Abstract
FecI, an extracytoplasmic-function σ factor, is required for initiation of transcription of the ferric citrate transport genes. A mutational analysis of the fecA promoter revealed that the nonconserved −10 region and a downstream regulatory element are important for fecA promoter activity. However, nucleotide substitutions in the well-conserved −35 region also have an effect on the fecA promoter activity. Titration of FecI suggests that the FecI-RNA polymerase holoenzyme does not bind strongly to the downstream regulatory element, which is therefore probably involved in a subsequent step of transcription initiation.
In Escherichia coli, transcription of the ferric citrate transport genes fecABCDE is controlled by a signal transduction mechanism that starts at the cell surface. (Fe3+ citrate)2 binds to the outer membrane protein FecA and without further transport into the cell induces transcription of the fec transport genes (9, 14). The signal transmitted by FecA loaded with (Fe3+ citrate)2 across the outer membrane is transmitted across the cytoplasmic membrane by FecR, a transmembrane protein (21, 28) that interacts with FecA in the periplasm and with the FecI extracytoplasmic-function (ECF) σ factor in the cytoplasm (7, 8, 18, 24). FecR enables FecI to bind to the RNA polymerase core enzyme; this complex then binds to the fecA promoter to initiate transcription of the fec transport genes (1, 2). The only promoter known to be recognized by FecI is that of the fecABCDE operon; no other σ factor of E. coli is endowed with such a narrow promoter specificity (17).
ECF σ factors belong to a subfamily of the σ70 class, based on their sequence conservation and function across bacterial species (3, 10, 16, 20). Comparisons of sequences indicate that the genomes of Caulobacter crescentus, Pseudomonas aeruginosa, Nitrosomonas europaea, and Streptomyces coelicolor are particularly rich in ECF σ factors and contain 13, 19, 22, and 50 predicted ECF σ factors, respectively. σ factors share four conserved regions which can be further subdivided. Region 4.2 recognizes the −35 element, and region 2.4 recognizes the −10 element of promoter DNA. A bacterial promoter consists of at least about 60 bp spanning the positions −40 to +20 in relation to the +1 start site of transcription (12). A comparison of ECF σ factors reveals that the −35 sequence and the spacing but not the sequence between the −35 and −10 regions are well conserved (Table 1) (4, 5, 10, 11, 19, 20, 23, 29). The −10 sequences show less homology (Table 1), which is reflected by the low homology of regions 2.4 in the ECF σ factors (16). The characteristic feature of region 2.4 of σ70, a set of hydrophobic residues that form an amphipatic α-helix, is not present in the corresponding region of ECF σ factors (16). The diversity of the −10 sequences and region 2.4 probably accounts for the coexistence of multiple members of the ECF σ-factor subfamily in the same species.
TABLE 1.
Promoter sequences recognized by ECF σ factors
| ECF σ factor | Nucleotide sequence
|
Reference | |
|---|---|---|---|
| −35 element | −10 element | ||
| FecI | GAAAAT | TGTCCT | |
| PA2468 | GAAAATa | TGTCGGa | 25 |
| PA3899 | GATATTa | TTTTTCa | 25 |
| SigR | GGGAAT | GTT | 10 |
| SigB | GGgaacb | cGTTab | 10 |
| AlgU/RpoE | GAAC | TCT | 29 |
| CarQ | GAAAC | CGT | 29 |
| SigW | TGAAAC | CGTA | 29 |
| SigX | TGTAACT | CGAC | 29 |
| PvdS | TAAAT | CGT | 29 |
Alignment of sequences of the putative pa2466 and pa3901 promoters with that of the fecA promoter. The amino acid sequences of the PA2468 and PA3899 σ factors in the region 4.2 are very similar to that of FecI.
Lowercase letters indicate sites of lower homology.
