Abstract
The xylanase gene cluster from the rumen anaerobe Prevotella bryantii B14 was found to include a gene (xynR) that encodes a multidomain regulatory protein and is downstream from the xylanase and β-xylosidase genes xynA and xynB. Additional genes identified upstream of xynA and xynB include xynD, which encodes an integral membrane protein that has homology with Na:solute symporters; xynE, which is related to the genes encoding acylhydrolases and arylesterases; and xynF, which has homology with the genes encoding α-glucuronidases. XynR includes, in a single 833-amino-acid polypeptide, a putative input domain unrelated to other database sequences, a likely transmembrane domain, histidine kinase motifs, response regulator sequences, and a C-terminal AraC-type helix-turn-helix DNA binding domain. Two transcripts (3.7 and 5.8 kb) were detected with a xynA probe, and the start site of the 3.7-kb transcript encoding xynABD was mapped to a position upstream of xynD. The DNA binding domain of XynR was purified after amplification and overexpression in Escherichia coli and was found to bind to a 141-bp DNA fragment from the region immediately upstream of xynD. In vitro transcription assays demonstrated that XynR stimulates transcription of the 3.7-kb transcript. We concluded that XynR acts as a positive regulator that activates expression of xynABD in P. bryantii B14. This is the first regulatory protein that demonstrates significant homology with the two-component regulatory protein superfamily and has been shown to be involved in the regulation of polysaccharidase gene expression.
Recent studies examining 16S ribosomal DNA sequence diversity have confirmed that the genera Prevotella and Bacteroides are among the most numerous bacteria present in the anaerobic ecosystems of the rumen and hind gut, respectively (16, 24, 25). Certain species, including Prevotella ruminicola and Prevotella bryantii from the rumen and Bacteroides ovatus from the human colon, are capable of utilizing a particularly wide variety of diet-derived polysaccharides as growth substrates (5, 18, 19). Although not known to be capable of breaking down crystalline cellulose, these organisms are thought to play an important role in the utilization and conversion of plant cell wall polysaccharides in the gastrointestinal tract, and they appear to act in concert with cellulolytic species in the rumen system (4). Rather little is known, however, about the organization and regulation of the enzyme systems responsible for utilization of plant cell wall polysaccharides in Bacteroides and Prevotella species.
In the rumen species P. bryantii much of the xylan-degrading activity of cultures has been found to be cell associated, but this activity was not detected in assays performed with whole cells, suggesting a periplasmic, membrane, or intracellular location for at least some of the enzymes involved (13). Similar observations have been made for xylanolytic enzymes in human colonic Bacteroides spp. (20), while detailed analyses of starch-degrading enzyme systems in Bacteroides thetaiotaomicron have demonstrated the involvement of periplasmic hydrolytic enzymes and starch-binding proteins (1, 17).
Several components of the xylan utilization system of P. bryantii B14 have now been identified. The linked genes xynA and xynB code for a family 10 xylanase and an oxygen-sensitive family 43 enzyme that shows β-xylosidase and exoxylanase activities, respectively (9, 10). Meanwhile, the unlinked gene xynC encodes a 66-kDa xylanase that has an unusual structure, in which the family 10 catalytic domain is interrupted by additional residues (6). Expression of xylanase activities in P. bryantii is known to be induced during growth on xylans (8-10, 13).
We show here that regulation of the xynABD xylanase gene cluster in P. bryantii B14 involves a multidomain regulator that is related to two-component regulatory proteins encoded by a gene situated immediately downstream of xynB. Two-component regulator systems are involved in coordinating a wide range of bacterial responses to environmental changes, including chemotaxis and osmoregulation (15, 22). This is the first example of a regulatory protein of this type that is involved in regulating genes concerned with polysaccharide utilization, however, and one of the first instances of such regulation to be analyzed in the Bacteroides-Prevotella group of bacteria.
MATERIALS AND METHODS
Organisms, media, and growth conditions.
P. bryantii B14 was grown anaerobically in RG medium containing (per liter) 150 ml of sterile rumen fluid, 0.9 g of K2HPO4, 0.9 g of KH2PO4, 1.8 g of (NH4)2SO4, 1.8 g of NaCl, 0.24 g of CaCl2 · 2H2O, 0.38 g of MgSO4 · 7H2O, 0.5 g of cysteine-HCl, 0.5 g of yeast extract (Difco Laboratories, Detroit, Mich.), 1 g of Trypticase (BBL Microbiology Systems, Cockeysville, Md.), and 2 g of glucose. Cultures were incubated at 39°C in 100-ml serum bottles for 20 h. Water-soluble xylan (WS-X) was prepared from oat spelt xylan by adding 10 g of oat spelt xylan to 90 ml of distilled water and shaking the preparation continuously at 39°C for 2 h. After centrifugation (5,000 × g, 30 min, 4°C), the supernatant was removed and transferred to a sterile 100-ml bottle to form a solution (WS-X). Escherichia coli clone 5/4a was grown in Luria-Bertani broth supplemented with 100 μg of ampicillin per ml. All medium components were purchased from Sigma-Aldrich, unless indicated otherwise.
Total RNA extraction.
WS-X (0.05%, vol/vol) was added to mid-exponential-phase cells of P. bryantii B14 grown in RG medium and incubated for 30 min. After 30 min of incubation the total microbial RNA was extracted by using Sepazol-RNA I Super (Nacalai Tesque Inc., Kyoto, Japan) according to the manufacturer's instructions. The total RNA was divided into aliquots and stored at −80°C until it was required for analysis.
PCR.
