Abstract
The patB gene product is required for growth and survival of the filamentous cyanobacterium Anabaena sp. strain PCC 7120 in the absence of combined nitrogen. A patB::gfp fusion demonstrated that this gene is expressed exclusively in heterocysts. patB mutants have a normal initial pattern of heterocyst spacing along the filament but differentiate excess heterocysts after several days in the absence of combined nitrogen. Expression of hetR and patS, two critical regulators of the heterocyst development cascade, are normal for patB mutants, indicating that patB acts downstream of them in the differentiation pathway. A patB deletion mutant suffers an almost complete cessation of growth and nitrogen fixation within 24 h of combined nitrogen removal. In contrast, a new PatB mutant that is defective in its N-terminal ferredoxin domain, or a previously described mutant that has a frameshift removing its C-terminal helix-turn-helix domain, grows very slowly and differentiates multiple contiguous heterocysts under nitrogen-deficient conditions.
Anabaena sp. strain PCC 7120 is a filamentous cyanobacterium capable of both nitrogen fixation and oxygenic photosynthesis. When deprived of combined nitrogen, approximately every 10th cell along the filament differentiates into a nitrogen-fixing heterocyst. Heterocysts provide the anaerobic environment in which nitrogenase can function. The oxygen-evolving photosystem II complex is inactivated in these cells, and a semipermeable barrier to gases is provided by the heterocyst envelope, which consists of an inner glycolipid layer and an outer polysaccharide layer (15). The morphological and biochemical changes that take place during heterocyst differentiation provide the enzyme nitrogenase with an anaerobic environment and the large supply of reductant and ATP that it requires.
Many mutant strains of Anabaena sp. strain PCC 7120 exhibit an altered heterocyst spacing pattern. One of these, the Pat-2 strain, has a normal initial heterocyst pattern but accumulates multiple contiguous heterocysts after several days in the absence of combined nitrogen (No) (14). The mutant strain has a single-base deletion at position 1342 of the patB gene, resulting in loss of the C-terminal portion of the protein, including an HTH-3/HTH-XRE family DNA-binding motif. The N-terminal domain of the protein contains two putative 4Fe-4S centers with a high degree of similarity to bacterial-type ferredoxin II proteins.
The Pat-2 strain was isolated in a screen for mutants that grow poorly in the absence of combined nitrogen. Consistent with the requirement for functional PatB under nitrogen-limiting conditions, the patB message was found to be strongly induced in the wild-type strain around 12 h after removal of nitrogen. The patB message continues to accumulate after the initial induction, although it never reaches a very high level (14). In this work, we have determined that patB is expressed specifically in heterocysts and that the heterocyst development regulators hetR and patS are expressed normally in patB mutants through the first round of heterocyst differentiation. We have also constructed two new mutants in patB to further explore its function. One of these carries a deletion of the entire patB gene. The other carries six Cys→Ala mutations predicted to disrupt the PatB Fe-S centers.
MATERIALS AND METHODS
Cloning and plasmid construction.
Cloning methods are described elsewhere (11, 19). All sequencing reactions were performed by the University of Chicago Cancer Research Center DNA Sequencing Facility.
The transcriptional reporter patB::gfp was constructed by PCR amplification of 1.0 kb upstream of the patB start codon using primers that were designed with, respectively, an XbaI site and a SmaI site at their 5′ ends. The PCR product was digested with these enzymes, and the XbaI site was filled with T4 DNA polymerase. The resulting blunt-ended fragment was cloned into the pAM1956 SmaI site directly upstream of gfp mut2, which was kindly provided by H. S. Yoon and J. W. Golden (Texas A&M University) (22). pAM1956 carries a neomycin-kanamycin resistance marker (Nmr/Kmr). The patS::gfp reporter was also provided by H. S. Yoon and J. W. Golden (22). A hetR::gfp reporter was described previously (4).
The patB open reading frame (ORF) deletion constructs ppatBΔΩRL271 and ppatBΔΩRL278 were made by PCR amplification of a 1.0-kb sequence upstream of patB and of a 1.0-kb segment downstream of patB. The upstream product was digested with XbaI and SmaI, and the downstream product was digested with SmaI and PstI, and both fragments were ligated into XbaI/PstI-digested BluescriptKS (Stratagene). The resulting construct was digested with SmaI and then ligated with a SmaI-digested streptomycin-spectinomycin resistance cassette (Smr/Spr) (9). A XhoI/SacI fragment containing this whole construct was moved from BluescriptKS to the sacB-containing suicide vectors pRL271 or pRL278 for conjugation into Anabaena sp. strain PCC 7120.
The patB ORF Fe-S center mutagenesis construct, pFeSpatBreplace, was made by first cloning the patB ORF into the pAlter-EX2 (Promega) vector. Cysteines 1 to 4 and 7 and 8 of the patB FeS centers were changed to alanines using mutagenic primers. The mutagenized patB ORF was cloned into ppatBΔΩRL278 (see above) at a BamHI site left behind by deletion of the Ω cassette. A 0.3-kb SfuI/XhoI fragment had also been deleted from this plasmid. The construct pwtpatBreplace was made by ligating the unmutagenized patB ORF into this modified ppatBΔΩRL278. The resulting constructs have the patB ORF separated from their native flanking sequences by a BamHI site and a SmaI half-site.
