Three cases of bilateral optic neuropathy were reported recently to the Netherlands Pharmacovigilance Centre Lareb. Patient 1, a 54 year old man, had blurred vision 34 days after he was given a third dose of infliximab for rheumatoid arthritis. He was also taking leflunomide, prednisone, naproxen, diazepam, fluoxetine, famotidine, and metoprolol, and acetaminophen or codeine. He had 20/30 vision in both eyes. Fundoscopy showed severe disc swelling, and perimetry showed visual field defects, which were most extensive in both lower quadrants. Fluorescein angiography showed capillary dilation and vascular leakage in both optic nerve heads. The patient was treated with steroids, but his vision did not recover.
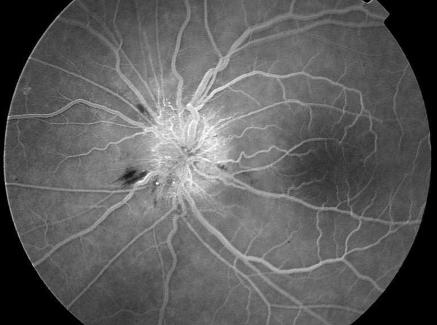
Patient 2, a woman aged 62, had blurred vision 40 days after her third infliximab infusion for rheumatoid arthritis. She was also taking atenolol, enalapril or hydrochlorothiazide, salicylic acid, terfenadine, and rofecoxib. Retinoscopy showed slight swelling of the optic disc in the right eye and marked disc swelling with a splinter haemorrhage on the disc margin in the left eye. In both eyes the capillaries of the optic nerve head were dilated, and fundus fluorescein angiography showed subsequent profuse vascular leakage (figure). The right eye had a normal visual field (as shown by Goldmann perimetry), but the left eye had a central scotoma. After three days the patient's vision in the left eye was 20/80. The optic nerve head of the left eye slowly turned pale, while oedema increased in the right eye. Seven weeks after receiving the infliximab infusion, the patient was given the drug for a fourth time. Twelve days later she started reporting symptoms in her right eye. Vision in this eye was 20/40 a week later and the eye had a cecocentral scotoma. The patient was subsequently treated with methylprednisolone, but her vision failed to improve.
Patient 3, a man aged 54, noticed a loss in the visual field of his right eye two weeks after he was given the last of three doses of intravenous infliximab for rheumatoid arthritis. He was also taking prednisone, diclofenac, and omeprazole. Fundoscopy showed disc swelling in both eyes and fundus fluorescein angiography showed capillary dilation and vascular leakage in the optic nerve heads. Perimetry of the right eye showed a large cecocentral scotoma; the left eye was normal. Within a few days the patient's vision in the right eye deteriorated to 20/400 and two months later the optic nerve head turned pale. At that time the vision in the left eye decreased to 20/100 and the visual field showed a central defect.
All patients were diagnosed as having anterior optic neuropathy. The defects in the central and cecocentral visual fields indicate that they had the toxic form of anterior optic neuropathy. (Altitudinal visual field defects, absent in our cases, indicate the ischaemic form.) All patients were treated with steroids to exclude temporal arteritis, but their condition did not improve.
All three patients reported symptoms after they had been given the third dose of infliximab, which suggests that the effects of the drug may have increased with cumulative dose or with time. None of the patients had Crohn's disease, but this may simply be due to a lower prescription rate for this indication. Alternatively, rheumatoid arthritis might be considered to be a risk factor for the anterior optic neuropathy.
In rare cases infliximab is associated with exacerbation of clinical symptoms or radiographic evidence of demyelinating disease suggestive of multiple sclerosis or optic neuritis, for example.1–2 In addition, optic neuritis has occurred in rare cases of rheumatoid arthritis.3 The clinical picture of demyelinating optic neuritis differs considerably from the clinical picture in our cases. Moreover, magnetic resonance imaging was normal in all patients.
Various drugs have been linked with optic neuropathy, but these drugs were not used by our patients, with the exception of omeprazole, which was taken by the third patient. It is questionable whether omeprazole causes optic neuropathy, however, and our patient has used omeprazole for a long time without ocular problems.4
The marketing authorisation holder, Centocor, has informed us that from the introduction of infliximab on the market (autumn 1999) until February 2002, about 5170 patients have been treated in the Netherlands with infliximab, including patients with rheumatoid arthritis and Crohn's disease.
Figure.
Fundus fluorescein angiography for patient 2. Capillaries on the optic nerve head are dilated and show vascular leakage. A splinter haemorrhage is present on the disc margin
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1. European Medicine Evaluation Agency. Summary of product characteristics of Remicade. www.eudra.org/humandocs/humans/epar/remicade/remicade.htm (accessed 5 Feb 2003).
- 2.Foroozan R, Buono LM, Sergott RC, Savino PJ. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol. 2002;120:985–987. [PubMed] [Google Scholar]
- 3.Agildere AM, Tutar NU, Yucel E, Coskun M, Benli S, Aydin P. Pachymeningitis and optic neuritis in rheumatoid arthritis: MRI findings. Br J Radiol. 1999;72:404–407. doi: 10.1259/bjr.72.856.10474506. [DOI] [PubMed] [Google Scholar]
- 4.Sachs G. Omepraxole and ocular damage. Lack of causality holds true. BMJ. 1998;316:67–68. [PMC free article] [PubMed] [Google Scholar]