Previously, it was shown that transcription of the fec transport genes starts at nucleotide 2741 (7) of the fec sequence (22), resulting in a polycistronic mRNA (7). Binding of the FecI RNA polymerase holoenzyme to a 75-bp DNA fragment (from positions −61 to +13) was demonstrated by DNA band shift experiments (2). The smallest plasmid-encoded DNA fragment studied that inhibited chromosomal fec transport gene expression by binding the FecI RNA polymerase extended from positions −82 to +62. In vitro transcription by FecI RNA polymerase was shown with a 650-bp promoter DNA fragment (2). Footprinting scans revealed that the Fur repressor protein loaded with Mn2+ (less prone to oxidation than Fe2+) covered 38 nucleotides (from positions −38 to −1) of the coding strand of the fecA promoter in which the Fur consensus sequence was localized (positions −36 to −17) (7). In addition, preliminary evidence was obtained for the involvement of the promoter downstream region for fecA transcription (2).
Since the ECF promoters deviate in several respects from the σ70 promoters, we examined the contribution of the −35, −10, and the downstream region for transcription of the fecA transport gene. Using site-directed mutagenesis, we constructed single-nucleotide changes in the fecA promoter (Fig. 1). To analyze the effects of the nucleotide substitutions, the mutated and the wild-type fecA promoters were fused to the gfp reporter gene. Plasmid pGFPA′ is a derivative of pFPV25 that contains a promoterless gfpmut3 gene (26) fused to the fecA promoter. Each construct was transferred into the fec deletion mutant E. coli AA93 transformed with plasmid pSV66 fecIRA, and the relative fluorescence was assayed in nutrient broth (NB) medium in the presence and absence of ferric citrate. The fluorescence was measured with the FL600 fluorescence reader (Biotek, Bad Friedrichshall, Germany) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
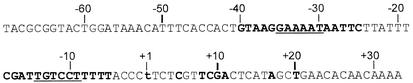
FIG. 1.
Nucleotide sequence of the fecA promoter analyzed. The −35 and −10 elements are underlined, and the sites of nucleotide substitutions are in bold letters.
Point mutations in the −35 region affect fecA promoter activity.
All mutated fecA promoters with nucleotide substitutions in the −35 element (−35 to −30) were less active than the fecA wild-type promoter, but none decreased fecA promoter activity more than threefold (Table 2). Mutations of the most conserved nucleotides A−34 and A−33 reduced the relative fluorescence no more than to 81 and 69%, respectively, of wild-type activity. Nucleotide substitutions next to the −35 element from positions −40 to −36 had a small effect on the activity of the fecA promoter, which shows that the putative −35 element with the conserved sequence GAAAAT begins at position G−35. Since none of the single mutations abolished fecA promoter activity, double mutations were introduced at a few sites. Combination of the single mutation G−35T (57% activity) with T−30G (41%) or A−34C (81%) reduced the activity to 32 and 29%, respectively. The double mutant C−32 G−30 was also less active (21%) than the C−32 (43%) and G−30 (41%) single mutants (Table 2). It appears that nucleotide substitutions in the −35 region are tolerated to some extent, which is not unexpected since different ECF σ factors bind to similar −35 regions. A comparable result was obtained with the sigX promoter of the SigX σ factor (13). However, the finding that the crtL promoter of Myxococcus xanthus to which the CarQ ECF σfactor binds is critically dependent on a pentanucleotide sequence centered at the −31 position (19) indicates differences in the structural requirements of ECF σ factor promoters. Upstream deletions in the fecA promoter extending to nucleotides −28 and −19 reduced β-galactosidase activity of a plasmid-encoded fecA-lacZ operon fusion to 23 and 25% of wild-type activity, respectively, whereas a deletion covering the entire promoter region to +1 completely abolished fecA-lacZ transcription (1). These data demonstrate that the −35 region can be partially replaced by other nucleotide sequences. This finding is consistent with the lack of complete promoter inactivation by the point mutations.
TABLE 2.
