Abstract
Peroxisomal disorders have been associated with malfunction of peroxisomal metabolic pathways, but the pathogenesis of these disorders is largely unknown. X-linked adrenoleukodystrophy (X-ALD) is associated with elevated levels of very-long-chain fatty acids (VLCFA; C>22:0) that have been attributed to reduced peroxisomal VLCFA β-oxidation activity. Previously, our laboratory and others have reported elevated VLCFA levels and reduced peroxisomal VLCFA β-oxidation in human and mouse X-ALD fibroblasts. In this study, we found normal levels of peroxisomal VLCFA β-oxidation in tissues from ALD mice with elevated VLCFA levels. Treatment of ALD mice with pharmacological agents resulted in decreased VLCFA levels without a change in VLCFA β-oxidation activity. These data indicate that ALDP does not determine the rate of VLCFA β-oxidation and that VLCFA levels are not determined by the rate of VLCFA β-oxidation. The rate of peroxisomal VLCFA β-oxidation in human and mouse fibroblasts in vitro is affected by the rate of mitochondrial long-chain fatty acid β-oxidation. We hypothesize that ALDP facilitates the interaction between peroxisomes and mitochondria, resulting, when ALDP is deficient in X-ALD, in increased VLCFA accumulation despite normal peroxisomal VLCFA β-oxidation in ALD mouse tissues. In support of this hypothesis, mitochondrial structural abnormalities were observed in adrenal cortical cells of ALD mice.
Peroxisomes are single membrane-bound subcellular organelles present in most eukaryotic cells (8). Peroxisomes are involved in several vital metabolic pathways, including β-oxidation of very-long-chain fatty acids (VLCFA; C>22:0), plasmalogen biosynthesis, oxidation of H2O2, α-oxidation of phytanic acid, bile acid synthesis, and cholesterol biosynthesis (40). Two major classes of peroxisomal disorders have been described. The first class, peroxisomal biogenesis disorders (PBDs; McKusick 601539), is a heterogeneous group of autosomal recessive diseases characterized by alterations in various peroxisomal proteins (called peroxins and encoded by PEX genes) involved in peroxisome biogenesis (38). PBDs include Zellweger syndrome (McKusick 214100), neonatal adrenoleukodystrophy (McKusick 202370), infantile Refsum's disease (McKusick 266510), and rhizomelic chondrodysplasia punctata (McKusick 215100). The second class of peroxisomal disorders, typified by X-linked adrenoleukodystrophy (X-ALD; McKusick 300100), includes disorders with a single peroxisomal enzyme or protein defect.
X-ALD is the most common peroxisomal disorder, with an incidence of approximately 1 in 17,000 (4, 9). It is a postnatal rapidly progressive disease that affects primarily the central nervous system white matter, the adrenal cortex, and the testis (23). The biochemical signature of X-ALD is increased levels of saturated unbranched VLCFA in plasma and tissues, particularly in the cholesterol ester, ganglioside, and proteolipid fractions of the brain white matter and cholesterol esters of the adrenal cortex (23). It has been clearly established that in fibroblasts, white cells, and amniocytes from X-ALD patients, there is a decrease in peroxisomal VLCFA degradation. Reduced activity of peroxisomal very-long-chain acyl coenzyme A (acyl-CoA) synthetase (VLCS), the enzyme that activates VLCFA to initiate their degradation, has been demonstrated in fibroblasts from X-ALD patients. However, the X-ALD gene, ABCD1, identified by positional cloning (24), encodes a protein (ALDP) that is a member of the ATP binding cassette (ABC) transporter superfamily of membrane proteins (16). ALDP is located in the peroxisomal membrane (25) but has no homology to any known VLCS (34) and no demonstrable VLCS activity (36). The role of ALDP in VLCFA metabolism, the pathophysiology of X-ALD, and its relationship to VLCS activity have yet to be determined.
