Abstract
The ywtD gene, which codes for an enzyme that degrades γ-polyglutamic acid (PGA), was cloned from Bacillus subtilis IFO16449. The gene is located immediately downstream of ywsC and ywtABC, a PGA operon involved in PGA biosynthesis, and it showed partial similarity to genes coding for dl-endopeptidase, a peptidoglycan-degrading enzyme. The ywtD gene, from which signal sequence is excised, was inserted into pET15b, and the recombinant plasmid was then transformed into Escherichia coli. Histidine-tagged YwtD was purified from sonicated cells of the transformant. The purified YwtD degraded PGA to yield two hydrolyzed products, a high-molecular-mass product (490 kDa with nearly 100% l-glutamic acid) and an 11-kDa product (with d-glutamic acid and l-glutamic acid in an 80:20 ratio). This finding and results of enzymatic analysis of the two products with carboxypeptidase G suggest that YwtD is a novel enzyme cleaving the γ-glutamyl bond only between d- and l-glutamic acids of PGA, and it may be designated γ-dl-glutamyl hydrolase.
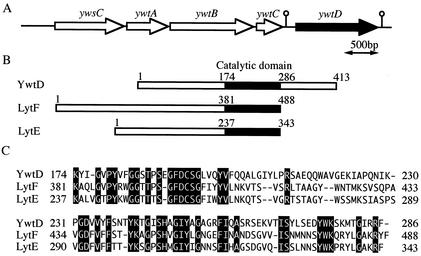
γ-Polyglutamic acid (PGA), an amino acid polymer that consists of only d-glutamic acid or d- and l-glutamic acids polymerized through γ-glutamyl bonds, is produced by several strains of Bacillus (4, 11, 19, 21). The genes required for PGA biosynthesis have also been cloned as ywsC and ywtABC (capBCA or pgsBCA) from Bacillus anthracis (14) and Bacillus subtilis (2, 26, 27), and ywsC was found to code for PGA synthetase (EC 6.3.2), which is a crucial enzyme in PGA production, catalyzing the biosynthesis of PGA from l-glutamate in the presence of ATP and Mn2+ ions (26). Sequencing of the complete genome of B. subtilis 168 (12) revealed that there is a gene (ywtD) located just downstream of ywsC and ywtABC that encodes a protein of 413 amino acids with a molecular mass of 45 kDa, and YwtD has been identified as an extracellular protein with a signal peptide of 32 amino acids, though its function remains unknown (6). Furthermore, a homology search revealed that a partial amino acid sequence of YwtD exhibits 40 and 37% identity with those of the catalytic domains of LytF and LytE, dl-endopeptidases that cleave the γ-glutamyl bond between d-glutamic acid and l-diamino acid of cell wall peptidoglycan (15, 20, 22), suggesting that YwtD functions as an enzyme that degrades PGA (Fig. 1). In this study, we purified and characterized the YwtD protein, and we demonstrated that YwtD is a novel PGA-hydrolyzing enzyme that cleaves only the γ-glutamyl bond between d-glutamic acid and l-glutamic acid of PGA.
FIG. 1.
Gene organization of ywtD and its upstream region (A), catalytic domains of YwtD and dl-endopeptidases of LytF and LytE (B), and amino acid sequence alignment of the catalytic domains (C). Numbers are amino acid residue numbers. Black shading indicates identity to YwtD.
Construction of a plasmid containing ywtD with a histidine-tag codon.
Plasmid pYWTD for expression of ywtD from which a signal sequence (6) was excised was constructed as follows. A sense primer, 5′-CTCGAGGATATACATCAGAATTG-3′ (XhoI site underlined), and an antisense primer, 5′-CTCGAGTTATTGGCACCCGTATACTTCC-3′ (XhoI site underlined), were designed on the basis of the sequence of the ywtD gene of B. subtilis 168 (11). A DNA fragment was amplified by PCR with the two primers and B. subtilis IFO16449 (26) chromosomal DNA as a template, and the amplified 1.2-kb fragment was inserted into the HincII site of pUC19, generating pUC-ywtD. After the sequence had been checked for amplification errors, it was inserted into the XhoI site of pET15b. The resulting plasmid, named pYWTD, was transformed into Escherichia coli BL21(DE3) by the method of Inoue et al. (8).