Site-directed mutations introduced at the −35 element (−40 to −25), the −10 element (−18 to −5), and the downstream regulatory elementa
| Plasmid | Sequences of the fecA promoter and the mutant fecA promoters | Relative fluorescence units (% of wild-type)
|
|
|---|---|---|---|
| NB + 50 μM dipyridyl | NB + 1 mM citrate | ||
| −35 element | |||
| pGFPA′ | GTAAGGAAAATAATTC | 403 (2) | 17,170 (100) |
| pGFP40 | TTAAGGAAAATAATTC | 363 (2) | 13,471 (78) |
| pGFP39 | GGAAGGAAAATAATTC | 247 (1) | 10,816 (61) |
| pGFP03 | GTCAGGAAAATAATTC | 669 (4) | 7,555 (44) |
| pGFP37 | GTACGGAAAATAATTC | 391 (2) | 16,101 (93) |
| pGFP36 | GTAATGAAAATAATTC | 958 (6) | 13,661 (79) |
| pGFP06 | GTAAGTAAAATAATTC | 704 (4) | 9,787 (57) |
| pGFP07 | TTAAGGCAAATAATTC | 1,287 (8) | 13,907 (81) |
| pGFP08 | TTAAGGACAATAATTC | 1,013 (6) | 11,847 (69) |
| pGFP09 | GTAAGGAACATAATTC | 635 (4) | 7,383 (43) |
| pGFP10 | GTAAGGAAACTAATTC | 446 (3) | 6,353 (37) |
| pGFP11 | GTAAGGAAAAGAATTC | 481 (3) | 7,039 (41) |
| pGFP29C | GTAAGGAAAATCATTC | 369 (2) | 6,122 (35) |
| pGFP388 | GTAAGGAAAATAGTTC | 351 (2) | 6,705 (39) |
| pGFP14 | GTAAGGAAAATAAGTC | 601 (4) | 9,272 (54) |
| pGFP26 | GTAAGGAAAATAATGC | 293 (2) | 6,745 (39) |
| pGFP25 | GTAAGGAAAATAATTA | 1,071 (6) | 8,035 (46) |
| pGFP3530 | GTAAGTAAAAGAATTC | 482 (3) | 5,736 (32) |
| pGFP3534 | GTAAGTCAAATAATTC | 287 (2) | 5,199 (29) |
| pGFP3230 | GTAAGGAACAGAATTC | 369 (2) | 3,764 (21) |
| pGFP3231 | GTAAGGAACCTAATTC | 262 (2) | 6,633 (37) |
| −10 element | |||
| pGFPA′ | CGATTGTCCTTTTT | 400 (2) | 17,926 (100) |
| pGFP19 | AGATTGTCCTTTTT | 244 (1) | 16,935 (94) |
| pGFP18 | CTATTGTCCTTTTT | 211 (1) | 12,934 (72) |
| pGFP17 | CGCTTGTCCTTTTT | 244 (1) | 13,639 (76) |
| pGFP16 | CGAGTGTCCTTTTT | 348 (2) | 7,147 (39) |
| pGFP27 | CGATGGTCCTTTTT | 430 (2) | 3,764 (21) |
| pGFP28 | CGATTTTCCTTTTT | 36 (0) | 179 (1) |
| pGFP28C | CGATTCTCCTTTTT | 235 (1) | 8,097 (45) |
| pGFP28A | CGATTATCCTTTTT | 525 (3) | 8,502 (47) |
| pGFP29 | CGATTGGCCTTTTT | 322 (2) | 2,151 (12) |
| pGFP30 | CGATTGTACTTTTT | 502 (3) | 4,660 (26) |
| pGFP31 | CGATTGTCATTTTT | 54 (0) | 717 (4) |
| pGFP32 | CGATTGTCCGTTTT | 72 (0) | 1,255 (7) |
| pGFP8G | CGATTGTCCTTGTT | 186 (1) | 11,228 (62) |
| pGFP7G | CGATTGTCCTTTGT | 208 (1) | 5,563 (31) |
| pGFP6G | CGATTGTCCTTTTG | 470 (3) | 8,869 (49) |
| Downstream regulatory element | |||
| pGFP41 | CTCTCGTTCGACTCATAGCT | 1,470 (8) | 15,954 (89) |
| pGFP44 | TTCCCGTTCGACTCATAGCT | 1,308 (7) | 14,162 (79) |
| pGFP48 | TTCTCGTCCGACTCATAGCT | 968 (5) | 11,831 (66) |
| pGFP49 | TTCTCGTTTGACTCATAGCT | 89 (0) | 896 (5) |
| pGFP50 | TTCTCGTTCTACTCATAGCT | 36 (0) | 179 (1) |
| pGFP50C | TTCTCGTTCCACTCATAGCT | 153 (1) | 9,793 (54) |
| pGFP50A | TTCTCGTTCAACTCATAGCT | 211 (1) | 220 (1) |
| pGFP51 | TTCTCGTTCGCCTCATAGCT | 107 (0) | 1,254 (7) |
| pGFP57 | TTCTCGTTCGACTCATCGCT | 609 (3) | 8,066 (45) |
| pGFP60 | TTCTCGTTCGACTCATAGCC | 753 (4) | 10,397 (58) |
The plasmids carry gfp fused to either the wild-type or mutated fecA promoter. Values (in Miller units) were determined for E. coli AA93 (Δfec) pSV66 (fecIRA) transformed with plasmids carrying the wild-type or mutant promoter fused to gfp. The bold letters indicate the changed nucleotides. The first base of each sequence given for the downstream regulatory element is at position +1.
Point mutations in the −10 region strongly reduce fecA promoter activity.