For all PCRs including those used for sequencing purposes (see below) the following conditions were used. To a 100-μl (total volume) reaction mixture were added 100 ng of template DNA, 50 pmol of forward primer, 50 pmol of reverse primer, 1 μM deoxynucleoside triphosphates, 2.5 U of rTaq polymerase (Toyobo Co. Ltd., Osaka, Japan), and 10 μl of 10× rTaq buffer (Toyobo Co. Ltd.). The volume of the reaction mixture was adjusted to 100 μl with sterile distilled H2O. PCR amplification was carried out by using a Takara thermal cycler (Takara Bio Inc., Shiga, Japan) and the following cycle conditions: cycle 1, 94°C for 4 min; and cycles 2 to 31, 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s, with a final elongation step of 72°C for 8 min and a ramp rate of 3°C/s. All oligonucleotides were purchased from Nisshinbo Industries, Inc., Tokyo, Japan.
DNA sequencing and analysis.
The dideoxy chain termination procedure for DNA sequencing was carried out with E. coli clone 5/4a by using an ABI Prism BigDye terminator cycle sequencing Ready Reaction kit (Applied Biosystems, Warrington, United Kingdom) and an ABI Prism 377 DNA sequencer. The upstream 1,621 bp of the xynR gene and upstream sequences were not found in the 5/4a clone and therefore were obtained from chromosomal DNA preparations (Wizard genomic DNA purification kit; Promega UK, Ltd., Southampton, United Kingdom) of P. bryantii B14 that were digested with appropriate restriction enzymes [all obtained from New England Biolabs (UK) Ltd., Hitchin, United Kingdom] and ligated into appropriately cut pUC18 (Amersham Biosciences UK Ltd., Little Chalfont, United Kingdom). PCR amplification with one gene-specific primer and the M13-20 or Reverse primer was carried out by using an MWG Primus 96 thermal cycler [MWG-Biotech (UK) Ltd., Milton Keynes, United Kingdom] and the following cycle conditions: cycle 1, 94°C for 4 min, 58°C for 60 s, and 72°C for 60 s; and cycles 2 to 32, 94°C for 60 s, 58°C for 60 s, and 72°C for 60 s, with a final elongation step of 72°C for 10 min and a ramp rate of 3°C/s. The PCR products were separated on 1.0% (wt/vol) agarose gels, and bands were excised from the gel and purified by using an agarose gel DNA extraction kit (Roche Diagnostics Ltd., Lewes, United Kingdom). Purified DNA was quantified spectrophotometrically (A260/A280) and sequenced as described above by using a combination of gene-specific primers and the M13-20 and Reverse primers. Sequencing primers were purchased from MWG-Biotech (UK), Ltd.
Computer-assisted DNA analysis was carried out by using online software available from the EMBL European Bioinformatics Institute website (http://www.ebi.ac.uk) or the EMBOSS sequence analysis package available through the Human Genome Mapping Project Resource Centre website (http://www.hgmp.mrc.ac.uk).
Northern blotting.
Template DNA for probing was prepared by PCR with primers FW-xynA and RV-xynB, which contained a 1,721-bp fragment of both xynA and xynB (Fig. 1). The probe was labeled with 2 MBq of [α-32P]dCTP (3,000 Ci/mmol; American Radiolabeled Chemicals Inc., St. Louis, Mo.) by using a Prime II random labeling kit (Stratagene, La Jolla, Calif.). Twenty micrograms of total RNA from P. bryantii B14, yeast RNA (Ambion Inc., Austin, Tex.), or mouse liver RNA (Ambion Inc.) was applied to a Northern blot. Northern blotting was performed as described by Sambrook et al. (20a), except that an ULTRAhyb solution (Ambion Inc.) was used in place of the prehybridization solution. Signals were detected by exposure to X-ray film (MXJB-1 medical X-ray film; Kodak, Rochester, N.Y.). The size of the target mRNA was estimated from the sizes of 16S and 23S rRNA, and the target RNA was detected by ethidium bromide staining before membrane transfer.
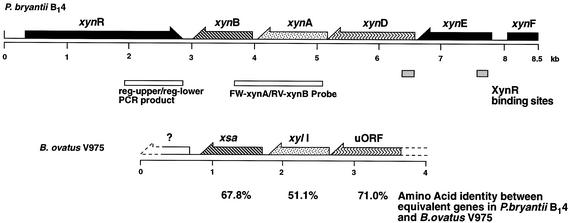
FIG. 1.
Diagram of the P. bryantii B14 xylan utilization operon. The available sequence of the related xylan utilization operon of B. ovatus V975 (26, 27) is shown beneath the equivalent genes in the P. bryantii B14 xylan utilization operon. Amino acid identities show the degrees of similarity for the three related genes. xynR (P. bryantii B14) and the gene indicated by the question mark (B. ovatus V975) show no sequence similarity. The recombinant protein fragment of XynR was synthesized from the PCR product obtained by using oligonucleotide primers reg-upper and reg-lower (Table 1). The probe used in Northern blotting was generated by PCR by using oligonucleotide primers FW-xynA and RV-xynB (Table 1). uORF, unidentified open reading frame.
Primer extension analysis.
