Abstract
Pathogenic Yersinia species use a type III secretion system to inhibit phagocytosis by eukaryotic cells. At 37°C, the secretion system is assembled, forming a needle-like structure on the bacterial cell surface. Upon eukaryotic cell contact, six effector proteins, called Yops, are translocated into the eukaryotic cell cytosol. Here, we show that a yscP mutant exports an increased amount of the needle component YscF to the bacterial cell surface but is unable to efficiently secrete effector Yops. Mutations in the cytoplasmic domain of the inner membrane protein YscU suppress the yscP phenotype by reducing the level of YscF secretion and increasing the level of Yop secretion. These results suggest that YscP and YscU coordinately regulate the substrate specificity of the Yersinia type III secretion system. Furthermore, we show that YscP and YscU act upstream of the cell contact sensor YopN as well as the inner gatekeeper LcrG in the pathway of substrate export regulation. These results further strengthen the strong evolutionary link between flagellar biosynthesis and type III synthesis.
There are three pathogenic species of Yersinia: Yersinia pestis, Yersinia enterocolitica, and Yersinia pseudotuberculosis. Yersinia pestis causes plague and is transmitted by flea bites or infectious aerosols, while Yersinia enterocolitica and Yersinia pseudotuberculosis are enteric pathogens that cause gastroenteritis after the ingestion of contaminated food or water (for reviews, see references 3 and 36). After reaching the intestine, enteropathogenic Yersinia cells are taken up by antigen-sampling M cells (1). This enables the bacteria to colonize the Peyer's patches, a gut-associated lymphoid tissue. Once in the Peyer's patches, the bacteria are able to inhibit phagocytosis by macrophages (10, 38) and polymorphonuclear leukocytes (52), which allows them to replicate extracellularly (44). In humans, such infections are typically self-limiting, while in rodents, the bacteria are able to colonize other organs, which results in a deadly systemic infection. The ability to cause infection is dependent on the presence of an approximately 70-kb plasmid encoding a type III secretion system (TTSS) that delivers Yop effectors into the cytosol of the target cell. The Yersinia TTSS is comprised of about 25 Ysc (Yop secretion) proteins. Nine of these proteins are conserved in the bacterial flagellar export apparatus and in the TTSSs found in a wide variety of gram-negative plant and animal pathogens (for a review, see reference 19). The Yersinia type III secretion apparatus assembles a needle-like structure comprised of the YscF protein on the bacterial cell surface prior to eukaryotic cell contact (18). The TTSSs of other gram-negative pathogens form similar structures (7, 21, 22, 48, 49). The concept of substrate specificity switching by TTSSs was first demonstrated in the flagellar system. The bacterial flagellum consists of three parts: the basal body, which is located in the cell wall and membranes of the bacterium; the hook, which is located on the cell surface; and the long flagellar filament, which is assembled onto the end of the hook and serves as a propeller during bacterial motility (for a review, see reference 26). The length of the hook is normally 55 nm. Yamaguchi and colleagues, however, showed that a fliK mutant exhibits a polyhook phenotype in which hook elongation proceeds to an abnormal extent but no flagellar filament is built (34). Mutations in fliK can be suppressed, with respect to filament assembly, by mutations in the export apparatus protein FlhB (24, 51), which is located in the bacterial inner membrane. Work by Minamino and Macnab has demonstrated that FliK, along with the hook and filament proteins, binds to the cytoplasmic domain of FlhB (30, 31). Therefore, upon the completion of hook assembly, it is possible that FliK switches the substrate specificity of the flagellar export apparatus by altering the conformation of FlhB in order to promote the export of the filament component flagellin. A similar phenomenon has been reported in the TTSS encoded by Salmonella pathogenicity island 1 (SPI1). Specifically, an invJ mutant assembles a type III secreton with abnormally long needles (23). Interestingly, an invJ mutant is unable to secrete effector proteins (5), which suggests that it is defective in substrate specificity switching. Recent findings by Tamano and coworkers (49) showed that Spa32 of Shigella spp. is involved in the control of needle length. Spa32 is homologous to InvJ and, interestingly, Spa32 is interchangeable with InvJ of Salmonella (49).
Here, we examined the phenotype of yscP mutants of the Yersinia TTSS. We demonstrate that a yscP mutant exports an increased amount of YscF to the bacterial cell surface prior to eukaryotic cell contact. Furthermore, the yscP mutant is able to secrete only low levels of the translocator proteins, YopB and YopD, and Yop effectors. Mutations in the cytoplasmic domain of the inner membrane protein YscU can restore a level of Yop effector secretion to the yscP mutant higher than that to the corresponding isogenic wild-type strain, while the amount of YscF present on the bacterial cell surface is reduced. These results suggest that YscP and YscU coordinately regulate secretion of the Yersinia TTSS.
MATERIALS AND METHODS
Media and growth conditions.
Yersinia strains were grown in brain-heart infusion (BHI) broth supplemented with either 5 mM EGTA and 20 mM MgCl2 (BHI minus Ca2+) or 2.5 mM CaCl2 (BHI plus Ca2+). Escherichia coli strains were grown in Luria-Bertani broth (LB) (8). Yersinia strains were grown in LB containing 50 μg of kanamycin ml−1 to maintain selection of the virulence plasmid. Yersinia strains carrying YscU-expressing plasmids were grown in LB supplemented with 100 μg of carbenicillin ml−1.
DNA methods.
DNA preparations and routine subcloning were performed as described by Sambrook et al. (42). DNA-sequencing reactions were performed by using the ThermoSequenase dye terminator cycle sequencing kit and analyzed with a SEQ4 × 4 sequencer (Amersham Pharmacia Biotech).
Construction of a yscF in-frame deletion mutant.