To determine the −10 element and its boundaries, single-nucleotide substitutions were introduced from −18 to −5. The mutated promoters were analyzed as described above for the −35 region. Mutations between positions −14 and −9 greatly reduced the activity of the fecA promoter (Table 2). Mutant fecA promoters with the nucleotide substitutions G−13T, C−10A, and T−9G almost completely abolished the activity of the fecA promoter, whereas mutations at C−12 strongly reduced fecA promoter activity. The G−13T substitution was replaced by the less extreme C and A substitutions. These mutants displayed a higher fecA promoter activity than did T−13. The mutations that completely inactivated the fecA promoter activity all had extreme nucleotide changes. The high fecA-gfp transcription rates of the −17 to −15 mutants define well the 5′ boundary of the −10 element, in contrast to the lower transcription of the −6 to −4 mutants (Table 2), which define less clearly the 3′ boundary. The data strongly indicate that the −10 region is essential for the activity of the fecA promoter and agree with data for SigX promoters and the crt promoter indicating that single-nucleotide replacements also abolished promoter activity.
Point mutations in the region downstream of the fecA transcription start site strongly reduce transcription.
The fecA downstream promoter element plays a critical role, as mutations C+9T and A+11C strongly reduced competition of a fecA promoter DNA fragment with the corresponding wild-type fecA promoter fragment for FecI-induced fecA-lacZ transcription. In addition, the T+7G mutation strongly reduced FecI-mediated binding of RNA polymerase to a 95-bp fecA DNA promoter fragment (2). In this study the level of fecA transcription was not determined. To further support this finding, we constructed by PCR mutagenesis single-nucleotide substitutions between positions +1 and +20. The promoter activity of the mutated downstream fragments was monitored by measuring gfp expression as indicated above. Mutants with the nucleotide substitutions C+9T, G+10T, and A+11C strongly reduced the relative fluorescence, resulting in 5, 1, and 7%, respectively, of wild-type promoter activity (Table 2). The mutation G+10A abolished fecA-gfp transcription as strongly as did G+10T (1% activity), whereas transcription by the G+10C promoter was 54% of wild-type activity (Table 2). Mutations at positions T+8, T+16, and T+20 affected fecA promoter activity much less, ranging between 45 and 66%.
DNA downstream of the transcription start site was shown to be important for other ECF σ factor-controlled promoters. DNA from nucleotides +50 to +120 of the crtL promoter is required for promoter activity (19). The DNA downstream of crtL contains an enhancer-like element that remains active when displaced to a site upstream of the promoter. The ECF σ factor PvdS is required for transcription of the pyoverdin synthesis genes of P. aeruginosa. For pvdF transcription the smallest fragment retaining promoter activity extended from nucleotides −91 to +34, indicating that a sequence element in the downstream region is required for maximal expression from this promoter (27). However, DNA downstream from the pvdE +1 site to nucleotide +195 did not increase the PvdS-dependent pvdE promoter activity (29). A study of the PvdS-dependent pvdA promoter finds that DNA downstream of the transcription start site is required for promoter activity in P. aeruginosa (15).