A 0.2-pmol portion of primer labeled at the 5′ end with fluorescein isothiocyanate, Trans-FITC (Table 1), was mixed with 50 μg of total RNA and 1 μl of RNase inhibitor (Toyobo Co. Ltd.) in 20 μl of ReverTra Ace buffer (Toyobo Co. Ltd.). The solution was incubated at 50°C for 1 h, and this was followed by hybridization at room temperature for 1.5 h. For reverse transcription, 1 μl of ReverTra Ace (Toyobo Co. Ltd.) was added, and the mixture was incubated at 42°C for 1 h. After ethanol precipitation, the cDNA transcript was dissolved in a solution containing 10 μl of distilled water and 10 μl of stop solution supplied in a Thermo Sequenase cycle sequencing kit (Shimadzu Corporation, Kyoto, Japan). The transcription initiation site was determined by examining a known sequence ladder.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′-3′)a | Position | Use | |
|---|---|---|---|---|
| FW-xynA | CAGCCTACGATGAAGGATG | 5040-5022 | Northern blotting | |
| RV-xynB | GGAGCCTCAGCAAACTGC | 3319-3336 | Northern blotting | |
| Trans-FITC | CGGTAGCATCAAGTCCATAG (5′ FITC labeled)b | 6379-6398 | Primer extension analysis | |
| reg-upper | CGGGATCCATGGACGATGATGCCAATATC (BamHI site for cloning) | 1907-1927 | Recombinant protein | |
| reg-lower | GGGGTACCTTTCTGATGTTCTCGGATATAG (KpnI site for cloning) | 2782-2803 | Recombinant protein | |
| gs5102 | CCATCGATTCCGAAGGATGAACAGC (BanIII site attached) | 6661-6645 | Gel shift assay | |
| gs4961 | CGGGATCCCGCTTGGGTTATTCATG (BamHI site attached) | 6520-6536 | Gel shift assay | |
| Reg-UP | GCATTATGGAACTATCAGCG | 1681-1700 | Nuclease protection assay | |
| Reg-DOWN | CTTGGCAGTAAGCAGAATGATAGG | 2267-2290 | Nuclease protection assay | |
| IVT-F | CCAGAACCTACAGCCAAGCC | 6030-6049 | In vitro transcription | |
| IVT-R | GCGTGCTAAGGCAAAGGGAC | 6829-6810 | In vitro transcription |
Restriction sites are indicated by boldface type.
FITC, fluorescein isothiocyanate.
Production of the recombinant regulatory protein.
An 896-bp fragment of xynR was amplified by PCR by using the reg-lower primer (BamHI site attached) and the reg-upper primer (KpnI site attached) (Table 1) and then was digested with BamHI and KpnI. After purification, this fragment was ligated into the expression vector pQE-30 (Qiagen GmbH, Hilden, Germany). Expression and purification of the recombinant protein were performed by using a QIAexpressionist kit (Qiagen GmbH) according to the supplier's instructions. The purity of the recombinant protein was checked by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with a 12% polyacrylamide gel.
Gel shift assay.
A DNA fragment containing the putative XynR binding site region was amplified by PCR with the gs5102 primer (BanIII site attached) and the gs4961 primer (BamHI site attached) (Table 1), and this was followed by digestion with BamHI and BanIII. This fragment was radiolabeled with 1 MBq of [α-32P]dCTP (see above) by the action of DNA polymerase I for 30 min at 37°C. The reaction mixture contained 5 U of the DNA polymerase I large fragment (New England Biolabs Inc., Beverly, Mass.), 2.5 nmol of a deoxynucleoside triphosphate mixture (dATP, dGTP, and dTTP), and 1 MBq of [α-32P]dCTP in 20 μl of DNA polymerase I buffer (New England Biolabs Inc., Beverly, Mass.). After the reaction was finished, unincorporated nucleotides were removed with a QIAquick nucleotide removal kit (Qiagen GmbH). Competitor DNA was also prepared by PCR and was purified with a QIAquick gel extraction kit (Qiagen GmbH). Then 0, 20, 40, or 80 ng of the recombinant regulator protein was added to 20 μl of binding buffer (40 mM Tris-HCl, 4 mM MgCl2, 100 mM NaCl, 2 mM EDTA, 20% [vol/vol] glycerol, 2 μg of bovine serum albumin, 5 mM spermidine, 1 μg of competitor DNA) and incubated at 20°C for 15 min. One microliter of 32P-labeled DNA (15,000 cpm) was added to the mixture, which was incubated for an additional 30 min. Polyacrylamide gel electrophoresis was performed with an 8% (wt/vol) polyacrylamide gel to determine the mobility of the labeled DNA. Signals were detected by exposure to X-ray film (MXJB-1 medical X-ray film; Kodak).
Preparation of cell extracts.
All procedures described below after cell growth were performed at 4°C. Cells in an 800-ml culture grown with pulse addition of WS-X (0.05%) until the mid-exponential phase were harvested by centrifugation (15,000 × g, 5 min). After the cell pellet was washed twice with cold 50 mM sodium phosphate buffer (pH 6.8), it was resuspended in 25 ml of cold M-0 buffer (10 mM Tris-HCl [pH 7.9], 1 mM EDTA, 5 mM dithiothreitol). The suspension was sonicated with a UD-201 ultrasonic disrupter (Tomy Ltd., Saitama, Japan), and then 25 ml of cold M-1 buffer (50 mM Tris-HCl [pH 7.9], 10 mM MgCl2, 2 mM dithiothreitol, 25% sucrose, 50% glycerol) and 6.5 ml of saturated ammonium sulfate solution were added. After the preparation was left on ice for 20 min, ultracentrifugation (100,000 × g, 3 h) was conducted. Then 5.9 g of ammonium sulfate and 0.01 ml of 1 M NaOH were added to the supernatant, and the solution was mixed continuously for 30 min. The solution was centrifuged (15,000 × g, 20 min), and the pellet was dissolved in 3 ml of cold M2 buffer [50 mM Tris-HCl (pH 7.9), 6 mM MgCl2, 40 mM (NH4)2SO4, 0.2 mM EDTA, 1 mM dithiothreitol, 15% (vol/vol) glycerol]. The solution was then dialyzed against M2 buffer for 6 h and kept frozen as a cell extract at −80°C until analysis.
In vitro transcription assay.