Fatty acids are activated by thioesterification to CoA by fatty acyl-CoA synthetases before they can participate in either catabolic or anabolic pathways (41). Fatty acyl-CoA synthetases capable of activating short-chain fatty acids (SCFA; C2 to C4), medium-chain fatty acids (MCFA; C6 to C10), long-chain fatty acids (LCFA; C12 to C20), or VLCFA (C>20) have been described. The protein encoded by the VLCS gene activates both VLCFA and LCFA, in contrast to long-chain acyl-CoA synthetase, which activates only LCFA. Long-chain acyl-CoA synthetase activity is found in peroxisomes, mitochondria, and microsomes, while VLCS activity is only found in peroxisomes and microsomes. Steinberg et al. (37) reported that for VLCS, the rate of activation of LCFA is 10- to 20-fold higher than the rate of activation of VLCFA. It has been suggested that ALDP is directly involved in VLCFA β-oxidation through transport of VLCS, VLCFA, or a required cofactor across the peroxisomal membrane. It should be noted, however, that the absence of ALDP results in the reduction, but not elimination, of VLCS activity and VLCFA β-oxidation in peroxisomes, suggesting either that there are compensatory activities in the peroxisome or that the effect of ALDP on peroxisomal VLCFA β-oxidation in fibroblasts is indirect.
There are an estimated 48 mammalian ABC proteins (7), located in cellular and subcellular membranes, that transport a wide variety of substrates, including ions, sugars, amino acids, proteins, and lipids (15, 16). Mammalian ABC transporter proteins typically consist of two hydrophobic transmembrane domains and two hydrophilic nucleotide-binding folds encoded by a single gene. Peroxisomal ABC transporters (7) comprise a subgroup (D) of related proteins that are encoded as half-transporters with a single transmembrane domain and a single nucleotide-binding fold. In mammals, there are four ABC subfamily D (ABCD) proteins, ALDP (encoded by the ABCD1 gene), the adrenoleukodystrophy-related protein ALDRP (encoded by the ABCD2 gene), the 70-kDa peroxisomal membrane protein PMP70 (encoded by the ABCD3 gene), and the PMP70-related protein PMP70R (encoded by the ABCD4 gene). Other mammalian ABC half-transporters identified to date dimerize to form functional transporters (33, 35). Homodimerization of ALDP and heterodimerization of ALDP with ALDR and PMP70 in vitro were demonstrated in our laboratory (34) and by Liu et al. (19). The functional significance of these dimers is unknown. However, differences in substrate transport among the various possible homo- and heterodimers could reflect the metabolic demands of various cell types since the peroxisomal ABC transporters are known to have differing tissue expression patterns in vivo (3, 12, 28, 39). This laboratory (6, 17) and others (10, 27) have demonstrated that overexpression of ALDR or PMP70, as well as ALDP, cDNA in fibroblasts from X-ALD patients improves peroxisomal β-oxidation. Thus, these peroxisomal ABC half-transporters, either specifically or nonspecifically, are able to facilitate peroxisomal VLCFA β-oxidation in fibroblasts lacking ALDP.
Previously, we reported the effect of a pharmacological agent, sodium 4-phenylbutyrate (4PBA), on fibroblasts from human PBD and X-ALD patients and ALD mice and showed that 4PBA treatment corrects VLCFA β-oxidation and VLCFA levels in these cells (17, 43). Initially, correction of VLCFA β-oxidation and VLCFA levels in these cells was attributed to an observed two- to threefold increase in ALDRP expression (17). However, more recent data suggest that increased histone acetylation, unrelated to increased ALDR expression, is associated with induction of VLCFA β-oxidation in cultured skin fibroblasts and indicates that induction of peroxisomal VLCFA β-oxidation by 4PBA and other pharmacological agents was associated with induction of mitochondrial LCFA β-oxidation (22). These data imply that there is intracellular communication between mitochondria and peroxisomes in human and mouse fibroblasts.
