Abstract
Background
The sequestration of Plasmodium falciparum–infected erythrocytes in capillary beds is a characteristic feature of severe malaria and is believed to be central to disease pathogenesis. Sequestration occurs in all P. falciparum infections, including those in asymptomatic individuals. Therefore, sequestration cannot be the sole determinant of severe disease; the intensity or distribution of infected erythrocytes may also contribute. Discerning the relationship between sequestration and well-defined clinical syndromes may enhance understanding of disease mechanisms.
Methods
We measured the concentration of parasite-derived lactate dehydrogenase (pLDH) in tissue samples obtained at autopsy from patients with clinically defined cerebral malaria. On the basis of the autopsy findings, patients were divided into 2 groups: those with an identifiable, nonmalarial cause of death and those without, who were presumed to have died of cerebral malaria. The concentration of pLDH, as determined by enzyme-linked immunosorbent assay, was used to estimate parasite load in different organs.
Results
When pLDH could be detected, the parasite load was higher in patients with presumed cerebral malaria than in parasitemic patients with assumed cerebral malaria with a nonmalaria cause of death identified at autopsy (P < .05 for brain, intestine, and skin).
Conclusions
These findings suggest that sequestration in patients with fatal cerebral malaria occurs in multiple organs and does not reflect a predilection in the parasite for the cerebral vasculature.
Malaria continues to be a major global affliction and has an enormous impact on human lives and on economic development in sub-Saharan Africa [1, 2]. Despite the overwhelming disease burden, many fundamental questions regarding the pathophysiological aspects of malaria remain unclear. For instance, cerebral malaria is characterized histologically by the sequestration of mature parasites in the capillaries and postcapillary venules of the brain, but whether and how this phenomenon contributes to disease pathogenesis is unclear [3].
One of the fundamental difficulties in assessing the relationship between the presence of parasites in the body and disease pathogenesis has been the inability to measure the presence of parasites in tissue during the disease process. The peripheral blood contains immature forms of Plasmodium falciparum (rings) and only rarely contains the life-cycle stages of the parasite thought to produce disease (trophozoites and schizonts). Erythrocytes containing these later stages are sequestered in various vascular beds throughout the body. This sequestration results from the expression of parasite proteins, which mediate the attachment of the parasitized erythrocyte to the endothelial cells of the microvasculature [4–6], on the surface of the erythrocyte. Currently, the only method of determining the presence, amount, and distribution of sequestered parasites is to examine tissues obtained after death. Although the presence of sequestered parasites in various organs has been documented for many years, their quantification has been less rigorously studied [7]. Traditionally, quantification has consisted of counting a number of vessels in a section of tissue and recording the percentage of vessels with parasitized erythrocytes. An important limitation to this method is that it fails to account for vessels containing different numbers of parasitized erythrocytes. This is particularly important in patients with marked sequestration, in whom, commonly, all vessels have some level of parasitemia. Assessing only the proportion of vessels parasitized would obscure differences related to the intensity of sequestration in each vessel.
Over the past 10 years, several tests with soluble parasite antigen have been developed, primarily for rapid field-based diagnosis of disease [8]. One of the more robust and sensitive of these assays measures parasite-derived lactate dehydrogenase (pLDH) [9]. There are 2 characteristics of pLDH that make it a suitable detection antigen. First, it is exquisitely species specific. Although the reaction it catalyzes is highly conserved, the structure of the P. falciparum enzyme reveals subtle differences that make it antigenically distinct from the human enzyme and from the enzyme of the 3 other malaria parasite species infecting humans [10]. Second, the half-life of the antigen in plasma is relatively short, allowing for the assay to measure only current or recent parasitemia. Results from assays that rely on antigens with longer half-lives can be more difficult to interpret because they measure an antigen load accumulated from multiple cycles of parasite replication. In contrast, the measurement of pLDH in erythrocyte lysates mimics more closely the parasite density in a peripheral smear made from the same blood, indicating that not a great deal of enzyme remains from earlier rounds of replication [11]. Although detection of pLDH has been used primarily for rapid diagnostic tests, several groups have also used the assay to detect the presence of viable parasites in vitro [12]. Commercial assays to detect pLDH are designed for qualitative use in diagnosis—to distinguish between the presence and absence of parasites—rather than to estimate parasite load.
