Abstract
The genome of MM1 (40,248 bp), a temperate bacteriophage from the Spain23F-1 multiresistant epidemic clone of Streptococcus pneumoniae, is organized in 53 open reading frames (ORFs) and in at least five functional clusters. Bioinformatic and N-terminal amino acid sequence analyses enabled the assignment of possible functions to 26 ORFs. Analyses comparing the MM1 genome with those of other bacteriophages revealed similarities, mainly with genomes of phages infecting gram-positive bacteria, which suggest recent exchange of genes between species colonizing the same habitat.
Streptococcus pneumoniae (the pneumococcus) is a gram-positive human pathogen that is the leading cause of pneumonia, meningitis, and bloodstream infections in the elderly and that is one of the main pathogens responsible of middle ear infections in children (23). A better knowledge of the molecular biology of the pneumococcus has been achieved through the study of pneumococcal phages (11). The abundance of temperate phages in clinical isolates of the pneumococcus was suggested some years ago (3), and it was recently proposed that up to 75% of the samples analyzed contained phages (26). Pneumococcal phages have been a subject of continuous interest in our laboratory since the isolation of these phages was reported (22, 36). We have recently isolated and partly characterized a temperate phage (MM1) belonging to the Siphoviridae family from a clinical isolate of the multiply antibiotic-resistant Spain23F-1 strain (12). This strain best illustrates the rapid spread of drug resistance, because it was originally detected in Spain and then was rapidly disseminated to other parts of the world (24). A study conducted in 38 states of the United States revealed that, of 328 isolates highly resistant to penicillin (MIC ≥ 2.0 μg/ml), about 40% belonged to the Spanish/American S. pneumoniae 23F clone Spain23F-1 (23).
Despite the observation that most recent clinical isolates of S. pneumoniae carry prophages, information on temperate pneumococcal phages is insufficient, and only a limited amount of data about gene expression and the function of the gene products is currently available. This, together with the documented interchanges between phage and host lytic genes that seem to play a role in pneumococcal virulence (19), prompted our interest in studying the genomics of these temperate phages. This approach appears to be a fundamental step in determining the contribution of phage genes to the virulence of this clinically important microorganism. Since DNAs highly similar to that of MM1 have been detected by Southern hybridization in several clinical isolates of pneumococci of different capsular types, a finding which indicates the widespread presence of closely related pneumococcal phages in pathogenic strains (12), this approach will also facilitate a comparative analysis of the genomes of these temperate phages.
Methods.
S. pneumoniae 949 (24) was grown in C medium (17) supplemented with yeast extract (0.8 mg/ml; Difco laboratories; C+Y medium) at 37°C without shaking, and growth was monitored with a Hach 2100N nephelometer. Phage MM1 was induced from the lysogenic strain 949 (12). At a concentration of 1.2 × 108 CFU/ml, mitomycin C was added to a final concentration of 75 ng/ml, and the culture was incubated in the dark at 37°C until lysis occurred. MM1 purification (9) and preparation of proteinase K-treated MM1 DNA (28) and DNA-protein complexes (9) were carried out as previously described. Amplified or restricted DNA fragments for cloning or sequencing were isolated from 0.7% (wt/vol) agarose gels with the Geneclean II kit (Bio 101). Restriction endonucleases (New England Biolabs, Beverly, Mass.) and T4 DNA ligase (Amersham-Pharmacia Biotech.) were used as recommended by the suppliers. DNA sequencing was carried out by using an ABI Prism 3700 DNA sequencer (Applied Biosystems, Inc.). DNA and protein sequences were analyzed with the Genetics Computer Group software package (version 10.0) (7) or with the programs at the Deambulum (http://www.infobiogen.fr), the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov), or the European Bioinformatics Institute (http://www.ebi.ac.uk) site. Sequence similarity searches were performed by using the EMBL/GenBank and SWISS-PROT databases. To localize putative functional motifs the PROSITE and Pfam databases were also employed (http://hits.isb-sib.ch/cgi-bin/PFSCAN). N-terminal sequence analyses were carried out according to a published procedure (31).
Determination of the complete nucleotide sequence of the MM1 genome.