The patB expression plasmid ppetE::patB contains the copper-inducible petE promoter fused to the complete patB ORF and is described in detail in reference 10.
Transformation and conjugation are described in detail in references 10, 14, and 19.
Cell culture.
Escherichia coli cultures were maintained by standard methods (19). Anabaena sp. strain PCC 7120 culture methods were as described previously (11, 18). Large cultures for RNA isolation, or cultures which required a high level of gassing, were grown in stirred bottles illuminated by cool white fluorescent bulbs at 30 to 40 μE/m2/s and gassed with a 2 to 3% CO2/air mixture. The pH of gassed cultures was maintained with 25 or 50 mM HEPES, pH 8.0. Culture maintenance and transfer from nitrogen-replete medium to No medium were as described previously (10). Cell growth was assessed by cell counting or by measuring the absorbance at 750 nm (20, 21).
Complementation of mutations in the patB ORF.
The ability of the patB ORF to complement patB mutants was tested by conjugating either the ppetE::patB copper-regulated patB-expression plasmid or the control ppetE plasmid into the mutant of interest (10). Exconjugants were selected on BG11-NO3 plates containing 30 μg of neomycin sulfate/ml and in the absence of added CuSO4. Isolates were then tested for their ability to grow on BG11 No, 30-μg/ml neomycin sulfate plates in the absence of added CuSO4. The trace amounts of copper derived from the glassware were sufficient to induce very low-level patB-cat hybrid message expression (data not shown). This level of expression was sufficient to complement all patB mutants tested.
Microscopy.
Cells were pipetted onto 1% agar cushions containing BG11 for photography. The strain carrying the patB::gfp construct was photographed with 400 ASA film in a Contax 167MT camera attached to a Zeiss Axioskop microscope. Images of green fluorescent protein (GFP) fluorescence were recorded by illuminating with 450- to 490-nm light from a Zeiss HBO100W/2 source and photographing emission through a 510-nm narrow-band-pass filter with a 16-s exposure. Red emission from chlorophyll was photographed without the 510-nm filter. Images of the strains carrying the patS::gfp reporter were captured using an Axiovision color charge-coupled device camera attached to a Zeiss Axioplan2 microscope. The CCD images of GFP fluorescence were acquired when the GFP was excited at 450 to 490 nm and viewed through the 500- to 550-nm filter of the Endow GFP filter set (Chroma).
Nitrogenase activity assays.
Reduction of acetylene to ethylene was measured by gas chromatography as described previously (7, 10).
Northern blotting and hybridization.
At selected times following nitrogen step-down, 300 ml was removed from a 4-liter culture and RNA was isolated using the Ambion Totally RNA kit protocol with modifications described elsewhere (10). RNA loading on formaldehyde-agarose gels was assessed by scanning baked blots with the blue laser of the Storm 860 phosphorimager at a photomultiplier voltage of 900. Under these conditions the imager detects ethidium bromide-stained rRNA bands (8). The doublet 23S rRNA bands of Anabaena sp. strain PCC 7120 were quantified and used to normalize the 32P signal from probes to specific mRNAs. Probes were labeled by random priming with the Ambion Strip-Easy DNA probe synthesis kit or the Roche Random primed DNA labeling kit, using [32P]dCTP or [32P]dATP (Amersham). Blots were imaged by exposure to a Type BAS-III Fuji phosphorimager screen for 2 h. The exposed screen was scanned with a Molecular Dynamics Storm 860 PhosphorImager and analyzed with Molecular Dynamics IQMac software.
RESULTS
patB::gfp is expressed exclusively in heterocysts, in the middle to late stages of heterocyst development.
