in the ascending loop of the AKT3 pore are represented by charged amino acids at the corresponding positions in the inward rectifiers (Figure 1A).
Figure 1.
Alignment of the Pore Region of Plant shaker-Like K+ Channels.
(A) Fifty-one amino acids from the end of the transmembrane region of S5 to the beginning of S6 are shown. The amino acids mutated in this study are indicated with arrows. Regions of interest are emphasized by boxes. Gray boxes indicate the predicted S5, pore, and S6 regions.
(B) Structural model of the KcsA channel depicting the equivalent positions of His-228 and Ser-271 in AKT3. An alignment of AKT3 with KcsA revealed that His-228 would reside in the descending loop and Ser-271 would reside in the ascending loop of the AKT3 channel.
His-228 Is a Key Element of the Proton Sensor
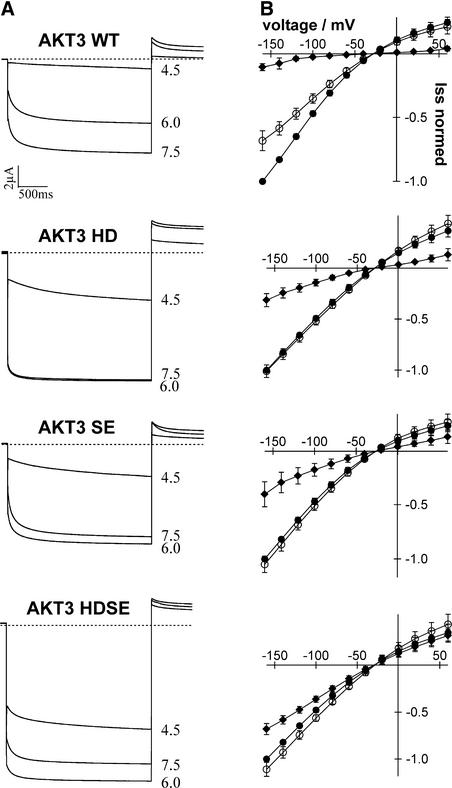
Marten et al. (1999) have shown that the macroscopic current in wild-type AKT3 (AKT3-WT) is decreased by external acidification. Although a 1.5-unit pH decrease from 7.5 to 6.0 resulted in a 32% ± 7.7% reduction of macroscopic current at −160 mV, the current was blocked almost completely at pH 4.5 (Figures 2A, 2B, and 3). To investigate the role of His-228 in pH sensing, we mutated this residue in AKT3 to Ala (H228A), Asn (H228N), Arg (H228R), and Asp (H228D). The macroscopic currents of AKT3 wild-type and mutant channels expressed in Xenopus oocytes were monitored in response to stepwise changes in extracellular pH (7.5, 6.0, and 4.5).
Figure 2.
pH Effect on Macroscopic Currents for AKT3-WT and Mutants.
(A) Whole-oocyte currents in 30 mM K+ of AKT3-WT and mutants in response to three different external pH values (7.5, 6.0, and 4.5) were studied. Currents were elicited by a test pulse to −160 mV from a holding potential of −30 mV. Tail currents were recorded at 0 mV. Traces depict representative cells from at least three independent experiments.
(B) Current-voltage relationships of AKT3-WT and mutants of steady state currents (Iss). Currents were normalized to the current recorded at −160 mV at pH 7.5 and plotted as a function of applied voltage at pH 7.5 (closed circles), pH 6.0 (open circles), and pH 4.5 (closed diamonds). Results shown are means ± sd of three or more experiments.
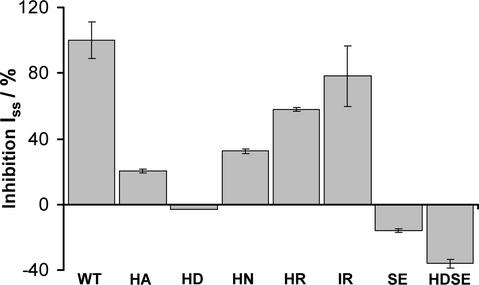
Figure 3.
Proton Sensitivity of AKT3-WT and Mutant Channels.
H+-dependent block of steady state currents at −160 mV in response to a stepwise change of the extracellular proton concentration from pH 7.5 to 6.0. The H+ sensitivity of the AKT3 mutants is given as relative inhibition compared with AKT3-WT. Data shown are means ± sd of three or more experiments. HA, H228A; HD, H228D; HN, H228N; HR, H228R; IR, I274R; SE, S271E.