Recently, Baumgart et al. reported mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model (PEX5−/−) of Zellweger syndrome (2). These included severe alterations of mitochondrial ultrastructure, changes in the expression and activity of mitochondrial respiratory chain complexes, and an increase in the heterogeneity of the mitochondrial component in various organs and specific cell types (2). Mitochondrial abnormalities have also been reported in seminal studies documenting a peroxisomal defect in Zellweger syndrome (14) and in X-ALD (30). There are precedents, therefore, for mitochondrial involvement in peroxisomal disorders.
In this study, we measured peroxisomal VLCFA β-oxidation activity and VLCFA levels in ALD mouse tissues before and after treatment with various pharmacological agents to investigate the relationship between peroxisomal VLCFA β-oxidation and VLCFA levels. The relationship between mitochondrial and peroxisomal fatty acid β-oxidation activities in cultured skin fibroblasts (i) with naturally occurring mutations in fatty acid metabolism and (ii) in the presence of drugs affecting mitochondrial activity was investigated. The role of mitochondria in the induction of peroxisomal VLCFA β-oxidation in fibroblasts by 4PBA was examined, and mitochondria in mouse tissue were examined ultrastructurally for morphological abnormalities.
MATERIALS AND METHODS
Fibroblast cell lines, cell culture, and drug treatment.
Primary fibroblast cell lines were derived from normal human controls and human X-ALD or mitochondrial mutant patients and from wild-type and ALD mice and grown at 37°C in 5% CO2 and Dulbecco modified Eagle medium supplemented with fetal calf serum (10%), penicillin (100 U/ml), and streptomycin (100 U/ml). Cell lines were grown to confluence in the presence or absence (control culture conditions) of antimycin A (0.4 μg/μl), sodium 4PBA (5 mM), or trichostatin A (TSA; 50 nM). 4PBA and TSA were from Sigma (St. Louis, Mo.).
Fatty acid analysis.
Total lipids in mouse tissues and human and mouse fibroblast cell lines were extracted, fractionated, purified by thin-layer chromatography, and subjected to capillary gas chromatography as previously described (20).
Fatty acid oxidation.
Fatty acid oxidation activity of mouse tissue and human and mouse fibroblast cell lines, postnuclear supernatants, and peroxisomal fractions was determined by measuring their capacity to degrade 1-14C-labeled fatty acids to water-soluble products (42). In some experiments, further metabolism of water-soluble products to CO2 by mitochondria was also measured. Reaction tubes were fitted with serum stoppers and plastic center wells (Kontes) containing 0.5-cm2 glass microfiber filters (presoaked in 10 μl of 20% KOH). After a 1-h fatty acid β-oxidation reaction, 200 μl of 3 N H2SO4 was added through the stopper to the bottom of the glass tube. After 2 h, the glass microfiber filter was removed and radioactivity was determined in a scintillation counter. Specific activity was expressed as nanomoles of 14CO2 released per hour per milligram of protein. [1-14C]palmitic acid (C16:0; American Radiolabeled Chemicals) and [1-14C]lignoceric acid (C24:0; synthesized from tricosanol [Sigma] and K14CN [American Radiolabeled Chemicals] by the method of Muralidharan and Kishimoto [26]) were solubilized by brief sonication and incubation at 37°C in 10 mM Tris Cl (pH. 8.0) containing 10 mg of α-cyclodextrin per ml. [1-14C]phytanic acid (synthesized from methyl pristanate and K14CN [26]) was solubilized by brief sonication and incubation at 37°C in 10 mM Tris Cl (pH. 8.0) containing 10 mg of β-cyclodextrin per ml. Fatty acid oxidation data were calculated as specific activity (nanomoles per hour per milligram).
Flow cytometric analysis of the effect of 4PBA on mitochondrial mass.