Production and purification of YwtD in E. coli.
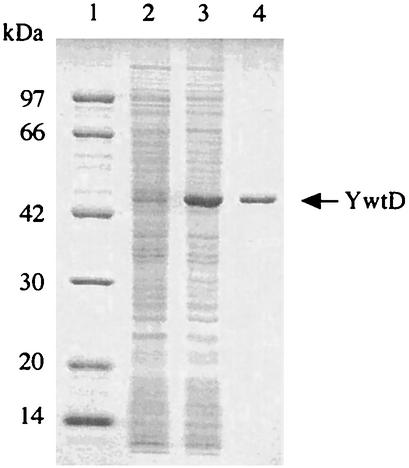
E. coli BL21(DE3) harboring pYWTD was grown at 37°C with shaking in Luria-Bertani medium containing 50 μg of ampicillin per ml. When cell growth had reached an optical density at 600 nm of 0.5, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mM, followed by cultivation for 5 h. Cells were harvested from 1,000 ml of culture by centrifugation, resuspended in 50 ml of 10 mM phosphate buffer (pH 7.0), and then disrupted by sonic oscillation for 20 min at 180 W. The sonicated cell suspension was centrifuged at 28,000 × g for 30 min, and the supernatant was applied to a nickel-nitrilotriacetic acid-agarose column (15 ml; Qiagen) equilibrated with 10 mM imidazole buffer (10 mM imidazole, 20 mM Tris-HCl, 0.3 M NaCl, 20% glycerol [pH 8.0]). The column was washed with 10 mM imidazole buffer, and then histidine-tagged protein was eluted with imidazole buffer (20 mM Tris-HCl, 0.3 M NaCl, 20% glycerol [pH 8.0]) containing a stepwise gradient of imidazole from 100 to 300 mM. The purified YwtD protein exhibited a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2), indicating that the molecular mass of 44 kDa is in agreement with that calculated from the deduced amino acid sequence of the ywtD gene from which signal sequence was omitted (6, 12).
FIG. 2.
SDS-PAGE of YwtD produced in E. coli BL21(DE3) harboring pYWTD and the purified YwtD protein. SDS-PAGE was done with a 12% polyacrylamide gel at pH 7.0 by the method of Laemmli (13). The gel was stained with Coomassie brilliant blue R-250. Lane 1, marker proteins; lane 2, cell extracts without IPTG induction; lane 3, cell extracts with 0.5 mM IPTG induction; lane 4, purified YwtD after nickel-nitrilotriacetic acid column chromatography.
Characterization of YwtD.
Purified YwtD (30 μg) and PGA (600 μg), prepared from B. subtilis IFO16449 as described previously (9), were mixed in 300 μl of 50 mM citrate buffer (pH 5.0) and then incubated at 37°C. Aliquots (30 μl each) were removed at various intervals for monitoring of the hydrolysis products by high-pressure liquid chromatography (HPLC) using a gel filtration column with authentic α-l-PGAs (Sigma Chemical Co.) as standards for molecular mass. The enzyme activity was also assayed by measuring free amino acid groups of the products (16). As shown in Fig. 3, PGA was degraded by YwtD to generate two separately hydrolyzed products: a high-molecular-mass product of about 490 kDa and a low-molecular-mass product of about 11 kDa. The latter product was gradually depolymerized to a lower-molecular-mass one by the enzyme reaction. Neither free glutamic acid nor γ-glutamyl dipeptide was detected through the reaction, indicating that YwtD cleaves the γ-glutamyl bond of PGA in an endo-type manner. The PGA hydrolase acts neither on α-l- and α-d-PGAs (Sigma) nor on cell wall peptidoglycan prepared from B. subtilis 168 by a previously described method (5).
FIG. 3.