Instantaneous and time-dependent activation, like AKT3-WT gating (Figure 2A), was conserved in all mutant channels studied (Figure 2A). This finding demonstrates that these residues are very unlikely to play a role in voltage-dependent gating of the AKT3 channel. In contrast to AKT3-WT, however, channel mutants at position His-228 were characterized by a pronounced reduction in pH sensitivity, indicating that this residue is involved in H+ sensing. When comparing macroscopic currents of wild-type and mutant channels in response to a pH shift from 7.5 to 6.0, the relative block by protons was still 57.86% ± 1.4% for AKT3-H228R, whereas the mutants AKT3-H228N and AKT3-H228A were inhibited by 32.47% ± 1.6% and 20.38% ± 1.09%, respectively (Figure 3).
The strongest effect, however, was obtained when His-228 was replaced with the negatively charged amino acid Asp. The AKT3-H228D mutant was completely insensitive to changes in external proton concentration in the pH range of 7.5 to 6.0. At more acidic pH (pH 4.5), almost no currents were recorded in oocytes injected with AKT3-WT, whereas AKT3-H228D still provoked inward as well outward potassium currents. The proton block at pH 4.5 compared with pH 7.5 was only 69% ± 6.3% (Figures 2A and 2B, second panel, and Figure 3).
His-228 and Ser-271 Work Together
To determine whether additional residues besides His-271 contribute to the H+ susceptibility of AKT3, we extended our studies to Ser-271 and Ile-274, which are distal to the selectivity filter GYGD (Figure 1, positions +4 and +7). Although these positions are occupied by charged amino acids in most of the inward-rectifying channels, noncharged or hydrophobic amino acids are present in members of the AKT3 subfamily. Therefore, these two positions in AKT3 were mutated by replacing Ser-271 with Glu (S271E) and Ile-274 with Arg (I274R) in the positions of acid-activated inward rectifiers.
The AKT3 channels carrying mutations at Ser-271 and Ile-274 distal to the pore region responded differentially to changes in extracellular pH. Although the AKT3-I274R mutant displayed pH sensitivity similar to that of the AKT3-WT channel (Figure 3), the mutant AKT3-S271E behaved like the AKT3-H228D mutant (Figures 2A and 2B, third panel). Again, a pH shift from 7.5 to 6.0 was ineffective at modulating macroscopic currents through AKT3-S271E, whereas at pH 4.5, steady state currents were reduced by 60% ± 1.5% (Figure 2, third panel).
To test the hypothesis that both residues, His-228 and Ser-289, contribute to the extracellular pH sensor of AKT3, we exposed the double mutant H228D-S289E (AKT3-HDSE) to pH changes (Figures 2A and 2B, bottom panel). In contrast to the single-mutant responses to a pH change from 7.5 to 6.0, K+ currents mediated by the double-mutant channel increased. Inhibition of steady state currents upon a shift from pH 7.5 to 4.5 was only 32% ± 5.9%. Thus, AKT3-HDSE displayed the strongest reduction in proton susceptibility among the mutants analyzed.
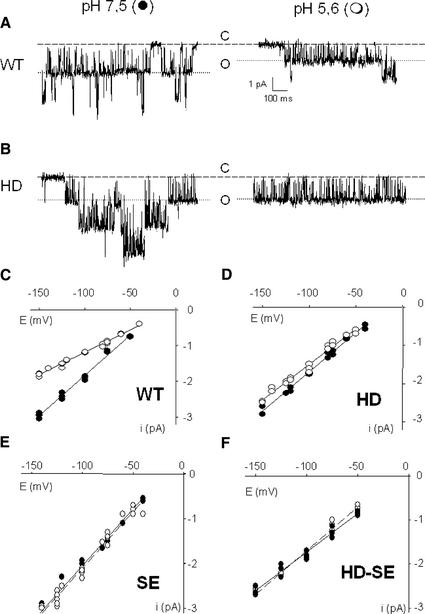
Single-Mutant Channels Are pH Insensitive
To analyze the altered pH dependence of the AKT3 mutants H228D, S289E, and HDSE in more detail, the single-channel conductance of the mutants in response to a pH change from 7.5 to 5.6 were compared with that of AKT3-WT (Figure 4). At neutral pH, the single-channel currents of all mutants were wild type like (Figure 3). In contrast to the AKT3-WT channel, however, a decrease in external pH from 7.5 to 5.6 did not change the single-channel conductance in any of the three mutants (Figures 4C to 4F). This behavior provides evidence that both residues, His-228 and Ser-271, seem to control the pH-dependent K+ permeation through AKT3.