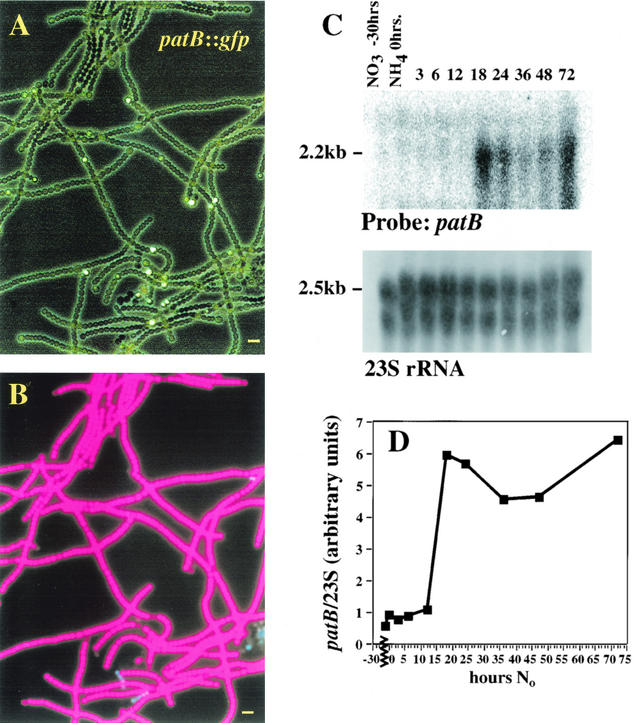
patB transcripts accumulate in cultures grown in the absence of combined nitrogen (14). Analysis of filaments carrying a patB::gfp transcriptional fusion shows that this expression occurs solely in heterocysts (Fig. 1A). Wild-type filaments carrying the patB::gfp fusion have no GFP fluorescence when grown with either NaNO3 or (NH4)2SO4 as a nitrogen source, but beginning at 16 h after transfer to No medium, fluorescence is visible in cells spaced at 10-cell intervals. This fluorescence increases in intensity for the next 2 days. Heterocysts can be distinguished by the loss of chlorophyll fluorescence (Fig. 1B) due to the breakdown of photosystem II. No GFP fluorescence was observed from the patB::gfp reporter strain earlier than 16 h when heterocyst differentiation was synchronized by growth in 1 mM (NH4)2SO4 prior to transfer to No medium. These data agree with the patB expression profile observed on Northern blots (Fig. 1C). The cultures used in previous Northern blot experiments, in which a low level of patB message was observed at 3 to 6 h after nitrogen step-down, were pregrown in nitrate, which permits a low level of differentiation (14).
FIG. 1.
(A) Composite image of Anabaena sp. strain PCC 7120 filaments carrying the patB::gfp transcriptional fusion at 51 h after nitrogen step-down, photographed under phase contrast, overlaid with the same field photographed with 450- to 490-nm excitation and emission through a 510-nm narrow-band-pass filter. Fluorescence is confined to the heterocysts. (B) Emission from chlorophyll visualized under 450- to 490-nm excitation, viewed without the emission filter. Heterocysts are the dim cells along the filament. The bars correspond to a length of 10 μm. (C) The upper panel shows a Northern blot of RNA prepared from wild-type Anabaena sp. strain PCC 7120 cells, following nitrogen step-down, probed with a patB internal fragment. The lower panel shows the bipartite 23S rRNA bands from a scan of the blots prior to hybridization, as described in Materials and Methods. These bands were quantified and used to normalize the phosphor signal for specific mRNAs from that lane (8). (D) The graph shows the patB signal from the time course (filled squares) normalized to 23S rRNA. The 2.2-kb message is induced 3.5-fold during the 12- to 18-h interval after removal of combined nitrogen.
There are no recognized DNA regulatory elements in the immediate 5′ region of patB. The nearest clearly identifiable upstream ORF is fdxB, which is 990 bp upstream of patB. This ORF is oriented in the divergent direction and is expressed primarily in heterocysts (data not shown). fdxB has ≥58% similarity at the amino acid level to ferredoxin III proteins from many bacteria (1).
Expression of the heterocyst regulators hetR and patS is the same in the wild type and in patB mutants.
Expression of the HetR master regulator of heterocyst development is both necessary and sufficient for this differentiation process (3, 4). However, the expression pattern of hetR can be regulated by other heterocyst-specific regulatory genes (such as hetN) that are themselves dependent upon hetR for expression (6). In the wild type, low-level GFP fluorescence from a hetR::gfp reporter is observed in whole filaments of nitrate-grown Anabaena sp. strain PCC 7120, although no fluorescence appears in cells in which heterocyst differentiation has been completely repressed by growth in 1 mM (NH4)2SO4 (data not shown). After transfer to No medium, hetR::gfp expression is up-regulated in single proheterocysts (6). In contrast, in a strain underexpressing the hetN gene, hetR is up-regulated in multiple contiguous cells that have the appearance of proheterocysts, within 20 h of nitrogen removal (6). It was hypothesized that hetR expression might not resolve to single cells in a patB mutant and might serve as an indicator of partial heterocyst differentiation of the heterocyst-adjacent cells, prior to development of multiple contiguous heterocysts. However, during heterocyst differentiation in the patBfr mutant, there was no expression of a hetR::gfp transcriptional fusion in heterocyst-adjacent cells in the first few days after transfer to No medium. Therefore, a defect in patB has no effect on the single proheterocyst-specific induction of hetR expression in nitrogen-deprived cultures.