HPLC analysis of hydrolyzed products from PGA by YwtD. PGA and the hydrolyzed products were analyzed by HPLC using an Asahipack GF-7 M HQ (300 by 7.6 mm; Showa Denko Co., Tokyo, Japan) with a refractive index detector. Samples were eluted with 50 mM phosphate buffer (pH 6.8) at a flow rate of 0.6 ml/min at 32°C. Peaks were assigned by coelution with authentic pullulan with a molecular mass of 200 kDa (Tokyo Kasei Co, Tokyo, Japan) and α-l-PGAs with molecular masses of 58, 32, and 14 kDa (Sigma).
The optimum pH for enzymatic activity was 5.0 in 50 mM citrate buffer, and the optimum temperature for the activity was 45°C. The enzyme was stable between pH 4 and 11 at 4°C for 16 h and retained 50% of its activity at 45°C for 1 h in 50 mM citrate buffer (pH 5.0). The addition of a divalent cation such as Ba2+, Ca2+, Mg2+, Mn2+, or Ni2+ at 5 mM had little effect on the enzymatic activity. Neither 5 mM EDTA nor 1 mM phenylmethylsulfonyl fluoride affected the activity, but pretreatment with 1 mM 4-(hydroxymercuri)benzoate, a sulfhydryl inhibitor, remarkably inhibited the enzymatic activity, indicating that YwtD may be a cysteine enzyme similar to dl-endopeptidase II (15, 22).
Analysis of hydrolyzed products.
PGA (20 mg) was completely hydrolyzed by purified YwtD (2.5 mg protein) at 37°C for 48 h in 5 ml of 50 mM citrate buffer (pH 5.0) solution. The reaction was stopped by boiling, and the mixture was subjected to ultrafiltration (Ultracent-30; Tosoh, Tokyo, Japan) for separation of a high-molecular-mass product (F-1) and a low-molecular-mass product (F-2). Each product was further purified by Sephacryl S-300HR gel filtration, and separation of both products was checked by HPLC analysis as described above. PGA, F-1, and F-2 were subjected to acid hydrolysis for measuring total glutamic acid content with an amino acid analyzer and glutamic acid optical isomer with an l-glutamic acid assay kit (Roche) as described previously (3). As shown in Table 1, PGA used as a substrate consisted of d- and l-glutamic acids in a ratio of 70:30, the molecular mass of which was estimated to be about 1,500 kDa. F-1, whose molecular mass was about 490 kDa, contained nearly 100% l-glutamic acid, whereas F-2, whose molecular mass was about 11 kDa, was composed of both d-glutamic acid and l-glutamic acid in a ratio of 80:20. The fact that F-1 consists almost only of l-glutamic acid and the fact that F-2 is rich in the d-isomer of glutamic acids suggest that the PGA hydrolase cuts neither between l- and l-glutamic acids nor between d- and d-glutamic acids of the γ-glutamyl bond of PGA. In order to elucidate which glutamic acid isomer exists at the carboxyl termini of the polymers, PGA, F-1, and F-2 (0.3 mg of each) were incubated with 0.3 U of carboxypeptidase G (γ-l-glutamyl hydrolase; Sigma) at 30°C for 12 h in 0.3 ml of 10 mM phosphate buffer (pH 7.2) solution. Free l-glutamic acid was released from PGA and F-1 but not from F-2, indicating that the carboxyl terminus of F-2 consists of d-glutamic acid (Table 1). It is conceivable that l-glutamic acid of F-2 may exist as a cluster at the amino terminus on account of the action of the enzyme. These results strongly suggest that the PGA hydrolase exclusively cleaves the γ-glutamyl bond between d- and l-glutamic acids, and the enzyme can be designated γ-dl-glutamyl hydrolase (EC class 3.4.19) according to the recommended nomenclature (17).
TABLE 1.
