Abstract
Cell cycle withdrawal associated with terminal differentiation is responsible for the incapability of many organs to regenerate after injury. Here, we employed a cell-free system to analyze the molecular mechanisms underlying cell cycle arrest in cardiomyocytes. In this assay, incubation of S phase nuclei mixed with cytoplasmic extract of S phase cells and adult primary cardiomyocytes results in a dramatic reduction of proliferating cell nuclear antigen (PCNA) protein levels. This effect was blocked by the proteasome inhibitors MG132 and lactacystin, whereas actinomycin D and cycloheximide had no effect. Immunodepletion and addback experiments revealed that the effect of cardiomyocyte extract on PCNA protein levels is maintained by p21 but not p27. In serum-stimulated cardiomyocytes PCNA expression was reconstituted, whereas the protein level of p21 but not that of p27 was reduced. Cytoplasmic extract of serum-stimulated cardiomyocytes did not influence the PCNA protein level in S phase nuclei. Moreover, the hypertrophic effect of serum stimulation was blocked by ectopic expression of p21 and the PCNA protein level was found to be upregulated in adult cardiomyocytes derived from p21 knockout mice. Our data provide evidence that p21 regulates the PCNA protein level in adult cardiomyocytes, which has implications for cardiomyocyte growth control.
The idea of eliciting tissue repair by regenerative growth has incited concerted effort within the past decade aimed at the identification of mechanisms which maintain cell cycle arrest in terminally differentiated cells. Progression of eukaryotic cells through the cell cycle is controlled by the specific activation of a series of cyclin-dependent kinases (cdk’s) (38). cdk’s are known to phosphorylate tumor suppressor pocket proteins (Rb, p107, and p130), resulting in a release of E2F transcription factors and thus enabling the transcription of E2F-dependent genes required for S phase entry (15). One mechanism that downregulates the activity of cdk’s leading to cell cycle arrest involves the binding of inhibitory proteins. Cyclin/cdk complexes are regulated by two families of cdk inhibitors (cki’s), the INK4 family and the CIP/KIP family, including p21 and p27. INK4 proteins and CIP/KIP proteins are structurally distinct and interact with cyclins and cdk’s in different ways. Whereas members of the INK4 family specifically inhibit cdk4 and cdk6, members of the CIP/KIP family are general inhibitors of cdk’s involved in the G1/S transition and S phase (36, 49). Furthermore, p21 and p27 are involved in the differentiation of intestinal epithelial cells, keratinocytes, PC12 cells, glioma cells, and skeletal and cardiac muscle cells (53). The biochemical activities of cki’s and their ability to promote differentiation implicate these proteins as mediators of cell cycle exit and differentiation. However, it is unclear why p21 and p27 are highly expressed in most differentiated cells, while cdk’s as their main targets are downregulated.
In a previous study we established a new myocardial cell-free system which is applicable to a variety of biochemical analyses aimed at the molecular dissection of cell cycle control in differentiated cardiomyocytes (17). During the neonatal period mammalian cardiomyocytes lose their ability to proliferate and exit the cell cycle in vivo (34, 45). It has been shown that the activity of cardiomyocyte DNA polymerase alpha (29) and the expression of proliferating cell nuclear antigen (PCNA) (33), cyclin A (62), and cyclin D1 (52) as well as the kinase activity and expression of cdk2 and cdk4 (7, 22) decrease during the first postnatal weeks. However, despite all these data the molecular mechanism underlying the cell cycle arrest in adult cardiomyocytes is still unclear.
Using a cell-free system we previously demonstrated that in principle adult cardiomyocyte nuclei are able to undergo DNA synthesis, if incubated with nuclear and cytoplasmic extracts derived from S phase cells (17). Moreover, there is cumulating evidence mainly from in situ studies indicating that adult human heart exhibits signs of increased DNA synthesis under certain pathophysiological conditions (5, 21). Also, viral protein simian virus 40 (SV40) large T antigen can act alone to induce proliferation in cardiomyocytes (47). It would seem, therefore, that differentiated cardiac muscle cells may be fully equipped to reenter the cell cycle. These observations raise the question what kind of mechanisms prevent differentiated adult cardiomyocytes from reentering S phase.
Transgenic mice that overexpress cyclin D1 under the control of the alpha-major histocompatibility complex promoter exhibit a 40% increase in heart weight and a twofold increase in cardiomyocyte number at 14 days. Enhanced DNA synthesis and multinucleation were described in adult cardiomyocytes of these mice. However, the absolute magnitude of DNA synthesis was small and no cell division was observed consistent with the need of additional cell cycle activators or the presence of cell cycle inhibitors (52). An important role of Rb in maintaining cell cycle arrest in adult cardiomyocytes has been suggested by overexpression studies of E2F transcription factors and viral proteins which inactivate pocket proteins like Rb. For example, reinduction of DNA synthesis in cultured primary cardiomyocytes could be achieved by overexpression of E1A or E2F1 in the presence of antiapoptotic survival factors (24, 25, 30, 58).
The loss of cardiomyocyte proliferative capacity coincides with a significant increase in protein levels and kinase inhibitory activity of the cki’s p21 and p27, which are abundantly expressed in adult cardiomyocytes (39), whereas cell cycle-perpetuating factors are downregulated. However, the precise role of these cki’s for cell cycle control in cardiomyocytes has not been addressed so far. This may be due to the lack of an overt cardiac phenotype in mice deficient for p21 (13) or p27 (40), although a prolonged duration of cardiomyocyte division could be demonstrated in p27-null mice by a recent study (40).
Collectively, all of these observations led us to hypothesize that in terminally differentiated cardiomyocytes the cell cycle is actively repressed. Here, we report that adult cardiomyocyte cytoplasmic extract negatively influences the PCNA protein level in H9c2 S phase nuclei in a cell-free system. The effect of this extract depends on endogenous p21 and is sensitive to proteasome inhibitors. In addition, we show that p21 is involved in the regulation of PCNA protein expression in vivo as evidenced by serum-stimulated isolated adult cardiomyocytes and cardiomyocytes derived from p21 knockout mice.
MATERIALS AND METHODS
Animals.
p21 knockout mice were kindly provided by Tyler Jacks (Massachusetts Institute of Technology, Cambridge) and were handled in accordance with National Institutes of Health institutional guidelines. The animals were backcrossed for eight generations to C57BL/6 mice. Thirteen-week-old male mice were anesthetized and sacrificed by CO2, and the hearts were excised and used as described below.
Cell culture and synchronization.
Animals were obtained from Moellegard, Schoenwalde, Germany, and their care and use were in accordance with approved animal care guidelines of the Max-Delbrück-Center, Berlin, Germany. Ventricular cardiomyocytes from 2-day-old Wistar rats or from 12- to 14-week-old male Wistar rats were isolated as described (17). Briefly, neonatal hearts were dissected, minced, and trypsinized. For selective enrichment of nonmyocytes (mainly primary cardiac fibroblasts), cells were preplated for 1.5 h. Adult hearts were rapidly excised and placed on a glass cannula for retrograde perfusion through the aorta. Perfusion was performed for 5 min with 1.7 mM CaCl2-Krebs Henseleit Solution (KHS) (KHS is 127 mM NaCl, 4.6 mM KCl, 24.8 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, 8.3 mM glucose, 2 mM Na-pyruvate, 10 mM creatine, 20 mM taurine), for 5 min with 0.5% fatty acid-free bovine serum albumin (FAFBSA; Sigma)-KHS and 30 min with 0.04% collagenase (selected type II; Worthington Biochemical Corp.)-0.23% FAFBSA-KHS. After mechanical dissection of the left ventricle cells were washed in 0.5% FAFBSA-KHS and used for extract preparation or were plated on laminin (Roche Molecular Biochemicals)-coated culture dishes. H9c2 cells (American Type Culture Collection, Manassas, Va., TCC CRL 1446) and nonmyocytes were synchronized in S phase using a single thymidine (2.5 mM; Sigma) block in culture medium for 18 h, followed by a release into medium lacking thymidine for 4 h as described (17).
Mammalian myocardial cell-free system.
The cell-free system used in our study is based on previously described methods using proliferating (26) or nondividing (17) mammalian cells. Briefly, cells were washed in 0.01 M HEPES (pH 7.4)-1.5 mM MgCl2 and swelled for 10 min on ice. Pellets were disrupted with a Dounce homogenizer and centrifuged, and cytoplasmic extracts were stored in liquid nitrogen after adding protease and phosphatase inhibitors. For preparation of nuclear extracts, pelleted nuclei were resuspended in phosphate-buffered saline (PBS), sonicated (Bachhofer Sonoplus HD70, MS 72/D), centrifuged, and mixed with inhibitors. Extracts contained 3 to 8 μg of protein per μl (determined by bicinchoninic acid [BCA] protein assay; Pierce). S phase nuclei were isolated using ethylhexadecyl-dimethylammonium bromide (Fluka) and immobilized on poly-l-lysine (Sigma)-coated coverslips. Nuclei were then washed with ice-cold PBS and subsequently covered with extracts, recombinant proteins (as indicated), and 20 μl of a buffered nucleotide mix (40 mM K-HEPES [pH 7.8], 7 mM MgCl2, 3 mM ATP, a 0.1 mM concentration [each] of GTP, CTP, and UTP; a 0.1 mM concentration [each[ of dATP, dGTP, dCTP, and dTTP; 0.5 mM dithiothreitol [DTT]; 40 mM creatine phosphate, and phosphocreatine kinase [0.5 μg/μl]; all from Roche). The reaction volume was kept at 60 μl by adding hypotonic buffer (10 mM HEPES [pH 7.4] and 1.5 mM MgCl2). For biotin-16-dUTP incorporation 7.5 μM biotin-16-dUTP was added after 30 min of incubation instead of dTTP.