Template DNA (799 bp) was amplified by PCR with the IVT-F and IVT-R primers (Table 1). The reaction mixture contained 16 nmol of each deoxynucleoside triphosphate and 2 μl of RNase inhibitor in 29 μl of RNA polymerase buffer and was kept on ice for 10 min. Then 10 μl of cell extract and 1 μl of recombinant regulatory protein (0, 400, or 800 ng/μl) were added to the reaction mixture. The reaction was started by addition of 2 μl of template DNA (1 μg/μl), and the mixture was incubated at 30°C for 45 min. After the incubation, the template DNA was digested with 20 U of RNase-free DNase (37°C, 15 min), and transcripts were purified with an RNAeasy kit (Qiagen GmbH). Digoxigenin (DIG)-labeled probe (799 bp) was prepared with a PCR DIG probe synthesis kit (Roche Diagnostics GmbH, Mannheim, Germany) by using the IVT-F and IVT-R primers (Table 1). Detection was performed with a nuclease protection assay (described below). The protected DNA probe (putative size, 523 bp) was electrophoresed on a 1% denatured agarose gel containing 9% formaldehyde.
Nuclease protection assay.
For the nuclease protection assay a 609-bp DIG-labeled probe was prepared by PCR by using a DIG probe synthesis kit (Roche Diagnostics GmbH) and primers Reg-UP and Reg-DOWN (Table 1). A total-RNA solution was mixed with 600 pg of DIG-labeled probe and 2 μg of yeast RNA (Ambion Inc.), and this was followed by ethanol precipitation. The nuclease protection assay was performed by using a Multi-NPA kit (Ambion Inc.) according to the supplier's instructions. Ten micrograms of total RNA from cells grown with pulse addition of 0.05% WS-X, xylose, or glucose and yeast RNA were used in this assay.
Detection of DIG-labeled probe.
After electrophoresis, nucleotides were transferred to a positively charged nylon membrane (Hybond-N+; Amersham Biosciences UK Ltd.) by capillary transfer with 20× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate). Nucleotides were fixed to the membrane by UV radiation cross-linking. The membrane was then washed with 0.1% SDS-2× SSC for 15 min at room temperature and with 0.1% SDS-0.2× SSC for 15 min at 50°C. Finally, it was shaken for 30 min in a skim milk solution (3 g of skim milk/100 ml of TBS [pH 7.4]; TBS contained 137 mM NaCl, 2.68 mM KCl, and 25 mM Tris-HCl). After the membrane was washed three times with TBS, it was incubated at 37°C for 1 h in an anti-DIG solution (1 μl of anti-Digoxigenin-AP [Roche Diagnostics GmbH] in 3 ml of TBS). The membrane was then shaken vigorously twice for 15 min in TBS containing 0.05% Tween 20. After the membrane was soaked for 5 min in AP 9.5 solution (100 mM Tris buffer [pH 9.5], 100 mM NaCl, 5 mM MgCl2 · 6H2O), it was incubated for 5 min with CDP-Star (Amersham Biosciences UK Ltd.). Chemiluminescent signals were detected by exposure to X-ray film (MXJB-1 medical X-ray film; Kodak).
RESULTS
Sequence analysis of regions flanking the xynAB xylanase genes in P. bryantii B14.
The sequences of two linked genes (xynA and xynB) that encode endoxylanase and β-xylosidase/exoxylanase activities, respectively, were reported previously (9, 10). Sequencing of the regions upstream from xynA and xynB revealed three additional open reading frames, designated xynD, xynE, and xynF (Fig. 1). The putative xynD gene product has a structure characteristic of membrane-spanning proteins and has homology with Na symport-type transporters (15, 22). XynD shows 71% amino acid identity with the product of an unidentified open reading frame from B. ovatus V975 (27). XynE shows significant similarities to the products of several genes recently identified from genome sequencing projects that are related to lipases and aryl esterases. XynF meanwhile exhibits similarities with α-glucuronidases (Table 2). The xynA, xynB, and xynD genes all show significant homology to members of a similar operon in B. ovatus V975 (Fig. 1).
TABLE 2.
P. bryantii B14 xylan utilization operon
| Open reading frame | Gene | Putative function | Closest database matcha | Open reading frame coordinates | Signal peptide | Stop codon |
|---|---|---|---|---|---|---|
| 1 | xynR | Two-component regulatory protein | 28.4% identity (599 amino acids) to CyaC of Anabaena sp. strain PCC7120 | 1302-2806 (833)b | ? | TAA |
| 2 | xynB | β-Xylosidase/exoxylanase | 67.8% identity (317 amino acids) to XylB of B. ovatus V975 | 3914-2955 (319) | ? | TAA |
| 3 | xynA | Endo-1,4-β-xylanase | 51.1% identity (350 amino acids) to XylI of B. ovatus V975 | 5106-3997 (369) | Yes | TAA |
| 4 | xynD | Sodium:solute symporter | 71.0% identity to an unidentified open reading frame of B. ovatus V975 | 6535-5138 (465) | Integral membrane protein | TAA |
| 5 | xynE | Putative arylesterase/lipase/ acylhydrolase (GDSL motif) | 34.5% identity (383 amino acids) to Sce6.29 of Streptomyces coelicolor | 7722-6538 (394) | Yes | TAA |
| 6 | xynF | α-Glucuronidase | 38.7% identity (106 amino acids) to BH1061 of Bacillus halodurans | 7987-8426 (>147) (incomplete) | Yes | ? |
The accession numbers for the most homologous genes are as follows: P74982 (XynR gene), P49943 (XynB gene), P49942 (XynA gene), AAD20252 (XynD gene), Q9KZR1 (XynE gene), and Q9KE00 (XynF gene). The three B. ovatus V975 gene homologues all fall within the same operon and are transcribed in the same direction (26).