In the present study, we adapted the pLDH ELISA so that we could apply it to known weights of various tissues and compare the quantity and distribution of parasitized erythrocytes between children who died of presumed cerebral malaria and children who met the clinical criteria for cerebral malaria but were found to have nonmalaria causes of death at autopsy.
PATIENTS, MATERIALS, AND METHODS
Patients
Tissues were obtained from pediatric autopsies that have been performed over the past 8 years as part of an ongoing study at the University of Malawi College of Medicine, Blantyre, Malawi. Patients were between the ages of 6 months and 6 years, and all satisfied the standard clinical case definition for cerebral malaria: Blantyre coma score of ≤2 on admission, no improvement in coma score after hypoglycemia was corrected or within the first 2 h after admission, P. falciparum parasitemia of any density, no evidence of meningitis (no pathogens cultured from cerebrospinal fluid collected on admission), and no other identifiable explanation for coma. All patients received intravenous (iv) quinine and were managed according to a standard protocol for iv fluids, antipyretics, anticonvulsants, and antibiotics. In the event of death, a local clinician requested permission for autopsy. If permission was granted, autopsies took place as soon as feasible at the Queen Elizabeth Central Hospital mortuary. Patients were classified as having assumed celebral malaria with a nonmalaria cause of death if another cause of death was identified at autopsy or by histological analysis; patients in whom no other cause of death could be identified were presumptively categorized as having died of cerebral malaria [13].
The series included 43 patients with presumed cerebral malaria and 17 patients with assumed cerebral malaria with a nonmalaria cause of death. Of those with a nonmalaria cause of death, 11 had sufficient numbers of adequately preserved tissue samples; their causes of death are listed in table 1. These patients were age matched with 11 patients with cerebral malaria. The study was approved by the ethics committees of the University of Malawi College of Medicine, Michigan State University, and the University of Liverpool.
Table 1.
Causes of death in patients who met the clinical case definition of celebral malaria in life but who had nonmalaria causes of death identified at autopsy.
| Patient | Diagnosis |
|---|---|
| 31 | Streptococcus pneumoniae pneumonia |
| 43 | Reye syndrome |
| 45 | S. pneumoniae pneumonia |
| 47 | Viral pneumonia |
| 49 | Ruptured arterio-venous malformation with subdural hematoma |
| 53 | Klebsiella oxytoca septicemia |
| 54 | Multiple skull fractures with subdural hematoma |
| 58 | Severe anemia |
| 67 | S. pneumoniae pneumonia |
| 71 | Subdural/intracranial hematomas |
| 73 | Severe pneumonia |
Tissue samples
Samples (~100 mg) from cerebral cortex, skin, kidney, liver, intestine, and lung were placed in frozen tissue matrix (optimal cutting temperature [OCT]), immediately frozen in liquid nitrogen, and subsequently stored at −80°C. The same tissues were fixed in 10% neutral buffered formalin. For the pLDH analysis, the frozen samples were thawed to room temperature, washed twice in PBS to remove excess OCT, and weighed. The sample was then placed in 1 mL of PBS and ground to a single-cell suspension in a sterile glass homogenizer. The resulting cell slurry was subjected to 3 freeze/thaw cycles to lyse the cells and then stored in liquid nitrogen until the ELISA was performed.