Mitotracker Green FM (Molecular Probes), a fluorescent dye, was used to measure mitochondrial mass in fibroblasts before and after 4PBA treatment. The cells were then incubated for 30 min with 50 nM Mitotracker Green FM under normal growth conditions. Cells were harvested, resuspended in cold phosphate-buffered saline, and analyzed by flow cytometry with a FACScalibur (Becton Dickinson) and CellQuest software. The autofluorescence of unstained cells was analyzed and subtracted from the analysis of fibroblasts stained with Mitotracker.
In vivo delivery of drugs to ALD mice.
4PBA (0.16 g/kg/day) and TSA (3.0 or 0.3 mg/kg/day) were delivered to ALD mice in their water supply on the basis of a water intake of 4 ml/day. Mice were sacrificed after 8 weeks, and the livers of wild-type, ALD, and 4PBA-treated ALD mice were collected. VLCFA levels and LCFA (C16:0) and VLCFA (C24:0) β-oxidation activity levels were measured as described above. Research experiments involving the use of mice complied with all relevant federal guidelines and institutional policies.
Auxotrophy.
Wild-type and ALD mouse fibroblasts were grown in glucose-free, delipidated medium alone (minimal medium) or in minimal medium supplemented with C24:0 (1 nmol/ml of medium) in the presence or absence of 4PBA (5 mM) or TSA (50 nM). Both 4PBA and TSA were tested because both of these pharmacological agents are known to induce VLCFA β-oxidation and decrease VLCFA levels in vitro. Before addition to the medium, C24:0 was dried under N2 and solubilized by sonication in α-cyclodextrin. Cells were incubated in 10-cm-diameter culture dishes for the times indicated, harvested, and counted in a hemocytometer.
Electron microscopy.
Adrenal glands were harvested from wild-type and ALD mice after perfusion with 4% glutaraldehyde. They were postfixed in osmium tetroxide and embedded in epoxy resin. The embedded tissues were thin sectioned and stained with uranyl acetate-lead citrate and processed for electron microscopic examination as previously described (20).
Statistical analysis.
P values were calculated by using the two-tailed Student t test. A P value < 0.01 was used as the criterion for statistical significance.
RESULTS
Fatty acid β-oxidation in X-ALD fibroblasts and tissues.
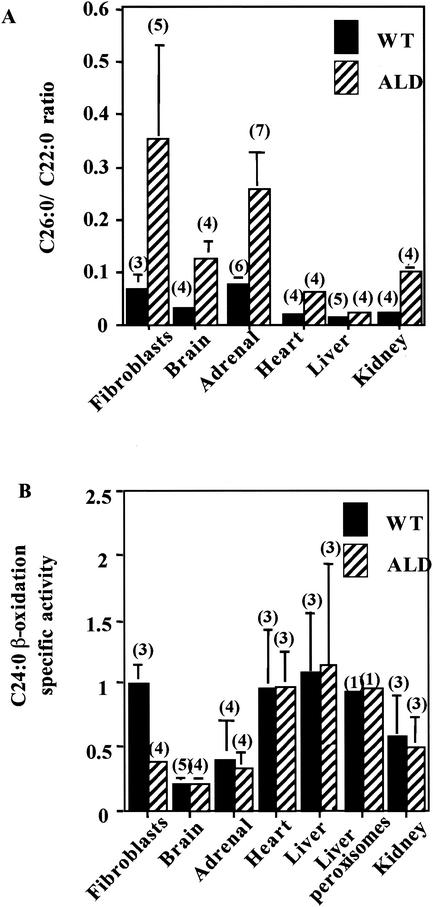
Previously, our laboratory (20) reported both a statistically significant increase in VLCFA levels in fibroblasts and tissues from X-ALD patients and ALD mice (Fig. 1A) and a decrease in the ability of fibroblasts from X-ALD patients and ALD mice to oxidize VLCFA (Fig. 1B). As shown in Fig. 1B, VLCFA β-oxidation in ALD mouse fibroblasts was approximately one-third of the level observed in wild-type mouse fibroblasts. This mirrors the findings obtained with fibroblasts, white blood cells, and amniocytes from human X-ALD patients compared with those obtained with normal controls (23). Surprisingly, no difference in VLCFA (C24:0 [Fig. 1B] and C26:0 [data not shown]) β-oxidation between wild-type and ALD mouse tissues (brain, adrenal gland, and liver [including purified liver peroxisomes]) was seen. This demonstrates that in mouse tissues, normal peroxisomal VLCFA β-oxidation occurs in the absence of ALDP. Taken together, these data suggest that ALDP is not required for peroxisomal VLCFA β-oxidation and that the elevated levels of VLCFA in ALD mouse tissues are not a consequence of impaired peroxisomal degradation.