Figure 4.
Effect of Extracellular pH on the Single-Channel Conductance of AKT3-WT and Mutants.
(A) and (B) Single-channel fluctuations at pH 7.5 (left) and pH 5.6 (right) for wild-type (WT) and H228D channels recorded in the cell-attached patch-clamp configuration at −100 mV. The closed state is marked with ticked lines (C), and the first open channel line is marked with a dotted line (O).
(C) to (F) Single-channel current-voltage relationship at pH 7.5 (closed circles) and pH 5.6 (open circles) for wild-type (C), H228D (D), S271E (E), and HDSE (F) channels. Linear regressions on three to six different patches in each condition revealed the following single-channel conductance values: wild-type (pH 7.5), 22.5 ± 0.3 pS; wild-type (pH 5.6), 12.3 ± 0.3 pS; H228D (pH 7.5), 21.1 ± 0.8 pS; H228D (pH 5.6), 18.3 ± 0.2 pS; S271E (pH 7.5), 25.4 ± 1.1 pS; S271E (pH 5.6), 25.4 ± 0.5 pS; HDSE (pH 7.5), 17.5 ± 0.8 pS; and HDSE (pH 5.6), 20.2 ± 0.8 pS. Data represent means ± se of three or more experiments.
AKT3 Is K+ Sensitive
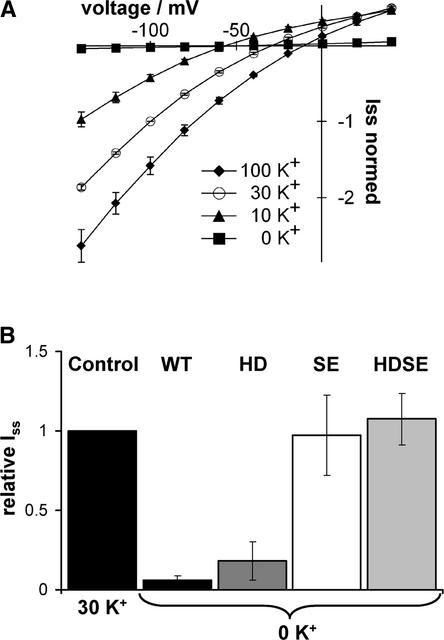
When studying the pH dependence of AKT3 at different K+ concentrations, we recognized a peculiar K+ dependence in the AKT3-WT channel. Therefore, we analyzed the macroscopic currents of AKT3-WT and mutants in response to varying external potassium concentrations. We found both K+ uptake and K+ release through AKT3 to depend strongly on the presence of external K+ ions (Figure 5A). A decrease of the K+ concentration from 100 to 30 mM and finally to 10 mM in the bath solution gradually decreased steady state inward currents but left outward currents at +40 mV unaffected. Omitting K+ from the perfusion solution and thereby maximizing the driving force for K+ release resulted in the complete loss of outward K+ currents through AKT3 (Figure 5A). In this context, it should be mentioned that the voltage-dependent gating of inward-rectifying shaker-like plant potassium channels is insensitive to changes in the external K+ concentration (Very et al., 1995; Blatt and Gradmann, 1997; Brüggemann et al., 1999).
Figure 5.
Potassium Sensitivity of AKT3-WT and Mutant Channels.
(A) Steady state current-voltage relationship of AKT3-WT channels at 100 mM (closed diamonds), 30 mM (open circles), 10 mM (closed triangles), and 0 mM (closed squares) external K+ concentrations. Currents were normalized to −100 mV in 30 mM at pH 7.5. Note that the reversal potential of AKT3 current is close to the K+ equilibrium potential in 100, 30, and 10 mM.
(B) Relative steady state outward K+ currents (Iss) at +40 mV in response to a shift from 30 to nominal 0 mM external K+ concentration. Currents were normalized to Iss of AKT3-WT recorded in 30 mM external K+ concentration. Results represent means ± sd of three or more experiments.