Indirect immunofluorescence staining.
Staining procedures were performed as previously described (17). Briefly, samples were fixed in methanol (−20°C, 5 min, for PCNA detection) or formaldehyde (3.7% [vol/vol], 10 min) and permeabilized (0.5% Triton X-100-PBS, 15 min). Slides were blocked and incubated with primary antibodies (mouse monoclonal antibody [MAb] to PCNA [PC10], diluted 1:40; Santa Cruz), rabbit polyclonal antibody (PAb) to p21 (H-164) or p27 (C-19) (diluted 1:50; all from Santa Cruz). Immune complexes were detected with fluorescein isothiocyanate (FITC)-conjugated antibody to mouse or rabbit immunoglobulins G (IgGs) (diluted 1:50; Dianova). Cardiomyocytes were costained with antiactin PAb (diluted 1:200; Sigma), antitropomyosin (1:200; Sigma) or antimyomesin MAb (1:5) (19). Immune complexes were detected with tetramethyl rhodamine isothiocyanate-conjugated antibody to mouse or rabbit IgGs (diluted 1:50; Dianova). Biotin-16-dUTP was visualized with FITC-conjugated streptavidin (diluted 1:100; Dianova) and DNA with 4′,6′-diamidino-2-phenylindole (DAPI) (0.5 μg/ml; Sigma). For the quantitative analyses 200 nuclei were counted in three random fields from 3 independent experiments (n = 3). Statistical significance was determined using the unpaired t test (StatView software).
Hearts obtained from p21 knockout mice were fixed in methanol (−20°C) at 4°C overnight, incubated in 30% sucrose solution (4°C, overnight), embedded in tissue freezing medium (Fisher Scientific), stored at least 24 h at −80°C and sectioned (7 μm thick) using a cryostat (Leica 3050S). Sections were stained as described above with minor modifications. Heart sections were blocked using donkey serum and stained with anti-PCNA rabbit PAb (FL-261) (diluted 1:100; Santa Cruz) and anti-GATA-4 goat PAb (C-20, diluted 1:50; Santa Cruz). Immune complexes were detected with Alexa Fluor 594 donkey anti-goat IgGs and Alexa Fluor 488 donkey anti-rabbit IgGs (diluted 1:200; Molecular Probes).
Immunodepletion.
Twenty microliters of blocked protein A-agarose (Roche Molecular Biochemicals) was incubated for 1.5 h at 4°C with 100 μl of cytoplasmic extract (350 μg of total protein) and 0.5 μg of PAb (p21 [H-164] and p27 [C-19]). Supernatant was transferred to freshly blocked beads, and antibodies were added and incubated for 1.5 h. To remove all antibodies from immunodepleted extracts, supernatant was incubated for 1.5 h with fresh beads, snap-frozen in liquid nitrogen, and stored at −80°C.
Immunoblotting.
Extracts containing 25 μg of protein were resolved by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE), blotted to polyvinylidene difluoride membranes (NEN Life Science Products), blocked, and incubated with PAb (p21 [C-19] and p27 [C-19]) (0.1 to 0.2 μg/ml; Santa Cruz). Antigen-antibody complexes were visualized using horseradish peroxidase-conjugated secondary antibody and the ECL system (Amersham).
For immunoblotting of whole heart protein extracts, hearts were homogenized on ice with a polytron homogenizer (PCU-11; kinematica AG) in a lysis buffer (20 mM HEPES [pH 7.2], 25 mM NaCl, 2 mM EGTA, 0.2 mM DTT, aprotinin [60 μg/ml], leupeptin [2 μg/ml], 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 1 mM Na3VO4, 25 mM β-glycerophosphate, and 0.1% Triton X-100). After incubation (4°C, 30 min), the homogenates were sonicated (Sonifier 250; Branson Ultrasonics Co.) on ice for 1 min and then centrifuged at 10,000 × g (4°C, 30 min) (20). The supernatants were stored at −80°C until use. Extracts containing 80 μg of protein were resolved by SDS-10% PAGE and visualized as described using anti-PCNA MAb (PC10) (diluted 1:1,000; Santa Cruz) and antiactin rabbit PAb (A-2066) (diluted 1:1,000; Sigma).
Protein expression and purification.
To overexpress glutathione-S-transferase (GST)-p27, human full-length p27 cDNA (Mitotix) was PCR cloned as a BamHI fragment in pGEX-1λT (Pharmacia). Insert orientation was verified by sequencing using the big dye terminator cycle sequencing kit (Abiprism; Perkin-Elmer). The plasmid for GST-p21 expression was kindly provided by Julian Blow (University of Dundee). Bacterial expression of GST, GST-p21, and GST-p27 was performed in Escherichia coli strain BL21 (Pharmacia) with 200 μM isopropyl-1 thio-β-d-galactopyranoside (3 h at 25°C, A600 = 0.6; Sigma). The bacterial pellet was lysed in 20 ml ice-cold NETN buffer (100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.6 μM aprotinin, 4 μM leupeptin, 2 mM Pefabloc SC, pepstatin [1 μg/ml], 20 mM Tris [pH 8.0]) containing lysozyme (1 mg/ml), sonicated (Bachhofer Sonoplus HD70, MS 72/D, cycle 70), and centrifuged (100,000 × g, 30 min, 4°C). GST fusion proteins were purified using glutathione-Sepharose beads (Pharmacia) blocked in 0.5% low-fat dried milk-NETN. After 40 min of incubation at 4°C on a wheeler, beads were washed six times with NETN, and bound GST or GST fusion proteins were eluted in 10 mM glutathione-50 mM Tris (pH 8.0).
The plasmid pET23a-CHisPCNA (wild type) for his-PCNA expression was kindly provided by Zophonias Jonsson (Brigham and Women's Hospital, Harvard Medical School). Bacterial expression of his-PCNA was performed in E. coli strain BL21(DE3)pLysS with 200 μM isopropyl-1 thio-β-d-galactopyranoside (3 h at 25°C; A600 = 0.6) (Novagen). The bacterial pellet was stored overnight at −80°C. Pellets were then thawed at 37°C for 7 min and resuspended in 50 mM NaH2PO4-300 mM NaCl-20 mM imidazole-2 mM Pefabloc (pH 8.0) and briefly sonicated. After centrifugation (15,000 × g, 15 min, 4°C) his-PCNA was purified from supernatant using an Ni-nitrilotriacetic acid (NTA) spin kit and columns (Qiagen).
Purified proteins were concentrated with a microconcentrator (centricon [Amicon]; nanosep 10 or 30 [Pall Gelman Laboratories]), diluted in hypotonic buffer to a concentration of 1 μg/μl (BCA protein assay; Pierce) and stored at −80°C and/or 4°C (his-PCNA). Purity was analyzed by SDS-PAGE.
Kinase assays.
Cells were lysed in RIPA+ buffer (300 mM NaCl, 0.1% SDS, 1% NP-40, 1% Na-deoxycholic acid, 2 mM EDTA, 1 mM DTT, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 4 μM leupeptin, 1.2 μM aprotinin, 10 mM NaH2PO4 [pH 7.0]) and incubated on ice for 15 min. Cell suspensions were sonicated (cycle 70; Bachhofer Sonoplus HD70, model MS 72/D) and centrifuged (13,000 × g, 15 min, 4°C), and the supernatant was snap-frozen in liquid nitrogen. cdk2, cyclin A and cyclin E were immunoprecipitated from these cell lysates (250 μg of adult cardiomyocytes or 100 μg of H9c2 cells) with anti-cdk2 (M2), anti-cyclin A (C-19) or anti-cyclin E (M-20) PAb (all from Santa Cruz). Twenty microliters of protein A-agarose beads (Roche Molecular Biochemicals) was washed, blocked (in 1% BSA-PBS at room temperature) and incubated with extract and 0.5 μg of PAb for 1.5 h at 4°C. After washing with kinase buffer (50 mM KCl, 8 mM MgCl2, 1 mM DTT, 3 mM ATP, 50 mM HEPES, pH 7.4) immune complexes were resuspended in 50 μl of kinase buffer containing histone H1 (0.2 μg/μl; Sigma). When recombinant proteins were used assays were incubated for 30 min on ice. [γ-32P]ATP (10 μCi; NEN) was added, and after shaking for 1 h at 37°C, the reaction was stopped by addition of 25 μl of SDS sample buffer. Samples were analyzed by SDS-PAGE and quantitated using a phosphorimager (Fuji) and the Tina software program.
Dedifferentiation model of adult cardiomyocytes.