ELISA for pLDH
Ninety-six-well plates were coated with 3 μg/mL MAb 19G7 (Flow) specific for pLDH. Plates were incubated overnight at 4°C, washed 3 times in PBS, and subsequently blocked with PBS with 2% nonfat milk (Shoprite) for 12 h. After 3 PBS washes, 100 μL of sample was applied in duplicate. Dilutions of a sample with a known number of parasites were also applied on each plate to serve as a standard. Samples were allowed to incubate at 4°C for 12 h, after which they were washed off and a 1:1000 dilution of biotinylated MAb 17E4 (Flow) was applied for 12 h at 4°C. This was followed by a 4-h room temperature incubation with a 1:10,000 dilution of streptavidin–horseradish peroxidase. Visualization was accomplished with Sure Blue Peroxidase substrate (Kirkegard & Perry) and with optical density read at 450 nm. There were good levels of reproducibility within this range, with <10% variability in readings performed on different days (figure 1). Samples were diluted differently to assure that at least 1 reading would fall within the linear range of the assay. Samples were grouped by tissue rather than by case, in an attempt to minimize variation between organs. pLDH levels were reported as optical density read at 450 nm per milligram of tissue. Values were not converted to parasite equivalents per milligram, because there are ~4-fold differences in pLDH levels, depending on parasite life-cycle stage (data not shown). Such a conversion would introduce variability related to the proportion of various life-cycle stages sequestered in the tissue.
Figure 1.
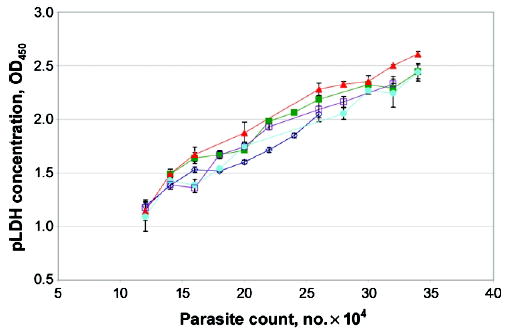
Reproducibility of parasite-derived lactate dehydrogenase (pLDH) ELISA assay. Twelve known quantities of parasites were assayed for pLDH concentrations on 5 consecutive days (as denoted by each line). Samples were measured in triplicate on each day.
Histological parasite counts
The formalin-fixed samples were paraffin embedded, sectioned, and stained with hematoxylin-eosin. Capillaries were identified on a tissue field by use of a 100× objective lens under oil immersion. A transected capillary was included in the assessment if the length of the transected section of the vessel did not exceed twice its transverse diameter and if it included either 1 or no endothelial cell nucleus. The capillary contents of 10 oil-immersion fields in each case were counted by a single observer. Parasites were counted as such if they were within a erythrocyte, had 1 or more nuclei, were surrounded by clear cytoplasm, and were not entirely refractile. The tissue field definitions for each organ observed under a 100× objective lens were as follows: lung, ≥3 complete alveoli; intestine, full field of the fibrovascular core at the tip of an intact microvillus; skin, full field of ≥6 adipocytes in the subcutaneous tissue. For the brain, capillaries and their contents were counted from the meninges to the border of the white/gray matter. Counting continued until at least 100 capillaries had been counted; for cerebral tissue, a complete run from the white/gray matter border to the meninges was performed. Levels of peripheral blood parasitemia were estimated as described elsewhere [13].
RESULTS
To evaluate the relationship of the pLDH assay to the histological evidence for sequestration, we first compared tissue parasite counts with the pLDH ELISA results. This comparison was performed in the 4 tissues in which sequestration was observed histologically and in which pLDH was consistently detected: cerebral cortex, lung, intestine, and skin. The Spearman correlation coefficient for brain was 0.63, resulting in a P value of <.005 (figure 2). The correlation coefficients for lung, intestine, and skin were 0.52, 0.63, and 0.46, respectively, resulting in P values of <.05 for each (data not shown).
Figure 2.