PCR was performed with the primer pairs YscF3-YscF4 and YscF5-YscF6, respectively, by using the plasmid pIB102 as template. YscF3 was tailed with an XbaI site, and YscF6 was tailed with a SacI site. The PCR yielded one fragment from the upstream region and one fragment from the downstream region of yscF. The 5′ ends of the primers YscF4 and YscF5 contain overlapping sequences between the two fragments, and a second PCR with the two fragments as templates and the primers YscF3 and YscF6 resulted in a fragment in which the yscF gene, deleted for codons 11 through 69, was flanked by upstream and downstream sequences. After digestion with XbaI and SacI, the fragment was cloned into the vector pDM4 (Table 1), resulting in pLS52. This plasmid was transformed into E. coli strain S17-1λpir, clones were selected on plates containing chloramphenicol, and the resulting transformants were verified by PCR. E. coli containing the plasmid was then used to introduce pLS52 by conjugation into the recipient Yersinia strain YPIII(pIB102). Clones in which the plasmid integrated by a single recombination event were selected on Yersinia selective agar base (YSAB) plates containing 50 μg of kanamycin/ml and 20 μg of chloramphenicol/ml. The insertion was verified by PCR with the primers from the second PCR. The resulting strain was then counterselected on sucrose to select for clones that had lost the plasmid. The yscF deletion was verified by PCR with primers YscF3 and YscF6. The resulting mutant was denoted YPIII(pIB202).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Recipient for cloning experiments | 17 |
| S17-1λpir | Recipient for suicide plasmids | 43 |
| BL21(DE3) | IPTG-inducible T7 RNA poly- merase | 47 |
| Yersinia | ||
| YPIII(pIB102) | wild-type, parental strain | 2 |
| YPIII(pIB69) | yscP-null strain | This study |
| YPIII(pIB75) | yscU-null strain | 25 |
| YPIII(pIB202) | yscF-null strain | This study |
| YPIII(pIB604) | yopB-null strain | 16 |
| YPIII(pIB82) | yopN-null strain | 39 |
| YPIII(pIB60469) | yopB yscP-double-null strain | This study |
| YPIII(pIB6975) | yscP yscU-double-null strain | This study |
| YPIII(pIB8769) | yopN yscP-double-null strain | This study |
| YPIII(pIB70169) | lcrG yscP-double-null strain | This study |
| YPIII(pIB2669) | lcrQ yscP-double-null strain | This study |
| YPIII(pIB62169) | yopD yscP-double-null strain | This study |
| Plasmid | ||
| pBAD33 | Contains PBAD promoter | 15 |
| pET-22b | T7 overexpression vector | Novagen |
| pDM4 | Suicide vector | 28 |
| pSL144 | ΔyscP in pDM4 | This study |
| pSL340 | ΔyopN in pDM4 | This study |
| pKK223-3 | Contains tac promoter | Amersham Phar- macia Biotech |
| pPE5 | yscU gene in pET-22b | This study |
| pPE33 | yscU gene in pKK223-3 | This study |
| pPE34 | yscP gene in pBAD33 | This study |
| pPE36 | yscU-A268F gene in pKK223-3 | This study |
| pPE37 | yscU-Y287G gene in pKK223-3 | This study |
| pPE38 | yscU-V292T gene in pKK223-3 | This study |
| pPE39 | yscU-Y317D gene in pKK223-3 | This study |
| pMMB66EH | Contains tac promoter | 14 |
| pJO33 | GST-YscF overexpression vector | This study |
| pGEX-5X-3 | Cloning vector | Amersham Phar- macia Biotech |
| pLS52 | ΔyscF in pDM4 | This study |
| pLS53 | YscF expression vector | This study |
For transcomplementation studies, the yscF gene was amplified by PCR with the primers YscF1 and YscF2 tailed with sites for EcoRI and PstI and cloned into the vector pMMB66EH in the strain E. coli S17-1λpir. After conjugation into the mutant strain on YSAB plates containing 100 μg of carbenicillin/ml and 50 μg of kanamycin/ml, the plasmid (pLS53) was verified by a plasmid mini preparation and PCR with the same primer pair. The primers used and their sequences were as follows: YscF1, 5′-GCT CAG AAT TCG ATG AGT AAC TTC TCT GGA TTT A-3′ (bp 4193 through 4214); YscF2, 5′-CTG ACT CTG CAG TTC ATA TTA TGG GAA CTT CTG T-3′ (bp 4462 through 4441); YscF3, 5′-GCT GAT CTA GAC GAA TTG AAT TTC GAG GTG CAA G-3′ bp 3801 through 3822); YscF4, 5′-GAT GCC TTG TCC TTT CGT AAA TCC AGA GAA G-3′ (bp 4222 through 4201); YscF5, 5′-ACG AAA GGA CAA GGC ATC CTA CAG AAG TTC-3′ (bp 4430 through 4450); YscF6, 5′-GCT CAC GAG CTC GAG ACG ATT TAA ACG TGA CTC-3′ (bp 4739 through 4719). All YscF primer sequences were from GenBank under accession no. M83225. Restriction sites are shown in boldface.
Construction of the yscP-null strain.
An in-frame deletion was made in the yscP gene by PCR amplifying YPIII(pIB102) genomic DNA with Pfx DNA polymerase and the primer pairs SL89-SL90 (which resulted in a fragment complementary to the upstream gene yscO and the first six codons of the yscP gene) and SL91-SL92 (which resulted in a fragment complementary to the last six codons of the yscP gene and the downstream gene yscQ). The two fragments were ligated by PCR with the primer pair SL89-SL92. The resulting PCR product was digested with XbaI and SphI and cloned into the same sites in the suicide vector pDM4. The resulting construct, pSL144, was then transformed into the E. coli strain S17-1λpir (43) and conjugated into the wild-type Yersinia strain YPIII(pIB102) by plating on Yersinia agar (Difco) plates containing 25 μg of chloramphenicol ml−1. Exconjugants were restreaked onto LB plates containing 5% sucrose in order to counterselect against bacteria still containing the pSL144 plasmid. Sucrose-resistant colonies were PCR amplified with the primer pair SL89-SL92 to confirm the presence of the deletion. The resulting strain, YPIII(pIB69), lacked codons 7 through 449 of the yscP gene. The primers used and their sequences were as follows: SL89, 5′-GCC TCT AGA TCA GCA AGC TTG CTT GCA GGC; SL90, 5′-CTC CCA CTC CTC ATA CTC AGG TTC TAA TGG GGA; SL91, 5′-GAA CCT GAG TAT GAG GAG TGG GAG GCT GAA GAA; SL92, 5′-GGC GCA TGC CCA GAA GGA GAT ATG CGC ATT. Restriction sites are shown in boldface.