The numbers in parentheses are the numbers of amino acids.
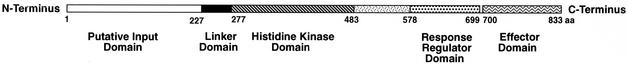
Downstream from xynB, on the opposite strand, is an open reading frame, designated xynR, which encodes a putative regulatory protein containing 833 amino acids. The C-terminal domain of XynR (residues 700 to 833) shows homology with helix-turn-helix (HTH) domains of the AraC family that are involved in DNA binding (2, 7). Residues 277 to 483 contain all five of the conserved motifs that are typically present in the histidine kinase portion of two-component regulators (12, 22) (Fig. 2). In between, residues 578 to 699 show homology with response regulator-type domains that are typically subject to phosphorylation (22, 23). The region preceding the histidine kinase domain includes a 9-amino-acid membrane-spanning motif (DAS transmembrane prediction server [3]) and represents a possible linker domain of the type that communicates conformational changes between the periplasm and the histidine kinase (14, 22). The N-terminal domain (residues 1 to 227) is unrelated to other database sequences. The XynR regulator from P. bryantii, however, differs from archetypal two-component regulatory proteins (12, 22) in that the sequences showing homology with sensor and response components are combined into a single polypeptide (Fig. 2).
FIG. 2.
Structure of the P. bryantii B14 two-component regulatory protein (XynR). The linker domain contains a characteristic membrane-spanning motif (9 amino acids; DAS transmembrane prediction server [3]). The histidine kinase domain contains the characteristic H, N, G1, F, and G2 boxes characteristic of histidine kinases of two-component regulatory proteins. The response regulator domain contains the characteristic aspartate residue that is normally phosphorylated in these domains. The effector domain contains an HTH region characteristic of the AraC family of DNA binding proteins. aa, amino acids.
Ribosome binding sites from members of the Cytophaga-Flavobacterium-Bacteroides (CFB) phylum do not resemble those from E. coli or most other gram-negative bacteria. We were, however, able to identify a putative consensus sequence (A-[At]-[atc]-[At]-[At]-[Tc]-[tC]-[at] 3 to 25 bp upstream of the start codon for all 11 of the known P. bryantii B14 catalytic genes (Fig. 3). The P. bryantii B14 two-component regulatory protein (xynR) and two B. ovatus V975 open reading frames also have similar upstream sequences. This sequence is, therefore, a possible candidate for a sequence that is involved in facilitating ribosome binding and initiation of translation.
FIG. 3.
Identification of a conserved sequence (boldface type and underlined) immediately upstream of P. bryantii B14 xylan utilization genes and other structural genes from Prevotella spp. The accession numbers for the genes are as follows: xynA and xynB, Z49241 and AJ428204; xynD, xynE, and xynF, AJ428204; ORF3, U96771; glnN, AF483911; xynC, Z79595; gdhA, U82240; cdxA, U35425; and pr23, M83379.
Transcription of xynABD.
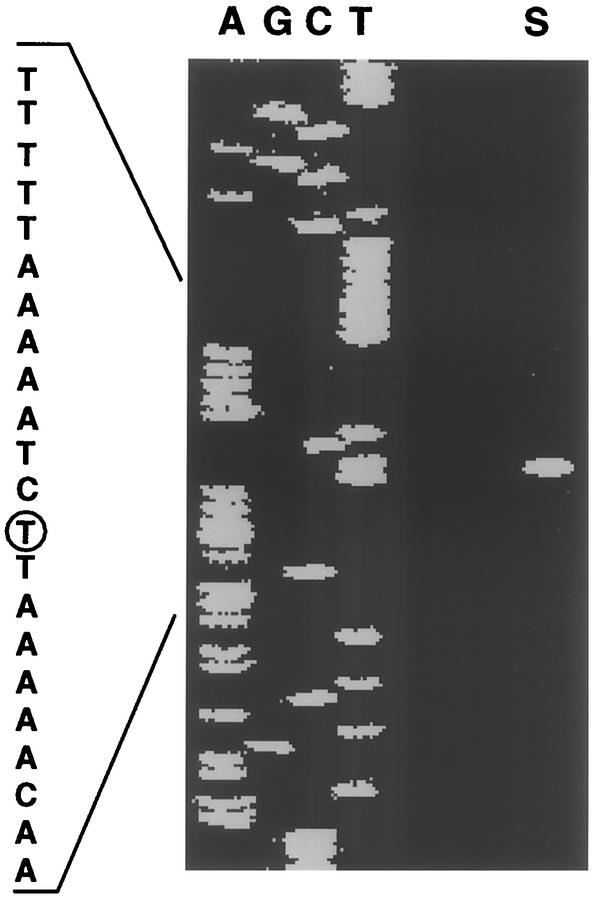
Northern blotting analysis with a 1,721-bp fragment of xynA and xynB (Fig. 1) as the probe detected two transcripts (approximately 3.7 and 5.8 kb) in P. bryantii B14 (Fig. 4). Primer extension analysis (Fig. 5) with primer Trans-FITC (residues 6379 to 6398) revealed that the transcription start site (T at residue 6551) of the 3.5-kb transcript (Fig. 4) was located 16 bp upstream of the xynD start codon (residue 6535). This is consistent with transcription of xynD, xynA, and xynB as a single polycistronic mRNA whose coding regions extend from residue 6535 to residue 2955 (Table 2 and Fig. 1). The larger (5.8-kb) transcript is consistent with transcription of the xynA, -B, -D, and -E genes as a single mRNA (residues 7722 to 2955).
FIG. 4.