Adult rat ventricular cardiomyocytes were cultured for 3 days in Dulbecco's modified Eagle medium F12 Nutrient Mix (DMEM/F12; GIBCO) containing 20% heat-inactivated fetal calf serum, 2% BSA, insulin (1 μg/ml), transferrin (1 μg/ml), sodium selenite (1 ng/ml) (all from Sigma), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM l-glutamine (all from GIBCO). Medium was changed every 24 h. To prevent proliferation of nonmyocytes, cells were cultured for the first 48 h in the presence of 10 μM cytosine β-d-arabinofuranoside (Sigma). For extract preparation cells were harvested using a cell scraper and processed as described.
Recombinant adenoviral constructs and infection.
The adenoviral constructs Ad-p21 and Ad-β-Gal were kindly provided by Carsten Brand (Max-Delbrück-Center). The recombinant Ad-p21 contained a cytomegalovirus promoter driving the human p21 full-length cDNA. The adenoviral construct Ad-p27 was kindly provided by P. D. Nisen (Abbott). HEK293 cells (American Type Culture Collection, Manassas, Va.) were used for homologous recombination and packaging. The virus titer was determined through direct immunofluorescence staining for adenovirus hexon protein (Imagen Adenovirus; DAKO). Cardiomyocyte cultures were infected with Ad-p21, Ad-β-Gal, or Ad-p27 at 200 PFU/cell overnight in medium 199 plus Earle's salt (Biochrom) containing 0.2% BSA, 5 mM creatine, 2 mM l-carnitine, 5 mM taurine (all from Sigma), insulin (15 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from GIBCO) before serum stimulation. Infection efficiency of ventricular cardiomyocyte cultures was >90% for each adenoviral construct as determined by indirect immunofluorescence staining.
RESULTS
Adult cardiomyocyte cytoplasmic extract downregulates PCNA protein levels.
PCNA, the auxiliary protein of DNA polymerase delta and epsilon, is involved in DNA replication and repair. This protein forms a homotrimeric structure which, encircling DNA, loads the polymerase onto the DNA template and is thus essential for cell cycle progression through S phase. A role for PCNA as a component of the cell cycle control apparatus is further recognized on the basis of the interaction with various enzymes and regulatory proteins like cyclins, cdk’s, the cki p21, and the growth arrest- and DNA damage-inducible proteins gadd45 and MyD118. PCNA is further involved in cell differentiation, and its expression is necessary for the transition of cells from quiescence to S phase (9, 41, 42, 60). In cardiomyocytes it has been shown that PCNA mRNA is expressed during all developmental stages. However, PCNA protein was found only in embryonic and neonatal but not adult cardiomyocytes (33). In this context we wondered whether adult cardiomyocyte cytoplasmic extract exhibits a negative effect on the PCNA protein level in S phase nuclei.
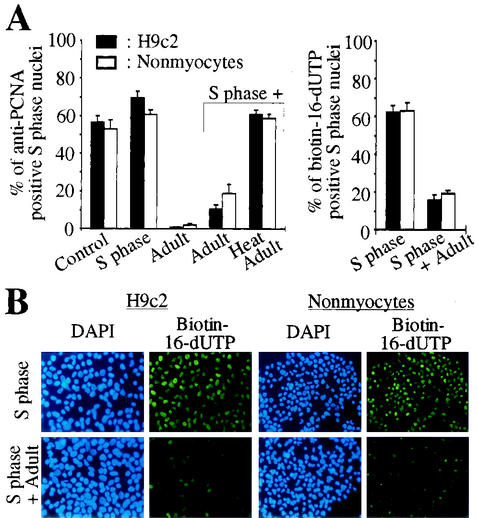
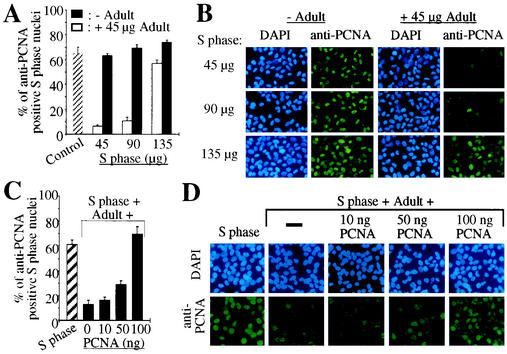
H9c2 cells, a muscle cell line derived from embryonic rat heart tissue, or primary cardiac nonmyocytes (mainly fibroblasts) were synchronized by a single thymidine block in S phase as previously described (17). Immobilized S phase nuclei were incubated with their own cytoplasmic extract and/or cytoplasmic extract of adult cardiomyocytes, and PCNA expression in the immobilized nuclei was monitored by indirect immunostaining. As a positive control S phase nuclei were analyzed before (>53% anti-PCNA positive nuclei, Fig. 1A) and after incubation with cytoplasmic extract of S phase cells demonstrating a stable PCNA protein level (>61% anti-PCNA positive nuclei, Fig. 1A). In contrast, incubation with adult cardiomyocyte cytoplasmic extract, even in the presence of S phase cytoplasmic extract, significantly reduced the percentage of anti-PCNA positive nuclei (<19%, Fig. 1A). Heat inactivation of the adult cytoplasmic extract abolished this inhibitory effect completely (>61% anti-PCNA positive nuclei, Fig. 1A). Moreover, the inhibitory effect of adult cardiomyocyte cytoplasmic extract on the PCNA protein level in S phase nuclei was reversible as shown by titration of adult cardiomyocyte cytoplasmic extract with S phase cytoplasmic extract (Fig. 2A and B). While the inhibitory effect of the adult cardiomyocyte extract was unaffected even in the presence of a twofold excess of S phase extract (<11% anti-PCNA positive nuclei versus >63% in the control), it was almost completely abolished when S phase extract was present at a threefold excess (57% anti-PCNA positive nuclei). The inhibitory effect of adult cytoplasmic extract was also abolished by titration with recombinant PCNA (Fig. 2C and D). While the inhibitory effect of adult cardiomyocyte extract (60 μg of total protein) was unaffected in the presence of S phase extract (45 μg of total protein) plus 10 ng of recombinant PCNA (12.5% ± 3.7% anti-PCNA positive nuclei versus 16.5% ± 2.5%), it was completely abolished when 100 ng of recombinant PCNA was added (69.7% ± 5.7% anti-PCNA positive nuclei versus 61.5% ± 3.4% in the control).
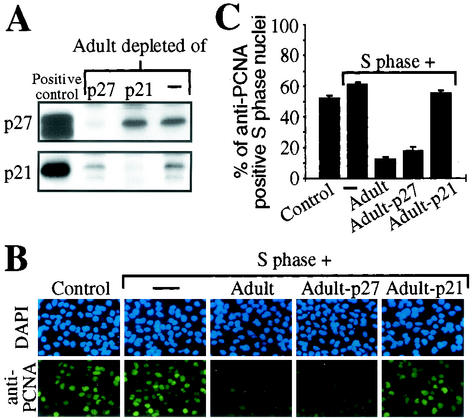
FIG. 1.
Adult cardiomyocyte cytoplasmic extract negatively influences the PCNA protein level in S phase nuclei. (A and B) Immobilized nuclei of H9c2 cells or primary cardiac nonmyocytes synchronized in S phase were incubated with cytoplasmic extracts (60 μg of total protein) from adult cardiomyocytes (Adult) and/or S phase cells (S phase) before or after heat inactivation (Heat) (3 min at 100°C) as indicated. Before (control) and after a 2-h incubation, nuclei were stained with monoclonal anti-PCNA antibody (PC10) or with FITC-coupled streptavidin against biotin-16-dUTP. Nuclear DNA was stained with DAPI, and experiments were quantitatively analyzed thereafter. Data are means + standard errors of the means (error bars) of three independent experiments.
FIG. 2.
The inhibitory effect of adult cardiomyocyte cytoplasmic extract is reversible. (A and B) Immobilized S phase nuclei were incubated with increasing amounts (total protein in μg) of S phase H9c2 cytoplasmic extracts (S phase) in the absence or presence of adult cardiomyocyte cytoplasmic extract (Adult) as indicated. (C and D) S phase nuclei were incubated with adult cardiomyocyte cytoplasmic extracts (60 μg of total protein) and S phase cytoplasmic extract (45 μg of total protein) in the presence or absence of increasing amounts of recombinant PCNA (10, 50, and 100 ng) as indicated. (A and C) Quantitative analysis (n = 3). Data are means + standard errors of the means (error bars). (B and D) Representative examples of the anti-PCNA immunofluorescence analysis, which was performed as described for Fig. 1.
Since PCNA is essential for DNA synthesis (41) the lack of PCNA staining might indicate the inhibition of DNA synthesis. Therefore, we repeated the experiments and monitored the incorporation of biotin-16-dUTP as a marker of newly synthesized DNA. Most S phase nuclei incubated with cytoplasmic extract of S phase cells incorporated biotin-16-dUTP during incubation (>62% biotin-16-dUTP positive nuclei, Fig. 1A and B), whereas the incorporation of biotin-16-dUTP was greatly inhibited in the presence of adult cytoplasmic extract (<19%) (Fig. 1).
Taken together, these data indicate that adult cardiomyocyte cytoplasmic extract exerts a negative effect on DNA synthesis and PCNA protein levels.
Downregulation of PCNA protein levels occurs in a proteasome-dependent manner.