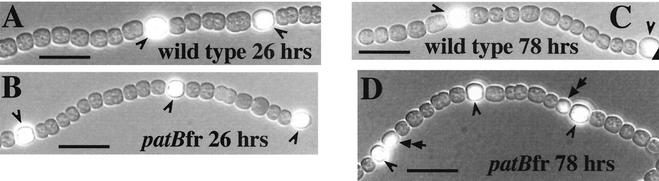
The patS gene encodes a heterocyst differentiation-inhibitory peptide (22). After removal of combined nitrogen from wild-type cultures, patS expression initially occurs in clusters of cells which are subsequently resolved to single proheterocysts spaced approximately 10 cells apart (22, 23). Expression of the patS::gfp reporter was examined in the patBfr mutants. Since, in the wild type, patS expression is up-regulated in clusters of cells and then resolved to single proheterocysts, it was thought that the patS::gfp reporter might serve as a finer indicator than hetR::gfp of partial heterocyst differentiation of the heterocyst-adjacent cells in patB mutants. However, it was determined that in the patBfr mutant, and all other patB mutants that have been constructed, patS::gfp expression is normal in the first 2 to 3 days after transfer to No medium, with expression resolving to single proheterocysts (Fig. 2B). After 3 days, patBfr carrying the patS::gfp reporter shows strong fluorescence from one or the other cell that is adjacent to a heterocyst (Fig. 2D). Expression of the patS::gfp reporter in the cell adjacent to filament-terminal heterocysts is seen in some wild-type filaments, but in patBfr, this heterocyst-adjacent-cell expression occurs frequently at intercalary heterocysts as well. This is consistent with a model in which the gene expression cascade of early heterocyst development is not altered in patB mutants, but a failure of heterocyst function (see below) leads to further heterocyst differentiation.
FIG. 2.
Composite images of wild-type Anabaena sp. strain PCC 7120 filaments (A and C) and patBfr mutants (B and D) carrying the patS::gfp reporter (22) photographed under phase contrast, combined with the same microscopic field photographed with excitation and emission wavelengths of 450 to 490 nm and 510 nm, respectively. After 26 h in No medium, in wild-type (A) or patBfr (B) filaments, patS::gfp is expressed exclusively in heterocysts or proheterocysts (single arrowheads). After 78 h in No medium, the reporter is expressed exclusively in heterocysts of the wild type (C), but in patBfr (D), expression also occurs in heterocyst-adjacent cells (double arrowheads).
Ectopic expression of patB from a heterologous promoter.
The heterocyst differentiation regulators patS and hetN inhibit heterocyst differentiation when overexpressed and result in an increased frequency of heterocysts when underexpressed (6, 22). Since a null mutant of patB forms multiple contiguous heterocysts, it was hypothesized that overexpression of patB might also negatively regulate heterocyst development. However, a heterocyst suppression phenotype was not observed for filaments overexpressing patB (data not shown) from the copper-regulated promoter petE carried on a plasmid (ppetE::patB plasmid) (data not shown) (10). Some cells in filaments ectopically expressing patB, in the absence of combined nitrogen, acquire some characteristics of heterocysts in an apparently random pattern. These cells form large polar granules and in some cases lose pigment fluorescence, events that occur during heterocyst development. However, these abnormal cells do not form the thickened double-layer cell walls characteristic of heterocysts.
Phenotypes of patB mutants.
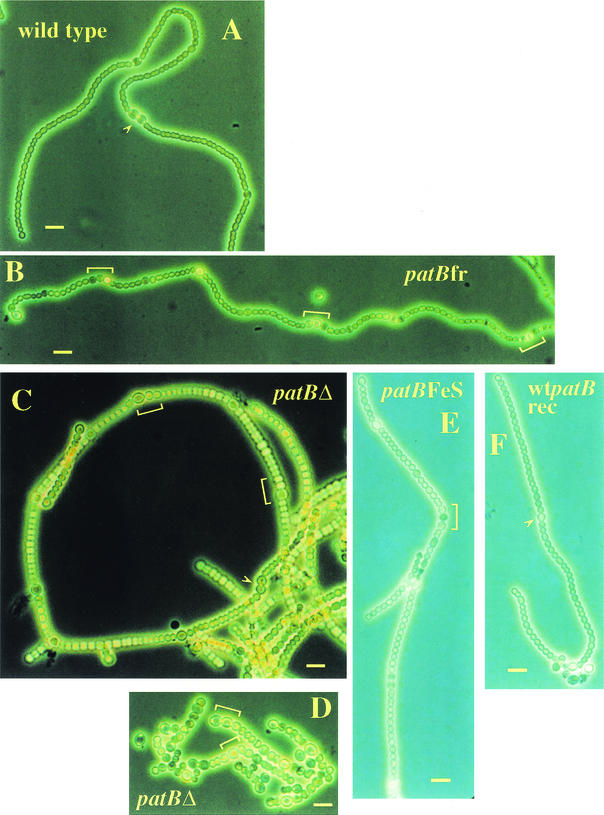
New mutants of Anabaena sp. strain PCC 7120 with defects in the patB gene were constructed, and their heterocyst pattern phenotypes (Fig. 3) and No growth phenotypes (Fig. 4) were analyzed. Three mutants were compared in this study: the previously characterized patB C-terminal frameshift mutant (patBfr), a deletion mutant of patB (patBΔ), and a mutant in which the putative iron-sulfur center cysteines were changed to alanines (patBFeS).
FIG. 3.
