Abstract
The NarX and NarQ sensor-histidine kinases control phosphorylation of the NarL and NarP response regulators in response to the respiratory oxidants nitrate and nitrite. Target operon transcription is activated by the Fnr protein in response to anaerobiosis, and it is further activated and/or repressed by the phospho-NarL and phospho-NarP proteins, which bind to heptamer DNA sequences. The location and arrangement of heptamers vary widely among different target operon control regions. We have constructed a series of monocopy lac operon control region constructs in which the primary operator O1-lac has been replaced by 7-2-7 heptamer pairs from the nrfA, nirB, napF, and fdnG operon control regions. These constructs provide tools for dissecting various aspects of ligand interactions with sensor-kinases, sensor interactions with response regulators, and phospho-response regulator interactions with DNA targets. Expression of the lacZ gene from these constructs was repressed to various degrees by nitrate and nitrite. In response to nitrate, the nrfA and nirB operon 7-2-7 heptamer pairs at operator O1 each mediated greater than 100-fold repression of lacZ gene expression, whereas the napF operon 7-2-7 heptamer pair mediated approximately tenfold repression. Introduction of narL, narP, narX, and narQ null alleles in various combinations allowed the in vivo interactions between different sensor-regulator pairs to be evaluated and compared.
Enterobacteria use a variety of compounds, including nitrate (NO3−) and nitrite (NO2−), as electron acceptors for anaerobic respiration (12). Anaerobic respiratory gene expression is induced by anaerobiosis, acting through the Fnr transcriptional activator (15), and is further controlled by nitrate and nitrite, acting through the Nar regulatory system (5, 31). In Escherichia coli, homologous interacting two-component regulatory systems comprise the membrane-spanning sensors NarX and NarQ and the response regulators NarL and NarP. Both sensors respond to nitrate and nitrite to control the phosphorylation of both response regulators. Phosphorylation increases the affinity of the NarL and NarP proteins for their specific DNA binding sites, from whence they activate and repress target operon expression. A key element for differential regulation in response to nitrate versus nitrite is ligand discrimination by the NarX sensor, which is hypothesized to act as a NarL kinase in response to nitrate but to act primarily as a phospho-NarL phosphatase in response to nitrite. By contrast, the NarQ sensor is hypothesized to act as a NarL and NarP kinase in response to both nitrate and nitrite (5, 31).
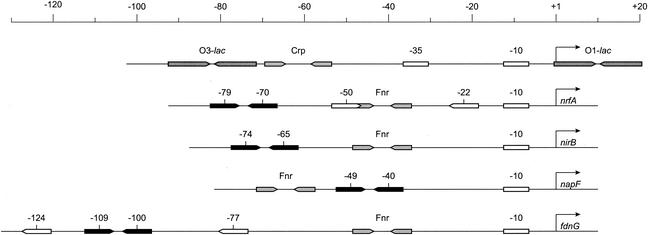
Specific DNA sites for binding phospho-NarL and phospho-NarP proteins are comprised of heptamer sequences (10, 17, 18, 33) for which a consensus sequence reads 5′-TACYYMT-3′ (where Y = C or T and M = A or C [7]). Nar heptamers are often present as pairs of inverted repeats with 2-nucleotide (nt) spacing (7-2-7 heptamer pairs), although other arrangements are also functional (2, 4, 7). The native locations of the heptamer pairs examined in this study are shown in Fig. 1. Individual heptamers are denoted by the position of the central base pair with respect to the transcription initiation site. For example, the two heptamers in the nirB operon control region are centered at −74 and at −65 (Fig. 1).
FIG. 1.
The lacZ, nrfA, nirB, napF, and fdnG operon control regions. The scale is in nucleotides. Arrows along the nucleotide sequence represent regulatory protein binding sites: black arrows, 7-2-7 heptamer pairs studied in this work; white arrows, adjacent heptamer sequences; dark gray arrows, lac operators; light gray arrows, Fnr or Crp protein binding sites. White rectangles represent promoter −35 and −10 elements. Nar heptamer sequences are denoted by the positions of the central nucleotide with respect to the transcription start sites, which are shown as the thin arrows at right above each sequence schematic.
The diversity of Nar heptamer organization in different control regions complicates attempts to compare phospho-NarL or phospho-NarP action at different sites. Some sites are used for transcription activation, some for repression, and some (e.g., the napF operon control region) for both. The NarL and NarP proteins exhibit different DNA-binding or transcription activation properties at different control regions (5, 31). Transcription of most known Nar-responsive operons is also dependent upon activation by the Fnr protein. We therefore sought to develop a versatile system that would permit in vivo analysis of the Nar regulatory circuit in isolation from these complicating factors.