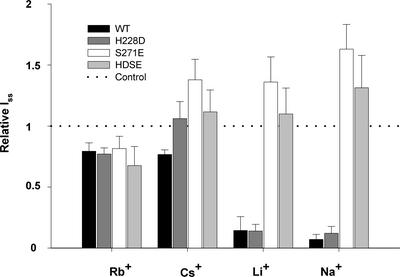
When we compared the different mutants with respect to K+ dependence, we recognized that the mutant AKT3-S271E, although reduced, even at nominally zero K+, carried outward currents (Figure 5B). The double mutant AKT3-HDSE, however, was completely insensitive to changes in external K+ concentrations. After the replacement of K+ with Rb+ or Cs+, we found that these monovalent cations were able to activate the AKT3 channel as well (Figure 6). In these experiments, outward currents through AKT3-WT and AKT3-H228D at +40 mV were of the same order of magnitude (Figure 6). In contrast, Na+ and Li+ were not able to restore outward currents.
Figure 6.
Sensitivity of AKT3-WT and Mutant Channels towards Monovalent Cations.
Relative steady state outward K+ currents (Iss) at +40 mV in response to a replacement of 100 mM external K+ by Rb+, Cs+, Li+, or Na+. Currents were normalized to Iss in 100 mM external K+ concentration (dotted line). Results represent means ± sd of three or more experiments. Note that in AKT3-WT and H228D, neither Li+ nor Na+ can substitute for K+, whereas mutants carrying a mutation at Ser-271 mediate outward currents irrespective of the nature of the cation present in the bath.
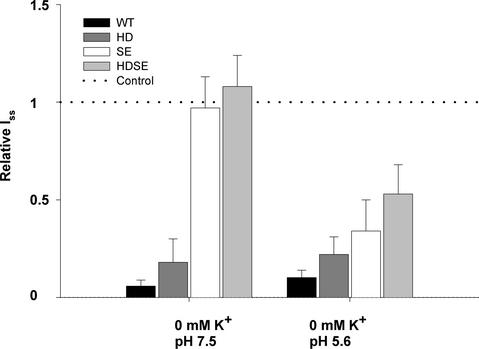
The distal pore mutant AKT3-S271E as well as the double mutant AKT3-HDSE, which is characterized by outward currents even at nominally zero external K+, mediated K+ efflux irrespective of the nature of the external cations present. These experiments suggest that the K+-dependent modulation of outward currents in AKT3 relies on potassium binding in the outer pore region rather than in the ion permeation pathway. However, when probing potassium sensitivity after a shift to pH 5.6, we found that the AKT3-S271E mutant regained its K+ sensitivity (Figure 7). Like AKT3-WT and the AKT3-H228D mutant, at pH 5.6, the outward K+ currents through channels harboring mutations at position Ser-271 declined significantly.
Figure 7.
AKT3 Mutants Regain K+ Sensitivity at Acidic pH.
Relative steady state outward K+ currents (Iss) at +40 mV in response to a shift from 30 to nominal 0 mM external K+ concentration at pH 7.5 compared with pH 5.6. Currents were normalized to Iss recorded in 30 mM external K+ concentration at the corresponding pH values (dotted line). Results represent means ± sd of three or more experiments.
DISCUSSION
pH Sensitivity
The performance of ion channels in response to both internal and external pH changes is of crucial physiological importance for plants (Dietrich et al., 2001; Felle, 2001). Here, we have studied the molecular basis of the proton block and K+ sensitivity of the Arabidopsis phloem channel AKT3 (Marten et al., 1999; Deeken et al., 2000; Lacombe et al., 2000b). Using site-directed mutagenesis followed by heterologous characterization in Xenopus oocytes, we showed that two titratable sites located in the outer mouth of the K+ channel pore are essential for the peculiar pH dependence of AKT3. A His residue at position 228 in the S5-P linker of AKT3, when replaced with Asp (H228D), was characterized by a loss of proton susceptibility (Figure 3). In addition, we determined that a second site on the ascending loop of the AKT3 pore (Figure 2B) was involved in proton sensing.