Phase contrast images of the wild type (A) and the patBfr mutant (B) after one week of growth in No medium. The wild type has a double heterocyst (arrowhead) that looks very different from the groups of heterocysts accumulated in the patBfr filaments (brackets). After 48 h in No medium, the patBΔ mutant (C) is already accumulating groups of immature heterocysts (brackets). After 1 week in No medium, the patBΔ mutant (D) forms multiple contiguous heterocysts, but due to the extent of fragmentation, they are mostly at the ends of filaments. The patBFeS mutant (E) is compared with its wild-type sibling wtpatBrec strain (F). Fewer clusters of multiple contiguous heterocysts (brackets) form in the patBFeS strain than in the other patB mutants. Bar, 10 μm.
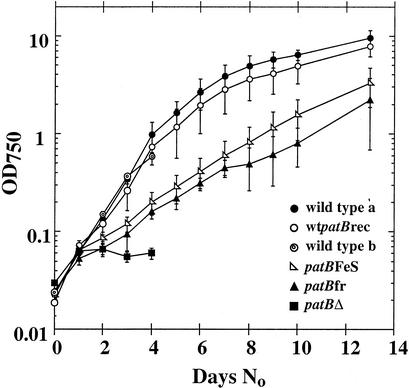
FIG. 4.
Growth of the patB mutants in the absence of combined nitrogen, measured by absorbance at OD750. The patBΔ mutant stops growing within 24 h of nitrogen removal. (The patBΔ results are an average of those for two replicates each of two separate isolates.) The growth of the patBfr mutant is less impaired than that of the patBΔ mutant, with growth continuing at a low rate for more than 2 weeks. (The patBfr results are averages of those for two replicates.) The growth of the patBFeS mutant is even closer to that of the wild type. This is consistent with the observed appearance of the filaments of this strain (see Fig. 3). (patBFeS results are averages for three replicates each of three separate isolates.) The wild-type wtpatBrec reconstituted strain that is the sibling of the patBFeS strain grows almost as well as wild-type Anabaena sp. strain PCC 7120. wild type a, average of results for two replicates grown at the same time as the patBfr and patBFeS cultures. wild type b, average of results for two replicates grown at the same time as the patBΔ cultures.
The patBfr mutant was previously determined to accumulate multiple contiguous heterocysts and to have delayed heterocyst formation, requiring 48 h rather than the normal 21 to 24 h for mature heterocysts to appear (14). The timing of heterocyst accumulation in the patBfr mutant was analyzed to determine whether multiple contiguous heterocysts develop immediately after the initial round of heterocyst formation or at a later time. The data presented in Table 1 demonstrate that the patBfr mutant does not accumulate a striking number of multiple contiguous heterocysts until 4 to 6 days after transfer to No medium. After 8 days, the patBfr mutant has >4-fold the number of multiple contiguous heterocysts as the wild type. Heterocysts that occur in clusters appear to be at different stages of development (Fig. 3). Older heterocysts of the patBfr mutant, as defined by apparently complete heterocyst walls and a large, rounded morphology, have a more light-colored, translucent appearance than normal heterocysts and are usually devoid of polar granules (Fig. 3B) (14). Filaments of this strain also fragment in the absence of combined nitrogen, as do those of many other mutants that are unable to grow in No (5, 14). Therefore, the initial round of heterocyst differentiation in the patBfr mutant has a normal pattern. The delay in the appearance of mature heterocysts in the patBfr mutant is the only abnormality in initial pattern formation.
TABLE 1.
Timing of heterocyst accumulation
| Genotype | % of heterocysts in clusters (n)a
|
||||
|---|---|---|---|---|---|
| 1.5 days | 3.5 days | 5.5 days | 6.5 days | 8.5 days | |
| Wild type | 4.3 (15/352) | 13.0 (85/645) | 8.1 (43/533) | 10.3 (57/555) | 5.8 (50/858) |
| patBfr | 7.2 (21/292) | 14.2 (91/642) | 20.5 (165/807) | 22.2 (197/886) | 25.3 (238/941) |
| patBΔ | 7.2 (13/181) | ||||
n, mutiplex heterocysts/total heterocysts × 100. A multiplex heterocyst is defined as a contiguous cluster of heterocysts. For example, a filament with one single heterocyst and a contiguous cluster of three heterocysts would be counted as 1 multiplex/1 multiplex + 1 single. Only intercalary heterocysts were counted. Filament terminal heterocysts were excluded because heterocyst pattern is often aberrant at filament termini in all Anabaena sp. strain PCC 7120 strains.