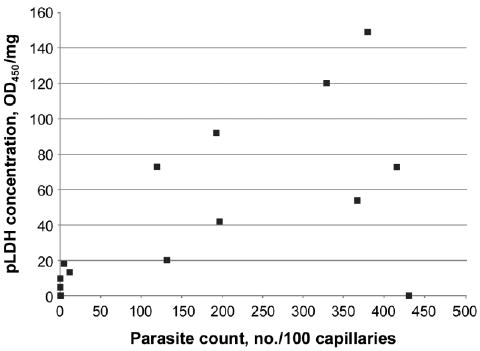
Comparison of parasite loads in the brain, estimated by parasite counting and parasite-derived lactate dehydrogenase (pLDH) ELISA methods. Parasite count reflects the no. of parasites seen per 100 cross-sectional capillaries, whereas pLDH ELISA represents parasite protein per milligram of tissue.
The 2 patient populations (presumed cerebral malaria [the CM group] and assumed cerebral malaria with nonmalaria causes of death [the NMCD group]) were comparable in terms of time spent in the hospital and duration of coma before death (table 2). The difference in mean peripheral parasitemia on admission was not statistically significant in this sample, although when the analysis was performed on the larger data set from which these samples were drawn (n = 43 for the CM group; n = 17 for the NMCD group), the CM group had a significantly higher mean parasite density (P < .05; data not shown).
Table 2.
Clinical features of the two patient groups: presumed cerebral malaria (CM) and assumed cerebral malaria with nonmalaria causes of death identified at autopsy (NMCD).
| Measurement | CM group, mean (range)a | NMCD group, mean (range)a | Pb |
|---|---|---|---|
| Parasitemia on admission, parasites/μL | 72,852 (463–782,320) | 13,384 (266–338,303) | .11 |
| Parasitemia at death, parasites/μL | 66,328 (307–280,000) | 24,258 (133–275,020) | .18 |
| Time in the hospital, h | 18.5 (4.0–38.0) | 20.2 (1.0–83.0) | .84 |
| Duration of coma, h | 20.1 (10.0–40.0) | 21.9 (5.0–84.0) | .77 |
NOTE. For both patient groups, n = 11.
Geometric mean for parasitemia on admission and parasitemia at death.
Mann-Whitney U test was used for parasitemia, and Student’s t test was used for durations.
Histologically, parasitized erythrocytes were seen sequestered in gray and white matter cerebral vessels of varying caliber, with margination evident in larger vessels. The smaller vessels (such as the capillaries used for quantitation) were more tightly packed. Within the skin and intestine, the very small capillaries of the adipocytic layer of the dermis and the tips of the intestinal villi demonstrated the majority of the sequestration burden. The sections of lung contained many fewer parasites than the other tissues, and these vascular beds appeared to be dominated by white blood cells, most containing parasite pigment. Similarly, the liver had a large burden of pigment-laden macrophages, but sequestered parasites and/or infected erythrocytes were rarely seen. The kidney occasionally had parasites sequestered in interstitial vessels; these were rarely present in glomeruli. pLDH was occasionally detected in all 6 of the tissues tested—cerebral cortex, skin, kidney, liver, intestine, and lung (figure 3). However, pLDH levels were most consistently detected in—and levels were higher in—the cerebral cortex, intestine, and skin. Figure 3 also demonstrates that patients with high concentrations of pLDH in 1 tissue were likely to have high concentrations elsewhere.
Figure 3.
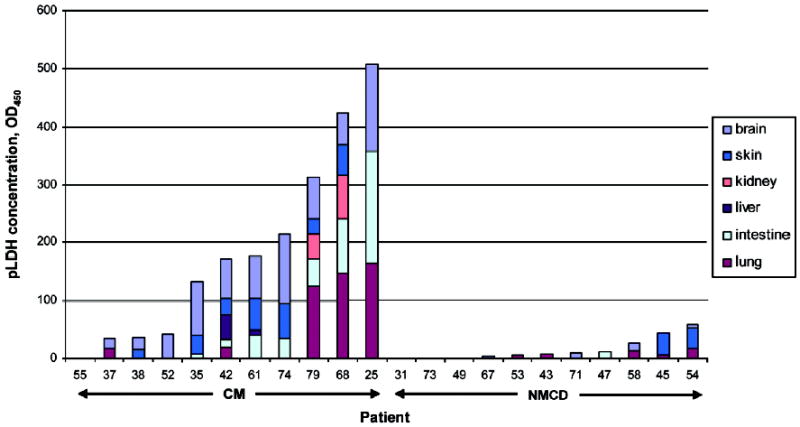
Comparison of concentrations of parasite-derived lactate dehydrogenase (pLDH) in various organs. Autopsy nos. are classified as presumed cerebral malaria (CM) or assumed cerebral malaria with nonmalaria causes of death (NMCD). Colors denote different tissues, and pLDH levels per milligram of each tissue are represented by lengths of bars.