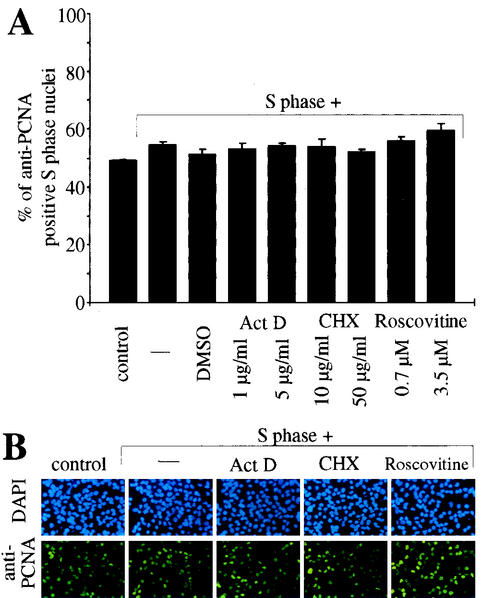
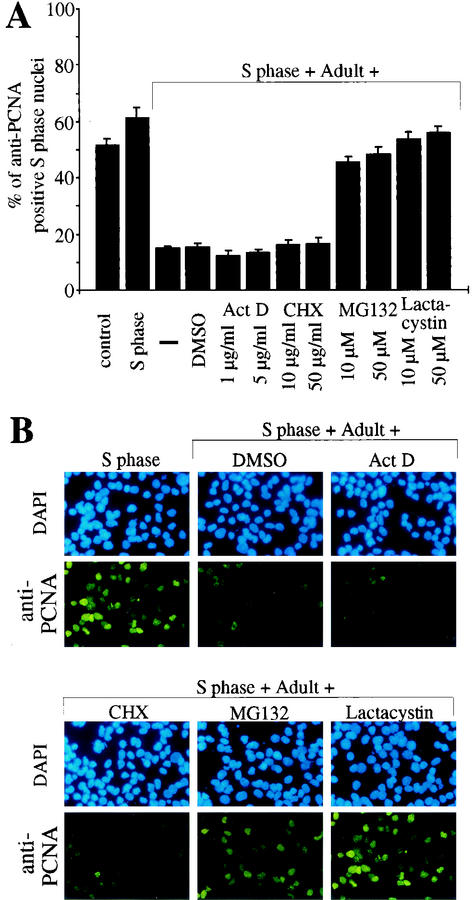
Previously, both transcriptional as well as posttranscriptional components in the regulation of PCNA mRNA levels after serum stimulation have been reported (1, 37, 46). Therefore, we next incubated S phase nuclei with S phase cytoplasmic extract in the presence of the transcriptional inhibitor actinomycin D or the translational inhibitor cycloheximide. None of these inhibitors had a significant effect on the PCNA protein level in S phase nuclei (Fig. 3). Also, inhibition of cdk2 activity with roscovitine had no effect on PCNA protein levels (Fig. 3). Therefore, we conclude that the inhibitory effect of adult cardiomyocyte cytoplasmic extract on PCNA expression involves processes other than transcription or translation of PCNA. Moreover, this effect seems not to be based on the inhibition of cdk2, which plays an important role during the G1 and S phases (16). This observation was corroborated by results obtained, when S phase nuclei were incubated with S phase cytoplasmic extract and inhibitors in the presence of adult cardiomyocyte cytoplasmic extract. Neither actinomycin D nor cycloheximide could influence the inhibitory effect of adult cardiomyocyte extract (Fig. 4). In contrast, addition of the proteasome inhibitors MG132 and lactacystin efficiently blocked the inhibitory effect on the PCNA protein level in S phase nuclei (>50% anti-PCNA positive nuclei versus <19%). These data indicate that adult cytoplasmic extract contains all proteins necessary to downregulate PCNA protein levels and that this effect could be mediated by a proteasome-dependent degradation of PCNA.
FIG. 3.
The effect of adult cardiomyocyte cytoplasmic extract on PCNA protein levels cannot be mimicked by transcriptional, translational, or cdk2 inhibitors. Immobilized S phase nuclei were incubated with S phase H9c2 cytoplasmic extracts (S phase) in the absence or presence of the transcriptional inhibitor actinomycin D (Act D), the translational inhibitor cycloheximide (CHX), or the cdk2 inhibitor roscovitine. (A) Quantitative analysis (n = 3). Data are means + standard errors of the means (error bars). (B) Representative examples of the anti-PCNA immunofluorescence analysis, which was performed as described for Fig. 1.
FIG. 4.
Proteasome inhibitors, but not inhibition of transcription or translation, can abolish the effect of adult cardiomyocyte cytoplasmic extract on PCNA protein levels. Immobilized S phase nuclei were incubated with cytoplasmic extracts from S phase H9c2 cells (S phase) and/or from adult cardiomyocytes in the absence or presence of the transcriptional inhibitor actinomycin D (Act D), the translational inhibitor cycloheximide (CHX) or proteasome inhibitors MG 132 and lactacystin as indicated. (A) Quantitative analysis (n = 3). Data are means + standard errors of the means (error bars). (B) Representative examples of the anti-PCNA immunofluorescence analysis, which was performed as described for Fig. 1.
The activity of adult cardiomyocyte cytoplasmic extract depends on p21.
Several observations indicate a role of the cki’s p21 and p27 in inducing and maintaining cell cycle arrest in differentiated cells. For instances, in cardiomyocytes forced expression of E2F1 (58) as well as stimulation of hypertrophic growth (8, 27, 43, 54) leads to an increase in DNA synthesis associated with a specific downregulation of p21 and p27, which is accompanied by elevated protein levels and kinase activities of cyclin/cdk complexes and reexpression of PCNA. In this context we hypothesized that p21 and p27 may exert effects other than the control of cdk’s which regulate cell cycle progression.
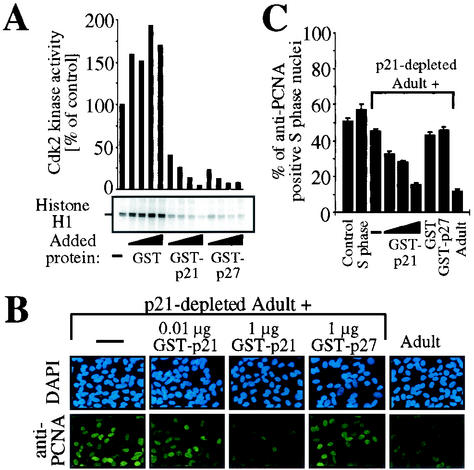
Therefore, we tested whether immunoprecipitation of p21 or p27 from adult cardiomyocyte cytoplasmic extracts influences the observed inhibitory effect on PCNA protein levels. Immunodepletion was confirmed by Western blot analysis (Fig. 5A). S phase H9c2 nuclei were incubated with p21- or p27-depleted adult cardiomyocyte cytoplasmic extract together with S phase H9c2 cytoplasmic extract. Incubation with untreated or p27-depleted adult cardiomyocyte cytoplasmic extract resulted in a decrease from 52% (control) to less than 18% anti-PCNA positive nuclei. In contrast, immunodepletion of p21 completely abolished the inhibitory effect of adult cardiomyocyte cytoplasmic extract (55% anti-PCNA positive nuclei) (Fig. 5B and C). Importantly, binding of p21 to PCNA does not interfere with detection of PCNA by the anti-PCNA antibody clone PC10 used in this study as previously demonstrated (9, 61). Our data suggest that the inhibitory effect of adult cardiomyocytes on the PCNA protein level in S phase nuclei may depend on p21. Since we cannot exclude the possibility that anti-p21 immune complexes contain other proteins responsible for the observed inhibitory effect, we next evaluated the inhibitory effect of p21-depleted adult cardiomyocyte extracts supplemented with recombinant p21. S phase H9c2 nuclei were incubated with S phase H9c2 cytoplasmic extract mixed with p21-depleted adult cardiomyocyte extract in the presence or absence of recombinant proteins. The biological activity of recombinant proteins was confirmed by assessment of their inhibitory effect on the activity of cdk2 immune complexes of S phase H9c2 cell extracts using a histone H1 kinase in vitro assay (Fig. 6 A). Incubation of p21-depleted adult extract together with S phase cytoplasmic extract caused only a weak decrease in the number of anti-PCNA positive nuclei (45%) compared to controls (>51%, Fig. 6B and C). In contrast, addition of recombinant p21 to this assay led to a dose-dependent decrease in anti-PCNA positive nuclei, which could not be observed with equal amounts of GST or recombinant p27 (Fig. 6B and C).
FIG. 5.
Immunodepletion of p21 but not of p27 abolishes the effect of adult cardiomyocyte cytoplasmic extract on PCNA protein levels. (A) Immunodepletion of adult cardiomyocyte cytoplasmic extract was confirmed by immunoblot analysis. p21 and p27 were immunoprecipitated with specific antibodies for p21 (H 164) and p27 (C-19). Extracts were then resolved by SDS-12% PAGE (25 μg of total protein) and immunoblotted with anti-p21 (C-19) and anti-p27 (C-19) antibodies. As positive control, total cellular extracts of Ad-p21- or Ad-p27-infected neonatal cardiomyocytes were used (58). (B) S phase nuclei were incubated with untreated or depleted extracts and/or S phase cytoplasmic extract (60 μg of total protein) as indicated. Representative examples of the anti-PCNA immunofluorescence analysis, which was performed as described for Fig. 1, are shown. (C) Quantitative analysis (n = 3). Data are means + standard errors of the means (error bars).