Analytical data for PGA and products hydrolyzed by YwtD
| Polymer | Proportion in total PGA (%) | Avg molecular mass (kDa)a | Composition of glutamic acid (%)
|
Hydrolysis by carboxypeptidase G (%)b | |
|---|---|---|---|---|---|
| d-Isomer | l-Isomer | ||||
| PGA | 100 | 1,500 | 70 | 30 | 8.21 (0.4) |
| F-1 | 18 | 490 | 0 | 100 | 24.5 (1.2) |
| F-2 | 82 | 11 | 80 | 20 | 0.0 (0) |
The apparent molecular masses of PGA and the hydrolyzed products were measured by gel filtration HPLC as described in the legend to Fig. 3.
Values are the nanomoles of free glutamic acid under the conditions described in the text. Percentages are quantitative ratios of free glutamic acid to each polymer.
Tanaka et al. purified an endo-type PGA-degrading enzyme that cleaves only the γ-glutamyl bond between l- and l-glutamic acids from a filamentous fungus (24), and they demonstrated by analysis of the enzymatic products that B. subtilis PGA is composed of an optically heterogeneous peptide in which each cluster of d- and l-glutamic acids is copolymerized into a single chain (23). Their finding is consistent with our results showing that the major product, F-2, is made up of both d- and l-glutamic acids in a ratio of 80:20, along with the occurrence of F-1, a high-molecular-mass product that consists almost entirely of l-glutamic acid (Table 1).
Other PGA-degrading enzymes have been isolated from bacteria (1, 10, 25, 28) and phage-infected cells of bacilli (7, 29), including an endo-type enzyme purified from phage-infected cells and B. licheniformis (10), but the enzyme actions on PGA were not analyzed in detail. γ-Glutamyltranspeptidase (GGT) from B. subtilis was reported to possess an exo-type PGA-hydrolyzing activity (1, 18). Dep of B. anthracis, the gene for which is located immediately downstream of capBCA, similar to ywtD following ywsC and ywtABC, has also been shown to be a PGA-degrading enzyme, and the amino acid sequence exhibited high similarity with that of GGT (25). Although its action on PGA has not yet been clarified, its high degree of similarity with GGT suggests that it hydrolyzes PGA in an exo-type manner. In conclusion, YwtD, which may belong to the dl-endopeptidase II family (22), is a novel PGA-hydrolyzing enzyme that cleaves only the γ-glutamyl bond between d- and l-glutamic acids of PGA, and it is useful for elucidating PGA structure as well as preparing an optically isomeric homogeneous PGA.
Nucleotide sequence accession number.
The sequence of ywtD has been submitted to the GenBank, EMBL, and DDBJ databases and can be found under accession number AB080748.
REFERENCES
- 1.Abe, K., Y. Ito, T. Ohmachi, and Y. Asada. 1997. Purification and properties of two isozymes of γ-glutamyltranspeptidase from Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 61:1621-1625. [DOI] [PubMed] [Google Scholar]
- 2.Ashiuchi, M., K. Soda, and H. Misono. 1999. A poly-γ-glutamate synthetic system of Bacillus subtilis IFO3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem. Biophys. Res. Commun. 263:6-12. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, C., Y. Asada, and T. Aida. 1989. Production of γ-polyglutamic acid by Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol. Chem. 53:2369-2375. [Google Scholar]
- 4.Cromwick, A. M., and R. A. Gross. 1995. Effects of manganese(II) on Bacillus licheniformis ATCC 9945A physiology and γ-poly(glutamic acid) formation. Int. J. Macromol. 17:259-267. [DOI] [PubMed] [Google Scholar]
- 5.Fein, J. E., and H. J. Rogers. 1976. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J. Bacteriol. 127:1427-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirose, I., K. Sano, I. Shinoda, M. Kumano, K. Nakamura, and K. Yamane. 2000. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146:65-75. [DOI] [PubMed] [Google Scholar]
- 7.Hongo, M., and A. Yoshimoto. 1970. Bacteriophages of Bacillus natto. Part III. Action of phage-induced γ-polyglutamic acid depolymerase on γ-polyglutamic acid and the enzymatic hydrolyzates. Agric. Biol. Chem. 34:1055-1063. [Google Scholar]