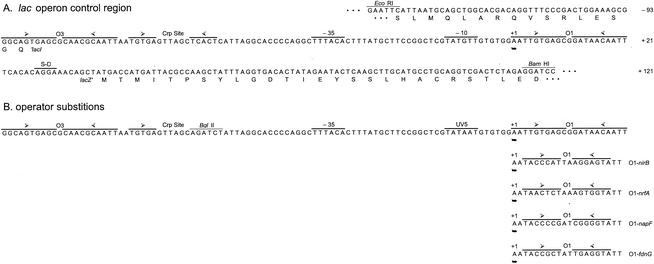
Transcription of the lacZYA operon for lactose catabolism is activated by the cyclic AMP (cAMP) receptor protein (Crp) and is repressed by the LacI protein. The primary operator (O1-lac) for LacI repressor binding consists of two inverted half-sites centered at position +11 with respect to the transcription initiation site (Fig. 1 and 2A). At least one of the two auxiliary operators, centered at +411 (O2-lac) and at −82 (O3-lac), is required for maximal repression (23). The requirement for Crp activation is suppressed by the lac UV5 alteration in the −10 promoter element (26).
FIG. 2.
Control region sequences. (A) lac operon control region from plasmid pALTER-1 (see also Fig. 1). (B) Modified lac operon control region used in this work. Substitutions at operator O1 are shown below the sequence. The transcription initiation site is labeled +1. Open arrowheads show centers of protein-binding half-sites.
Replacement of the operator O1-lac with a binding site for Crp protein results in Crp-repressible lacZYA operon expression (14). Thus, the lac operators can be engineered to analyze heterologous regulatory systems. For this study, we replaced the primary operator O1-lac with 7-2-7 heptamer pairs from the nrfA, nirB, napF, and fdnG operon control regions (Fig. 1). These operator substitution constructs provide in vivo tools for evaluating signal ligand interactions with sensor-kinases, examining sensor interactions with response regulators, and analyzing response regulator-DNA interactions.
MATERIALS AND METHODS
Strains and plasmids.
Strains and plasmids are listed in Table 1. Control region sequences are depicted in Fig. 2. Genetic crosses were performed by P1kc-mediated generalized transduction (22). Null alleles of nar regulatory genes (Table 1) have been described previously (25). Standard methods were used for restriction endonuclease digestion, ligation, transformation, and PCR amplification of DNA (19).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype and/or phenotype | Reference or source |
|---|---|---|
| E. coli | ||
| DHB6521 | Δlac mel gyrA supF λInCh1 (Kmr) | 3 |
| VJS632 | F− λ− prototroph | 30 |
| VJS676 | As VJS632 but Δ(argF-lacIZYA)U169 | 30 |
| Derivatives of strain VJS676 (λRS45-derived lysogens) | ||
| VJS6959 | λ [O3-lac O1-lac lacZ+Y+A+] | This study |
| VJS6934 | λ [O3-lac O1-nrfA lacZ+Y+A+] | This study |
| VJS6311 | λ [O3-lac O1-nirB lacZ+Y+A+] | This study |
| VJS6313 | λ [O3-lac O1-nirB lacZ+Y+A+]/narL215::Tn10 | This study |
| VJS6315 | λ [O3-lac O1-nirB lacZ+Y+A+]/narP253::Tn10d(Cm) | This study |
| VJS6317 | λ [O3-lac O1-nirB lacZ+Y+A+]/narL215::Tn10 narP253::Tn10d(Cm) | This study |
| VJS6813 | λ [O3-lac O1-napF lacZ+Y+A+] | This study |
| VJS6814 | λ [O3-lac O1-napF lacZ+Y+A+]/narL215::Tn10 | This study |
| VJS6815 | λ [O3-lac O1-napF lacZ+Y+A+]/narP253::Tn10d(Cm) | This study |
| VJS6816 | λ [O3-lac O1-napF lacZ+Y+A+]/narL215::Tn10 narP253::Tn10d(Cm) | This study |
| VJS6945 | λ [O3-lac O1-fdnG lacZ+Y+A+] | This study |
| VJS6978 | λ [O3-lac O1-fdnG lacZ+Y+A+]/narL215::Tn10 | This study |
| VJS6979 | λ [O3-lac O1-fdnG lacZ+Y+A+]/narP253::Tn10d(Cm) | This study |
| VJS6980 | λ [O3-lac O1-fdnG lacZ+Y+A+]/narL215::Tn10 narP253::Tn10d(Cm) | This study |
| Derivatives of strain VJS676 (λInCh-derived segregants) | ||
| VJS7450 | λ− Δ(attλ-lom)::bla [O3-lac O1-lac lacZ+] | This study |
| VJS7445 | λ− Δ(attλ-lom)::bla [O3-lac O1-nirB lacZ+] | This study |
| VJS7484 | λ− Δ(attλ-lom)::bla [O3-lac O1-fdnG lacZ+] | This study |
| VJS7489 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+] | This study |
| VJS7906 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/narL249::Ω-Sp | This study |
| VJS7907 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/narP253::Tn10d(Cm) | This study |
| VJS7905 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/narQ251::Tn10d(Tc) | This study |
| VJS7911 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/narQ251::Tn10d(Tc) narL249::Ω-Sp | This study |
| VJS7912 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/narQ251::Tn10d(Tc) narP253::Tn10d(Cm) | This study |
| VJS7904 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/ΔnarX242 | This study |
| VJS7909 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/Δ(narXL)235 | This study |
| VJS7910 | λ− Δ(attλ-lom)::bla [O3-lac O1-nrfA lacZ+]/ΔnarX242 narP253::Tn10d(Cm) | This study |
| VJS7449 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+] | This study |
| VJS7475 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/narL215::Tn10 | This study |
| VJS7476 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/narP253::Tn10d(Cm) | This study |
| Plasmids | ||
| pRS414 | Apr ′lacZ gene fusion vector | 28 |
| pVJS3253 | Apr Δ(lacY lacA cynX tet) derivative of pRS414 | This study |
Culture media and conditions.