Construction of the yscP yopB-double-null strain.
Plasmid pSL144 was conjugated into the yopB-null strain YPIII(pIB604) (19) as described above. The resulting strain was the yscP yopB-double-null strain YPIII(pIB60469).
Construction of the yscP yscU-double-null strain.
Plasmid pSL144 was conjugated into the yscU-null strain YPIII(pIB75) as described above.
Construction of the yopN yscP-double-null strain.
An in-frame deletion in the yopN gene was constructed by PCR amplifying YPIII(pIB102) genomic DNA with Pfx DNA polymerase and the primer pairs SAL226-SAL227 (which resulted in a fragment complementary to the yopN promoter region and the first six codons of the yopN coding sequence) and SAL228-SAL229 (which resulted in a fragment complementary to the last four codons of the yopN gene and the downstream gene tyeA). The two fragments were ligated by PCR with the primer pair SAL226-SAL229. The resulting PCR product was digested with SphI and XbaI and cloned into the same sites of the suicide vector pDM4. The resulting construct, pSL340, was conjugated into the yscP-null strain YPIII(pIB69) as described above. The resulting strain, YPIII(pIB8769), lacked codons 7 through 289 of the yopN gene. The primers used and their sequences were as follows: SAL226, 5′-GCC GCA TGC GGC GGC TAC CTA CAA TGC CAT GAC; SAL227, 5′-GAA AGG TCG TAC GTT ATG AAG CGT CGT CAT AAC TAC; SAL228, 5′-ACG CTT CAT AAC GTA CGA CCT TTC TGA GTT TAT GGG; SAL229, 5′-GGC TCT AGA GCC AGA TTG AGC CAT CTC TAA TTG. Restriction sites are shown in boldface.
Preparation of YscF antiserum.
A fragment of yscF generated by PCR with primer pair JO214-JO217 and strain YPIII(pIB102) as template was cloned into pGEX-5X-3 (Amersham Pharmacia Biotech) with the BamHI and NotI restriction sites, generating plasmid pJO33. Fusion proteins were expressed, purified, and cleaved at the factor Xa cleavage site according to the manufacturer's instructions. The resulting polypeptide, approximately 8 kDa in size, was used as antigen to raise a polyclonal rabbit antiserum (Agrisera, Umeå, Sweden), which was used in Western blots without further purification. The primers used were JO214 (5′-CG ACA GGG ATC CAG ATG AGT AAC TTC TCT GGA TTT) and JO217 (5′-CGA CAG GCG GCC GCG TTA TGG GAA CTT CTG TAG GA). Restriction sites are shown in boldface.
Construction of mutations in the cytoplasmic domain of YscU.
YPIII(pIB102) genomic DNA was PCR amplified with Pfx DNA polymerase and the primer pair SL53-SL83. The resulting PCR product, consisting of the yscU gene, was digested with EcoRI and PstI and cloned into the same sites in the pKK223-3 vector (Amersham Pharmacia Biotech) such that the expression of YscU was under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible tac promoter. The resulting construct, pPE33, was used as the template for site-directed mutagenesis, which was carried out according to the procedures in the GeneEditor in vitro site-directed mutagenesis kit (Promega).
The mutagenic oligonucleotides used were as follows: the A268F mutation (PE40), the Y287G mutation (PE41), the V292T mutation (PE43), and the Y317D mutation (PE46). The primers used and their sequences were as follows: SL53, 5′-GGC CTG CAG TTA TAA CAT TTC GGA ATG TTG; SL83, 5′-GCC GAA TTC ATG AGC GGA GAA AAG ACA GAG; PE40, 5′-CCG ACC CAT ATT TTC ATT GGT ATT CTT TAC; PE41, 5′-GTA ACA TTC AAA GGT ACC GAT GCC CAA G; PE43, 5′-ACC GAT GCC CAA ACT CAG ACT GTG CGC; PE46, 5′-GCC CGT GCT CTT GAT TGG GAT GCG CTC G. Restriction sites are depicted in boldface.
Yop secretion assay.
Overnight cultures of Y. pseudotuberculosis strains were grown in BHI medium lacking Ca2+ which contained 50 μg of kanamycin ml−1 at 26°C. The cultures were diluted to an optical density at 600 nm of 0.2 into 10 ml of fresh medium and grown at 26°C for 1 h and then for an additional 2 h at 37°C to induce secretion. The cultures were then centrifuged at 3,000 × g for 15 min. The supernatants containing the secreted Yops were passed through a 0.45-μm-pore-size filter and precipitated with 10% trichloroacetic acid (TCA). TCA precipitates were centrifuged at 3,000 × g for 20 min, the supernatants were discarded, and the remaining pellets were dried at room temperature. The pellets were resuspended in 250 μl of 2% sodium dodecyl sulfate (SDS) and precipitated with acetone at −20°C for 30 min. Samples were centrifuged at 20,800 × g for 10 min, the supernatants were discarded, and the pellets were air-dried. The pellets were then resuspended in 100 μl of 8 M urea and an equal amount of 2× sample buffer. Equal amounts of culture supernatant and cell pellet fractions were separated by SDS-PAGE gels stained with Coomassie blue or transferred to a nitrocellulose membrane. Yop proteins were detected by using a polyclonal anti-Yop antiserum raised against cell-secreted Yops (11).
Immunoblotting.
Samples were separated by SDS-12% PAGE and electroblotted (Trans Blot SD; Bio-Rad) onto a nitrocellulose transfer membrane (Protran; Schleicher and Schuell) by using a transfer buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. The membrane was blocked for 1 h with Tris-buffered saline plus 0.1% Tween 20 (TBS-T) and 5% nonfat dry milk. The membrane was probed for 1 h with the appropriate polyclonal antiserum in 10 ml of the blocking buffer and was then washed three times at 5 min each with TBS-T. The membrane was incubated for 1 h with an anti-rabbit antibody (Amersham Pharmacia Biotech) in 10 ml of blocking buffer, followed by washing with TBS-T. Proteins were detected by using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Surface localization of YscF.