Northern blot identification of two mRNA transcripts from 20 μg of P. bryantii B14 total RNA hybridized to 1,721-bp DNA PCR probes (FW-xynA and RV-xynB) containing fragments of xynA and xynB (residues 3319 to 5040). The size of the 3.5-kb transcript corresponds to the expected size of a single polycistronic transcript comprising the xynA, xynB, and xynD genes. The size of the 5.8-kb transcript corresponds to the expected size of a single transcript comprising the xynA, xynB, xynD, and xynE genes. No hybridization of the xynA-xynB probe was observed when it was used with 20 μg of either mouse liver or Saccharomyces cerevisiae total RNA (data not shown). The sizes of the transcripts were determined by comparison with P. bryantii B14 16S and 23S ribosomal DNA.
FIG. 5.
Primer extension analysis with primer Trans-FITC (residues 6379 to 6398) revealed that the transcription start site (T at residue 6551) of the 3.5-kb transcript identified in Fig. 4 was located 16 bp upstream of the xynD start codon (residue 6535). Lanes A, G, C, and T contained sequencing ladders with the Trans-FITC primer. Lane S shows the transcription initiation site from P. bryantii B14 cells after pulse addition of 0.05% (vol/vol) WS-X.
Binding of XynR to DNA upstream from xynD.
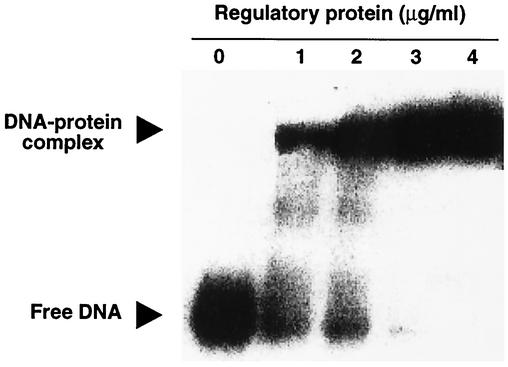
The coding sequences for the C-terminal domain of XynR (containing the HTH DNA binding domain [Fig. 2]) were amplified with primers reg-upper and reg-lower (Table 1 and Fig. 1) and cloned into the expression vector pQE-30. The His-tagged recombinant protein was purified and tested for the ability to bind to a 141-bp DNA fragment containing the transcriptional start site identified for xynABD and the immediate upstream sequences identified by Northern blotting (see above). A gel shift assay (Fig. 6) confirmed binding of the XynR domain to this DNA fragment.
FIG. 6.
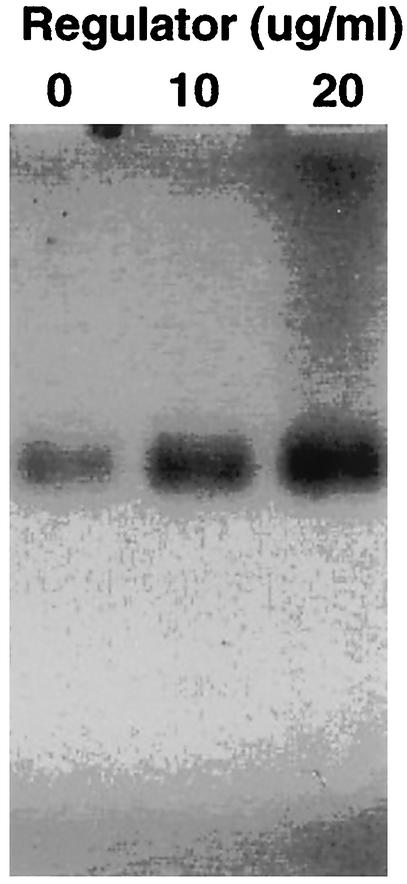
Gel shift assay revealing binding of a 299-amino-acid recombinant protein fragment of XynR containing the HTH DNA binding domain (Fig. 2) to a 32P-dCTP-labeled 141-bp DNA fragment (residues 6520 to 6661) containing the transcriptional start site identified for the xynABD transcript by Northern blot analysis and primer extension analysis (Fig. 4 and 5). As the concentration of the recombinant XynR polypeptide was increased, there was a concomitant decrease in the amount of free DNA. Samples were electrophoresed on 8% (wt/vol) polyacrylamide gels.
An in vitro transcription assay (Fig. 7) revealed that the recombinant fragment of XynR binds to the transcription start site identified by primer extension analysis (Fig. 5). The nuclease protection assay demonstrated that the DNA sequence immediately upstream of the transcription initiation site was degraded, whereas the DNA sequence downstream of the XynR binding site was protected from degradation. Addition of increasing concentrations of recombinant XynR demonstrated that this protein activated transcription of the xynABD transcript, thus confirming that XynR is a transcriptional activator.
FIG. 7.
In vitro transcription assay revealing that XynR acts as a transcriptional activator of the xynABD transcript. A 780-bp PCR product containing 275 bp of sequence upstream of xynD and 505 bp of the xynD gene itself (primers IVT-F and IVT-R) was hybridized with P. bryantii B14 cell extract (lane 0) and different concentrations (10 and 20 μg/ml) of the recombinant XynR polypeptide (lanes 10 and 20). A nuclease protection assay revealed the presence of an undegraded 523-bp fragment of DNA that corresponds to the xynD coding sequence from the PCR product (505 bp) and the 16 bp upstream of this fragment that corresponds to the transcription initiation site (Fig. 5). Electrophoresis was carried out under denaturing conditions (1% agarose and 9% formaldehyde)
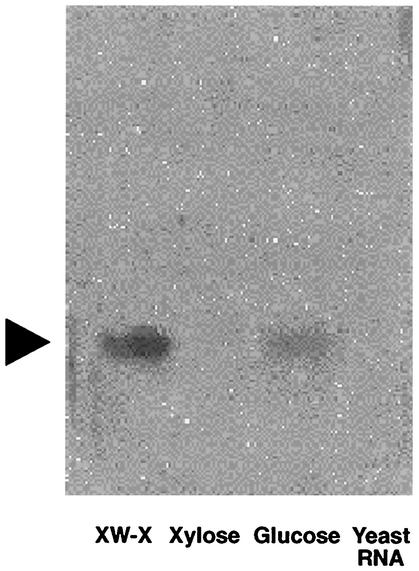
A nuclease protection assay (Fig. 8) indicated that significantly larger amounts of xynR mRNA were detected in the cells grown on xylan than in the cells grown on any other substrate tested. This demonstrated that expression or activity of xynR was itself regulated in response to the growth substrate.