Mutations at the second site, S271E, like H228D, significantly shifted the pKa of the proton-mediated block toward more acidic pH values. Thus, our findings are in agreement with previous studies that have shown that the molecular basis of the proton sensitivity of ion channels can be attributed to the protonation of titratable amino acids such as His, Cys, and Lys (Guy and Durell, 1995; Jäger and Grissmer, 2001). However, different molecular mechanisms have been proposed to account for the proton sensitivity of ion channels such as the inward-rectifying plant potassium channel KST1 or animal K+ channels such as hKir3.4, hKv1.3, and rKv1.5 (Coulter et al., 1995; Hoth et al., 1997; Jäger et al., 1998; Steidl and Yool, 1999).
KST1 activation by acidic pH involves the protonation of two extracellular His residues. Although one His is located within the KST1 pore, the second resides in the S3-S4 linker, which very likely contributes to the formation of the outer pore (Hoth and Hedrich, 1999b). Protonation of these His residues leads to a shift in the voltage-dependent open probability of KST1 toward less negative membrane potentials and thereby increases K+ uptake (Hoth et al., 1997). In contrast, hKir3.4, like AKT3, undergoes proton-induced reductions in single-channel conductance. Structure–function analyses revealed that in hKir3.4, upon protonation of a His near the pore, a titratable Cys residue influences ion conductance (Coulter et al., 1995). In line with the molecular mechanism proposed for the proton-induced block of hKir3.4, the pH-mediated decrease in single-channel conductance observed in AKT3-WT is lost in the AKT3 mutants H228D and S271E and in the double mutant AKT3-HDSE.
Potassium Sensitivity
The current amplitude and magnitude of inactivation of hKv1.3 and rKv1.5 are reduced by acidic extracellular pH, an effect hypothesized to be induced by the protonation of a His residue located near the channel pore (Busch et al., 1991; Jäger et al., 1998; Steidl and Yool, 1999). These studies have shown that pH sensitivity interferes with K+-dependent gating of these voltage-dependent outward-rectifier channels. Although this phenomenon is well known for vertebrate shaker-like K+ channels (Yellen, 1997), this behavior was demonstrated only recently for plant outward rectifiers (Gaymard et al., 1998; Ache et al., 2000; Lacombe et al., 2000a).
The Arabidopsis delayed rectifiers SKOR and GORK are affected by external K+ in a dual fashion: (1) the activation potential is sensitive to EK; and (2) K+ release through these channels requires external potassium (Gaymard et al., 1998; Ache et al., 2000; Ivashikina et al., 2001). In this context, it should be noted that the gating of TOK1, the yeast outward rectifier, is sensitive to external changes in K+ ions as well. TOK1 comprises four transmembrane domains (S1 to S4) followed by two pore motifs (S5-P1-S6 and S7-P2-S8) in tandem, and Vergani and colleagues (Vergani et al., 1998; Vergani and Blatt, 1999) have shown that mutations on either site of the selectivity filter affect the K+-dependent gating of this channel.
Here, we have shown that K+ efflux mediated by the weak inward rectifier AKT3 is sensitive to extracellular potassium. As in GORK, SKOR, and the animal shaker-like potassium channels, removal of potassium from the bath solution abolished outward currents through AKT3. S271E and the HDSE double mutant, however, which have been shown to be involved in proton sensing, have counterparts in the potassium-insensitive shaker-like plant inward rectifiers (Brüggemann et al., 1999). In contrast to the AKT3-WT channel and the H228D mutant, these mutants still conduct outward potassium currents in the absence of external potassium.
Future experiments replacing Ser-271 with other amino acids and the test of the role of neighboring positions will allow us to determine if the elimination of potassium dependence is caused by the absence of Ser or just by the presence of any negatively charged residue at position 271. In agreement with the behavior of rKv1.4, outward K+ currents could be restored by replacing external potassium with rubidium or cesium but not with sodium or lithium (Pardo et al., 1992). The fact that the well-known voltage-dependent K+ channel blocker Cs+ is able to maintain the outward current suggests the existence of an external K+ lock-in site in the outer mouth of the AKT3 channel (Vergara et al., 1999; Jäger and Grissmer, 2001). However, this site is not abolished in the AKT3-S271E mutant, because this mutant, like its animal counterparts, regains its potassium sensitivity at acidic extracellular pH (Jäger and Grissmer, 2001).
In recent work on the KcsA potassium channel, it has been shown that a K+ ion is present at the outer month of the pore (Morais-Cabral et al., 2001; Zhou et al., 2001). Thus, the amino acids His-228 and Ser-271 could play a major role in the maintenance of the electrostatic field that stabilized this K+ ion at this position (Figure 1B).