In order to relate promoter activity to promoter binding of FecI RNA polymerase, in vivo titration experiments were carried out. An excess of plasmid-encoded wild-type fecA promoter DNA binds the FecI-RNA polymerase complex, resulting in lesser availability of the holoenzyme for transcription of the chromosomal fecB-lacZ operon fusion in E. coli ZI418 (1). Mutations in the −10, −35, and downstream regions were examined. The wild-type fecA promoter and mutant fecA promoters fused to gfp were cloned into the high-copy-number vector pBCSK+. The β-galactosidase activity of E. coli ZI418 transformed with pBCSK+ and grown in the presence of the inducer (Fe3+ citrate)2 amounted to 325 Miller units (Table 3). β-Galactosidase activity was reduced to 192 Miller units in cells with plasmid-encoded wild-type fecA promoter. Even though the plasmid-encoded fecA promoter did not completely reduce expression of chromosomal fecB-lacZ, this reduction was taken as 100%, in relation to which the reduction caused by the mutated fecA promoters was determined. The G+10T mutation reduced β-galactosidase activity only by 63% (Table 3). The T−12G mutation reduced β-galactosidase activity by 14%, while the A−31C mutation reduced β-galactosidase activity by 60%. The mutation in the −10 region most strongly impaired binding of the FecI RNA polymerase complex to the plasmid-encoded fecA promoter.
TABLE 3.
FecI-binding and induction activity of the wild-type and the mutated fecA promoter regionsa
| Plasmid | Base changes | β-Galactosidase activity (percent FecI-binding)
|
Relative fluorescence (percent induction)
|
||
|---|---|---|---|---|---|
| NB medium | NB medium + 1 mM citrate | NB medium | NB medium + 1 mM citrate | ||
| pBCSK+ | 108 | 325 (0) | 181 | 224 | |
| pHCA | Wild type | 94 | 192 (100) | 258 | 2,636 (100) |
| pHC10 | A−31C | 105 | 246 (60) | 255 | 1324 (50) |
| pHC29 | T−12G | 138 | 307 (14) | 178 | 909 (34) |
| pHC50 | G+10 T | 125 | 241 (63) | 160 | 234 (9) |
Values (in Miller units) were determined for E. coli ZI418 fecB-lacZ transformed with high-copy-number plasmids carrying the fecA wild-type promoter or fecA mutant promoter fused to gfp.
Activity of the wild-type and the mutated fecA promoter fragments on the high-copy-number plasmids in ZI418 was determined by gfp expression (Table 3). Despite the relatively high FecI RNA polymerase binding ability of the G+10T promoter mutant (63%), the induction activity was low (9%) (Table 3). The relative activities of the promoter mutants on the high-copy-number plasmids were higher (Table 3) than on the low-copy-number plasmids (Table 2). However, the absolute fluorescence values listed in Table 3 are lower than those listed in Table 2, which may be caused by the reduced availability of FecI RNA polymerase due to its binding to the chromosomal fecA promoter of ZI418, which is not present in the AA93 fec deletion mutant used for the data presented in Table 2. Induction levels by (Fe3+ citrate)2 via the wild-type promoter were comparable (10-fold) in the two E. coli strains.
The results described here confirm and extend previous findings on the importance of the downstream region of the fecA promoter for fec transport gene transcription (2). Since binding of the FecI-RNA polymerase complex to the downstream region did not play a major role, the downstream region may be involved in a subsequent step of transcription initiation. For the initiation process in E. coli the closed RNA polymerase holoenzyme (RPc) turns into an open complex conformation. DNase I footprint assays showed that several E. coli promoters exist in two forms; one form displays protection of the promoter from −55 to −5 (RPc1) against DNase I, and the other displays it from −55 to + 20 (RPc2) (6). Although it is not clear how the conformational changes of RPc1 to RPc2 occur, it seems that the downstream DNA is placed into its binding site (12).