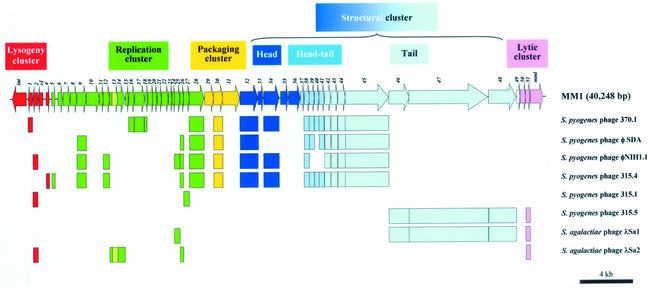
The DNA from mature phage particles appears to contain a covalently bound protein, as reported for other pneumococcal phages (9, 10, 28). Moreover, preliminary assays (restriction analyses, denaturation-renaturation analyses combined with electron microscopy, etc.) suggested that MM1 DNA is circularly permuted, terminally redundant, and packaged via a headful mechanism (data not shown). Initially, we decided to determine the nucleotide sequence of the entire genome of MM1 by using a shotgun sequencing approach, and the overlapping sequences were assembled into several contiguous stretches. The remaining gaps were closed by PCR amplification, with the entire phage genome as the template, and sequencing with specific primers. Our analyses revealed the presence of 53 open reading frames (ORFs) in a unit genome size of 40,248 bp in the prophage state (Fig. 1). The correctness of the sequence assembly was confirmed by comparing the predicted map from this sequence with that experimentally obtained by using restriction enzymes. The average G+C content of MM1 DNA was calculated as 38.4%, which is only slightly lower than the 39.7% reported for the host genome (14, 35).
FIG. 1.
Schematic representation of the proposed functional organization of MM1 DNA. Genes likely to belong to the same cluster were marked with the same colors. Genes from S. pyogenes or S. agalactiae phages similar to MM1 ORFs are also shown. In all cases the corresponding gene products showed amino acid identities ranging from 24 to 73% (see Table 1).
Analysis and organization of the MM1 genome.
The 53 ORFs analyzed potentially code for polypeptides with more than 50 amino acid (aa) residues. Every ORF is preceded by a putative ribosome binding site and begins with either an ATG, GTG, or TTG initiation codon. The most common stop codon used was TAA (33 ORFs). The MM1 genome is apparently organized into five major clusters (schematically represented in Fig. 1) in the prophage state and starts with the int gene (Table 1). The five leftmost genes (from int to orf4) comprise the lysogeny cluster and are organized in a way characteristic of temperate bacteriophages of the Siphoviridae family from low-G+C-content gram-positive bacteria (20). orf2 codes for a protein that is 54% identical to a protein of Streptococcus agalactiae phage λSa2. These proteins share the Zn recognition motif (V-X-X-H-E[I]-G-H) characteristic of metalloproteinases (16). This sequence is named the HD domain and defines a new superfamily of metal-dependent phosphohydrolases (Pfam database accession no. PF01966). The cI gene codes for a 120-aa protein that exhibits high similarity to several phage-related transcriptional repressors and that represents a λ CI analogue. Most probably, Orf4 represents the Cro-like repressor of MM1.
TABLE 1.
Comparative analysis of the genes from S. pneumoniae phage MM1 with proteins included in the databases
| Gene | Nucleotide
|
No. of amino acids encoded | Function | Best match (% amino acid identity) | log10E | Comment and/or further matches (% amino acid identity) | |
|---|---|---|---|---|---|---|---|
| Start | Stop | ||||||
| int | 1230 | 103 | 375 | Integrase | See reference 12 | ||
| orf1 | 1699 | 1349 | 116 | —a | L. lactis phage TPW22 (76) | −15 | S. thermophilus phage O1205 (55); S. pyogenes phage 370.1 (42) |
| orf2 | 2093 | 1713 | 126 | — | S. agalactiae phage λSa2 (54) | −26 | S. thermophilus phage Sfi21 (43); S. pyogenes phages φNIH1.1 (43) and 315.1 (38) |