FIG. 8.
Effect of growth substrate on the expression of XynR, as determined by a nuclease protection assay. Exponentially growing cultures of P. bryantii B14 were pulsed with either 0.05% WS-X (XW-X), 0.05% xylose, or 0.05% glucose for 30 min, and then total RNA was extracted and 10 μg was transferred onto a Hybond-N+ positively charged nylon membrane. Two micrograms of S. cerevisiae total RNA (Yeast RNA) was used as a negative control. A 609-bp PCR product (obtained with primers Reg-UP and Reg-DOWN) corresponding to 398 bp of the xynR upstream sequence and 211 bp of xynR (residues 1681 to 2290) was labeled with DIG and used as a probe in the nuclease protection assay.
DISCUSSION
P. bryantii B14 is a rumen anaerobe belonging to the gram-negative CFB phylum that is thought to make an important contribution to the utilization of xylans and other hemicellulosic polysaccharides in the rumen ecosystem. In the human colon, another member of this phylum, B. ovatus, is also thought to play a significant role in xylan utilization. Strong parallels are evident between the xylan cluster studied here from P. bryantii B14 and a shorter sequenced region reported from the human colonic strain B. ovatus V975 (26, 27) (Fig. 1). The products of the xynA, xynB, and xynD genes exhibit between 51 and 71% amino acid sequence identity with the products of xsa, xylI, and an unidentified open reading frame which occur in the same order in the B. ovatus chromosome. XynA and XylI are both endoxylanases, while XynB and Xsa and are β-xylosidases (9). Our analysis suggests that XynD and its homologue in B. ovatus are membrane proteins that are likely to be involved in oligosaccharide transport. Previous knockout studies with B. ovatus V975 showed that disruption of the xynD homologue, in the Ω K mutant, severely reduced the growth rate on xylan compared with the growth rate of the wild-type strain (26). This effect was ascribed in part to the reduced expression of β-xylosidase and xylanase activities due to a polar effect on the downstream xsa and xylI genes. Our results suggest that alterations in oligosaccharide transport could also have accounted for the reduced growth rate observed. This is consistent with observations described above which indicate that xynA, xynB, and xynD are likely to be transcribed as a single mRNA.
Previous work has demonstrated that xylanolytic activities are up-regulated in response to xylan as the growth substrate in P. bryantii B14 (8-11). The XynR regulatory gene product is therefore an obvious candidate for a regulatory protein that could be involved in this response. Our results show that the HTH-type DNA binding domain of this protein binds specifically to sequences within the xylanase gene cluster. This regulatory protein is of interest for several reasons. It is the first example of a regulator from a Prevotella strain that shows homology with two-component regulators described for other bacteria, and it is also the first example of a regulator of this type that has been found to govern expression of genes involved in polysaccharide utilization. Single polypeptides that combine multiple elements of these regulators are known to occur in other cases, including the rteC tetracycline resistance gene regulator in Bacteroides spp. (21). Such organization in the P. bryantii XynR regulatory protein is particularly intriguing, however. If we assume that an input domain responds directly to the availability of oligosaccharide signals, these signals must presumably be detected in the periplasm and the response must be transmitted to a cytoplasmically located DNA binding domain via a membrane-spanning portion of the protein. Alternatively, it is perhaps more likely that the regulator does not respond directly to substrate signals but is the final step in a more complex regulatory cascade. Indeed, this alternative is strongly suggested by the observation made in this study that xynR expression itself responds at a transcriptional level to the growth substrate.
The smaller transcript detected in this study with the 1,721-bp probe whose initiation site was located just upstream of xynD corresponds to the xynABD genes. The DNA binding domain of the XynR regulator was shown to bind a sequence immediately upstream of the xynD gene in a position that makes it very likely to regulate production of this transcript. Such regulation was confirmed by an in vitro transcription assay which showed that XynR acts as a positive regulator of xynABD transcription. It is not known whether additional binding sites for XynR occur upstream of xynE, but similar regulation of the larger 5.8-kb transcript might also occur.
The functions of xynE and xynF (encoding a 107-amino-acid partial sequence) have not been investigated experimentally, but the products of both of these genes are possible candidates for enzymes involved in the debranching of xylan polysaccharides or oligosaccharides through the removal of esterified acetyl or phenolic acid groups and in the debranching of glucuronic acid residues. The region downstream from xsa in B. ovatus (Fig. 1) does not contain any sequences homologous to P. bryantii xynR. Rather, this region includes a sequence that shows homology with the putative α-glucuronidase-encoding sequence of xynF (31.9% amino acid identity for a 119-amino-acid overlap [ClustalW]). Thus, the organization of the xylan utilization genes other than xynABD is clearly markedly different in B. ovatus than it is P. bryantii, and it remains to be established whether B. ovatus V975 also possesses a transcriptional regulator similar to XynR that governs xylanase gene expression.