FIG. 6.
Recombinant p21 is sufficient to restore the inhibitory effect of p21-depleted adult extract. (A) Biological activity of recombinant proteins. Cdk2 was immunoprecipitated from S phase H9c2 cells and kinase assays were performed in the absence (−) or presence of increasing amounts (0.02, 0.04, 0.2, and 2 μg) of GST, GST-p21, or GST-p27 using histone H1 as a substrate. Quantifications are shown above and are normalized for signal of Coomassie staining. (B) Immunofluorescence analysis. S phase nuclei were incubated with untreated or p21-depleted adult cardiomyocyte cytoplasmic extracts and/or S phase cytoplasmic extract (60 μg of total protein) in the presence or absence of increasing amounts (0.01, 0.1, and 1 μg) of GST-p21, 1 μg of GST, or 1 μg of GST-p27 as indicated. Representative examples of the anti-PCNA immunofluorescence analysis are shown, which were performed as described for Fig. 1. (C) Quantitative analysis (n = 3) of experiments described for panel B. Data are means + standard errors of the means (error bars).
In summary, these experiments demonstrate that the observed inhibitory effect of adult cardiomyocyte cytoplasmic extract on PCNA protein levels is based on p21 and not on p27.
Adult cardiomyocyte cytoplasmic extract contains factors acting synergistically with p21.
We tested further whether p21 alone exerts an inhibitory effect on PCNA protein expression which is comparable to that of adult cardiomyocyte cytoplasmic extract. S phase H9c2 nuclei were incubated with their own S phase cytoplasmic extract in the presence of recombinant p21 or p27 protein. However, the presence of GST or that of recombinant p27 did not result in a significant reduction in the number of anti-PCNA positive nuclei (>50.4%) compared to the controls (<63.6%). In contrast, addition of recombinant p21 showed a dose-dependent decrease in the number of anti-PCNA positive nuclei (Fig. 7B). Notably, several nuclei still exhibited a bright anti-PCNA staining after incubation with recombinant p21 and the decrease in the number of anti-PCNA positive nuclei was less pronounced (32%) (Fig. 7A and B) compared to cytoplasmic extract of adult cardiomyocytes (<19%) (Fig. 1A). Adding recombinant p21 to S phase extracts mimics the inhibitory effect of adult cardiomyocyte extract on PCNA expression, though to a lower extent, indicating that probably other factors act in synergy with p21 to regulate PCNA expression.
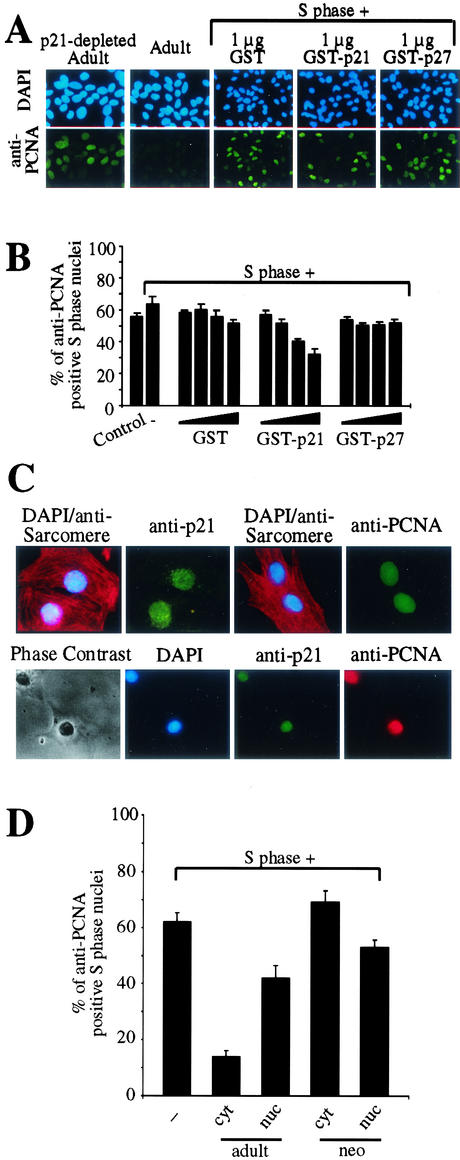
FIG. 7.
Adult cardiomyocyte cytoplasmic extract contains factors acting synergistically with p21 in the control of PCNA expression. (A) Immunofluorescence analysis. S phase nuclei were incubated with untreated or p21-depleted adult cardiomyocyte cytoplasmic extracts or S phase cytoplasmic extract (60 μg of total protein) in the presence or absence of increasing amounts of recombinant proteins (0.01, 0.02, 0.1, and 1 μg) as indicated. Representative examples of the anti-PCNA immunofluorescence analysis are shown, which were performed as described for Fig. 1. (B) Quantitative analysis (n = 3) of experiments described for panel A. Data are means + standard errors of the means(error bars). (C) Expression profiles of p21 and PCNA in neonatal cardiomyocytes. Expression was determined by immunocytological staining. Cardiomyocytes were costained with anti-p21 (H-164) and anti-PCNA (PC10) together with antisarcomere (antitropomyosin or antiactin) antibodies (red). Nuclear DNA was stained with DAPI (blue). (D) Immobilized nuclei of H9c2 cells synchronized in S phase were incubated with cytoplasmic (cyt) or nuclear (nuc) extracts from adult (adult) or neonatal (neo) cardiomyocytes as described in Fig. 1. Data are means + standard errors of the means (error bars) of three independent experiments.
Neonatal cardiomyocytes which still retain some proliferative capacity coexpress both p21 and PCNA in the nucleus (Fig. 7C), indicating that p21 localization and function are unique to adult cardiomyocytes. Notably, cytosolic but not nuclear extract from adult and neither nuclear nor cytosolic extract from neonatal cardiomyocytes were able to inhibit PCNA expression in our cell-free system (Fig. 7D). These data together with the data obtained using recombinant p21 suggest that p21 has to be localized to the cytoplasm to act in synergy with other factors resulting in PCNA degradation.
p21 blocks PCNA expression during serum stimulation of adult cardiomyocytes.
Cardiomyocytes display two distinct developmentally programmed forms of growth. In utero, ventricular mass is augmented by cardiac myocyte proliferation, hyperplasia, which ceases soon after birth. In postnatal life, ventricular cardiomyocytes exit the cell cycle and respond to an increased hemodynamic burden with an adaptive growth characterized by an enlargement in cell size known as hypertrophy (57). Interestingly, it has been reported that p21 and p27 expression is downregulated in cardiac hypertrophy in vivo (27), whereas PCNA expression is upregulated (43). However, it is unclear whether p21 and/or p27 downregulation is responsible for PCNA upregulation.
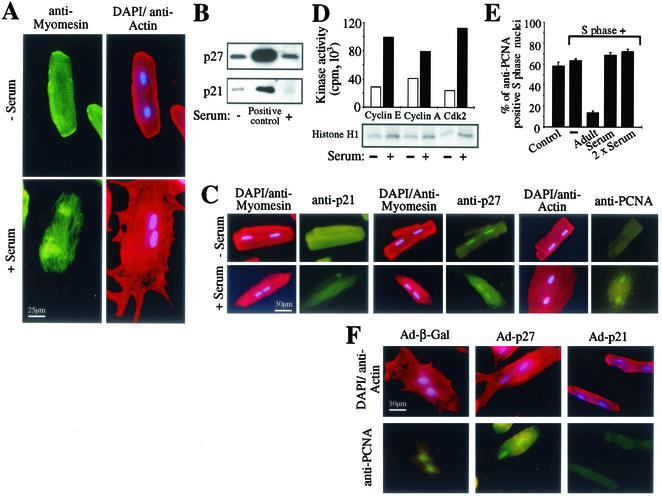
Serum stimulation is an established model to induce and to investigate hypertrophic growth of adult cardiomyocytes (65). Adult cardiomyocytes respond to serum stimulation with an increase in cell size and reorganization of myofilaments (Fig. 8A). The protein level of p21 was significantly reduced in adult cardiomyocytes during serum-induced hypertrophic growth, whereas the protein level of p27 was unchanged (Fig. 8B and C). Moreover, serum-stimulated cardiomyocytes expressed nuclear PCNA (67.3% ± 5.4%) (Fig. 8C), which was detergent soluble (data not shown). In proliferating cells, PCNA cycles between a chromatin-bound, detergent-insoluble state in S phase and a diffuse soluble state when DNA is not being synthesized (6). These data indicate that adult cardiomyocytes do not enter the S phase after serum stimulation despite an induction of cdk2 and cyclin A/E-associated histone H1 kinase activities (Fig. 8D). As an additional control for the role of p21 in our cell-free system we tested the effect of cytoplasmic extract from serum-stimulated cardiomyocytes. Cytoplasmic extract of serum-stimulated adult cardiomyocytes lacking p21 did not exhibit an inhibitory effect on PCNA protein level in our cell-free system (>69% anti-PCNA positive nuclei, Fig. 8E). Furthermore, concerning the morphological changes and PCNA expression, we could block the effect of serum on cardiomyocytes by viral overexpression of p21 but not of p27 or β-Gal (Fig. 8F). The localization pattern of ectopically expressed p21 or p27 was identical to that of the endogenous counterparts with p21 being localized predominantly to the cytosol and p27 to both nuclei and cytoplasm (data not shown).