| cI | 2477 | 2115 | 120 | Repressor | S. pyogenes phage φ speA (51) | −18 | Many phage repressors: 315.1, 315.4, NIH1.1, PSA, PS1, bIL310, TPW22, φgle |
| orf4 | 2774 | 2965 | 63 | Cro-like repressor | Lactobacillus gasseri phage φadh (45) | −8 | S. pyogenes phages 315.4 (44) and 315.6 (42); L. lactis prophage PS2 (42) |
| orf5 | 3140 | 3406 | 88 | Replication | S. pyogenes phage 315.4 (39) | −8 | |
| orf6 | 3638 | 3904 | 88 | — | |||
| orf7 | 3985 | 4509 | 174 | — | |||
| orf8 | 4561 | 5013 | 150 | — | |||
| orf9 | 5019 | 5726 | 235 | Recombination | S. thermophilus phage 7201 (39) | −25 | S. pyogenes phages φNIH1.1, φ SDA, and 315.4 (24) |
| orf10 | 5729 | 6721 | 330 | DNA binding | S. thermophilus phage 7201 (38) | −35 | L. lactis phage ul36 (26); S. pyogenes phage 370.3 (27) |
| orf11 | 6743 | 6919 | 58 | — | S. pyogenes phage similar to φ370.2 (39) | −5 | |
| orf12 | 6909 | 7454 | 181 | single-stranded-DNA binding | S. pneumoniae phage VO1 (98) | −73 | S. agalactiae Ssb-3 (64); S. pyogenes phages 315.4 and φNIH1.1 (63); S. thermophilus phage 7201 (60); several SSB proteins from gram-positive bacteria |
| orf13 | 7480 | 8637 | 385 | C5-MTase (α subunit) | S. agalactiae phage λSa2 (62) | −74 | S. pneumoniae transposon Tn5252 (55), S. pneumoniae type 4 (53), and many other modification methylases from phages and bacteria (30-35) |
| orf14 | 7631 | 8056 | 141 | C5-MTase (β subunit) | S. agalactiae phage λSa2 (62) | −28 | L. lactis AAM27270 (48); S. agalactiae (44); S. pneumoniae transposon Tn5252 (43); S. pneumoniae type 4 (41); Lactococcus phage 4268 (46); E. coli EcoHK31I polypeptide β (35) |
| orf15 | 8672 | 8875 | 67 | — | |||
| orf16 | 8929 | 9363 | 144 | — | S. pyogenes phage 370.1 (60) | −27 | |
| orf17 | 9365 | 10102 | 245 | — | S. pyogenes phage 370.1 (61) | −55 | |
| orf18 | 10119 | 10331 | 70 | — | S. pyogenes phage 370.1 (64) | −15 | |
| orf19 | 10342 | 10743 | 133 | — | L. lactis phage TP901-1 (46) | −20 | Several L. lactis phages: φ31.1 (45), ul36.1 (45), bIL309 (38), pi1 (37); L. monocytogenes phage A118 (32) |
| orf20 | 10745 | 10942 | 68 | — | |||
| orf21 | 11000 | 11317 | 105 | — | Lactobacillus phage φadh (36) | −6 | |
| orf22 | 11319 | 11816 | 165 | — | S. thermophilus phage O1205 (39) | −11 | S. pyogenes phage 370.3 (36); S. thermophilus phages Sfi11 (37) and Sfi19 (32); L. monocytogenes phage A118 (34) |
| orf23 | 11816 | 12103 | 95 | — | |||
| orf24 | 12103 | 12276 | 57 | — | |||
| orf25 | 12291 | 12710 | 139 | — | S. pyogenes phage φ speA (52) | −23 | L. lactis phage Tuc2009 (37); S. agalactiae phage λSa1 (43); S. pyogenes phage φNIH1.1 (33) |
| orf26 | 12770 | 13012 | 80 | — | S. pyogenes phage φ SDA (63) | −17 | S. agalactiae phage λSa2 (69); S. pyogenes phages 315.4, 315.5, and φNIH1.1 (63); L. monocytogenes phage PSA (41); Staphylococcus aureus phage φ 12 (44) |
| orf27 | 13009 | 13401 | 130 | — | S. pneumoniae SP1142 (54) | −20 | S. pyogenes phages φ speA and 315.1 (27) |
| orf28 | 13467 | 14561 | 364 | Nuclease | Mycobacteriophage L5 gp1 (36) | −25 | Mycobacteriophage D29 (36); S. pyogenes phages φ SDA, 315.4, φNIH1.1 (50), and 370.1 (47) |
| orf29 | 14542 | 15288 | 248 | — | |||
| orf30 | 15492 | 15947 | 151 | — | S. pyogenes phage 370.1 (38) | −9 | S. pyogenes phages 315.4, φ SDA, and φNIH1.1 (36) |
| orf31 | 15937 | 17247 | 436 | Terminase large subunit | L. monocytogenes phage A118 (53) | −96 | Many phage terminases: φ gle, SPP1, Lj771, 370.3, PBSX |