The frameshift mutation in the patBfr mutant results in the loss of only the C-terminal portion of the protein. This C-terminal segment includes a domain with similarity to the HTH-3/HTH-XRE family of phage DNA-binding proteins. It was hypothesized that if PatB functions primarily as a DNA-binding protein with regulatory activity, the patBfr strain might be essentially a null mutant. To test this possibility, the deletion mutant (patBΔ), in which the entire ORF has been replaced by a Smr/Spr Ω cassette, was constructed. The defects of the patBΔ mutant differ from those of the patBfr mutant in several respects. The patBΔ mutant does not experience the delay in heterocyst differentiation of the patBfr strain. The patBΔ mutant resembles the patBfr mutant in that it has a normal initial heterocyst pattern, but it accumulates multiple contiguous heterocysts more rapidly than the patBfr mutant. After only 48 h in No medium, the patBΔ mutant begins to form what appear to be heterocyst clusters (Fig. 3C). After 7 days, the filaments in these cultures are so fragmented that very few filaments longer than 10 cells remain, and multiple contiguous heterocyst frequencies cannot be counted (Fig. 3D and Table 1). The defects of patBΔ can be complemented by expression of patB from the copper-regulated promoter petE carried on a plasmid (ppetE::patB plasmid) (10).
Comparison of the severe effect that deletion of patB has on No-grown cultures with the milder phenotype of the C-terminal patB frameshift suggested that an N-terminal or central domain of the protein might be involved in an essential function. The N-terminal 58-amino-acid segment of PatB contains two putative 4Fe-4S centers and is 53% similar to several bacterial-type ferredoxin II proteins (1, 14). To test the possibility that the mutant phenotypes of patBΔ strains are caused primarily by the loss of these Fe-S centers, site-directed mutant strains were constructed in which the four cysteines of the first putative Fe-S center and the two 3′ cysteines of the second center were all changed to alanines. Constructs (pFeSpatBreplace or pwtpatBreplace) containing either the mutagenized patB ORF or the wild-type patB ORF, with 1 kb of wild-type flanking homology on either side, were conjugated into the patBΔ6 strain. Following initial selection with neomycin and sucrose counterselection against the sacB gene contained in the vector, the exconjugants were screened for loss of the Smr/Spr Ω cassette of the deletion mutant. patBFeS filaments do not exhibit the delayed heterocyst formation seen in patBfr filaments, and although they accumulate multiple contiguous heterocysts, the clusters are not as extensive as those of the patBfr mutant (Fig. 3E). The appearance of older heterocysts of the patBFeS and patBfr mutants is similar. The heterocysts are light-colored, translucent, and usually lack polar granules. The defects of patBFeS can be complemented by expression of patB from the ppetE::patB plasmid (10). Also, the sibling control strain of the patBFeS mutant in which the Ω cassette of the deletion mutant was replaced with a wild-type patB ORF (wtpatBrec) appears similar to wild-type Anabaena sp. strain PCC 7120 (Fig. 3F). These data indicate that the defects in the patBFeS mutant are due to the mutation of the cysteines to alanines. The ferredoxin-like domain of PatB is therefore required for full function of the protein, although the lesion in the patBFeS strain is much less severe than that of the deletion mutant and slightly less severe than that of the patBfr mutant.
The severity of the growth phenotypes of the patB mutants correlates with the degree of heterocyst clustering and filament fragmentation of these strains. The patBfr and patBFeS mutants are able to grow slowly in the absence of combined nitrogen for at least 2 weeks, while growth of the patBΔ mutant ceases after only one day (Fig. 4) (14). At the time that growth of patBΔ cultures stops, multiple contiguous heterocysts have not yet formed (Fig. 3C and 4).
A small amount of acetylene reduction activity (0.79 ± 0.34 pmol of acetylene reduced/h/optical density at 750 nm (OD750) of culture) can be detected in the patBΔ mutant, indicating that a nitrogenase enzyme with at least some function is produced in this strain. This is a >390-fold reduction from the wild-type rate of 309 ± 53 pmol of acetylene reduced/h/OD750 of culture. Nitrogenase function in the patBfr mutant is reduced only sevenfold (41.7 ± 3.8 pmol of acetylene reduced/h/OD750 of culture).
Expression of a heterocyst-specific cytochrome c oxidase operon in patB mutants.
An operon encoding a heterocyst-specific cytochrome c oxidase (coxBACII) is located 1.8 kb upstream of the patB start codon (11). The proximity of an operon encoding a putative heterocyst-specific terminal oxidase to patB, whose domain structure suggests that it might encode a redox-sensitive transcription factor, led us to investigate the mRNA levels of coxBACII in the patB mutants.