Defined, complex, and indicator media for genetic manipulations were used as described previously (19). Ampicillin was used at 25 mg/ml for selecting λInCh transductants (3).
Defined medium to grow cultures for enzyme assays was buffered with 3-[N-morpholino]propanesulfonic acid (MOPS) as previously described (30). The initial pH of this medium is set at 8.0 in order to ameliorate nitrite toxicity (32). Because the pKa′ of MOPS is 7.2, the buffering capacity of this medium continually increases as acidic fermentation products accumulate; at harvest, cultures typically had a pH value of about 7.5.
Medium for batch cultures grown to the mid-exponential phase contained glucose (80 mM) as the carbon source, and the respiratory oxidants NaNO3 and NaNO2 were added to 40 mM and 5 mM, respectively. Medium for overnight cultures arrested in the mid-exponential phase (13) contained glucose (6 mM), glucose plus NaNO3 (4 mM and 10 mM, respectively), or glucose plus NaNO2 (6 mM and 8 mM, respectively) as indicated. These concentrations were determined empirically to support growth to the mid-exponential phase (about 35 to 40 Klett units).
Cultures were grown at 37°C. Culture densities were monitored with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, N.Y.) equipped with a number 66 (red) filter. Anaerobic cultures for enzyme assays were grown in screw-cap tubes as described previously (30).
Enzyme assay.
β-Galactosidase activities were determined at room temperature (approximately 21°C) by following the hydrolysis of o-nitrophenyl-β-d-galactoside in CHCl3-sodium dodecyl sulfate-permeabilized cells. Specific activities are expressed in arbitrary units (22). All cultures were assayed in duplicate, and reported values are averages from at least two independent experiments.
Construction of operator-substituted lac control regions.
Oligonucleotide-directed site-specific mutagenesis was used to introduce substitutions into the lac operon control region. Mutagenesis followed either the ampicillin selection protocol (16) or the QuickChange protocol (Stratagene Cloning Systems, La Jolla, Calif.), as described previously (1). The high-fidelity thermostable DNA polymerase was Accuzyme (Bioline USA, Reno, Nev.).
Our starting point was the lac operon control region in plasmid pALTER-1 (16), which contains the wild-type lac sequence from codon Ala-331 of lacI through codon Thr-5 of lacZ (Fig. 2A). A 111-nt in-frame segment containing bacteriophage SP6 and T7 promoters and sites for several restriction endonucleases (including BamHI) lies immediately downstream of codon Thr-5. We used site-specific mutagenesis to make three changes into the lac control region (Fig. 2). The first change introduced an upstream EcoRI site spanning lacI codons 343 to 345 (5′-GCC GAT TCA changed to 5′-GCG AAT TCA). This allows the lac control region to be released as a 260-nt EcoRI-BamHI fragment. The second change introduced a BglII site spanning positions −58 through −53 (5′-TCACTC changed to 5′-AGATCT), which also destroys the promoter-proximal half-site for binding the cAMP-bound Crp protein. The third change is the UV5 substitution at the promoter −10 element (5′-TATGTT changed to 5′-TATAAT), which suppresses the Crp-binding site alteration (26). Together, these changes resulted in a lac control region cassette that includes the Crp-independent lac UV5 promoter and operators O3-lac and O1-lac. This plasmid served as template for subsequent site-specific alterations.
Following each round of mutagenesis, the DNA sequence for the 260-nt EcoRI-BamHI fragment encompassing the lac control region was determined in order to eliminate isolates with spurious nucleotide substitutions. The control region cassettes were then recloned into the lacZ gene fusion vector pRS414 or its Δ(lacYA) derivative pVJS3253, which permit in-frame cloning of EcoRI-BamHI fragments proximal to lacZ codon Val-10. This reconstructs a functional lacZ+ gene whose transcription is governed by the upstream control region.
We experienced considerable difficulty cloning the O1-fdnG version into the lacZYA vector pRS414. As our preliminary analysis indicated that this 7-2-7 sequence effected only weak Nar-dependent repression of lacZ expression, we hypothesized that LacY protein overproduction in these constructs was deleterious to the host cells. We therefore deleted the region from position DraI-3127 (at codon 4 of lacY) to NgoMIV-6815 in plasmid pRS414. This deletion removed the ′lacY, lacA, cynX′ and ′tet sequences, thereby reducing the 10.6-kb plasmid pRS414 to the 7.0-kb plasmid pVJS3253. This vector proved suitable for cloning all constructs, including one that retains the O1-lac operator sequence, and therefore was employed for all subsequent experiments.
Plasmid pRS414-based constructs were crossed into bacteriophage λRS45 (27), and monocopy lysogens were identified by a whole-colony PCR test (24). The smaller size of plasmid pVJS3253 makes it compatible with the packaging limit of bacteriophage λInCh (3). These constructs were placed in monocopy in the host chromosome as described previously (3).
RESULTS
Comparison of bacteriophage λ vectors and culture conditions.