| orf32 | 17260 | 18828 | 522 | Minor capsid protein | S. pyogenes phage 315.4 (62) | −70 | S. pyogenes phages φNIH1.1, 370.1, φ SDA (62); Lactobacillus phage φ gle (43) |
| orf33 | 18761 | 18988 | 75 | — | |||
| orf34 | 19031 | 20182 | 383 | Minor capsid protein | S. pyogenes phage φNIH1.1 (49) | −71 | S. pyogenes phage 315.4 and 370.1 (50); L. monocytogenes phage A118 (32) |
| orf35 | 20321 | 20884 | 187 | Scaffolding | L. monocytogenes phage A118 (37) | −11 | Lactobacillus phages φ gle (33) and LL-H (30); other phages (SPP1, mv4) |
| orf36 | 20902 | 21789 | 295 | Major capsid protein | L. lactis phage ul36 (37) | −29 | N-terminal amino acid sequence determination (see text) |
| orf37 | 21793 | 22026 | 77 | — | |||
| orf38 | 22069 | 22461 | 130 | — | S. pyogenes phage 370.1 (51) | −20 | S. pyogenes phages 315.4, φ SDA (50), and φNIH1.1 (48) |
| orf39 | 22451 | 22822 | 123 | Minor capsid protein | S. pyogenes phage φ SDA (42) | −12 | S. pyogenes phages 315.4 and 370.1 (42) |
| orf40 | 22822 | 23166 | 114 | Minor capsid protein | S. pyogenes phage 370.1 (36) | −12 | S. pyogenes phage 315.4 (36); Lactobacillus phages φ gle (39) and LL-H (35); L. monocytogenes phage A118 (29) |
| orf41 | 23166 | 23573 | 135 | Minor capsid protein | S. pyogenes phage 315.4 (44) | −21 | S. pyogenes phages 370.1 and φ SDA (44); Lactobacillus phage φ gle (31) |
| orf42 | 23570 | 24019 | 149 | Tail protein | S. pyogenes phage φ SDA (73) | −45 | S. pyogenes phages 315.4, φNIH1.1 (72), and 370.1 (71); L. monocytogenes phage A118 (44); N-terminal amino acid sequence determination (see text) |
| orf43 | 24084 | 24572 | 162 | — | S. pyogenes phage φ SDA (54) | −25 | S. pyogenes phages 370.1, 315.4, and φNIH1.1 (54) |
| orf44 | 24585 | 25154 | 189 | — | S. pyogenes phage 370.1 (52) | −33 | S. pyogenes phages φNIH1.1, 315.4 (52), and φ SDA (51); Lactobacillus phage φ gle (30); L. monocytogenes phage A118 (33) |
| orf45 | 25147 | 28428 | 1,093 | Tail protein | S. pyogenes phage 315.4 (59) | −170 | S. pyogenes phages φNIH1.1,φ SDA, and 370.1 (59); L. lactis phage ul36 (32); S. thermophilus phages Sfi11 (21) and O1205 (21) |
| orf46 | 28425 | 29939 | 504 | Minor structural protein | S. thermophilus phage O1205 (43) | −79 | Several S. thermophilus phages: Sfi11, 7201, Sfi19, and Sfi21; S. pyogenes phage 315.5 (43); S. agalactiae phage λSa1 (33) |
| orf47 | 29941 | 35910 | 1,989 | Antireceptor | S. thermophilus phage Sfi21 (35) | −77 | Several S. thermophilus phages: DT2, MD2, Sfi11, and O1205; S. pyogenes phage 315.5 (32); S. agalactiae phage λSa1 (32) |
| orf48 | 35921 | 37999 | 692 | Minor tail protein | S. pneumoniae phage Dp-1 (69) | −147 | Several S. thermophilus phages: Sfi11, O1205, DT1, and 7201; S. pyogenes phage 315.5 (37); S. agalactiae phage λSa1 (33) |
| orf49 | 38053 | 38322 | 89 | — | S. pneumoniae phage VO1 (96) | −28 | S. pneumoniae phage Dp-1 (62) |
| orf50 | 38332 | 38748 | 138 | Holin | S. pneumoniae phage VO1 (91) | −48 | Bacillus subtilis phages GA-1 (33), B103, φ29, and pZA (22) |
| orf51 | 38752 | 39084 | 110 | Holin/antiholin? | S. pneumoniae phage VO1 (80) | −29 | S. mitis phage SM1 (75); S. agalactiae phages λSa2 (67) and λSa1 (48); S. pyogenes phage 315.5 (54) |
| mml | 39088 | 40044 | 318 | Lytic amidase | S. pneumoniae phage HB-3 (97) | −141 | S. pneumoniae LytA (89) and other phage and bacterial choline-binding proteins |
—, function unknown.