- 8.Inoue, H., H. Nojima, and H. Okayama. 1990. High-efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 9.Ito, Y., T. Tanaka, T. Ohmachi, and Y. Asada. 1996. Glutamic acid independent of poly(γ-glutamic acid) by Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 60:1239-1242. [Google Scholar]
- 10.King, E. C., A. J. Blacker, and T. D. H. Bugg. 2000. Enzymatic breakdown of poly-γ-d-glutamic acid in Bacillus licheniformis: identification of a polyglutamyl γ-hydrolase enzyme. Biomacromolecules 1:75-83. [DOI] [PubMed] [Google Scholar]
- 11.Kunioka, M., and A. Goto. 1994. Biosynthesis of poly(γ-glutamic acid) from l-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 40:867-872. [Google Scholar]
- 12.Kunst, F., et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Makino, S., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margot, P., M. Pagni, and D. Karamata. 1999. Bacillus subtilis 168 gene lytF encodes a γ-d-glutamate-meso-diaminopimelate muropeptidase expressed by the alternative vegetative sigma factor, σD. Microbiology 145:57-65. [DOI] [PubMed] [Google Scholar]
- 16.Moore, S., and W. H. Stein. 1954. A modified ninhydrin reagent for the photometric determination of amino acid and related compounds. J. Biol. Chem. 211:907-913. [PubMed] [Google Scholar]
- 17.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. 1992. Enzyme nomenclature. Academic Press, Inc., New York, N.Y.
- 18.Ogawa, Y., D. Sugiura, H. Motai, K. Yuasa, and Y. Tahara. 1997. DNA sequence of Bacillus subtilis (natto) NR-1 γ-glutamyltranspeptidase gene, ggt. Biosci. Biotechnol. Biochem. 61:1596-1600. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa, Y., F. Yamaguchi, K. Yuasa, and Y. Tahara. 1997. Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci. Biotechnol. Biochem. 61:1684-1687. [DOI] [PubMed] [Google Scholar]
- 20.Onishi, R., S. Ishikawa, and J. Sekiguchi. 1999. Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis. J. Bacteriol. 181:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih, I. L., and Y. T. Van. 2001. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 79:207-225. [DOI] [PubMed] [Google Scholar]
- 22.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzyme with multiple functions. Microbiology 146:249-262. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka, T., K. Fujita, S. Takenishi, and M. Taniguchi. 1997. Existence of an optically heterogeneous peptide unit in poly(γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 84:361-364. [Google Scholar]
- 24.Tanaka, T., O. Hiruta, T. Futamura, K. Uotani, A. Saitoh, M. Taniguchi, and S. Oi. 1993. Purification and characterization of poly(γ-glutamic acid) hydrolase from a filamentous fungus, Myrothecium sp. TM-4222. Biosci. Biotechnol. Biochem. 57:2148-2153. [Google Scholar]
- 25.Uchida, I., S. Makino, C. Sasakawa, M. Yoshikawa, C. Sugimoto, and N. Terakado. 1993. Identification of a novel gene, dep, associated with depolymerization of the capsular polymer in Bacillus anthracis. Mol. Microbiol. 9:487-496. [DOI] [PubMed] [Google Scholar]
- 26.Urushibata, Y., S. Tokuyama, and Y. Tahara. 2002. Characterization of the Bacillus subtilis ywsC gene, involved in γ-polyglutamic acid production. J. Bacteriol. 184:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urushibata, Y., S. Tokuyama, and Y. Tahara. 2002. Difference in transcription levels of cap genes for γ-polyglutamic acid production between Bacillus subtilis IFO16449 and Marburg 168. J. Biosci. Bioeng. 93:252-254. [DOI] [PubMed] [Google Scholar]
- 28.Volcani, B. E., and P. Margalith. 1957. A new species (Flavobacterium polyglutamicum) which hydrolyzes the γ-l-glutamyl bond in polypeptides. J. Bacteriol. 74:646-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimoto, A., S. Nomura, and M. Hongo. 1973. γ-Polyglutamic acid depolymerase induced by infection of natto and Bacillus subtilis phages and its further properties. Agric. Biol. Chem. 37:83-90. [Google Scholar]