Acknowledgments
The Rowett Research Institute is supported by the Scottish Executive Rural Affairs Department.
Derry Mercer, Kohji Miyazaki, Hiroyuki Miyamoto, and Tatsuaki Hirase contributed equally to this work.
REFERENCES
- 1.Anderson, K. L., and A. A. Salyers. 1989. Biochemical evidence that starch breakdown by Bacteroides thetaiotaomicron involves outer-membrane starch-binding sites and periplasmic starch-degrading enzymes. J. Bacteriol. 171:3192-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan, R. G., and B. W. Matthews. 1989. The helix-turn-helix DNA-binding motif. J. Biol. Chem. 264:1903-1906. [PubMed] [Google Scholar]
- 3.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 4.Dehority, B. A. 1991. Effects of microbial synergism on fiber digestion in the rumen. Proc. Nutr. Soc. 50:149-159. [DOI] [PubMed] [Google Scholar]
- 5.Flint, H. J., and C. S. Stewart. 1999. Bacteroides and Prevotella, p. 198-202. In R. K. Robinson, C. A. Batt, and P. Patel (ed.), Encyclopedia of food microbiology. Academic Press, New York, N.Y.
- 6.Flint, H. J., T. R. Whitehead, J. C. Martin, and A. Gasparic. 1997. Interrupted catalytic domain structures in xylanases from two distantly related strains of Prevotella ruminicola. Biochim. Biophys. Acta 1337:161-165. [DOI] [PubMed] [Google Scholar]
- 7.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner, R. G., J. E. Wells, J. B. Russell, and D. B. Wilson. 1995. The effect of carbohydrates on the expression of the Prevotella ruminicola 1,4-β-d-endoglucanase. FEMS Microbiol. Lett. 125:305-310. [DOI] [PubMed] [Google Scholar]
- 9.Gasparic, A., R. Marinsek-Logar, J. Martin, R. J. Wallace, F. V. Nekrep, and H. J. Flint. 1995. Isolation of genes encoding β-d-xylanase, β-d-xylosidase and α-l-arabinofuranosidase activities from the rumen bacterium Prevotella ruminicola B14. FEMS Microbiol. Lett. 125:135-141. [DOI] [PubMed] [Google Scholar]
- 10.Gasparic, A., J. Martin, A. S. Daniel, and H. J. Flint. 1995. A xylan hydrolase gene cluster in Prevotella ruminicola B14: sequence relationships, synergistic interactions, and oxygen sensitivity of a novel enzyme with exoxylanase and β-(1,4)-xylosidase activities. Appl. Environ. Microbiol. 61:2958-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hespell, R. B., and T. R. Whitehead. 1990. Physiology and genetics of xylan degradation by gastrointestinal-tract bacteria. J. Dairy Sci. 73:3013-3022. [DOI] [PubMed] [Google Scholar]
- 12.Kofoid, E. C., and J. S. Parkinson. 1991. Tandem translation starts in the cheA locus of Escherichia coli. J. Bacteriol. 173:2116-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki, K., J. C. Martin, R. Marinsek-Logar, and H. J. Flint. 1997. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B14. Anaerobe 3:373-381. [DOI] [PubMed] [Google Scholar]
- 14.Park, H., and M. Inouye. 1997. Mutational analysis of the linker region of EnvZ, an osmosensor in Escherichia coli. J. Bacteriol. 179:4382-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 16.Ramsak, A., M. Peterka, K. Tajima, J. C. Martin, J. Wood, M. E. A. Johnston, R. I. Aminov, H. J. Flint, and G. Avgustin. 2000. Unraveling the genetic diversity of ruminal bacteria belonging to the CFB phylum. FEMS Microbiol. Ecol. 33:69-79. [DOI] [PubMed] [Google Scholar]
- 17.Reeves, A. R., G. R. Wang, and A. A. Salyers. 1997. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J. Bacteriol. 179:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salyers, A. A. 1984. Bacteroides of the human lower intestinal tract. Annu. Rev. Microbiol. 38:293-313. [DOI] [PubMed] [Google Scholar]
- 19.Salyers, A. A. 1985. Breakdown of polysaccharides by human intestinal bacteria. J. Environ. Pathol. Toxicol. Oncol. 5:211-231. [PubMed] [Google Scholar]
- 20.Salyers, A. A., J. R. Balascio, and J. K. Palmer. 1982. Breakdown of xylan by enzymes from human colonic bacteria. J. Food Biochem. 6:39-55. [Google Scholar]
- 20a.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Stevens, A. M., N. B. Shoemaker, L. Y. Li, and A. A. Salyers. 1993. Tetracycline regulation of genes on Bacteroides conjugative transposons. J. Bacteriol. 175:6134-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 23.Stock, J. B., A. M. Stock, and J. M. Mottonen. 1990. Signal transduction in bacteria. Nature 344:395-400. [DOI] [PubMed] [Google Scholar]
- 24.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajima, K., R. I. Aminov, T. Nagamine, K. Ogata, M. Nakamura, H. Matsui, and Y. Benno. 1999. Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol. Ecol. 29:159-169. [Google Scholar]
- 26.Weaver, J., T. R. Whitehead, M. A. Cotta, P. C. Valentine, and A. A. Salyers. 1992. Genetic analysis of a locus on the Bacteroides ovatus chromosome, which contains xylan utilization genes. Appl. Environ. Microbiol. 58:2764-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitehead, T. R. 1995. Nucleotide sequences of xylan-inducible xylanase and xylosidase arabinosidase genes from Bacteroides ovatus V975. Biochim. Biophys. Acta 1244:239-241. [DOI] [PubMed] [Google Scholar]