As in other pac site phages, the replication cluster of MM1 follows the lysogeny cluster. In MM1 DNA, there is a very A+T-rich sequence (76% A+T within 231 bp) located between orf5 and orf6 that contains many direct and reverse repeats (not shown) and that may correspond to the origin of replication (ori) of the phage genome. The ori region includes, among others, three copies of a tandem, 15-bp direct repeat (TTTTACAAATCTGTA) as well as two copies of a 25-bp direct repeat (AATAAATACTAACTAACAACAAGTA). Moreover, this area contains three palindromes capable of forming weak stem-loop structures with free energies ranging between −8.5 and −9.2 kcal/mol. These structures may be involved in the melting of the DNA strands and most likely represent the starting points of DNA replication. The orf9 and orf10 gene products are similar to two proteins that are also encoded by adjacent genes in the genome of the Streptococcus thermophilus phage 7201 (32). The protein similar to Orf9 is assumed to be a recombinase, whereas Orf10 appears to be a histone-like protein since it contains a Pfam PF00216-like motif that binds DNA. orf13 and orf14 correspond to two overlapped genes since they are transcribed from two different frames and encode proteins that are very similar to two proteins encoded by the S. pneumoniae transposon Tn5252, identified as components of a 5-cytosine-specific DNA methyltransferase (C5-MTase) (PF00145) (29). The presence and significance of genes coding for C5-MTases in MM1 and related phages have been recently discussed (25). These genes as well as that coding for the lytic amidase (mml) are examples of high sequence similarities between genes of the host bacterium and those of a pneumococcal temperate phage. Note especially that the G+C contents of orf13 (41.4%) and orf14 (44.1%) are clearly different from the average for the MM1 genome, 38.4% (see above). Most of the proteins encoded by the ORFs identified in this cluster strongly resemble proteins of unknown function encoded by phage genomes of the Siphoviridae family from low-G+C-content gram-positive bacteria although a ParB-like nuclease domain (PF02195) has been found in Orf28. ParB preferentially cleaves single-stranded DNA. Interestingly, orf27 is very similar to the SP1142 gene (62% identity) from the genome of S. pneumoniae strain TIGR4 (35). SP1142 is part of a 10.5-kb cluster of 19 contiguous ORFs (from SP1129 to SP1147) that is absent in the pneumococcal R6 genome and that likely corresponds to a phage remnant. As examples, SP1129 potentially codes for a protein 42% identical to the integrase of the phage 370.4 from Streptococcus pyogenes, whereas the products of genes SP1130, SP1131, and SP1134 were 27, 52, and 42% identical, respectively, to the products of ORFs from the Lactococcus lactis prophage ps3 (data not shown).
orf31 encodes a 436-aa protein exhibiting 53% identity with the large subunit of the terminase identified in Listeria monocytogenes phage A118 (18). The product of orf30 is a 151-aa protein that is 38% identical to the protein encoded by an ORF from the S. pyogenes phage 370.1 and that may represent the small subunit of the terminase (Table 1). Comparison of the gene organization of the MM1 genome (Fig. 1) with those of several Siphoviridae phage DNAs (6) suggested that the structural cluster includes 17 genes (from orf32 to orf48) that should participate in the formation of the head and tail of the MM1 phage (Table 1). The orf35 gene product is a protein of 187 aa that has significant similarity (37% identity) to the putative scaffold protein of L. monocytogenes phage A118; orf35 is located immediately upstream of the major capsid protein gene (orf33), which is characteristic of genes encoding scaffolding proteins (13). Sequencing the N-terminal amino acids of the major structural MM1 protein yielded M-P-S-N-Q-N, and sequencing the second-largest protein band produced M-T-R-Q-K-N, corresponding to Orf36 and Orf42, respectively. The large Orf47 (1,989 aa) may be the protein recognizing the phage receptor at the pneumococcal surface. Antireceptor proteins from Siphoviridae phages infecting low-G+C-content gram-positive bacteria usually contain repeated G-X-Y motifs at their C moieties (21). This motif appears to be characteristic of tropocollagen molecules, and its biological function is to provide elasticity and resistance.