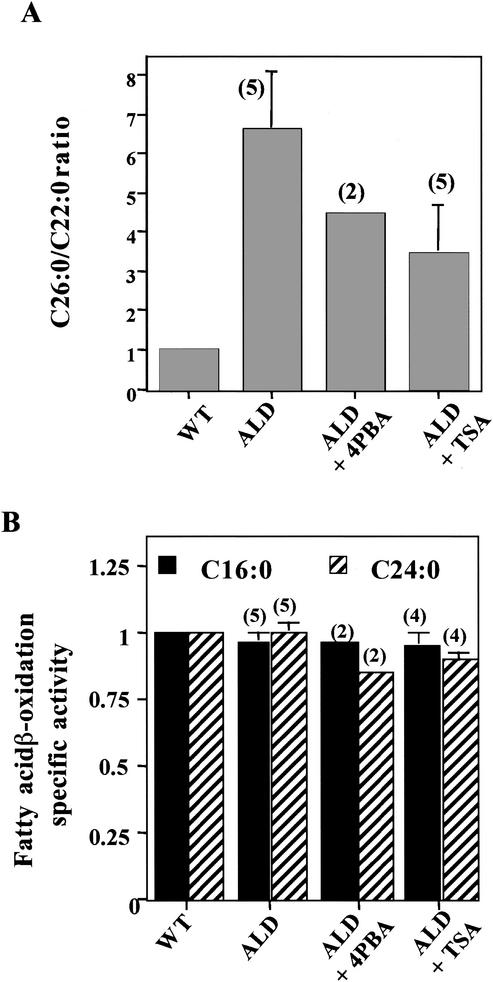
FIG. 1.
ALD mouse tissues have normal VLCFA (C24:0) β-oxidation but accumulate VLCFA. (A) Total lipids were extracted, fractionated, and analyzed by gas chromatography as previously described (20). C26:0/C22:0 ratios are shown. These data were taken from Table 2 of reference 20 with permission. (B) VLCFA (C24:0) β-oxidation activity (nanomoles per hour per milligram) was measured in wild-type (WT) and ALD mouse fibroblasts and tissues. Isolated peroxisomes were determined to be intact if most of the peroxisomal catalase activity was recovered in the peroxisomal pellet. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Since this laboratory (6, 17) and others (10, 27) have demonstrated that overexpression of ALDR or PMP70, as well as ALDP, cDNA following transfection into fibroblasts from X-ALD patients restores peroxisomal β-oxidation, its increased expression in the ALD mouse might be compensatory. However, measurement of the level of expression of these other peroxisomal half-transporters is unchanged in ALD mouse tissues (J.-F. Lu et al., unpublished data). Treatment of human and mouse wild-type and X-ALD fibroblasts with various pharmacological agents, including 4PBA, increases mitochondrial LCFA β-oxidation, as well as peroxisomal VLCFA β-oxidation (22). Therefore, the influence, if any, of mitochondrial activity on peroxisomal VLCFA β-oxidation was investigated by using cells with naturally occurring mutations in fatty acid metabolism and drugs affecting mitochondrial function.
Mitochondrial mutant fibroblast β-oxidation.