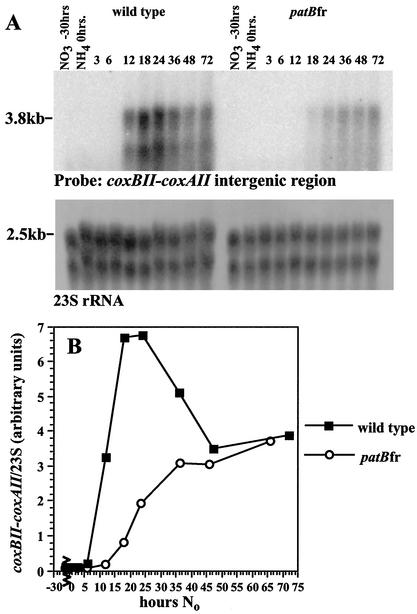
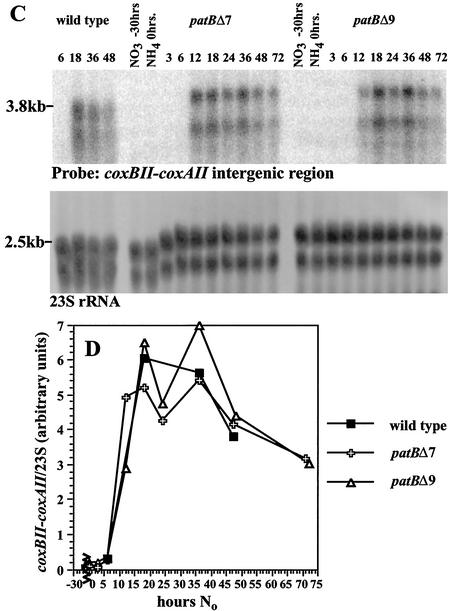
RNA was prepared from wild-type and patBfr cultures following transfer to No and hybridized with a gene-specific probe (a 0.16-kb coxBII-coxAII intergenic segment). The 3.8-kb message recognized by this probe increases 35-fold in the wild type, between 6 and 18 h after transfer (Fig. 5A and B). The coxBACII message level is depressed significantly in the patBfr mutant, especially at the earlier times. In contrast to these results obtained with the patBfr mutant, the coxBII-coxAII message levels in the deletion mutants of patB closely resemble those of the wild type. The results for patBΔ mutants 7 and 9 are shown in Fig. 5C and D. Unlike the patBfr mutant, the patBΔ mutants have an expression pattern of the 3.8-kb coxBACII message that is nearly identical to that of the wild type.
FIG. 5.
Expression of the heterocyst-specific coxBACII operon in patB mutants. (A and B) RNA from nitrogen-starved cultures of the wild type and the patBfr mutant were probed with a 0.16-kb coxBII-coxAII intergenic fragment. The signal from this 3.8-kb message was normalized to the 2.5-kb 23S rRNA band. (C and D) RNA from two different patBΔ mutants was compared to that of the wild type, using the coxBII-coxAII probe and rRNA normalization described above. hours No, hours grown in medium free of combined nitrogen.
DISCUSSION
The patB frameshift mutant strain was originally identified on the basis of its very slow growth in the absence of fixed nitrogen (14). The sequence of the gene suggested that the protein had ferredoxin-like domains near the N terminus and a DNA-binding domain near the C terminus. These features suggested further that PatB might be a redox-sensitive transcription factor. In the present work, we show that patB expression is confined to heterocysts, that a mutant with a deletion of patB has a more severe growth and nitrogen fixation defect than the original frameshift mutant, and that mutation of the putative ferredoxin domain also produces a growth and nitrogen fixation defect.
Expression of the master heterocyst regulator hetR is the same in patB mutants and in the wild type (data not shown), implying that unlike the hetN regulator, patB does not exert an effect on hetR expression. Also, expression of the heterocyst differentiation inhibitor patS is the same in the wild type and in patB mutants during the first 2 to 3 days after removal of combined nitrogen.
Although patB does not appear to be involved in the early-to-middle stages of the heterocyst differentiation process, it is required for survival in the absence of combined nitrogen. A deletion of the patB ORF results in cessation of growth in No medium within 24 h, only trace amounts of nitrogenase activity, rapid accumulation of multiple contiguous heterocysts, and fragmentation. These defects are more severe than those suffered in No medium by either a patB C-terminal frameshift mutant lacking the putative helix-turn-helix domain or an N-terminal mutant lacking the cysteine residues that would be required to form the putative Fe-S centers. Both of these mutants are capable of slow growth and slowly accumulate multiple contiguous heterocysts. The fact that these defects can be caused by site-directed mutations in six of the eight cysteines that would be critical for formation of an Fe-S center gives weight to the proposal that this is indeed an Fe-S domain.
The pairing of an Fe-S domain and a helix-turn-helix DNA binding motif in a single ORF initially suggested that PatB might be a functional analog of FixK, a member of the fumarate and nitrate reductase (FNR) family of redox regulators that serves as an activator of genes required for symbiotic nitrogen fixation, including genes encoding a respiratory terminal oxidase (2, 16). However, PatB has significant differences from FNR family proteins in both its C-terminal helix-turn-helix domain and its N-terminal 4Fe-4S domain (1, 2, 12, 17). Also, the completion of the Anabaena sp. strain PCC 7120 genome has revealed that there are five ORFs in this organism (Anabaena sp. strain PCC 7120 genome database, Kazusa DNA Research Institute) with more extensive similarity to E. coli FNR and rhizobial FixK proteins than that possessed by PatB (1, 13).