orf50 codes for a protein of 138 aa, and preliminary cloning experiments with Escherichia coli suggest that this protein corresponds to the holin of phage MM1 (M. Gindreau, R. López, and P. García, unpublished observations). Orf51 is 75% identical to the predicted holin from the Streptococcus mitis temperate phage SM1 recently isolated (1). Finally, the mml gene codes for a protein of 318 aa highly similar (97% identity) to the lytic amidase characterized in phage HB-3 of S. pneumoniae (27). It has been proposed that holin is the protein that provokes unspecific lesions into the cytoplasmic membrane that allow the murein hydrolase to escape and hydrolyze the cell wall (37). Interestingly, the G+C content of the mml gene (47.2%) is noticeably higher than the average content of MM1 genome (38.4%), suggesting a possible acquisition of this gene by horizontal transfer since the G+C content of a whole genome is characteristic for a given species or group (33). Differences between the codon usage of a gene and the codon bias of the host organism are additional criteria for identifying horizontal transfers. Table 2 shows the codon usage of mml compared to those of the MM1 and S. pneumoniae genomes. The codon usages of MM1 and S. pneumoniae were similar, whereas that of mml was noticeably different. Thus, in mml the codons CGC, AAC, GAC, AUC, and AAG are the most frequently used for Arg, Asn, Asp, Ile, and Lys, respectively, whereas in phage MM1 the codons primary utilized for the same amino acids are AGA, AAU, GAU, AUU, and AAA, respectively. The most frequent codon used for Arg in S. pneumoniae is CGU. Table 2 also shows that the codon usage for orf14 differs from the average usage for MM1 genes although this was not evident for orf13.
TABLE 2.
Partial codon usage for the MM1 genes orf13, orf14, and mml
| Amino acid | Codon | Codon usagea for:
|
||||
|---|---|---|---|---|---|---|
| MM1 | Spn | orf13 | orf14 | mml | ||
| Arg | AGG | 0.12 | 0.06 | 0.22 (5) | 0.57 (4) | 0.00 (0) |
| AGA | 0.33 | 0.19 | 0.48 (11) | 0.00 (0) | 0.18 (2) | |
| CGG | 0.04 | 0.05 | 0.04 (1) | 0.00 (0) | 0.09 (1) | |
| CGA | 0.14 | 0.12 | 0.17 (4) | 0.14 (1) | 0.09 (1) | |
| CGU | 0.26 | 0.43 | 0.04 (1) | 0.29 (2) | 0.18 (2) | |
| CGC | 0.11 | 0.16 | 0.04 (1) | 0.00 (0) | 0.45 (5) | |
| Asn | AAU | 0.62 | 0.67 | 0.48 (10) | 0.17 (1) | 0.37 (7) |
| AAC | 0.38 | 0.33 | 0.52 (11) | 0.83 (5) | 0.63 (12) | |
| Asp | GAU | 0.72 | 0.67 | 0.70 (14) | 0.38 (3) | 0.37 (10) |
| GAC | 0.28 | 0.33 | 0.30 (6) | 0.62 (5) | 0.63 (17) | |
| Gln | CAG | 0.29 | 0.34 | 0.26 (5) | 0.56 (5) | 0.50 (5) |
| CAA | 0.71 | 0.66 | 0.74 (14) | 0.44 (4) | 0.50 (5) | |
| Ile | AUA | 0.19 | 0.10 | 0.23 (7) | 0.33 (2) | 0.00 (0) |
| AUU | 0.51 | 0.55 | 0.47 (14) | 0.17 (1) | 0.27 (3) | |
| AUC | 0.31 | 0.35 | 0.30 (9) | 0.50 (3) | 0.73 (8) | |
| Lys | AAG | 0.29 | 0.36 | 0.38 (9) | 0.29 (2) | 0.62 (13) |
| AAA | 0.71 | 0.64 | 0.62 (15) | 0.71 (5) | 0.38 (8) | |
Data for Spn were compiled from reference 35 (http://www.tigr.org/tigr-scripts/CMR2/codon_tables.spl). Numbers in parentheses are the numbers of amino acid residues present in the corresponding protein.
MM1 is the first temperate phage infecting S. pneumoniae for which the complete nucleotide sequence has been determined and whose genome has been analyzed in detail. These data are a starting point to carry out further studies on clinical isolates of the pneumococcus, where as many as 75% of the isolates have been reported to be lysogenic (26), since phage conversion might play an important role in the evolution of many pathogenic bacteria (5). The modular organization of the MM1 genome is similar to those of other temperate streptococcal phages, where genes belonging to the lysogeny cluster were the only genes carried in the opposite DNA strand of the phage genome (4). Twenty-six out of the 53 proteins deduced from the MM1 DNA sequence have significant similarities to products of ORFs reported in the databases (Table 1), and this enabled ascription of putative functions to some of them on the grounds of homologies to defined proteins. Most of these proteins belong to temperate phages of the Siphoviridae family infecting gram-positive bacteria and, particularly, to S. pyogenes phages, namely, 370.1, φ SDA, φNIH1.1, 315.1, 315.4, and 315.5 (Fig. 1). DNAs from nearly all of these phages were found when the genomes of three virulent S. pyogenes strains were sequenced (2, 8, 15, 30). The noticeable similarity between genes of phages infecting S. pyogenes and S. pneumoniae suggests a frequent genetic interchange between both species or a recent divergence from a common ancestor phage. From the evolutionary viewpoint the MM1 genome appears to be organized in at least two different regions (Fig. 1). The first one (from int to orf45) has many similarities in sequence and organization with the S. pyogenes phages except phage 315.5, whereas the right part of the MM1 prophage (with the noticeable exception of mml), that is, from orf46 to orf51, is closely related to phages 315.5 and λSa1 from S. pyogenes and S. agalactiae (34), respectively. As shown above, the mml gene encoding the lytic enzyme of the phage might have been introduced into the MM1 genome by horizontal transfer.