Overnight cultures of the wild-type Y. pseudotuberculosis strain YPIII(pIB102), the yscF-null mutant YPIII(pIB202), and the yscP-null mutant YPIII(pIB69), expressing different YscU constructs, were grown at 26°C in BHI medium containing calcium (2.5 mM) supplemented with 50 μg of kanamycin ml−1 (to select for the virulence plasmid) and 100 μg of carbenicillin ml−1 (to select for YscU-expressing constructs). The cultures were diluted to an optical density at 600 nm of 0.2 in 10 ml of fresh medium and grown for 1 h at 26°C, after which the cultures were shifted to 37°C for 2 h to induce secretion. Whole cultures were sheared by five passages through a hypodermic needle (23Gx1, 0.6 by 25 mm; B. Braun), which released surface proteins and organelles from the bacterial surface. The cultures were centrifuged at 1,800 × g for 15 min. The pellets were resuspended in 100 μl of H2O and an equal amount of 2× sample buffer. The supernatants were precipitated with 10% TCA and incubated on ice for 30 min and thereafter centrifuged at 1,800 × g for 20 min. Next, the supernatants were discarded and the pellets were resuspended in 250 μl of 2% SDS and precipitated with acetone at −20°C for 30 min. The samples were then centrifuged at 15,000 × g for 10 min, the supernatants were discarded, and the pellets were dried, followed by resuspension in 100 μl of 8 M urea and an equal amount of 2× sample buffer. Two microliters of the pellet, diluted 1:10, and 5 μl of undiluted supernatant were separated by 15% Tris-tricine SDS-PAGE and transferred to a nitrocellulose membrane. YscF was detected with a polyclonal anti-YscF antibody.
Cytotoxicity assay.
Overnight cultures of the wild-type strain YPIII(pIB102), the yscP-null mutant YPIII(pIB69), and the yopB yscP-double-null mutant YPIII(pIB60469) were grown in LB containing 50 μgof kanamycin ml−1; for strains expressing different YscU constructs, 100 μg of carbenicillin ml−1 was also added. Cytotoxicity was assayed as described previously (39). Pictures were taken with a phase-contrast microscope (Zeiss).
RESULTS
Phenotypic characterization of a yscP mutant.
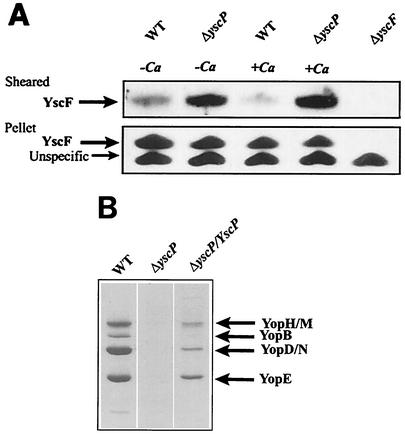
To investigate the possible role of YscP in regulating the secretion of the Yersinia type III needle protein YscF, a yscP-null mutant, YPIII(pIB69), and the isogenic wild-type strain YPIII(pIB102) (Table 1) were grown at 37°C in either the presence or absence of calcium. The bacterial supernatants were first investigated for the presence of YscF. No YscF could be detected in these fractions, showing that YscF was not released to the culture medium during growth (data not shown). The bacterial pellets were sheared to release surface proteins and organelles from the bacterial surface. When the sheared fraction of the bacterial pellet was investigated, the yscP-null mutant was found to release larger amounts of YscF to the bacterial surface than the wild-type strain, irrespective of the calcium concentration of the medium (Fig. 1A). Since no YscF was found in the culture supernatants, we concluded that the YscF protein recovered from the sheared fractions originated from the bacterial surface. In addition, the levels of YscF in the bacterial pellets recovered after shearing were similar (Fig. 1A), which suggested that the increased level of YscF released by shearing that was found in the yscP-null mutant was not due to increased expression of the YscF protein. Moreover, we could also confirm the findings of others that the yscP mutant secreted much lower levels of Yops than the wild-type strain (Fig. 1B) (35, 46).
FIG. 1.
YscP regulates the substrate specificity of the Yersinia TTSS. (A) Surface localization of YscF in the yscP-null strain. The wild-type strain YPIII(pIB102) (WT) and the yscP-null mutant YPIII(pIB69) were grown at 37°C in BHI medium with or without calcium. The bacteria were harvested by centrifugation, and the pellets were sheared to release surface organelles from the bacterial surface. The bacteria were then centrifuged to separate cell pellet and sheared fractions. Two microliters of the pellet, diluted 1:10, and 5 μl of undiluted supernatant were separated on SDS-PAGE gels, and Western blots were performed by using an anti-YscF antiserum. (B) Yop secretion by the yscP-null strain. Bacteria were grown at 37°C in BHI medium without calcium to induce secretion. Bacteria were centrifuged to separate cell pellet and supernatant fractions. Equal amounts of supernatant fractions were separated by SDS-PAGE and stained with Coomassie brilliant blue. The yscP-null strain was transcomplemented by pPE34, which expresses YscP under the control of an arabinose-inducible promoter.