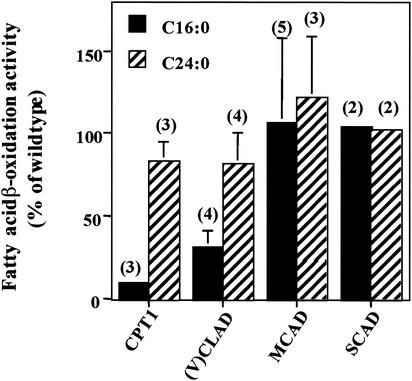
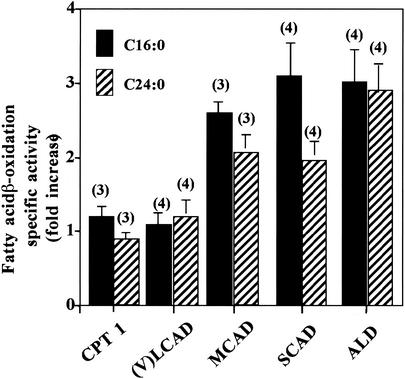
Human mitochondrial mutant fibroblasts deficient in (i) carnitine palmitoyltransferase 1 (CPT1), a protein involved in import of LCFA into mitochondria, or (ii) mitochondrial long-chain fatty acyl-CoA dehydrogenase [(V)LCAD], an enzyme involved in mitochondrial β-oxidation of saturated unbranched LCFA, were assayed for the ability to oxidize fatty acids. Both cell lines showed reduced LCFA (C16:0) β-oxidation activity, as expected, and, surprisingly, also showed reduced VLCFA (C24:0) β-oxidation (Fig. 2). VLCFA β-oxidation was approximately 75% of normal control activity. Fibroblasts deficient in MCAD (mitochondrial medium-chain fatty acyl-CoA dehydrogenase) and SCAD (mitochondrial short-chain fatty acyl-CoA dehydrogenase), which are necessary for mitochondrial β-oxidation of saturated unbranched MCFA and SCFA, respectively, demonstrated normal LCFA and VLCFA β-oxidation activity. These data suggest that the level of peroxisomal VLCFA β-oxidation activity is related to LCFA β-oxidation activity or LCFA levels. The level of MCFA or SCFA β-oxidation activity or MCAD or SCAD levels does not affect peroxisomal VLCFA β-oxidation.
FIG. 2.
LCFA (C16:0) and VLCFA (C24:0) β-oxidation activities were measured in fibroblasts with a reduced capacity for mitochondrial fatty acid β-oxidation. Fibroblasts lacking either (i) CPT1, i.e., the ability to transport LCFA across the mitochondrial membrane, or (ii) (V)LCAD, i.e., the ability to metabolize LCFA, had reduced rates of LCFA (C16:0) and peroxisomal VLCFA (C24:0) β-oxidation. Fibroblasts with mutations affecting MCAD and SCAD β-oxidation had normal rates of both LCFA (C16:0) and peroxisomal VLCFA (C24:0) β-oxidation. Wild-type fatty acid β-oxidation was determined in each experiment as described in Materials and Methods, and this value was used to calculate the percentage of wild-type activity in the fibroblast cell lines being studied. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Effect of LCFA level on VLCFA β-oxidation.
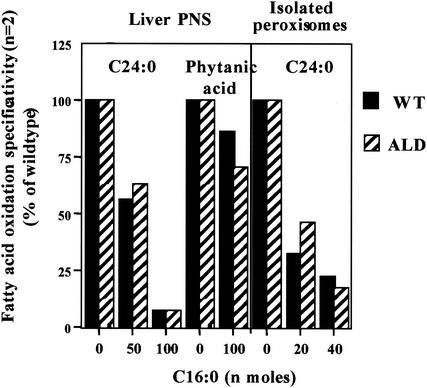
To test the hypothesis that peroxisomal VLCFA β-oxidation activity is related to LCFA β-oxidation and/or levels, LCFA (C16:0) loading experiments were performed. Wild-type and ALD mouse liver postnuclear supernatants or isolated peroxisomes were incubated with increasing concentrations of C16:0, and the effects on VLCFA β-oxidation were measured. An inverse relationship between the amount of C16:0 and the rate of VLCFA (C24:0) β-oxidation in postnuclear supernatants was observed (Fig. 3). This effect was also seen when wild-type and ALD mouse liver peroxisomes isolated from Nycodenz density gradients were incubated with increasing amounts of C16:0 (Fig. 3). Peroxisomal phytanic acid α-oxidation was only mildly affected by the addition of C16:0 (Fig. 3), suggesting that the effect of C16:0 loading is specific for VLCFA (C24:0) β-oxidation and not simply due to limitation of cofactor availability resulting from excess unlabeled C16:0.