Still-unresolved issues are the impact of phages on the evolution of host genomes and the links between phages of pathogenic and nonpathogenic strains in gram-positive bacteria exhibiting similar organizations; these issues raise questions as to the contribution of the phages to survival in different environments (4, 6, 23). Since there is a large incidence of lysogeny among clinical strains of S. pneumoniae (26), the report of the first complete genome of a temperate pneumococcal phage of this bacterium provides an important tool to facilitate the study of potential virulence genes carried by pneumococcal viruses that might infect different species colonizing the same habitat. Studies in progress in our laboratory will expand our observations on the importance of prophages for shaping the natural population of S. pneumoniae as well as on the evolutionary diversification of the bacterial host.
Nucleotide sequence accession number.
The MM1 genome sequence has been deposited in the EMBL, GenBank, and DDBJ databases and appears under accession no. AJ302074.
Acknowledgments
We thank H. Brüssow for critical reading of the manuscript and for helpful suggestions, A. Fenoll for providing the 949 lysogenic strain, M. Rejas for electron microscopy preparation, and E. Cano and M. Carrasco for technical assistance.
This work was supported by grants from the Dirección General de Investigación Científica y Técnica (BCM2000-1002) and from Programa de Grupos Estratégicos de la Comunidad Autónoma de Madrid.
REFERENCES
- 1.Bensing, B. A., I. R. Siboo, and P. M. Sullam. 2001. Proteins PblA and PblB of Streptococcus mitis, which promote binding to human platelets, are encoded within a lysogenic bacteriophage. Infect. Immun. 69:6186-6192. [DOI] [PMC free article] [PubMed]
- 2.Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M.-Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheimer, H. P. 1979. Lysogenic pneumococci and their bacteriophages. J. Bacteriol. 138:618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüssow, H. 2001. Phages of dairy bacteria. Annu. Rev. Microbiol. 55:283-303. [DOI] [PubMed] [Google Scholar]
- 5.Cheetham, B. F., and M. E. Katz. 1995. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol. Microbiol. 18:201-208. [DOI] [PubMed] [Google Scholar]
- 6.Desiere, F., W. M. McShan, D. van Sinderen, J. J. Ferretti, and H. Brüssow. 2001. Comparative genomics reveals close genetic relationships between phages from dairy bacteria and pathogenic streptococci: evolutionary implications for prophage-host interactions. Virology 288:325-341. [DOI] [PubMed] [Google Scholar]
- 7.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García, E., A. Gómez, C. Ronda, C. Escarmís, and R. López. 1983. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5′ termini of its DNA. Virology 128:92-104. [DOI] [PubMed] [Google Scholar]
- 10.García, P., J. M. Hermoso, E. García, J. L. García, and R. López. 1986. Formation of a covalent complex between the terminal protein of pneumococcal bacteriophage Cp-1 and 5′-dAMP. J. Virol. 58:31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García, P., A. C. Martín, and R. López. 1997. Bacteriophages of Streptococcus pneumoniae: a molecular approach. Microb. Drug Resist. 3:165-176. [DOI] [PubMed] [Google Scholar]
- 12.Gindreau, E., R. López, and P. García. 2000. MM1, a temperate bacteriophage of the 23F Spanish/USA multiresistant epidemic clone of Streptococcus pneumoniae: structural analysis of the site-specific integration system. J. Virol. 74:7803-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrix, R. W., and R. L. Duda. 1998. Bacteriophage HK97 head assembly: a protein bullet. Adv. Virus Res. 50:235-288. [DOI] [PubMed] [Google Scholar]
- 14.Hoskins, J., W. E. Alborn, J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D.-J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoje, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P.-M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, R. Jaskunas, P. R. J. Rosteck, P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikebe, T., A. Wada, Y. Inagaki, K. Sugama, R. Suzuki, D. Tanaka, A. Tamaru, Y. Fujinaga, Y. Abe, Y. Shimizu, and H. Watanabe. 2002. Dissemination of the phage-associated novel superantigen gene speL in recent invasive and noninvasive Streptococcus pyogenes M3/T3 isolates in Japan. Infect. Immun. 70:3227-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongeneel, C. V., J. Bouvier, and A. Bairoch. 1968. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 242:211-214. [DOI] [PubMed] [Google Scholar]