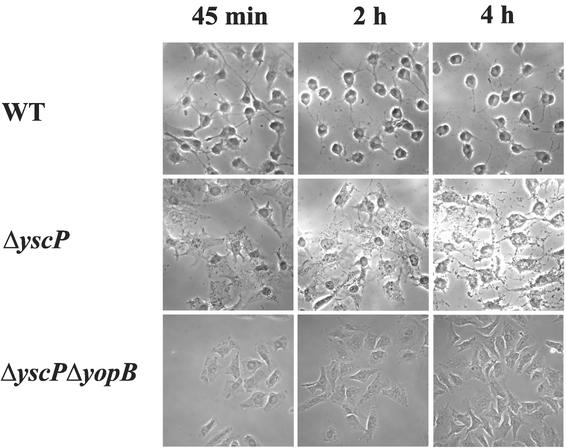
YopE is a Rho-GTPase-activating protein (53) that, when translocated into the eukaryotic cell cytosol, induces a cytotoxic effect by disrupting the actin cytoskeleton of host cells (40). Since the yscP mutant was defective in Yop secretion, we tested whether it was capable of inducing a cytotoxic response in eukaryotic cells. HeLa cells were infected with either the yscP mutant YPIII(pIB69) or the wild-type strain YPIII(pIB102), and cytotoxicity was monitored by phase-contrast microscopy (Fig. 2). The results demonstrate that, consistent with the yscP mutant's defect in Yop secretion, the cytotoxic effect induced by the yscP mutant was delayed relative to that induced by the wild-type strain. The control strain, a yscP yopB double mutant which lacks the essential translocator protein YopB (16, 33, 45), did not induce a cytotoxic response. These results suggest that YscP is not required for the translocation of effector proteins into the cytosols of eukaryotic cells but rather exerts its effect at the level of Yop secretion.
FIG. 2.
Cytotoxicity of the yscP-null strain. HeLa cells were infected with either the wild-type strain YPIII(pIB102) (WT), the yscP-null mutant YPIII(pIB69), or the yopB yscP-double-null mutant YPIII(pIB60469) as described in Materials and Methods, and the effect on HeLa cells was recorded. Photographs were taken at the indicated times. A delay in cytotoxicity was seen in the yscP-null mutant compared to that seen in the wild-type strain. The yopB yscP-double-null strain was used as a negative control, since it lacks the essential translocator protein YopB.
Mutations in YscU partly suppress the yscP mutant phenotype.
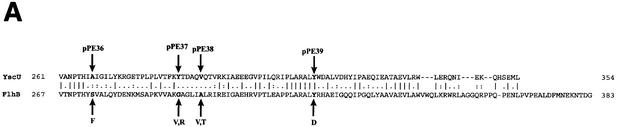
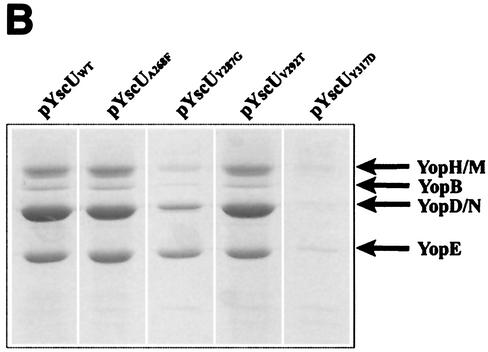
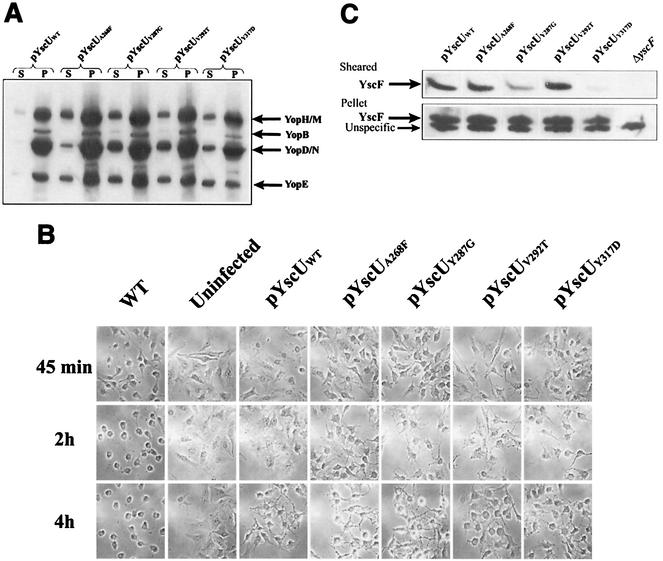
Previous studies demonstrated that a fliK mutant, which exhibits a polyhook phenotype, could be partially suppressed by mutations in the cytoplasmic domain of the inner membrane protein FlhB (24, 51). Homologues of FlhB are essential components of all TTSSs (for a review, see reference 19), and the Yersinia FlhB homologue is YscU. Guided by the suppressor mutations isolated by Macnab and colleagues, we used site-directed mutagenesis to introduce four individual missense mutations into the cytoplasmic domain of YscU (Fig. 3A). The mutations included the following: A268F(pPE36), Y287G(pPE37), V292T(pPE38), and Y317D(pPE39). The YscU mutants were first expressed in trans under the control of the IPTG-inducible tac promoter in the yscU-null strain YPIII(pIB75). Bacteria were grown at 37°C in the absence of calcium in order to induce Yop secretion. The results show that the level of Yop secretion by the A268F and V292T mutants was equivalent to that of wild-type YscU, while the Y317D mutant secreted much lower levels of Yop (Fig. 3B). The Y287G mutant secreted Yops at an intermediate level (Fig. 3B).
FIG. 3.
yscU mutants. (A) Alignment of the C-terminal-most amino acid sequences of YscU of Y. pseudotuberculosis and of FlhB of Salmonella enterica serovar Typhimurium. A pairwise alignment was performed with the EMBOSS alignment tool at the homepage of the European Bioinformatics Institute (http://www.ebi.ac.uk/emboss/align). Indicated in boldface are the extragenic suppressors in flhB of polyhook first-site mutations in fliK as described by Williams et al. (51) and the single-site mutations in yscU generated and utilized in this study. The mutations in FlhB are S274F (F), G293V and G293R (V,R), A298V and A298T (V,T), and Y323D (D). In YscU, the mutations are A268F(pPE36), Y287G(pPE37), V292T(pPE38), and Y317D(pPE39). (B) Yop secretion by different yscU mutants. Mutations in the cytoplasmic region of YscU were introduced in trans into the yscU-null strain YPIII(pIB75). Expression of YscU was under the control of the tac promoter, which was not induced with IPTG in these experiments. Bacteria were grown at 37°C in BHI medium lacking calcium to induce secretion. Cultures were centrifuged to separate the supernatant and cell pellet fractions. Equal amounts of supernatant fractions were separated by SDS-PAGE and stained with Coomassie brilliant blue. The mutations introduced into the cytoplasmic region of YscU were based on those studied by Macnab and colleagues (Fig. 3A) (51). The positions of these sites are indicated in the figure. WT, wild type.