Mutations in the fec-specific −10 region strongly reduce FecI polymerase binding and fecA transcription. FecI RNA polymerase specifically binds to the −10 fecA promoter region, and for this reason mutations in the −10 region reduce promoter activity. This may apply to other ECF σ factors for which different −10 regions exist. In contrast, the fecA −35 region tolerates nucleotide replacements.
Acknowledgments
We thank Monica Ogierman for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (BR 330/19-1).
REFERENCES
- 1.Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169:483-490. [DOI] [PubMed] [Google Scholar]
- 2.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of σ70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 3.Brutsche, S., and V. Braun. 1997. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol. Gen. Genet. 256:416-425. [DOI] [PubMed] [Google Scholar]
- 4.Cao, M., P. A. Kobel, M. M. Morshedi, M. F. Wu, C. Paddon, and J. D. Helmann. 2002. Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443-457. [DOI] [PubMed] [Google Scholar]
- 5.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli sigma E regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 6.deHaseth, P. L., M. L. Zupancic, and M. T. Record, Jr. 1998. RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 180:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enz, S., V. Braun, and J. H. Crosa. 1995. Transcription of the region encoding the ferric dicitrate transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene 163:13-18. [DOI] [PubMed] [Google Scholar]
- 8.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Härle, C., J. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 11.Hershberger, C. D., R. W. Ye, M. R. Parsek, Z. D. Xie, and A. M. Chakrabarty. 1995. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (sigma E). Proc. Natl. Acad. Sci. USA 92:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, L. M. 2002. Open season on RNA polymerase. Nat. Struct. Biol. 9:502-504. [DOI] [PubMed] [Google Scholar]
- 13.Huang, X., and J. D. Helmann. 1998. Identification of target promoters for the Bacillus subtilis sigma X factor using a consensus-directed search. J. Mol. Biol. 279:165-173. [DOI] [PubMed] [Google Scholar]
- 14.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 15.Leoni, L., A. Cervo, N. Orsi, and P. Visca. 1996. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J. Bacteriol. 178:2299-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda, H., M. Jishage, T. Nomura, N. Fujita, and A. Ishihama. 2000. Two extracytoplasmic function sigma subunits, sigma(E) and sigma(FecI), of Escherichia coli: promoter selectivity and intracellular levels. J. Bacteriol. 182:1181-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahren, S., S. Enz, and V. Braun. 2002. Functional interaction of region 4 of the extracytoplasmic function sigma factor FecI with the cytoplasmic portion of the FecR transmembrane protein of the Escherichia coli ferric citrate transport system. J. Bacteriol. 184:3704-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Argudo, I., R. M. Ruiz-Vazquez, and F. J. Murillo. 1998. The structure of an ECF-sigma-dependent, light-inducible promoter from the bacterium Myxococcus xanthus. Mol. Microbiol. 30:883-893. [DOI] [PubMed] [Google Scholar]
- 20.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 21.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 22.Pressler, U., H. Staudenmaier, L. Zimmermann, and V. Braun. 1988. Genetics of the iron dicitrate transport system of Escherichia coli. J. Bacteriol. 170:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sexton, R., P. R. Gill, Jr., D. N. Dowling, and F. O'Gara. 1996. Transcriptional regulation of the iron-responsive sigma factor gene pbrA. Mol. Gen. Genet. 250:50-58. [DOI] [PubMed] [Google Scholar]
- 24.Stiefel, A., S. Mahren, M. Ochs, P. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 26.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 27.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 28.Welz, D., and V. Braun. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J. Bacteriol. 180:2387-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson, M. J., B. J. McMorran, and I. L. Lamont. 2001. Analysis of promoters recognized by PvdS, an extracytoplasmic-function sigma factor protein from Pseudomonas aeruginosa. J. Bacteriol. 183:2151-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]