pLDH measurements in the cerebral cortex, intestine, and skin were statistically significantly higher in the patients with presumed cerebral malaria than in patients with assumed cerebral malaria with nonmalaria causes of death (P < .005, P = .05, and P = .04, respectively) (figure 4).
Figure 4.
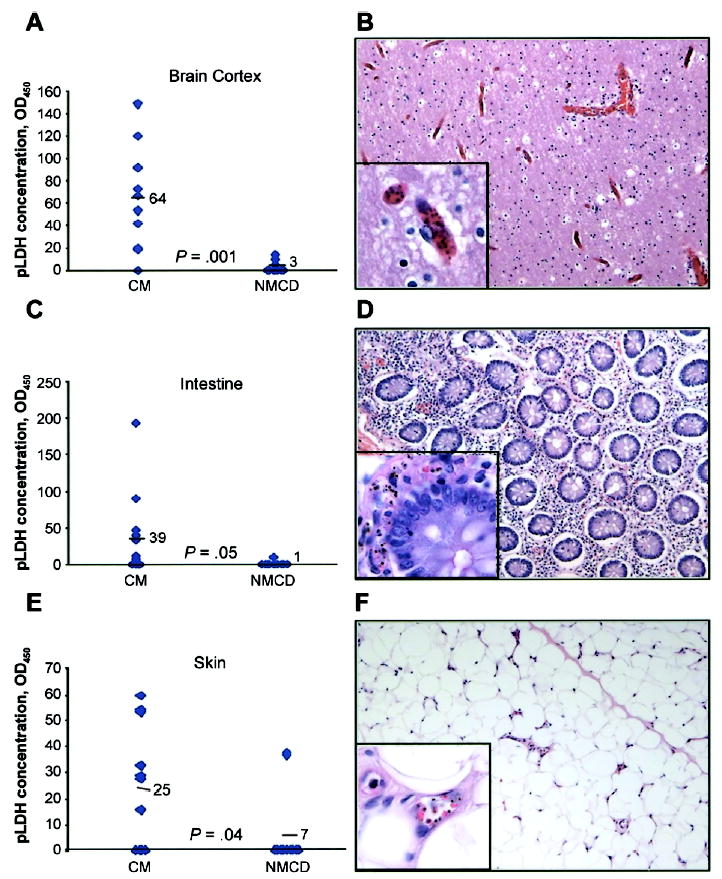
Dot plots comparing parasite-derived lactate dehydrogenase concentrations in brain cortex (A), intestine (C), and skin (E) of patients with presumed cerebral malaria (CM) or with assumed cerebral malaria with nonmalaria causes of death (NMCD). The means are represented numerically (i.e., 64, 39, and 25) and by horizontal bars. Shown are hematoxylin-eosin–stained sections of brain cortex (B), intestine (D), and skin (F) of a patient with cerebral malaria showing abundant sequestration (×200, with ×1000 inset).
DISCUSSION
Although the cytoadherence of parasitized erythrocytes to endothelial surfaces has been studied extensively in vitro, there are many basic questions regarding sequestration in vivo that remain unanswered. These include questions as fundamental as the location and relative intensities of sequestration in various organs of children dying of cerebral malaria. One of the major difficulties inherent in answering these questions is the challenge of obtaining a broad spectrum of organs from children who have died of cerebral malaria. The choice of proper controls for such experiments is also important, because many children in malarious regions are parasitemic without being symptomatic. Organs from these children are rarely available unless they have an alternative disease process present that leads to death. The autopsy-based severe malaria study that has been taking place in Malawi over the past 8 years has provided an appropriate sample of organs from children dying of a variety of clinically well-defined syndromes and, thus, meets this challenge.