Based on our observations that (1) two peripheral residues modulate the pH sensitivity of the AKT3 channel and (2) one of these residues confers potassium sensitivity to AKT3, we conclude that protonation of these amino acids in the outer pore controls K+-dependent K+ currents through the phloem K+ channel. The role of H+ and K+ sensitivity of the AKT2/3 channels will now be addressed in planta by expressing the mutant channels under the control of the AKT2/3 promoter in the akt2/3-1 background.
METHODS
AKT3 mutants were generated using the Quick-Change site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands) as described in Hoth and Hedrich (1999a). The complementary RNAs of AKT3 wild-type and mutant channels were generated by in vitro transcription (T7-Megascript kit; Ambion, Austin, TX) and injected into oocytes of Xenopus laevis (Centre de Recherche en Biochimie Macromoléculaire, Centre National de la Recherche Scientifique, Montpellier, France) using a PicospritzerII microinjector (General Valve, Fairfield, NJ). Two to 6 days after injection, double-electrode voltage-clamp recordings were made with a Turbotec-01C amplifier (NPI Instruments, Tamm, Germany). The electrodes were filled with 3 M KCl and had typical input resistances of ∼2 MΩ.
Solutions for pH measurements were composed of 30 mM KCl, 2 mM MgCl2, 1 mM CaCl2, and 10 mM Tris/Mes, pH 7.5, Mes/Tris, pH 6.0, or citrate/Tris, pH 4.5. The solution used to determine the sensitivity toward extracellular cations contained 100 mM XCl (where X = K, Na, Li, Rb, or Cs), 2 mM MgCl2, 1 mM CaCl2, and 10 mM Tris/Mes, pH 7.5. Solutions for Figures 5 and 7 were composed of 100, 30, and 10 mM KCl, 2 mM MgCl2, 1 mM CaCl2, and 10 mM Tris/Mes, pH 7.5, or Mes/Tris pH 5.6. The ionic strength was kept constant by replacing K+ with N-methyl-d-glucamine. All media were adjusted to a final osmolality of 215 to 235 mosmol/kg with d-sorbitol.
For patch-clamp experiments, devitellinized oocytes were placed in a bath solution containing 100 mM KCl, 2 mM MgCl2, 1 mM CaCl2, and 10 mM Tris/Mes, pH 7.5. Pipettes were filled with solution containing 100 mM KCl, 2 mM MgCl2, 1 mM CaCl2, and 10 mM Tris/Mes, pH 7.5, or Mes/Tris, pH 5.6. Currents were recorded in the cell-attached configuration using an EPC-9 amplifier (HEKA, Lambrecht, Germany) as described previously (Marten et al., 1999).
Upon request, all novel material described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial purposes.
Accession Numbers
The GenBank accession numbers for the sequences shown in Figure 1 are as follows: KST1, X79779; KAT1, M86990; KAT2, NP_193563; AKT1, X62907; AKT3, U44745; VFK1, CAC29435; ZMK1, CAA68912; and SKT2, CAA70870.
Acknowledgments
We are grateful to Kerstin Neuwinger for technical assistance. This work was founded by a European Molecular Biology Organization long-term fellowship to B.L. and Deutsche Forschungsgemeinschaft grants to R.H.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003244.