We modified the lac control region as described in Materials and Methods to make transcription initiation independent of the cAMP-responsive Crp protein (26). Four different Nar 7-2-7 sequences, from the nrfA, nirB, napF, and fdnG control regions, were then substituted in place of the O1-lac primary operator (Fig. 1 and 2B). We made monocopy derivatives of most lac O1 substitution control regions as both λRS45 lysogens and as λInCh segregants as described in Materials and Methods. The λInCh procedure provides antibiotic selection for specialized transducing phage, and it also provides a more direct route to isolating monocopy constructs. The final step of the λInCh procedure results in segregation of the λ prophage (3).
We used two methods for growing cultures in defined MOPS-buffered medium. The first was to culture strains to the mid-exponential phase in medium containing excess glucose, following past practice (30). The second method was to culture strains overnight in medium containing limiting glucose, such that growth arrested in the mid-exponential phase (13). We empirically adjusted the glucose, nitrate, and nitrite concentrations so that growth arrested at approximately the same density irrespective of added electron acceptor (see Materials and Methods). Because we used different culture methods and different bacteriophage λ derivatives at different times during the course of this work, we compared the expression of O1-lac constructs (in lacI null strains) in all four combinations of phage systems and growth conditions used. The levels of lacZ gene expression were very similar in the λRS45 and λInCh versions and under the two different culture conditions (Table 2). Overall, we judge the two monocopy methods and two culture conditions to yield essentially identical results.
TABLE 2.
Expression of the lacZ gene from O3-lac O1-lac constructsa
| Strain | LacZ sp act
|
Repression
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Excess Glcb with:
|
Limiting Glcc with:
|
Excess Glc with:
|
Limiting Glc with:
|
|||||||
| No addition | NO3− | NO2− | No addition | NO3− | NO2− | NO3− | NO2− | NO3− | NO2− | |
| VJS6959 (λRS45) | 1,910 | 1,990 | 1,730 | 2,240 | 1,720 | 1,820 | 1.0 | 1.1 | 1.3 | 1.2 |
| VJS7450 (λInCh) | 2,070 | 2,120 | 1,800 | 2,040 | 1,390 | 1,380 | 1.0 | 1.2 | 1.5 | 1.5 |
Specific activity is indicated in Miller units.
Strains were cultured to the mid-exponential phase in MOPS defined medium with excess glucose, as described in Materials and Methods.
Strains were cultured overnight in MOPS defined medium with limiting glucose, as described in Materials and Methods.
The glucose-limited overnight cultures exhibited a slight (1.5-fold or less) decrease in lacZ gene expression during growth with the electron acceptors nitrate or nitrite; this decrease was less pronounced in the glucose-excess cultures (Table 2). Previous studies have demonstrated that catabolite repression of lac operon expression is relieved during anaerobic growth and restored upon addition of nitrate as an alternative electron acceptor (9). We therefore attribute the slight decrease in lacZ gene expression in nitrate- or nitrite-supplemented cultures to residual catabolite repression elicited by the electron acceptor during anaerobic growth.
Effects of nitrate and nitrite on lacZ gene expression from O1 substitution control regions.
We used site-specific mutagenesis to replace the primary operator O1-lac with 7-2-7 heptamer pairs from four different Nar-responsive control regions (Fig. 2B). Each construct in this series retains the O3-lac operator. Two of the constructs, O1-nrfA and O1-nirB, exhibited about 100-fold repression of lacZ gene expression in response to nitrate and five- to tenfold repression in response to nitrite (Table 3). By contrast, the O1-napF construct exhibited only about tenfold repression of lacZ gene expression in response to nitrate (Table 3). Finally, the O1-fdnG construct exhibited less than twofold repression of lacZ gene expression in response to nitrate (Table 3). Similar results were obtained for all four constructs with both excess glucose and glucose-limited overnight cultures (data not shown; see Tables 4 and 5).
TABLE 3.
Expression of the lacZ gene from O3-lac O1 substitution constructsa
| Construct | LacZ sp actb with:
|
Repression with:
|
|||
|---|---|---|---|---|---|
| No addition | NO3− | NO2− | NO3− | NO2− | |
| O3-lac O1-lac | 2,700 | 2,660 | 2,360 | 1.0 | 1.1 |
| O3-lac O1-nrfA | 3,920 | 33 | 420 | 120 | 9.3 |
| O3-lac O1-nirB | 2,700 | 23 | 430 | 120 | 6.3 |
| O3-lac O1-napF | 810 | 74 | 360 | 11 | 2.3 |
| O3-lac O1-fdnG | 2,140 | 1,100 | 1,860 | 1.9 | 1.2 |
Specific activity is indicated in Miller units.
Strains were cultured to the mid-exponential phase in MOPS defined medium with excess glucose, as described in Materials and Methods. All carry λRS45-derived prophages.
TABLE 4.