Children dying of cerebral malaria have many sequestered parasites present in the brain [13], but the presence of parasites in the vasculature of other organs has not been systematically studied. Basic mechanistic questions such as whether cerebral malaria is the result of increased sequestration throughout the entire vasculature or whether there is an increased affinity of parasitized erythrocytes specifically for brain vasculature remain unanswered. Resolution of these questions could lead to further insight into the specific interactions that take place between parasitized erythrocytes and the vascular endothelial cells that support their sequestration.
The presence of sequestered parasitized erythrocytes in tissues other than the brain has been documented for more than a century [7]. These initial studies found a predominance of parasitized erythrocytes in the brains, intestines, and hearts of soldiers dying of malaria on campaigns in malaria-endemic areas [14]. Subsequent studies of P. falciparum infection in the Aotus-monkey model confirmed the ability of parasites to sequester in a variety of tissues [15, 16].
Several autopsy studies in adults have shown a gradation of sequestration levels among organs, with a brain > heart > lung > liver > kidney > skin hierarchy [17, 18]. One such study was able to distinguish between complicated and uncomplicated adult malaria on the basis of the level of sequestration in these noncerebral tissues [17]. A second was unable to find significant differences in tissue parasite densities between complicated and uncomplicated malaria [18]. Neither of these studies investigated sequestration in the intestinal tract. Multiorgan system failure is common in adults with severe malaria; in children, the predominant syndromes are anemia, metabolic derangements (acidosis and hypoglycemia), and encephalopathy [19, 20]. Therefore, extrapolations to children from adult autopsy studies should be made with care.
To our knowledge, this is the largest study of the pathological findings in human malaria to focus on the population group at highest risk of death, African children. In addition to the usual histological method of quantification, which consists of determining the proportion of vessels in a given organ that contain parasitized erythrocytes, we also counted parasite elements within these vessels and used an ELISA-based method to detect an enzyme unique to P. falciparum, pLDH. The histological assessment of percentage of vessels parasitized may not be a good reflection of parasite load, especially when nearly all vessels show evidence of sequestration. In that situation, which is commonly seen in children dying of cerebral malaria [13], the density of sequestration within vessels, rather than the mere presence or absence of parasites, determines the parasite load. Furthermore, it has been shown that, in adults with cerebral malaria, there is extensive heterogeneity in the intensity of parasitization, even within very small areas [14]. The ELISA-based method addresses this spectrum because it includes parasites from a large volume of tissue, much larger than can be addressed histologically. The ELISA-based method of quantification also provides additional information: an estimate of total parasite load in the tissue (a product of the proportion of vessels with parasites, the density of parasites per vessel, and the vascularity of the tissue). Within a specific organ, comparisons made with the histological counting method are clearly valid, but, when comparison between different tissues with different levels of vascularity is attempted, this approach is flawed. In assessing tissue-specific parasite load, the ELISA-based method measures the absolute amount of pLDH present in a given amount of tissue from any organ, thus obviating the need to correct for vascularity.
A concern with the measurement of parasites in tissue samples has been the problem of distinguishing sequestered parasites from the ring stages in the peripheral blood that were merely flowing through that organ at the time of death. This is unlikely to be a problem in this study, given the detection level of the pLDH assay (figure 1). Even the highest peripheral parasitemia at death (2.8 × 105 parasites/μL) would produce an OD450 of only 2.0, far below the average OD450 of 60 seen in 1 mg of brain cortex. The likelihood of detecting the nonsequestering ring stages of the parasite in tissues is further decreased by the fact that rings express ~4-fold less pLDH than trophozoites (data not shown). Finally, there was no correlation between peripheral parasitemia at the time of death and the measurement of pLDH in any of the tissue samples (P = .35, data not shown). For these reasons, our findings are unlikely to have been affected by any ring-stage parasites flowing through the tissues at the time of death.