References
- Ache, P., Becker, D., Deeken, R., Dreyer, I., Weber, H., Fromm, J., and Hedrich, R. (2001). VFK1, a Vicia faba K+ channel involved in phloem unloading. Plant J. 27, 571–580. [PubMed] [Google Scholar]
- Ache, P., Becker, D., Ivashikina, N., Dietrich, P., Roelfsema, M.R., and Hedrich, R. (2000). GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 486, 93–98. [DOI] [PubMed] [Google Scholar]
- Bauer, C.S., Hoth, S., Haga, K., Philippar, K., Aoki, N., and Hedrich, R. (2000). Differential expression and regulation of K+ channels in the maize coleoptile: Molecular and biophysical analysis of cells isolated from cortex and vasculature. Plant J. 24, 139–145. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R., and Gradmann, D. (1997). K+-sensitive gating of the K+ outward rectifier in Vicia guard cells. J. Membr. Biol. 158, 241–256. [DOI] [PubMed] [Google Scholar]
- Brüggemann, L., Dietrich, P., Becker, D., Dreyer, I., Palme, K., and Hedrich, R. (1999). Channel-mediated high-affinity K+ uptake into guard cells from Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 3298–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, A.E., Hurst, R.S., North, R.A., Adelman, J.P., and Kavanaugh, M.P. (1991). Current inactivation involves a histidine residue in the pore of the rat lymphocyte potassium channel RGK5. Biochem. Biophys. Res. Commun. 179, 1384–1390. [DOI] [PubMed] [Google Scholar]
- Cao, Y., Ward, J.M., Kelly, W.B., Ichida, A.M., Gaber, R.F., Anderson, J.A., Uozumi, N., Schroeder, J.I., and Crawford, N.M. (1995). Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 109, 1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter, K.L., Perier, F., Radeke, C.M., and Vandenberg, C.A. (1995). Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron 15, 1157–1168. [DOI] [PubMed] [Google Scholar]
- Deeken, R., Sanders, C., Ache, P., and Hedrich, R. (2000). Developmental and light-dependent regulation of a phloem-localised K+ channel of Arabidopsis thaliana. Plant J. 23, 285–290. [DOI] [PubMed] [Google Scholar]
- Dennison, K.L., Robertson, W.R., Lewis, B.D., Hirsch, R.E., Sussman, M.R., and Spalding, E.P. (2001). Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 127, 1012–1019. [PMC free article] [PubMed] [Google Scholar]
- Dietrich, P., Sanders, D., and Hedrich, R. (2001). The role of ion channels in light-dependent stomatal opening. J. Exp. Bot. 52, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Dreyer, I., Michard, E., Lacombe, B., and Thibaud, J.B. (2001). A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 505, 233–239. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, T., Zimmermann, S., and Müller-Röber, B. (1997). Association of plant K+in channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett. 409, 166–170. [DOI] [PubMed] [Google Scholar]
- Felle, H.H. (2001). pH: Signal and messenger in plant cells. Plant Biol. 3, 577–591. [Google Scholar]
- Gaymard, F., Pilot, G., Lacombe, B., Bouchez, D., Bruneau, D., Boucherez, J., Michaux-Ferriere, N., Thibaud, J.B., and Sentenac, H. (1998). Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. [DOI] [PubMed] [Google Scholar]
- Guy, H.R., and Durell, S.R. (1995). Structural models of Na+, Ca2+, and K+ channels. Soc. Gen. Physiol. Ser. 50, 1–16. [PubMed] [Google Scholar]
- Hedrich, R., Moran, O., Conti, F., Busch, H., Becker, D., Gambale, F., Dreyer, I., Küch, A., Neuwinger, K., and Palme, K. (1995). Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators. Eur. Biophys. J. 24, 107–115. [DOI] [PubMed] [Google Scholar]
- Hoth, S., Dreyer, I., Dietrich, P., Becker, D., Müller-Röber, B., and Hedrich, R. (1997). Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. USA 94, 4806–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, S., Geiger, D., Becker, D., and Hedrich, R. (2001). The pore of plant K+ channels is involved in voltage and pH sensing: Domain-swapping between different K+ channel α-subunits. Plant Cell 13, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, S., and Hedrich, R. (1999. a). Distinct molecular bases for pH sensitivity of the guard cell K+ channels KST1 and KAT1. J. Biol. Chem. 274, 11599–11603. [DOI] [PubMed] [Google Scholar]
- Hoth, S., and Hedrich, R. (1999. b). Susceptibility of the guard-cell K+-uptake channel KST1 to Zn2+ requires histidine residues in the S3-S4 linker and in the channel pore. Planta 209, 543–546. [DOI] [PubMed] [Google Scholar]
- Ivashikina, N., Becker, D., Ache, P., Meyerhoff, O., Felle, H.H., and Hedrich, R. (2001). K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 508, 463–469. [DOI] [PubMed] [Google Scholar]
- Jäger, H., and Grissmer, S. (2001). Regulation of a mammalian Shaker-related potassium channel, hKv1.5, by extracellular potassium and pH. FEBS Lett. 488, 45–50. [DOI] [PubMed] [Google Scholar]