If PatB is a transcriptional regulator binding DNA via the helix-turn-helix motif, the frameshift mutant would be expected to be a null. One possible explanation for the greater viability of patBfr than of patBΔ is that the frameshift mutation of patBfr that removes the putative DNA-binding domain might be leaky, allowing a low level of read-through, full-length protein. Even in the wild type, induction of the patB message in No cultures never attains a high level, requiring long exposure times of Northern blots to detect it. Also, the defects of the patB mutants can be complemented by the very low level of patB message that is produced from the copper-regulated petE::patB construct when no extra copper has been added to the growth medium (data not shown). A control construct containing the petE promoter without the patB ORF is not able to complement (data not shown). Therefore, the production of even a few copies of full-length protein might provide sufficient PatB function.
It is also possible that PatB has a function(s) that it is still able to perform in the absence of this C-terminal DNA-binding motif. If the N-terminal, putative Fe-S center is responsible for such a function, a patBfr/patBFeS double mutant would be expected to have an additive effect and result in a phenotype similar to that of the deletion mutant. Alternatively, another segment of the patB ORF might be required for its proper function. While there are no strongly conserved domains in the central 390 amino acids of the patB ORF, there are three short segments each of which has a low degree of similarity to protein sequences in GenBank (1). However, these similarities do not provide clues to PatB function (1). Further definition of the critical domains of PatB may be provided by a comparison of a patBfr/patBFeS double mutant with the patBΔ mutant and by the creation of mutations in the central portion of the patB ORF.
Acknowledgments
This work was supported by research grant GM 21823 from the NIH and training grant GM O7183 from the NIH.
We thank James Golden and Ho Sung Yoon for DNA constructs and advice about GFP, Jeffrey Elhai for shuttle vectors, Jennifer Moran and Sean Callahan for helpful discussions, and Sheeba Thomas, Helene Callahan, Rama Malik, Anthony Gray, and Karalee Ensing for DNA sequencing.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Batut, J., M. L. Daveran-Mingot, M. David, J. Jacobs, A. M. Garnerone, and D. Kahn. 1989. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 8:1279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buikema, W. J., and R. Haselkorn. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321-330. [DOI] [PubMed] [Google Scholar]
- 4.Buikema, W. J., and R. Haselkorn. 2001. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA 98:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buikema, W. J., and R. Haselkorn. 1991. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan, S. M., and W. J. Buikema. 2001. The role of HetN in maintenance of the heterocyst pattern in Anabaena sp. PCC 7120. Mol. Microbiol. 40:941-950. [DOI] [PubMed] [Google Scholar]
- 7.Colon-Lopez, M. S., D. M. Sherman, and L. A. Sherman. 1997. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 179:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eykholt, R. L., M. D. Mitchell, and K. W. Marvin. 2000. Direct imaging of Northern blots on an optical scanner using ethidium bromide. BioTechniques 28:864-866, 868, 870. [DOI] [PubMed]
- 9.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 10.Jones, K. M. 2001. Ph.D. thesis. University of Chicago, Chicago, Ill.
- 11.Jones, K. M., and R. Haselkorn. 2002. Newly identified cytochrome c oxidase operon in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 specifically induced in heterocysts. J. Bacteriol. 184:2491-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan, S. R., and C. O. Pabo. 1988. Structure of the lambda complex at 2.5 A resolution: details of the repressor-operator interactions. Science 242:893-899. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205-213. [DOI] [PubMed] [Google Scholar]
- 14.Liang, J., L. Scappino, and R. Haselkorn. 1993. The patB gene product, required for growth of the cyanobacterium Anabaena sp. strain PCC 7120 under nitrogen-limiting conditions, contains ferredoxin and helix-turn-helix domains. J. Bacteriol. 175:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murry, M. A., and C. P. Wolk. 1989. Evidence that the barrier to the penetration of oxygen into heterocysts depends upon two layers of the cell envelope. Arch. Microbiol. 151:469-474. [Google Scholar]
- 16.Nellen-Anthamatten, D., P. Rossi, O. Preisig, I. Kullik, M. Babst, H. M. Fischer, and H. Hennecke. 1998. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J. Bacteriol. 180:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padmanabhan, S., M. A. Jimenez, C. Gonzalez, J. M. Sanz, G. Gimenez-Gallego, and M. Rico. 1997. Three-dimensional solution structure and stability of phage 434 Cro protein. Biochemistry 36:6424-6436. [DOI] [PubMed] [Google Scholar]
- 18.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Sigalat, C., and Y. Kouchkowski. 1975. Fractionnement et caracterisation de l'algue bleue unicellulaire Anacystis nidulans. Physiol. Veg. 13:243-258. [Google Scholar]
- 21.Tandeau de Marsac, N., and J. Houmard. 1988. Complementary chromatic adaptation: Physiological conditions and action spectra. Methods Enzymol. 167:318-328. [Google Scholar]
- 22.Yoon, H. S., and J. W. Golden. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935-938. [DOI] [PubMed] [Google Scholar]
- 23.Yoon, H. S., and J. W. Golden. 2001. Pats and products of nitrogen fixation control heterocyst pattern. J. Bacteriol. 183:2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]