Effects of narL and narP null alleles on expression of the lacZ gene from O3-lac O1 substitution constructsa
| Construct | Genotype
|
LacZ sp actb with:
|
Repression with:
|
||||
|---|---|---|---|---|---|---|---|
| narL | narP | No addition | NO3− | NO2− | NO3− | NO2− | |
| O3-lac O1-nirB | + | + | 3,390 | 30 | 360 | 110 | 9.4 |
| + | − | 3,350 | 39 | 520 | 86 | 6.4 | |
| − | + | 3,470 | 680 | 1,010 | 5.1 | 3.4 | |
| − | − | 3,270 | 2,330 | 2,860 | 1.4 | 1.1 | |
| O3-lac O1-napF | + | + | 1,150 | 85 | 290 | 14 | 4.0 |
| + | − | 1,160 | 100 | 650 | 12 | 1.8 | |
| − | + | 1,210 | 140 | 170 | 8.6 | 7.1 | |
| − | − | 1,170 | 760 | 1,050 | 1.5 | 1.1 | |
Specific activity is indicated in Miller units.
Strains were cultured overnight in MOPS defined medium with limiting glucose. All carry λRS45-derived prophages.
TABLE 5.
Effects of narX, narQ, narL, and narP null alleles on expression of the lacZ gene from the O3-lac O1-nrfA constructa
| Genotype
|
LacZ sp actb with:
|
Repression with:
|
||||||
|---|---|---|---|---|---|---|---|---|
| narL | narP | narX | narQ | No addition | NO3− | NO2− | NO3− | NO2− |
| + | + | + | + | 6,660 | 60 | 470 | 110 | 14 |
| + | − | + | + | 6,460 | 65 | 1,140 | 100 | 5.7 |
| − | + | + | + | 6,580 | 180 | 340 | 36 | 19 |
| + | + | + | − | 6,180 | 90 | 1,590 | 69 | 3.9 |
| + | − | + | − | 6,500 | 90 | 1,520 | 72 | 4.3 |
| − | + | + | − | 3,420 | 520 | 660 | 6.6 | 5.2 |
| + | + | − | + | 6,840 | 40 | 60 | 170 | 110 |
| + | − | − | + | 6,700 | 60 | 60 | 110 | 110 |
| − | + | − | + | 6,580 | 50 | 60 | 130 | 110 |
Specific activity is indicated in Miller units.
Strains were cultured overnight in MOPS defined medium with limiting glucose. All are λInCh-derived segregants.
The nonrepressed level of lacZ gene expression from the O1-napF construct was about threefold lower than that from the other constructs (Table 3). Conversely, the nonrepressed level of lacZ gene expression from the O1-nrfA construct was about 1.5-fold higher than that from the other constructs (Table 3). Mutations at positions +6 and +10 within O1-lac affect lac promoter function (20). Thus, it is possible that certain of the operator substitutions we studied had mild effects on lacUV5 promoter strength.
Effects of narL and narP null alleles on lacZ gene expression from O1-substitution control regions.
We next examined repression of lacZ gene expression from the O1-nirB, O1-napF, and O1-nrfA constructs in strains carrying null alleles of the narL and narP genes encoding the two Nar response regulators. Expression of the lacZ gene from the O1-nirB construct was strongly repressed by nitrate in the narL+ narP null strain (86-fold) (Table 4) but only weakly repressed in the narP+ narL null strain (5.1-fold). By contrast, lacZ gene expression from the O1-napF construct was repressed to a similar degree by nitrate in both the narL+ narP null and the narP+ narL null strains (12- versus 8.6-fold) (Table 4). Expression of the lacZ gene from the O1-nrfA construct was strongly repressed by nitrate in both the narL+ narP null and the narP+ narL null strains (100- versus 36-fold) (Table 5, lines 1 to 3).
Maximal nitrite repression of lacZ gene expression from the O1-nirB construct required both narL+ and narP+ (Table 4). By contrast, nitrite repression of lacZ gene expression from the O1-napF construct was due almost exclusively to narP+ (7.1- versus 1.8-fold) (Table 4). Indeed, the narL+ allele antagonized the narP+-dependent nitrite repression of lacZ gene expression from the O1-napF construct (4.0- versus 7.1-fold) (Table 4). Nitrite repression of lacZ gene expression from the O1-nrfA construct was likewise dependent largely upon narP+ (19- versus 5.7-fold) (Table 5, lines 1 to 3).
Effects of narX and narQ null alleles on lacZ gene expression from the O1-nrfA control region.
We next wished to examine the effects of null alleles of the narX and narQ genes encoding the two Nar sensors. We chose the O1-nrfA construct because it exhibited robust nitrate repression of lacZ gene expression in both narL null and narP null strains, as described in the preceding section.
In the narX+ narQ null strain, effective nitrate repression of lacZ gene expression from the O1-nrfA construct required narL+, and nitrite repression of lacZ gene expression was weak irrespective of narL+ or narP+ (Table 5). These observations are consistent with the idea that the NarX protein responds preferentially to nitrate. Conversely, the narQ+ narX null strain exhibited very strong repression of lacZ gene expression by nitrate or nitrite irrespective of narL+ or narP+ (Table 5). This indicates that the NarX protein acts to inhibit the influence of the NarQ protein with respect to nitrite signaling.
DISCUSSION
Replacing the O1-lac operator with a binding site for Crp protein results in Crp-dependent repression of lacUV5 promoter transcription (14). Other examples in which the lac promoter (or artificial promoters based on lac) have been used to examine repression by heterologous regulators include the anaerobic activator protein Fnr (38) and the quorum-sensing regulatory protein LuxR (11).