The cellular mechanisms responsible for the selective sequestration pattern seen in this study remain to be elucidated. One possibility is that the 3 tissues in which the maximum sequestration takes place (brain, intestine, and skin) share a common receptor on the vascular endothelial cells. Alternatively, sequestered parasites in each of these tissues could be qualitatively distinct. It is known that most infections in areas of high transmission, such as Malawi, are a result of multiple parasite clones; if these were expressing alternative ligands, this could account for the differential organ sequestration [21, 22].
The presence of an abundance of sequestered parasites in organs other than the brain raises the question of whether this level of parasitization leads to the clinical symptoms involving these organs. Because the children admitted to this study were comatose, there was never any reported abdominal pain or pruritus at the time of admission. The presence of abdominal pain or symptoms relating to parasite sequestration in the skin has not been formally investigated in children emerging from coma at the Malawi study site. Our own autopsy data have shown that 56% of children dying of histologically proven cerebral malaria have small bowel intussusceptions (authors’ unpublished data). Studies of children with less-severe forms of malaria in Nigeria do, however, report that as many as 50% of children report some form of gastrointestinal disturbance [23]. The authors speculate that the alternative forms of distress (i.e., vomiting vs. diarrhea) result from different levels of sequestration in the vascular beds that supply different sections of the intestinal tract. Such a hypothesis would be difficult to confirm, because tissue samples are available only at time of death, making it impossible to quantify tissue-specific sequestration and correlate it with the dynamic progression and resolution of symptoms.
Using the average weight of the brain (1200 g) and the intestine (340 g) of a 5-year-old [24] and calculating the parasite load on the basis of a standard pLDH curve generated from absolute levels of in vitro–cultured parasites, we estimated the absolute levels of sequestration in each organ from our data. In some of the patients dying of cerebral malaria, the absolute number of parasites sequestered in the intestines approached the absolute number found in the brain. This heavy sequestration in noncerebral tissues emphasizes that cerebral malaria is a syndrome involving sequestration in multiple organs. The involvement of organs in addition to the brain is consonant with the recent findings of Clark et al. from this same patient series in which markers of inflammation could be identified throughout the body [24, 25]. The fact that these children present with predominantly central nervous system symptoms, despite significant parasite sequestration in other organs, suggests that the brain may respond in a unique way to sequestered parasites, that it may be more susceptible than other tissues to the effects of sequestration, or that sequestration is not responsible for tissue dysfunction.
The results of the present study provide an increased understanding of the basic biology of cerebral malaria and suggest that estimates of total parasite load might help to distinguish true cerebral malaria from cases of incidental parasitemia. The sharp distinction observed between the patients with presumed cerebral malaria and the patients with assumed cerebral malaria with other causes of death in this study suggests that these patients with assumed malaria are indeed a distinct entity—that, although they satisfied the standard clinical case definition of cerebral malaria, autopsies revealed nonmalaria anatomical causes of death. A recent study has investigated possible methods of determining total parasite load before death [26]. The authors evaluated plasma pLDH, along with several other parasite and host markers, as estimators of total-body parasite load. They compared concentrations of the potential markers with a statistical estimate of parasite load derived from several serial measures of peripheral parasitemia. No correlation was found between plasma pLDH and their estimate of total-body parasite load. Our research setting and archived samples will allow us to investigate the relationship between plasma pLDH and parasite load by measuring sequestration at the time of death and also by correlating marker levels with disease outcome. Until pLDH is further evaluated as an indicator of total parasite load and disease outcome, our attention will focus on discerning the mechanisms involved in the organ-specific pattern of sequestration seen in children dying of presumed cerebral malaria, compared with those seen in children with assumed cerebral malaria who have nonmalaria causes of death.