Each of the YscU mutants was then expressed in the yscP yscU-double-null mutant to investigate whether it could partially suppress the yscP phenotype in comparison with FlhB. Bacteria were grown at 37°C in medium lacking calcium, and Yop secretion was measured. The results indicate that all the YscU mutants restored an increased level of Yop secretion when expressed in the yscP yscU double mutant compared to wild-type YscU (Fig. 4A). Consistent with these results, these same yscU mutants also restored a more rapid cytotoxic response to the yscP yscU strain (Fig. 4B). Thus, these yscU mutants could suppress the yscP mutation and, similar to the corresponding flhB mutants, the suppression was only partial. Next, the surface localization of YscF was examined. Bacteria were grown at 37°C in calcium-containing medium and sheared to release surface-located proteins. The results showed that expression of both YscU-Y287G and YscU-Y317D reduced the amount of surface-localized YscF in the yscP yscU double mutant, whereas the other YscU mutations did not (Fig. 4C). The YscU-Y317D suppressor mutation exhibited the strongest suppressor phenotype with respect to both Yop and YscF secretion (compare Fig. 3 with 4A and C). Together, these results confirmed that mutations in YscU can suppress the phenotype of the yscP-null mutant, which strongly argues for the fact that YscP and YscU coordinately regulate substrate export by the Yersinia TTSS.
FIG. 4.
Mutations in yscU suppress the yscP mutant phenotype. (A) Mutations in YscU increase Yop secretion by the yscP mutant. Mutations in the cytoplasmic region of YscU were introduced in trans into the yscP yscU-double-null strain. Expression of YscU was under the control of the tac promoter, which was not induced with IPTG in these experiments. Bacteria were grown at 37°C in BHI medium lacking calcium to induce secretion. Cultures were centrifuged to separate cell pellet (P) and culture supernatant (S) fractions. Equal percentages of each fraction were separated by SDS-PAGE, and Western blots were performed by using an anti-total Yops antiserum. WT, wild type. (B) Mutations in YscU increase the cytotoxicity of the yscP mutant. Mutations in the cytoplasmic domain of YscU were introduced in trans into the yscP yscU-double-null strain. HeLa cells were infected with these strains as described in Materials and Methods, and the effect on HeLa cells was recorded. Photographs were taken at the indicated times. (C) Mutations in YscU reduce the surface localization of YscF by the yscP mutant. Mutations in the cytoplasmic region of YscU were introduced in trans into the yscP yscU-double-null strain. Bacteria were grown at 37°C in BHI medium plus calcium and were sheared to release surface organelles from the bacterial surface as described in Materials and Methods. Cultures were centrifuged to separate cell pellet and sheared fractions. Two microliters of the pellet, diluted 1:10, and 5 μl of undiluted supernatant were separated by SDS-PAGE, and Western blots were performed by using an anti-YscF antiserum.
YscP acts upstream of YopN, LcrG, LcrQ, and YopD.
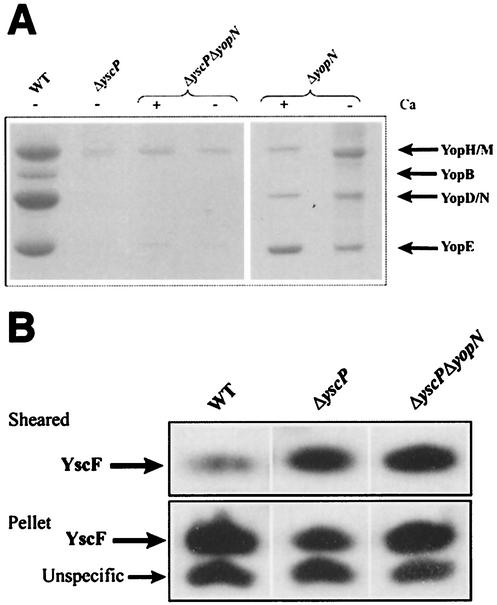
Previous studies indicated that the LcrG (9, 27, 32) and YopN (4, 12) proteins might act as stop valves or gatekeepers to inhibit Yop secretion prior to eukaryotic cell contact. This is based on the fact that mutations in any of these genes result in a calcium-blind derepressed phenotype in which Yops are secreted in the presence of calcium. To determine the order in which YscP, YscU, and YopN act during the regulation of substrate export by the Yersinia TTSS, we constructed a yopN yscP double mutant. This strain was grown at 37°C in either the presence or absence of calcium, and Yop secretion was measured. The results showed that Yop secretion was impaired in both the presence and absence of calcium (Fig. 5A). The amount of the needle component YscF exported to the bacterial cell surface by the yopN yscP-null strain was also examined. Bacteria were grown at 37°C in the presence of calcium and were sheared to release YscF from the bacterial cell surface. The results showed that the yopN yscP strain exported an abnormally large amount of YscF to the bacterial cell surface (Fig. 5B). The fact that the phenotype of the yopN yscP double mutant is similar to that of the yscP mutant alone, with regards to the levels of YscF and Yop secretion, indicates that YopN did not directly regulate the substrate specificity of the Yersinia TTSS but rather acted downstream of YscP and YscU to regulate substrate export in response to eukaryotic cell contact.
FIG. 5.
YscP acts upstream of YopN. (A) The yopN yscP double mutant is impaired in Yop secretion. The yopN yscP-null strain YPIII(pIB8769) and the yopN-null strain YPIII(pIB82) were grown at 37°C in BHI medium with or without calcium to induce secretion. Bacteria were centrifuged to separate cell pellet and supernatant fractions. Equal amounts of supernatant fractions were separated by SDS-PAGE and stained with Coomassie brilliant blue. WT, wild type. (B) Surface localization of YscF in the yopN yscP mutant. Bacteria were grown at 37°C in BHI medium plus calcium and were sheared to release surface organelles from the bacterial surface as described in Materials and Methods. Cultures were centrifuged to separate cell pellet and sheared fractions. Two microliters of the pellet, diluted 1:10, and 5 μl of undiluted supernatant were separated on SDS-PAGE gels, and Western blots were performed by using an anti-YscF antiserum.