We describe here a series of monocopy lacUV5-based promoter constructs in which the native O1-lac and O3-lac operators are replaced by 7-2-7 heptamer pairs from the Nar-regulated nrfA, nirB, napF, and fdnG operons. Repression of lacZ gene expression from these O1-substitution constructs reflects at least four parameters: (i) signal ligand (nitrate or nitrite), (ii) ligand interactions with sensor-kinases (NarX and NarQ), (iii) sensor interactions with response regulators (NarL and NarP), and (iv) phospho-response regulator interactions with DNA targets.
Relative affinities for binding phospho-NarL and phospho-NarP proteins.
The inverted sequence symmetry of 7-2-7 heptamers pairs, coupled with analysis of single- and double-nucleotide substitutions, strongly suggests that they are bound by dimers of phospho-NarL or phospho-NarP protein (33). This conclusion is supported by recent X-ray analysis of the NarL protein carboxyl-terminal DNA-binding domain cocrystallized with a 7-2-7 heptamer pair oligonucleotide (21). Although phospho-NarL can also bind to heptamers that are deployed in other configurations (2, 4, 7, 17), we focus here on the 7-2-7 heptamer pairs that comprise the sole or principal NarL and NarP protein binding sites in their respective control regions (Fig. 1).
The tetrameric LacI repressor effects about 20-fold repression of lacZYA operon expression in constructs lacking both auxiliary operators O2-lac and O3-lac (23). The greater-than-100-fold repression of lacZ gene expression by nitrate from the O1-nrfA and O1-nirB constructs (Table 3) therefore constituted a robust response that suggests high-affinity protein-DNA interactions. The O1-napF construct exhibited about tenfold repression of lacZ gene expression by nitrate, whereas the O1-fdnG construct exhibited less than twofold repression of lacZ gene expression by nitrate (Table 3). These results imply relative in vivo binding affinities, from high to low, of nrfA, nirB > napF > fdnG. This is consistent with the conclusions from previous studies, as described below.
The O1-nrfA construct.
During growth in batch cultures, expression of the nrfABCDEFG operon encoding periplasmic nitrite reductase is induced by nitrite and repressed by nitrate (25, 29, 34, 35). Full-level nitrite induction is observed in either narL+ narP null or narP+ narL null strains and requires the 7-2-7 heptamer pair at −79 and −70 (25, 34). Qualitative DNase I protection studies revealed maximal protection of the nrfA 7-2-7 heptamer pair at relatively low concentrations of either maltose binding protein (MBP)-NarL or MBP-NarP protein phosphorylated with acetyl phosphate (7). Thus, previous in vivo and in vitro studies indicate that phospho-NarL and phospho-NarP proteins both bind the nrfA operon 7-2-7 heptamer pair with relatively high affinities. During growth with nitrate, phospho-NarL protein (but not phospho-NarP protein) binds also to lower-affinity heptamers at positions −50 and −22 (Fig. 1) to repress nrfA operon expression (7, 34).
Comparison of lacZ gene expression from the O1-nrfA construct in narL+ narP null and narP+ narL null strains suggests that the nrfA operon 7-2-7 heptamer pair was bound with similar affinities by either phospho-NarL or phospho-NarP protein during growth with nitrate (Table 5), fully consistent with these previous studies.
The O1-nirB construct.
During growth in batch cultures, expression of the nirBDC operon encoding NADH-nitrite reductase is induced by both nitrate and nitrite (33-35). Nitrite induction and full-level nitrate induction require the 7-2-7 heptamer pair at −74 and −65 and the narL+ gene, whereas the narP+ gene is required only for full-level nitrate induction (33, 34). The phospho-NarL and phospho-NarP proteins are not direct activators of nirB operon expression, but rather counter the effects of negative regulatory proteins (39). In qualitative DNase I protection studies, maximal protection of the nirB 7-2-7 heptamer sequence was observed at relatively low concentrations of MBP-NarL protein but at considerably higher concentrations of MBP-NarP protein (7). Thus, previous in vivo and in vitro studies indicate that phospho-NarL protein binds the nirB operon 7-2-7 heptamer pair with higher affinity than phospho-NarP protein.
The O1-nirB construct exhibited strong repression of lacZ gene expression by nitrate in the narL+ narP null strain but only weak repression in the narP+ narL null strain (Table 4). This is fully consistent with conclusions drawn from the studies summarized above. The DNA sequence determinants of this differential binding by phospho-NarL and phospho-NarP protein remain to be determined.
The O1-napF construct.
During growth in batch cultures, expression of the napFDAGHBC operon encoding periplasmic nitrate reductase is induced by nitrite and to a lesser degree by nitrate (6, 25, 36). Nitrite and nitrate induction require the narP+ gene and the 7-2-7 heptamer pair at −49 and −40. The phospho-NarL protein antagonizes phospho-NarP-dependent transcription activation by competing for binding to the 7-2-7 heptamer pair (6, 8). In qualitative DNase I protection studies, maximal protection of the napF 7-2-7 heptamer sequence was observed at relatively low concentrations of MBP-NarL protein but at considerably higher concentrations of MBP-NarP protein (6).