- 17.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme activity in Pneumococcus. Biochim. Biophys. Acta 39:508-517. [DOI] [PubMed] [Google Scholar]
- 18.Loessner, M. J., R. B. Inman, P. Lauer, and R. Calendar. 2000. Complete nucleotide sequence, molecular analysis and genome structure of bacteriophage A118 of Listeria monocytogenes: implications for phage evolution. Mol. Microbiol. 35:324-340. [DOI] [PubMed] [Google Scholar]
- 19.López, R., M. P. González, E. García, J. L. García, and P. García. 2000. Biological roles of two new murein hydrolases of Streptococcus pneumoniae representing examples of module shuffling. Res. Microbiol. 151:437-443. [DOI] [PubMed] [Google Scholar]
- 20.Lucchini, S., F. Desiere, and H. Brüssow. 1999. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J. Virol. 73:8647-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucchini, S., F. Desiere, and H. Brüssow. 1998. The structural gene module in Streptococcus thermophilus bacteriophage φ Sfi11 shows a hierarchy of relatedness to Siphoviridae from a wide range of bacterial hosts. Virology 246:63-73. [DOI] [PubMed] [Google Scholar]
- 22.McDonnell, M., C. Ronda-Laín, and A. Tomasz. 1975. “Diplophage”: a bacteriophage of Diplococcus pneumoniae. Virology 63:577-582. [DOI] [PubMed] [Google Scholar]
- 23.McGee, L. K., K. P. Klugman, and A. Tomasz. 2000. Serotypes and clones of antibiotic-resistant pneumococci, p. 375-379. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanism of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 24.Muñoz, R., T. J. Coffey, M. Daniels, C. G. Dowson, G. Laible, J. Casal, R. Hakenbeck, M. Jacobs, J. M. Musser, B. G. Spratt, and A. Tomasz. 1991. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J. Infect. Dis. 164:302-306. [DOI] [PubMed] [Google Scholar]
- 25.Obregón, V., P. García, R. López, and J. L. García. VO1, a temperate bacteriophage of the type 19A multiresistant epidemic 8249 strain of Streptococcus pneumoniae: analysis of variability of lytic and putative C5 methyltransferase genes. Microb. Drug Resist., in press. [DOI] [PubMed]
- 26.Ramirez, M., E. Severina, and A. Tomasz. 1999. A high incidence of prophage carriage among natural isolates of Streptococcus pneumoniae. J. Bacteriol. 181:3618-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero, A., R. López, and P. García. 1990. Sequence of the Streptococcus pneumoniae bacteriophage HB-3 amidase reveals high homology with the major host autolysin. J. Bacteriol. 172:5064-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero, A., R. López, R. Lurz, and P. García. 1990. Temperate bacteriophages of Streptococcus pneumoniae that contain protein covalently linked to the 5′ ends of their DNA. J. Virol. 64:5149-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampath, J., and M. N. Vijayakumar. 1998. Identification of a DNA cytosine methyltransferase gene in conjugative transposon Tn5252. Plasmid 39:63-76. [DOI] [PubMed] [Google Scholar]
- 30.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Ricklefs, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campbell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speicher, D. W. 1994. Methods and strategies for the sequence analysis of proteins on PVDF membranes. Methods 6:262-273. [Google Scholar]
- 32.Stanley, E., L. Walsh, A. van der Zwet, G. F. Fitgerald, and D. van Sinderen. 2000. Identification of four loci isolated from two Streptococcus thermophilus phage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol. Lett. 182:271-277. [DOI] [PubMed] [Google Scholar]
- 33.Sueoka, N. 1988. Directional mutation pressure and neutral molecular evo-lution. Proc. Natl. Acad. Sci. USA 85:2653-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 99:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelber, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 36.Tiraby, J. G., E. Tiraby, and M. S. Fox. 1975. Pneumococcal bacteriophages. Virology 68:566-569. [DOI] [PubMed] [Google Scholar]
- 37.Wang, I.-N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]