FIG. 8.
p21 regulates PCNA expression in adult cardiomyocytes. (A) Adult cardiomyocytes were costained for actin (red) and myomesin (green) before and after serum stimulation. Nuclear DNA was stained with DAPI. (B) Immunoblot analysis. Cytoplasmic extract of untreated or serum-stimulated adult cardiomyocytes (25 μg of total protein) was resolved by SDS-12% PAGE and immunoblotted with antibodies specific for p21 (C-19) and p27 (C-19). As positive control, total cellular extracts of Ad-p21- or Ad-p27-infected neonatal cardiomyocytes were used (58). (C) Immunofluorescence analysis. Before and after serum stimulation cardiomyocytes were costained with anti-p21 (H-164), anti-p27 (C-19), and anti-PCNA (PC10) (all green) together with antimyomesin or antiactin antibodies (red). Nuclear DNA was stained with DAPI (blue). (D) Induction of kinase activities. Cell lysates (250 μg of total protein) of adult cardiomyocytes before (white bars) and after (black bars) serum stimulation were prepared, and cdk2, cyclin A, and cyclin E were immunoprecipitated with anti-cdk2 (M2), anti-cyclin A (C-19), and anti-cyclin E (M-20) antibodies. Kinase activities of the resulting immune complexes were measured using histone H1 as substrate. (E) Quantitative analysis. Immobilized nuclei of S phase H9c2 cells were incubated with cytoplasmic extracts (60 μg of total protein) from untreated or serum-stimulated adult cardiomyocytes (adult) or S phase cells (S phase) as indicated. Immunofluorescence analysis was performed as described for Fig. 1 and experiments were quantitatively analyzed thereafter (n = 3). Data are means + standard errors of the means (error bars). (F) Immunofluorescence analysis of PCNA after ectopic expression of p21 and p27 or β-Gal in cardiomyocytes and serum stimulation. Before serum stimulation cardiomyocytes were infected with Ad-p21, Ad-p27, or Ad-β-Gal. After serum stimulation cardiomyocytes were costained with anti-PCNA (PC10) (green) together with antiactin antibodies (red). Nuclear DNA was stained with DAPI (blue).
These data indicate that p21 is required for the maintenance of differentiation and is regulating the expression of PCNA. In contrast, p27, whose expression in adult cardiomyocytes is not affected by serum stimulation, might be involved in the control of later cell cycle stages and be responsible for the lack of further progression of these cells into S phase.
p21−/− adult cardiomyocytes possess elevated levels of PCNA.
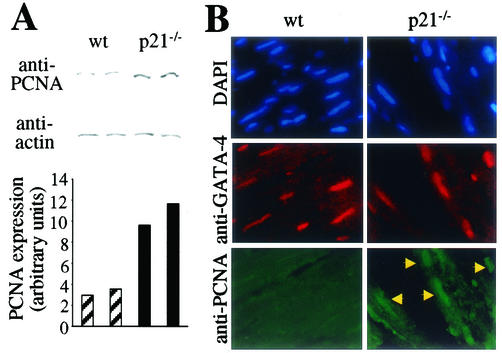
Our data obtained by using a cell-free system as well as primary cell culture indicate clearly that adult cardiomyocytes possess a p21-dependent activity that regulates the protein level of PCNA. These data implicate that adult cardiomyocytes of p21 knockout mice should have an increased PCNA protein level. As predicted by our in vitro data the protein level of PCNA is significantly increased (> threefold) in adult hearts of p21 knockout mice compared to that in hearts of wild-type control animals (Fig. 9A). In order to detect the origin of increased PCNA levels, we costained heart sections for PCNA and the transcription factor GATA-4, the latter of which is specific for striated muscle. In contrast to hearts from wild-type littermates we could detect PCNA in cardiomyocyte nuclei exclusively in sections derived from p21 knockout hearts (Fig. 9B).
FIG. 9.
p21 regulates PCNA expression in adult cardiomyocytes in vivo. (A) Immunoblot analysis. Whole extracts of wild-type and p21 knockout hearts (80 μg of total protein) were resolved by SDS-10% PAGE and immunoblotted with antibodies specific for PCNA (PC10) and actin (A-2066). Western blot results were quantitated by Quantity One (Bio-Rad, Hercules, Calif.), and the signal for PCNA was normalized by actin staining. (B) Immunofluorescence analysis. Tissue sections of wild-type or p21 knockout hearts were costained with anti-PCNA rabbit PAb (FL-261, green) and anti-GATA-4 goat PAb (C-20, red, cardiac specific). Nuclear DNA was stained with DAPI (blue). Arrows mark examples for nuclei of cardiomyocytes that are anti-PCNA positive.
These data demonstrate that p21 participates in the regulation of PCNA protein levels in adult cardiomyocytes in vivo.
DISCUSSION
The usage of viral proteins like SV40 large T antigen or E1A as well as transgenic mice models suggested that pocket proteins might play an important role in maintaining the cell cycle arrest in terminally differentiated adult cardiomyocytes. However, upon cardiomyocyte differentiation, the expression of cdk’s dramatically decreases (7, 22), whereas the expression of cki’s increases (39). In this context, the function of p21 and p27 in differentiated cells may be other than that of controlling cdk activity. Recently, it has been shown that p21 is a positive regulator in differentiation of oligodendrocytes independent of cell cycle control (63) and that transient overexpression of p21 in U937 cells results in cell surface expression of monocytic markers (2). In contrast, forced expression of p21 inhibits the expression of terminal differentiation markers in keratinocytes (14). Reportedly, p21 binds to and inhibits stress-activated protein kinases (50), which may mechanistically be involved in the regulation of hypertrophic growth in cardiomyocytes (11, 50). In addition, interaction of p21 with gadd45 (23) or STAT3 (12) might participate in differentiation-regulating pathways and data are accumulating that cytoplasmic p21 plays an important role in protecting cells against apoptosis (3, 64). However, up to this end few data are available suggesting a role of cki’s in maintaining cell cycle arrest in differentiated cells. It has been shown in cardiomyocytes that forced expression of E2F1 (58) as well as stimulation of hypertrophic growth (8, 27) leads to an increase in DNA synthesis associated with a specific downregulation of p21 and p27. A role for p21 in maintaining the postmitotic state was further supported by the observation that p21 and Rb control the absence of DNA replication in terminally differentiated muscle cells (32). However, the mechanistic details of how p21 contributes to the maintenance of the cell cycle arrest are poorly understood.
Previously, several studies indicated that p21 might be involved in the regulation of PCNA expression in cardiomyocytes. Whereas p21 is upregulated during differentiation (39), PCNA is downregulated (33). However, hearts of mice lacking p21 exhibit no overt myocardial phenotype, and so far no data are available about cell cycle changes in p21−/− adult cardiomyocytes (13). However, it has been shown that in the renal epithelium PCNA protein expression is more than 5-fold increased after renal ablation in the p21−/− mice compared to that in p21+/+ mice, supporting a role for p21 in the regulation of PCNA protein level (35). In ventricular tissue obtained from 6-day-old p27−/− mice, p21 is weakly expressed, whereas PCNA protein expression level is significantly elevated in comparison to that in p27+/+ mice. However, when p21 expression is increased in the adult stage PCNA protein expression is decreased to control levels (40).
We have shown that cytoplasmic extract of adult cardiomyocytes influences the PCNA protein level in S phase nuclei in a mammalian myocardial cell-free system (Fig. 1 and 2). Recently, it has been reported that activation of Rb in S phase cells disrupts the chromatin tethering of PCNA. This effect was dependent on downregulation of cdk2 activity (48). Since nuclear membranes of the S phase nuclei in the cell-free system are permeabilized (data not shown), it might be possible that PCNA is leaking out after release from chromatin. However, inhibition of cdk2 activity had no influence on the PCNA protein level in the S phase nuclei as shown by using roscovitine and recombinant p27.
It has been shown that PCNA mRNA is expressed in cardiomyocytes from all developmental stages. However, PCNA protein was found only in embryonic and neonatal but not adult cardiomyocytes (33). Similar results were obtained by studying the PCNA gene expression in rat brain. PCNA was found to be expressed at a significant level in both cerebellum and cerebrum of adult rat brain. It was noted that the cell bodies of Purkinje cells contained PCNA transcripts. The Purkinje cells are nerve cells of the cerebellum and generally thought to be nonproliferative. Regardless of the high level of PCNA mRNA, PCNA protein was not detected (31). This suggests that regulation of PCNA gene expression may be at the translational level. The data obtained from our studies using chemical inhibitors indicate that the decreased PCNA protein level is due to proteasome-dependent degradation and not due to the inhibition of the transcription of the PCNA gene or translation of its mRNA (Fig. 3 and 4).