In response to nitrate, lacZ gene expression from the O1-napF construct was repressed to a similar degree in both narL+ narP null and narP+ narL null strains (Table 4). In response to nitrite, however, effective repression required the narP+ allele (Table 4). These results are consistent with conclusions drawn from the in vivo studies summarized above. However, the relative affinities for phospho-NarL and phospho-NarP inferred from the in vivo data reported here do not correlate with the previous qualitative DNase I protection results (6). Certainly, phospho-NarP protein is an effective activator of napF operon transcription (6, 8, 25), suggesting that it probably has a relatively high affinity for the napF operon 7-2-7 heptamer pair (Table 4).
The O1-fdnG construct.
During growth in batch cultures, expression of the fdnGHI operon encoding respiratory formate dehydrogenase is induced by nitrate and to a much lesser degree by nitrite (18, 25). Nitrite induction requires the narL+ gene, the 7-2-7 heptamer pair at −109 and −100, and an additional heptamer at −77 (4, 17, 18). In qualitative DNase I protection studies, maximal protection of the fdnG operon 7-2-7 heptamer sequence required relatively high concentrations of MBP-NarL protein compared to that of the nirB and nrfA operon 7-2-7 heptamer sequences (7).
Expression of the lacZ gene from the O1-fdnG construct was repressed by only twofold during growth in nitrate (Table 3). Although congruent with the qualitative DNase I protection results summarized above, this result surprised us because fdnG operon expression is induced about 100-fold by nitrate (4, 18, 25). Further analysis is required to understand the determinants for the affinities, both in vivo and in vitro, of different 7-2-7 heptamer pairs for binding the phospho-NarL and phospho-NarP proteins.
Sensor-regulator interactions.
The O1-nrfA construct exhibited strong repression of lacZ gene expression by either phospho-NarL or phospho-NarP protein (Table 5). Therefore, we chose this construct to further examine the effects of null alleles of the narX and narQ genes encoding the sensor-kinases. Previous studies have suggested that the NarX sensor responds preferentially to nitrate, whereas the NarQ sensor responds equally well to both nitrate and nitrite (5, 31).
In the narX+ narQ null strain, strong nitrate repression of lacZ gene expression from the O1-nrfA construct required the narL+ allele. Furthermore, nitrite repression of lacZ gene expression was significantly decreased by the narQ null allele irrespective of narL+ or narP+ (Table 5). These results support the idea that NarX-NarL forms a cognate two-component pair that responds preferentially to nitrate (5, 31).
By striking contrast, the narQ+ narX null strain exhibited strong repression of lacZ gene expression by either nitrate or nitrite irrespective of narL+ and narP+ (Table 5). This suggests that the NarQ sensor can partner effectively with either the NarP or the NarL response regulator in response to either nitrate or nitrite. Thus, the NarQ sensor appears to be less selective than the NarX sensor with respect to both signal ligand and response regulator.
Previous studies led to the conclusion that negative regulation (i.e., phospho-NarL phosphatase activity) by the NarX protein is a critical aspect of differential target operon expression in response to nitrate versus nitrite (25, 29, 37). The results summarized in Table 5 support this idea. In narX+ narQ+ strains, nitrate, but not nitrite, elicited strong repression of lacZ gene expression from the O1-nrfA construct. By contrast, in narQ+ narX null strains, both nitrate and nitrite were equally strong signals for repression. Therefore, the NarX protein serves to antagonize NarQ function, especially in response to nitrite (5, 31).
Acknowledgments
We are grateful to Jiarong Shi and Hui-Chung Wu for their important contributions to early phases of this work and to Alex Appleman and Janine Lin and the reviewers for helpful critique of the manuscript. We thank Dana Boyd for providing the λInCh strains in advance of publication.
This study was supported by Public Health Service Grant GM36877 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Appleman, J. A., and V. Stewart. 2003. Mutational analysis of a conserved signal-transducing element: the HAMP linker of the Escherichia coli nitrate sensor NarX. J. Bacteriol. 185:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearson, S. M., J. A. Albrecht, and R. P. Gunsalus. 2002. Oxygen and nitrate-dependent regulation of dmsABC operon expression in Escherichia coli: sites for Fnr and NarL protein interactions. BMC Microbiol. 2:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, D., D. S. Weiss, J. C. Chen, and J. Beckwith. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwin, A. J., J. Li, and V. Stewart. 1996. Analysis of nitrate regulatory protein NarL-binding sites in the fdnG and narG operon control regions of Escherichia coli K-12. Mol. Microbiol. 20:621-632. [DOI] [PubMed] [Google Scholar]
- 5.Darwin, A. J., and V. Stewart. 1996. The Nar modulon systems: nitrate and nitrite regulation of anaerobic gene expression, p. 343-359. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R. G. Landes Biomedical Publishers, Austin, Tex.