LcrG has been suggested to act as an inner gatekeeper, working at the cytosolic side to regulate substrate export via the TTSS (32), and a lcrG mutant expresses and secretes Yops also in the presence of Ca2+. An interesting question was whether LcrG acted upstream or downstream of YscP in the regulatory hierarchy. Therefore, a double lcrG yscP mutant was constructed and analyzed. In comparison with the yopN yscP double mutant, the lcrG yscP mutant did not secrete Yops but instead exported YscF in large amounts to the surface of the cell (data not shown). Thus, YscP and YscU are epistatic over LcrG.
Two additional proteins, LcrQ and YopD, have also been implicated in Yop regulation, since the corresponding mutants in contrast to the wild-type strain showed high levels of Yop expression when incubated at 37°C in the presence of Ca2+ (13, 37, 50). We wanted to investigate the possibility that these two proteins affected the YscP-YscU substrate switch. Therefore, we analyzed the phenotypes of the lcrQ yscP and yopD yscP double mutants. Both double mutants had lost their ability to secrete Yops but had retained their ability to secrete YscF in large amounts to the bacterial surface (data not shown), indicating that neither LcrQ nor YopD affects the substrate specificity switch of the Yersinia TTSS.
DISCUSSION
In this work, we have confirmed and extended previous findings regarding the role of YscP in the TTSS by Yersinia (35, 46) and shown that yscP-null mutants were severely affected in their ability to secrete Yop effector proteins. In contrast, the YscF protein was secreted in elevated amounts. YscF subunits build up a surface-located pilus-like structure, the needle structure, which protrudes from the Yersinia TTSS (18). Similar structures have also been identified in Salmonella and in Shigella (21, 22, 49). YscP shows homology to InvJ of Salmonella and Spa32 of Shigella. Mutants in either invJ or spa32 assemble extended needles, and these mutants are also unable to secrete effector molecules to the culture supernatant (23, 49). Thus, yscP-null mutants show a phenotype similar to that of the invJ and spa32 mutants, indicating that YscP, InvJ, and Spa32 exhibit similar functions in regulating the secretion of effector proteins in the three different species. Work by Kutsukake, Macnab, and coworkers has shown that FliK, which is essential for regulating the hook length of the flagellum, is involved in regulating the subunit secretion of the flagellum export apparatus (24, 51). Interestingly, FliK shows homology with YscP, InvJ, and Spa32 (19), and Galan and coworkers have suggested that InvJ is, in comparison to FliK, involved in regulating substrate specificity (23). Our findings support this view, and we suggest that YscP has a similar function by regulating a switch in secretion from YscF to Yop effectors after the TTSS has been activated by eukaryotic cell contact. How this switch is regulated is at present unclear. Our results show, however, that the inactivation of YscP results in elevated YscF secretion and reduced Yop secretion. This suggests that YscP is inactive prior to contact between the pathogen and the eukaryotic cell. Consequently, YscP is activated upon target cell contact, which results in elevated Yop secretion and subsequent Yop translocation. A potential problem with this model is the fact that YscP is secreted (unpublished results) (35, 46). However, both InvJ and FliK are secreted, and it seems therefore likely that these proteins fulfill their regulatory functions prior to or during secretion and that secretion per se is not a prerequisite for their function (6, 29). The yscP-null mutant shows a leaky phenotype with regards to Yop secretion, since the mutant is still cytotoxic for HeLa cells. This is in contrast to fliK flagellar mutants and invJ mutants of Salmonella, which are impaired in substrate secretion (41, 51). The reason behind this difference is unclear, but it is possible that our cytotoxic assay was more sensitive than the assays used to determine the phenotypes of the fliK and invJ mutants. Another possibility is that the other type III systems of Yersinia encoded by chromosomal genes can partially complement the loss of secretion by a yscP-null mutant and thus cause an artificial situation.
Interestingly, Kutsukake, Macnab, and coworkers have isolated suppressor mutants that partially restore the fliK phenotype with regards to filament assembly. These extragenic suppressors were localized to flhB and were found to be substitutions in the carboxy-terminal end of FlhB (Fig. 3A) (24, 51). FlhB is homologous to YscU of Yersinia, and when the corresponding amino acids were changed in YscU, partial suppression of the yscP-null mutant was obtained. This was evidenced by an increased level of Yop secretion in vitro and by the induction of a more rapid cytotoxic response in infected HeLa cells in vivo. The suppression of the yscP mutant phenotype by the YscU-Y317D mutant was particularly impressive given that this mutant secreted much lower levels of Yop than wild-type YscU when expressed in the yscU-null strain (compare Fig. 3B with 4A). In addition, the YscU-A268F and YscU-Y317D mutations reduced the amount of YscF present on the bacterial surface in a calcium-containing medium. Given that most of the mutations in YscU involve the replacement of hydrophobic residues with polar residues or larger hydrophobic residues, we suspect that these mutant proteins may have slightly altered conformations that serve to mimic the conformation of YscU that normally occurs when YscP is present. In any event, this suppression suggests that YscU, together with YscP, has a role in regulating the secretion of Yop effectors as well as YscF in the Yersinia TTSS. Like FlhB (30), the C-terminal cytoplasmic part of YscU is organized into two domains, as it was demonstrated that YscU was specifically proteolyzed in vivo (25). Although there is no evidence that the proteolysis of YscU per se is important for the function of the Yersinia TTSS, it clearly reflects the conformation of the YscU cytoplasmic domains. Specifically, deletion of the conserved proteolytic site of YscU, comprised of amino acid residues 263 through 266, did not abolish the proteolysis of YscU but resulted in a larger C-terminal proteolytic fragment. This result is consistent with the YscU-Δ263-266 mutant having an altered conformation (25). Significantly, this mutant restored only minimal Yop secretion to the yscU-null strain, which suggests that the conformation of the cytoplasmic domains of YscU is important for Yop secretion (25). Since YscP and YscU coordinately regulate secretion by the Yersinia TTSS, we suggest that YscP may serve to regulate the conformation of YscU in the cytosol of the bacterial cell.