Acknowledgments
We thank the patients’ families for the privilege of conducting these autopsies, Krupa Bhojani for technical expertise, and Louis Miller for his continued interest and support.
Footnotes
Presented in part: American Society of Tropical Medicine and Hygiene annual meeting, Miami, Florida, 7–11 November 2004 (abstract 2023).
Potential conflicts of interest: none reported.
Financial support: US National Institutes of Health (grant RO1 AI34969); Wellcome Trust, UK (grant 042390/Z/94); National Institute of Allergy and Infectious Diseases intramural research funds.
References
- 1.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–94. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull World Health Organ. 1999;77:624–40. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 4.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–10. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 7.Marchiafava E, Bignami A. Monographs on malaria: on summer-autumn malarial fevers. Publications Sydenham N Soc. 1894;150:1. [Google Scholar]
- 8.Craig MH, Bredenkamp BL, Williams CH, et al. Field and laboratory comparative evaluation of ten rapid malaria diagnostic tests. Trans R Soc Trop Med Hyg. 2002;96:258–65. doi: 10.1016/s0035-9203(02)90092-1. [DOI] [PubMed] [Google Scholar]
- 9.Piper R, Lebras J, Wentworth L, et al. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH) Am J Trop Med Hyg. 1999;60:109–18. doi: 10.4269/ajtmh.1999.60.109. [DOI] [PubMed] [Google Scholar]
- 10.Dunn CR, Banfield MJ, Barker JJ, et al. The structure of lactate dehydrogenase from Plasmodium falciparum reveals a new target for anti-malarial design. Nat Struct Biol. 1996;3:912–5. doi: 10.1038/nsb1196-912. [DOI] [PubMed] [Google Scholar]
- 11.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–10. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 12.Makler MT, Ries JM, Williams JA, et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–41. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 13.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 14.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudgeon LS, Clarke C. A contribution to the microscopical histology of malaria, as occurring in the Salonika force in 1916, and a comparison of these findings with certain clinical phenomena. Lancet. 1917;190:153–6. [Google Scholar]
- 16.Miller LH. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969;18:860–5. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- 17.Jervis HR, Sprinz H, Johnson AJ, Wellde BT. Experimental infection with Plasmodium falciparum in Aotus monkeys. II. Observations on host pathology. Am J Trop Med Hyg. 1972;21:272–81. doi: 10.4269/ajtmh.1972.21.272. [DOI] [PubMed] [Google Scholar]
- 18.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 19.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–75. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 20.Newton CR, Taylor TE, Whitten RO. Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg. 1998;58:673–83. doi: 10.4269/ajtmh.1998.58.673. [DOI] [PubMed] [Google Scholar]
- 21.Waller D, Krishna S, Crawley J, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- 22.Arnot D. Unstable malaria in Sudan: the influence of the dry season. Clone multiplicity of Plasmodium falciparum infections in individuals exposed to variable levels of disease transmission. Trans R Soc Trop Med Hyg. 1998;92:580–5. doi: 10.1016/s0035-9203(98)90773-8. [DOI] [PubMed] [Google Scholar]
- 23.Sowunmi A, Ogundahunsi OA, Falade CO, Gbotosho GO, Oduola AM. Gastrointestinal manifestations of acute falciparum malaria in children. Acta Trop. 2000;74:73–6. doi: 10.1016/s0001-706x(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 24.Clark IA, Awburn MM, Whitten RO, et al. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar J. 2003;2:6. doi: 10.1186/1475-2875-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark IA, Awburn MM, Harper CG, Liomba NG, Molyneux ME. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malar J. 2003;2:41. doi: 10.1186/1475-2875-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochola LB, Marsh K, Lowe B, Gal S, Pluschke G, Smith T. Estimation of the sequestered parasite load in severe malaria patients using both host and parasite markers. Parasitology. 2005;131:449–58. doi: 10.1017/S0031182005008085. [DOI] [PubMed] [Google Scholar]