Previously, analysis of the interaction between human p21 and PCNA identified a small 20-amino-acid peptide derived from the C terminus of p21 that was sufficient to interact with PCNA and to inhibit SV40 DNA replication in vitro (10, 61). Further studies have shown that either full-length p21 or p21-derived regions can inhibit DNA replication in a PCNA-dependent manner, and although p21 does not appear to inhibit DNA repair, a synthetic PCNA-binding p21 peptide can affect nucleotide excision repair in vitro (18, 28, 51, 59). Our data concur with data obtained from these studies of the SV40 in vitro DNA replication system, wherein recombinant p21 but not p27 inhibits DNA replication by interaction with PCNA by its C-terminal moiety. While the inhibitory effects of p21 on PCNA-dependent DNA synthesis were expected, although studied in a mammalian system, the possibility that p21 controls PCNA expression provides new mechanistic insight. So far no studies describe a direct regulatory function of p21 related to the proteasome or the ubiquitin ligases and PCNA degradation. However, it is known that p21 regulates casein kinase II, which is known to copurify with proteasome preparation and which has previously been shown to be responsible for the phosphorylation of the C8 subunit of the 20S proteasome (44). Furthermore, it is believed that proper phosphorylation of the PTEN C terminus by casein kinase II is important for PTEN protein stability to proteasome-mediated degradation (55). Notably, PCNA contains two predicted casein kinase II phosphorylation sites (ScanProsite). Thus, p21 might have a regulatory function regarding the proteasome or the PCNA stability by regulating the activity of casein kinase II. By which mechanism p21 regulates PCNA protein levels in detail and which other factors may be involved present challenging issues for future studies.
In addition to the in vitro system we also studied the role of p21 in intact cells (Fig. 8 and 9). Serum stimulation is an established model to induce and to investigate hypertrophic growth of adult cardiomyocytes (65). We observed that during serum stimulation p21 expression is downregulated whereas PCNA expression is upregulated. These data indicated that p21 influences the PCNA protein level in adult cardiomyocytes. Importantly, ectopic expression of p21 not only could reverse this effect, but also antagonized serum-dependent modification of the cardiomyocyte phenotype. These data concur with previous data obtained with neonatal cardiomyocytes (54). In contrast, p27 protein level was not affected by serum stimulation and its overexpression could not block serum induced morphological changes.
Since hypertrophy is paralleled by cell growth, the increase of cdk activity and protein expression as well as downregulation of cki’s might be some kind of a forme fruste of hyperplasia and could represent cell cycle reentry from G0 into G1 phase (27, 54). This cell cycle reentry might be necessary for the growth of cells. Our data obtained from serum stimulation studies suggest that p21 controls this G0/G1 cell cycle exit.
Addition of recombinant PCNA abolished the effect of adult cardiomyocyte extract on PCNA protein level in S phase nuclei in our cell-free system but did not restore the ability to synthesize DNA (data not shown). These data suggest that besides degradation of PCNA adult cardiomyocytes have additional control mechanisms to avoid S phase entry and proliferation of adult cardiomyocytes, one of which could include p27. This might explain why primary cardiac tumors are extremely rare (reviewed in references 4 and 56) and why hearts of mice lacking p21 exhibit no overt myocardial phenotype like tumor growth (13). However, by examining the hearts derived from p21 knockout mice in more detail, we found that they are characterized by a significant increase in cardiomyocyte PCNA expression (Fig. 9), supporting our in vitro cell-free system.
Taken together, we propose a new function for p21. Using this methodological approach we suggest that endogenous p21 of adult cardiomyocytes mediates the proteasome-dependent degradation of PCNA and thus inhibits PCNA-dependent DNA replication. Moreover, we conclude that p21 and p27 repress proliferation by blocking cell cycle progression at distinct stages in cardiomyocytes with p21 controlling serum-induced dedifferentiation through inhibition of G0/G1 transition. p27 might control later stages of cell cycle progression and possibly inhibits progression into S phase.
Acknowledgments
We thank C. Brand (Max-Delbrück-Center) and P. D. Nisen (Abbott) for providing viruses, Julian Blow and Alan Score (University of Dundee) for sharing their GST-p21CIP1 expression plasmid, Tyler Jacks (Massachusetts Institute of Technology) for the p21 knockout mice, and Zophonias Jonsson (Brigham and Women's Hospital, Harvard Medical School) for the pET23a-CHisPCNA plasmid and instructions. We also thank M. Gossen for critical reading of the manuscript.
This work was supported by a grant-in-aid by the Max-Delbrück-Center for Molecular Medicine and by a grant from the Deutsche Forschungsgemeinschaft (Ha-1777/9-1) given to R. von Harsdorf.
REFERENCES
- 1.Alder, H., M. Yoshinouchi, M. B. Prystowsky, P. Appasamy, and R. Baserga. 1992. A conserved region in intron 1 negatively regulates the expression of the PCNA gene. Nucleic Acids Res. 20:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asada, M., T. Yamada, K. Fukumuro, and S. Mizutani. 1998. p21Cip1/WAF1 is important for differentiation and survival of U937 cells. Leukemia 12:1944-1950. [DOI] [PubMed] [Google Scholar]
- 3.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, A. E. 2000. Primary heart tumors in the pediatric age group: a review of salient pathologic features relevant for clinicians. Pediatr. Cardiol. 21:317-323. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami, A. P., K. Urbanek, J. Kajstura, S. M. Yan, N. Finato, R. Bussani, B. Nadal-Ginard, F. Silvestri, A. Leri, C. A. Beltrami, and P. Anversa. 2001. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 344:1750-1757. [DOI] [PubMed] [Google Scholar]
- 6.Bravo, R., and H. Macdonald-Bravo. 1987. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 105:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, G., R. A. Poolman, C. J. McGill, and J. M. Li. 1997. Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J. Mol. Cell Cardiol. 29:2261-2271. [DOI] [PubMed] [Google Scholar]
- 8.Capasso, J. M., S. Bruno, W. Cheng, P. Li, R. Rodgers, Z. Darzynkiewicz, and P. Anversa. 1992. Ventricular loading is coupled with DNA synthesis in adult cardiac myocytes after acute and chronic myocardial infarction in rats. Circ. Res. 71:1379-1389. [DOI] [PubMed] [Google Scholar]
- 9.Chen, I. T., M. L. Smith, P. M. O'Connor, and A. J. Fornace, Jr. 1995. Direct interaction of Gadd45 with PCNA and evidence for competitive interaction of Gadd45 and p21Waf1/Cip1 with PCNA. Oncogene 11:1931-1937. [PubMed] [Google Scholar]
- 10.Chen, J., P. K. Jackson, M. W. Kirschner, and A. Dutta. 1995. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374:386-388. [DOI] [PubMed] [Google Scholar]
- 11.Choukroun, G., R. Hajjar, S. Fry, F. del Monte, S. Haq, J. L. Guerrero, M. Picard, A. Rosenzweig, and T. Force. 1999. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH(2)-terminal kinases. J. Clin. Investig. 104:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coqueret, O., and H. Gascan. 2000. Functional interaction of STAT3 transcription factor with the cell cycle inhibitor p21WAF1/CIP1/SDI1. J. Biol. Chem. 275:18794-18800. [DOI] [PubMed] [Google Scholar]
- 13.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675-684. [DOI] [PubMed] [Google Scholar]
- 14.Di Cunto, F., G. Topley, E. Calautti, J. Hsiao, L. Ong, P. K. Seth, and G. P. Dotto. 1998. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 280:1069-1072. [DOI] [PubMed] [Google Scholar]
- 15.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 16.Ekholm, S. V., and S. I. Reed. 2000. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12:676-684. [DOI] [PubMed] [Google Scholar]
- 17.Engel, F. B., L. Hauck, M. C. Cardoso, H. Leonhardt, R. Dietz, and R. von Harsdorf. 1999. A mammalian myocardial cell-free system to study cell cycle reentry in terminally differentiated cardiomyocytes. Circ. Res. 85:294-301. [DOI] [PubMed] [Google Scholar]
- 18.Flores-Rozas, H., Z. Kelman, F. B. Dean, Z. Q. Pan, J. W. Harper, S. J. Elledge, M. O'Donnell, and J. Hurwitz. 1994. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc. Natl. Acad. Sci. USA 91:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grove, B. K., V. Kurer, C. Lehner, T. C. Doetschman, J. C. Perriard, and H. M. Eppenberger. 1984. A new 185,000-dalton skeletal muscle protein detected by monoclonal antibodies. J. Cell Biol. 98:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi, Y., S. Kim, T. Murakami, S. Yamanaka, and H. Iwao. 1998. Cardiac mitogen-activated protein kinase activities are chronically increased in stroke-prone hypertensive rats. Hypertension 31:50-56. [DOI] [PubMed] [Google Scholar]