It has been suggested that the LcrG (9, 27, 32) and YopN (4, 12, 20) proteins function as gatekeepers to prevent Yop effector secretion prior to eukaryotic cell contact. Specifically, these mutants display a calcium-independent phenotype in which Yop secretion occurs, even when the bacteria are grown in a medium that contains calcium. LcrG is thought to act intracellularly (9, 27, 32), while YopN (12) is thought to act on the bacterial cell surface. The question is whether these proteins act downstream of YscP and YscU or whether they have a direct role in regulating the substrate specificity of the Yersinia TTSS. Our results showed that both a yopN yscP and a lcrG yscP double mutant exhibit a phenotype essentially the same as that of the yscP mutant alone. That is, the double mutants exported an increased amount of the needle component YscF to the bacterial cell surface in the presence of calcium but were unable to efficiently secrete Yops in either the presence or absence of calcium. These results suggest that YopN and LcrG act downstream of YscP and YscU. Thus, these proteins may serve as a safety mechanism to prevent the premature secretion of Yop effectors until eukaryotic cell contact occurs.
Acknowledgments
This work was supported by the Swedish Research Council and the Swedish Foundation of Strategic Research.
REFERENCES
- 1.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. [DOI] [PubMed] [Google Scholar]
- 2.Bölin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collazo, C. M., and J. E. Galan. 1996. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect. Immun. 64:3524-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collazo, C. M., M. K. Zierler, and J. E. Galan. 1995. Functional analysis of the Salmonella typhimurium invasion genes invl and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol. Microbiol. 15:25-38. [DOI] [PubMed] [Google Scholar]
- 7.Daniell, S. J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F. P. Booy, R. K. Shaw, S. Knutton, G. Frankel, and S. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3:865-871. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. W., D. Botstein, J. R. Roth, and the Cold Spring Harbor Laboratory. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.DeBord, K. L., V. T. Lee, and O. Schneewind. 2001. Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183:4588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fällman, M., K. Andersson, S. Håkansson, K. E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsberg, A., I. Bölin, L. Norlander, and H. Wolf-Watz. 1987. Molecular cloning and expression of calcium-regulated, plasmid-encoded proteins of Y. pseudotuberculosis. Microb. Pathog. 2:123-137. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg, A., A. M. Viitanen, M. Skurnik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977-986. [DOI] [PubMed] [Google Scholar]
- 13.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 14.Fürste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 15.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Håkansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 98:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iriarte, M., M. P. Sory, A. Boland, A. P. Boyd, S. D. Mills, I. Lambermont, and G. R. Cornelis. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimbrough, T. G., and S. I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA 97:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 23.Kubori, T., A. Sukhan, S. I. Aizawa, and J. E. Galan. 2000. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA 97:10225-10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutsukake, K., T. Minamino, and T. Yokoseki. 1994. Isolation and characterization of FliK-independent flagellation mutants from Salmonella typhimurium. J. Bacteriol. 176:7625-7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavander, M., L. Sundberg, P. J. Edqvist, S. A. Lloyd, H. Wolf-Watz, and A. Forsberg. 2002. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J. Bacteriol. 184:4500-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 27.Matson, J. S., and M. L. Nilles. 2001. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183:5082-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minamino, T., B. Gonzalez-Pedrajo, K. Yamaguchi, S. I. Aizawa, and R. M. Macnab. 1999. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol. Microbiol. 34:295-304. [DOI] [PubMed] [Google Scholar]
- 30.Minamino, T., and R. M. Macnab. 2000. Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J. Bacteriol. 182:4906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minamino, T., and R. M. MacNab. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052-1064. [DOI] [PubMed] [Google Scholar]
- 32.Nilles, M. L., A. W. Williams, E. Skrzypek, and S. C. Straley. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordfelth, R., and H. Wolf-Watz. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect. Immun. 69:3516-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson-Delafield, J., R. J. Martinez, B. A. Stocker, and S. Yamaguchi. 1973. A new fla gene in Salmonella typhimurium—flaR—and its mutant phenotype-superhooks. Arch. Mikrobiol. 90:107-120. [DOI] [PubMed] [Google Scholar]
- 35.Payne, P. L., and S. C. Straley. 1999. YscP of Yersinia pestis is a secreted component of the Yop secretion system. J. Bacteriol. 181:2852-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 38.Rosqvist, R., I. Bolin, and H. Wolf-Watz. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 40.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russmann, H., T. Kubori, J. Sauer, and J. E. Galan. 2002. Molecular and functional analysis of the type III secretion signal of the Salmonella enterica InvJ protein. Mol. Microbiol. 46:769-779. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Simon, R., U. Preifer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1:784-791. [Google Scholar]
- 44.Simonet, M., S. Richard, and P. Berche. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 46.Stainier, I., S. Bleves, C. Josenhans, L. Karmani, C. Kerbourch, I. Lambermont, S. Totemeyer, A. Boyd, and G. R. Cornelis. 2000. YscP, a Yersinia protein required for Yop secretion that is surface exposed, and released in low Ca2+. Mol. Microbiol. 37:1005-1018. [DOI] [PubMed] [Google Scholar]
- 47.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 48.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamano, K., E. Katayama, T. Toyotome, and C. Sasakawa. 2002. Shigella Spa32 is an essential secretory protein for functional type III secretion machinery and uniformity of its needle length. J. Bacteriol. 184:1244-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams, A. W., S. Yamaguchi, F. Togashi, S.-I. Aizawa, I. Kawagishi, and R. M. Macnab. 1996. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J. Bacteriol. 178:2960-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Visser, L. G., A. Annema, and R. van Furth. 1995. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect. Immun. 63:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]