- Jäger, H., Rauer, H., Nguyen, A.N., Aiyar, J., Chandy, K.G., and Grissmer, S. (1998). Regulation of mammalian Shaker-related K+ channels: Evidence for non-conducting closed and non-conducting inactivated states. J. Physiol. 506, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum, K.A., and Slayman, C.W. (1996). Isolation of an ion channel gene from Arabidopsis thaliana using the H5 signature sequence from voltage-dependent K+ channels. FEBS Lett. 378, 19–26. [DOI] [PubMed] [Google Scholar]
- Lacombe, B., Pilot, G., Gaymard, F., Sentenac, H., and Thibaud, J.B. (2000. a). pH control of the plant outwardly-rectifying potassium channel SKOR. FEBS Lett. 466, 351–354. [DOI] [PubMed] [Google Scholar]
- Lacombe, B., Pilot, G., Michard, E., Gaymard, F., Sentenac, H., and Thibaud, J.B. (2000. b). A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12, 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten, I., Hoth, S., Deeken, R., Ache, P., Ketchum, K.A., Hoshi, T., and Hedrich, R. (1999). AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. USA 96, 7581–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser, P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Zhou, Y., and MacKinnon, R. (2001). Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414, 37–42. [DOI] [PubMed] [Google Scholar]
- Müller-Röber, B., Ellenberg, J., Provart, N., Willmitzer, L., Busch, H., Becker, D., Dietrich, P., Hoth, S., and Hedrich, R. (1995). Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 14, 2409–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, R.L., McKendree, W.L., Jr., Hirsch, R.E., Sedbrook, J.C., Gaber, R.F., and Sussman, M.R. (1995). Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, L.A., Heinemann, S.H., Terlau, H., Ludewig, U., Lorra, C., Pongs, O., and Stühmer, W. (1992). Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. USA 89, 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar, K., Fuchs, I., Luthen, H., Hoth, S., Bauer, C.S., Haga, K., Thiel, G., Ljung, K., Sandberg, G., Bottger, M., Becker, D., and Hedrich, R. (1999). Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 96, 12186–12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot, G., Lacombe, B., Gaymard, F., Cherel, I., Boucherez, J., Thibaud, J.B., and Sentenac, H. (2001). Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221. [DOI] [PubMed] [Google Scholar]
- Roelfsema, M.R.G., and Hedrich, R. (1999). Plant ion transport. In Encyclopedia of Life Sciences (Macmillan Reference Ltd., www.els.net).
- Schachtman, D.P., Schroeder, J.I., Lucas, W.J., Anderson, J.A., and Gaber, R.F. (1992). Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258, 1654–1658. [DOI] [PubMed] [Google Scholar]
- Schönherr, R., and Heinemann, S.H. (1996). Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J. Physiol. 493, 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl, J.V., and Yool, A.J. (1999). Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol. Pharmacol. 55, 812–820. [PubMed] [Google Scholar]
- Szyroki, A., Ivashikina, N., Dietrich, P., Roelfsema, M.R., Ache, P., Reintanz, B., Deeken, R., Godde, M., Felle, H., Steinmeyer, R., Palme, K., and Hedrich, R. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 98, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani, P., and Blatt, M.R. (1999). Mutations in the yeast two pore K+ channel YKC1 identify functional differences between the pore domains. FEBS Lett. 458, 285–291. [DOI] [PubMed] [Google Scholar]
- Vergani, P., Hamilton, D., Jarvis, S., and Blatt, M.R. (1998). Mutations in the pore regions of the yeast K+ channel YKC1 affect gating by extracellular K+. EMBO J. 17, 7190–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara, C., Alvarez, O., and Latorre, R. (1999). Localization of the K+ lock-in and the Ba2+ binding sites in a voltage-gated calcium-modulated channel: Implications for survival of K+ permeability. J. Gen. Physiol. 114, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Very, A.A., Gaymard, F., Bosseux, C., Sentenac, H., and Thibaud, J.B. (1995). Expression of a cloned plant K+ channel in Xenopus oocytes: Analysis of macroscopic currents. Plant J. 7, 321–332. [DOI] [PubMed] [Google Scholar]
- Yellen, G. (1997). Single channel seeks permeant ion for brief but intimate relationship. J. Gen. Physiol. 110, 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Morais-Cabral, J.H., Kaufman, A., and MacKinnon, R. (2001). Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414, 43–48. [DOI] [PubMed] [Google Scholar]
- Zimmermann, S., and Sentenac, H. (1999). Plant ion channels: From molecular structures to physiological functions. Curr. Opin. Plant Biol. 2, 477–482. [DOI] [PubMed] [Google Scholar]