- 21.Kajstura, J., A. Leri, N. Finato, C. Di Loreto, C. A. Beltrami, and P. Anversa. 1998. Myocyte proliferation in end-stage cardiac failure in humans. Proc. Natl. Acad. Sci. USA 95:8801-8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, M. J., and G. Y. Koh. 1997. Differential and dramatic changes of cyclin-dependent kinase activities in cardiomyocytes during the neonatal period. J. Mol. Cell Cardiol. 29:1767-1777. [DOI] [PubMed] [Google Scholar]
- 23.Kearsey, J. M., P. J. Coates, A. R. Prescott, E. Warbrick, and P. A. Hall. 1995. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene 11:1675-1683. [PubMed] [Google Scholar]
- 24.Kirshenbaum, L. A., M. Abdellatif, S. Chakraborty, and M. D. Schneider. 1996. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev. Biol. 179:402-411. [DOI] [PubMed] [Google Scholar]
- 25.Kirshenbaum, L. A., and M. D. Schneider. 1995. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein- and p300-binding domains. J. Biol. Chem. 270:7791-7794. [DOI] [PubMed] [Google Scholar]
- 26.Krude, T., M. Jackman, J. Pines, and R. A. Laskey. 1997. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell 88:109-119. [DOI] [PubMed] [Google Scholar]
- 27.Li, J. M., and G. Brooks. 1997. Downregulation of cyclin-dependent kinase inhibitors p21 and p27 in pressure-overload hypertrophy. Am. J. Physiol. 273:H1358-H1367. [DOI] [PubMed] [Google Scholar]
- 28.Li, R., S. Waga, G. J. Hannon, D. Beach, and B. Stillman. 1994. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 371:534-537. [DOI] [PubMed] [Google Scholar]
- 29.Limas, C. J., and C. Limas. 1978. DNA polymerases during postnatal myocardial development. Nature 271:781-783. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., and R. N. Kitsis. 1996. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J. Cell Biol. 133:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y. C., and Y. W. Liu. 1992. Gene expression of proliferating cell nuclear antigen in rat brain. Biochem. Int. 28:129-136. [PubMed] [Google Scholar]
- 32.Mal, A., D. Chattopadhyay, M. K. Ghosh, R. Y. Poon, T. Hunter, and M. L. Harter. 2000. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J. Cell Biol. 149:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marino, T. A., S. Haldar, E. C. Williamson, K. Beaverson, R. A. Walter, D. R. Marino, C. Beatty, and K. E. Lipson. 1991. Proliferating cell nuclear antigen in developing and adult rat cardiac muscle cells. Circ. Res. 69:1353-1360. [DOI] [PubMed] [Google Scholar]
- 34.McGill, C. J., and G. Brooks. 1995. Cell cycle control mechanisms and their role in cardiac growth. Cardiovasc. Res. 30:557-569. [PubMed] [Google Scholar]
- 35.Megyesi, J., P. M. Price, E. Tamayo, and R. L. Safirstein. 1999. The lack of a functional p21(WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proc. Natl. Acad. Sci. USA 96:10830-10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374:131-134. [DOI] [PubMed] [Google Scholar]
- 37.Ottavio, L., C. D. Chang, M. G. Rizzo, S. Travali, C. Casadevall, and R. Baserga. 1990. Importance of introns in the growth regulation of mRNA levels of the proliferating cell nuclear antigen gene. Mol. Cell. Biol. 10:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pines, J. 1995. Cyclins, CDKs and cancer. Semin. Cancer Biol. 6:63-72. [DOI] [PubMed] [Google Scholar]
- 39.Poolman, R. A., R. Gilchrist, and G. Brooks. 1998. Cell cycle profiles and expressions of p21CIP1 and P27KIP1 during myocyte development. Int. J. Cardiol. 67:133-142. [DOI] [PubMed] [Google Scholar]
- 40.Poolman, R. A., J. M. Li, B. Durand, and G. Brooks. 1999. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ. Res. 85:117-127. [DOI] [PubMed] [Google Scholar]
- 41.Prelich, G., M. Kostura, D. R. Marshak, M. B. Mathews, and B. Stillman. 1987. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature 326:471-475. [DOI] [PubMed] [Google Scholar]
- 42.Prosperi, E. 1997. Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control. Prog. Cell Cycle Res. 3:193-210. [DOI] [PubMed] [Google Scholar]
- 43.Quaini, F., E. Cigola, C. Lagrasta, G. Saccani, E. Quaini, C. Rossi, G. Olivetti, and P. Anversa. 1994. End-stage cardiac failure in humans is coupled with the induction of proliferating cell nuclear antigen and nuclear mitotic division in ventricular myocytes. Circ. Res. 75:1050-1063. [DOI] [PubMed] [Google Scholar]
- 44.Rivett, A. J., S. Bose, P. Brooks, and K. I. Broadfoot. 2001. Regulation of proteasome complexes by gamma-interferon and phosphorylation. Biochimie 83:363-366. [DOI] [PubMed] [Google Scholar]
- 45.Rumyantsev, P. P. 1977. Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. Int. Rev. Cytol. 51:186-273. [PubMed] [Google Scholar]
- 46.Sell, C., C. D. Chang, J. Koniecki, H. M. Chen, and R. Baserga. 1992. A cryptopromoter is activated in the proliferating cell nuclear antigen gene of growth arrested cells. J. Cell. Physiol. 152:177-184. [DOI] [PubMed] [Google Scholar]
- 47.Sen, A., P. Dunnmon, S. A. Henderson, R. D. Gerard, and K. R. Chien. 1988. Terminally differentiated neonatal rat myocardial cells proliferate and maintain specific differentiated functions following expression of SV40 large T antigen. J. Biol. Chem. 263:19132-19136. [PubMed] [Google Scholar]
- 48.Sever-Chroneos, Z., S. P. Angus, A. F. Fribourg, H. Wan, I. Todorov, K. E. Knudsen, and E. S. Knudsen. 2001. Retinoblastoma tumor suppressor protein signals through inhibition of cyclin-dependent kinase 2 activity to disrupt PCNA function in S phase. Mol. Cell. Biol. 21:4032-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 50.Shim, J., H. Lee, J. Park, H. Kim, and E. J. Choi. 1996. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature 381:804-806. [DOI] [PubMed] [Google Scholar]
- 51.Shivji, M. K., S. J. Grey, U. P. Strausfeld, R. D. Wood, and J. J. Blow. 1994. Cip1 inhibits DNA replication but not PCNA-dependent nucleotide excision-repair. Curr. Biol. 4:1062-1068. [DOI] [PubMed] [Google Scholar]
- 52.Soonpaa, M. H., G. Y. Koh, L. Pajak, S. Jing, H. Wang, M. T. Franklin, K. K. Kim, and L. J. Field. 1997. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J. Clin. Investig. 99:2644-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Studzinski, G. P., and L. E. Harrison. 1999. Differentiation-related changes in the cell cycle traverse. Int. Rev. Cytol. 189:1-58. [DOI] [PubMed] [Google Scholar]
- 54.Tamamori, M., H. Ito, M. Hiroe, Y. Terada, F. Marumo, and M. A. Ikeda. 1998. Essential roles for G1 cyclin-dependent kinase activity in development of cardiomyocyte hypertrophy. Am. J. Physiol. 275:H2036-H2040. [DOI] [PubMed] [Google Scholar]
- 55.Torres, J., and R. Pulido. 2001. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276:993-998. [DOI] [PubMed] [Google Scholar]
- 56.Vander Salm, T. J. 2000. Unusual primary tumors of the heart. Semin. Thorac. Cardiovasc. Surg. 12:89-100. [DOI] [PubMed] [Google Scholar]
- 57.Vikstrom, K. L., T. Bohlmeyer, S. M. Factor, and L. A. Leinwand. 1998. Hypertrophy, pathology, and molecular markers of cardiac pathogenesis. Circ. Res. 82:773-778. [DOI] [PubMed] [Google Scholar]
- 58.von Harsdorf, R., L. Hauck, F. Mehrhof, U. Wegenka, M. C. Cardoso, and R. Dietz. 1999. E2F-1 overexpression in cardiomyocytes induces downregulation of p21CIP1 and p27KIP1 and release of active cyclin-dependent kinases in the presence of insulin-like growth factor I. Circ. Res. 85:128-136. [DOI] [PubMed] [Google Scholar]
- 59.Waga, S., G. J. Hannon, D. Beach, and B. Stillman. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574-578. [DOI] [PubMed] [Google Scholar]
- 60.Warbrick, E. 2000. The puzzle of PCNA's many partners. Bioessays 22:997-1006. [DOI] [PubMed] [Google Scholar]
- 61.Warbrick, E., D. P. Lane, D. M. Glover, and L. S. Cox. 1995. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol. 5:275-282. [DOI] [PubMed] [Google Scholar]
- 62.Yoshizumi, M., W. S. Lee, C. M. Hsieh, J. C. Tsai, J. Li, M. A. Perrella, C. Patterson, W. O. Endege, R. Schlegel, and M. E. Lee. 1995. Disappearance of cyclin A correlates with permanent withdrawal of cardiomyocytes from the cell cycle in human and rat hearts. J. Clin. Investig. 95:2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zezula, J., P. Casaccia-Bonnefil, S. A. Ezhevsky, D. J. Osterhout, J. M. Levine, S. F. Dowdy, M. V. Chao, and A. Koff. 2001. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
- 65.Zuppinger, C., M. C. Schaub, and H. M. Eppenberger. 2000. Dynamics of early contact formation in cultured adult rat cardiomyocytes studied by N-cadherin fused to green fluorescent protein. J. Mol. Cell Cardiol. 32:539-555. [DOI] [PubMed] [Google Scholar]