- 6.Darwin, A. J., and V. Stewart. 1995. Nitrate and nitrite regulation of the Fnr-dependent aeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site. J. Mol. Biol. 251:15-29. [DOI] [PubMed] [Google Scholar]
- 7.Darwin, A. J., K. L. Tyson, S. J. Busby, and V. Stewart. 1997. Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol. Microbiol. 25:583-595. [DOI] [PubMed] [Google Scholar]
- 8.Darwin, A. J., E. C. Ziegelhoffer, P. J. Kiley, and V. Stewart. 1998. Fnr, NarP, and NarL regulation of Escherichia coli K-12 napF (periplasmic nitrate reductase) operon transcription in vitro. J. Bacteriol. 180:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrogosz, W. 1965. The influence of nitrate and nitrite reduction on catabolite repression in Escherichia coli. Biochim. Biophys. Acta 100:553-566. [DOI] [PubMed] [Google Scholar]
- 10.Dong, X. R., S. F. Li, and J. A. DeMoss. 1992. Upstream sequence elements required for NarL-mediated activation of transcription from the narGHJI promoter of Escherichia coli. J. Biol. Chem. 267:14122-14128. [PubMed] [Google Scholar]
- 11.Egland, K. A., and E. P. Greenberg. 2000. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol. 182:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 13.Haldimann, A., L. L. Daniels, and B. L. Wanner. 1998. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J. Bacteriol. 180:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin, N., and M. Ptashne. 1987. Mutants of the catabolite activator protein of Escherichia coli that are specifically deficient in the gene-activation function. Proc. Natl. Acad. Sci. USA 84:8315-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 16.Lewis, M. K., and D. V. Thompson. 1990. Efficient site directed in vitro mutagenesis using ampicillin selection. Nucleic Acids Res. 18:3439-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J., S. Kustu, and V. Stewart. 1994. In vitro interaction of nitrate-responsive regulatory protein NarL with DNA target sequences in the fdnG, narG, narK and frdA operon control regions of Escherichia coli K-12. J. Mol. Biol. 241:150-165. [DOI] [PubMed] [Google Scholar]
- 18.Li, J., and V. Stewart. 1992. Localization of upstream sequence elements required for nitrate and anaerobic induction of fdn (formate dehydrogenase-N) operon expression in Escherichia coli K-12. J. Bacteriol. 174:4935-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Maquat, L., K. Thornton, and W. Reznikoff. 1980. lac promoter mutations located downstream from the transcription start site. J. Mol. Biol. 139:537-549. [DOI] [PubMed] [Google Scholar]
- 21.Maris, A. E., M. R. Sawaya, M. Kaczor-Grzeskowiak, M. R. Jarvis, S. M. Bearson, M. L. Kopka, I. Schröder, R. P. Gunsalus, and R. E. Dickerson. 2002. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 9:771-778. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Oehler, S., E. R. Eismann, H. Kramer, and B. Müller-Hill. 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9:973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. (Erratum, 23:1278, 1995.) [DOI] [PMC free article] [PubMed]
- 25.Rabin, R. S., and V. Stewart. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J. Bacteriol. 175:3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverstone, A., R. Arditti, and B. Magasanik. 1970. Catabolite-insensitive revertants of lac promoter mutants. Proc. Natl. Acad. Sci. USA 66:773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, G. S. A. B., S. Lubinsky-Mink, C. G. Jackson, A. Kassel, and J. Kuhn. 1986. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid 15:172-181. [DOI] [PubMed] [Google Scholar]
- 29.Stewart, V., Y. Lu, and A. J. Darwin. 2002. Periplasmic nitrate reductase (NapABC enzyme) supports anaerobic respiration by Escherichia coli K-12. J. Bacteriol. 184:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart, V., and J. Parales, Jr. 1988. Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J. Bacteriol. 170:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart, V., and R. S. Rabin. 1995. Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli, p. 233-252. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 32.Tomsett, A. B., and R. H. Garrett. 1980. The isolation and characterization of mutants defective in nitrate assimilation in Neurospora crassa. Genetics 95:649-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyson, K. L., A. I. Bell, J. A. Cole, and S. J. W. Busby. 1993. Definition of nitrite and nitrate response elements at the anaerobically inducible Escherichia coli nirB promoter: interactions between FNR and NarL. Mol. Microbiol. 7:151-157. [DOI] [PubMed] [Google Scholar]
- 34.Tyson, K. L., J. A. Cole, and S. J. W. Busby. 1994. Nitrite and nitrate regulation at the promoters of two Escherichia coli operons encoding nitrite reductase: identification of common target heptamers for both NarP- and NarL-dependent regulation. Mol. Microbiol. 13:1045-1055. [DOI] [PubMed] [Google Scholar]
- 35.Wang, H., and R. P. Gunsalus. 2000. The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. J. Bacteriol. 182:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, H., C. P. Tseng, and R. P. Gunsalus. 1999. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J. Bacteriol. 181:5303-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams, S. B., and V. Stewart. 1997. Discrimination between structurally related ligands nitrate and nitrite controls autokinase activity of the NarX transmembrane signal transducer of Escherichia coli K-12. Mol. Microbiol. 26:911-925. [DOI] [PubMed] [Google Scholar]
- 38.Williams, S. M., H. J. Wing, and S. J. W. Busby. 1998. Repression of transcription initiation by Escherichia coli FNR protein: repression by FNR can be simple. FEMS Microbiol. Lett. 163:203-208. [DOI] [PubMed] [Google Scholar]
- 39.Wu, H., K. L. Tyson, J. A. Cole, and S. J. W. Busby. 1998. Regulation of transcription initiation at the Escherichia coli nir operon promoter: a new mechanism to account for codependence on two transcription factors. Mol. Microbiol. 27:493-505. [DOI] [PubMed] [Google Scholar]