FIG. 3.
Effect of LCFA concentration on VLCFA (C24:0) β-oxidation and phytanic acid α-oxidation in wild-type (WT) and ALD mouse liver postnuclear supernatants (PNS) and VLCFA (C24:0) β-oxidation in isolated peroxisomes. Increasing amounts of unlabeled LCFA (C16:0) were added to mouse liver postnuclear supernatants and isolated peroxisomes, and peroxisomal VLCFA (C24:0) β-oxidation and phytanic acid α-oxidation were measured.
Effect of mitochondrial inhibitor on fibroblast fatty acid β-oxidation.
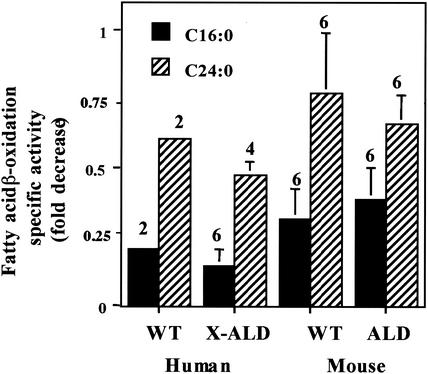
When human and mouse fibroblasts (wild-type and X-ALD) were treated with the mitochondrial inhibitor antimycin A (a complex III inhibitor; 31), mitochondrial activity was totally inhibited, as determined by a CO2 release assay (data not shown). Presumably, the remaining LCFA β-oxidation activity seen in Fig. 4 is peroxisomal, suggesting that peroxisomal LCFA β-oxidation is not affected by the absence of ALDP or by the presence of antimycin A. Treatment with the mitochondrial inhibitor antimycin A results in a decrease (approximately 25%) in peroxisomal VLCFA (C24:0) β-oxidation activity, in agreement with the data in Fig. 2 and 3, where the rate of VLCFA β-oxidation appears to be dependent on the rate of LCFA β-oxidation.
FIG. 4.
Effect of the mitochondrial complex III inhibitor antimycin A on fibroblast fatty acid β-oxidation. Human and mouse fibroblasts from wild-type (WT) and affected individuals were grown for 2 days in medium containing antimycin A (0.4 μg/ml). Wild-type LCFA β-oxidation (C16:0) and VLCFA (C24:0) fatty acid β-oxidation were determined in each experiment as described in Materials and Methods and set to 1. These values were used to calculate the fold decrease in activity in the fibroblast cell lines being studied. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Collectively, the data shown in Fig. 2 to 4 suggest that decreases in VLCFA β-oxidation activity associated with X-ALD could be a secondary effect of elevated intracellular LCFA levels and that ALDP may not participate directly in peroxisomal fatty acid β-oxidation.
In vivo effect of 4PBA on VLCFA β-oxidation and levels.
Previously, we reported that 4PBA treatment of ALD mice in vivo resulted in a decrease in VLCFA levels in tissue and, on the basis of results obtained with 4PBA-treated fibroblasts, proposed that this was attributable to the increase in ALDRP expression (17). More recently, we evaluated the effects of 4PBA and other pharmacological agents on fatty acid β-oxidation in ALD mouse fibroblasts and showed that pharmacological induction of VLCFA and LCFA fatty acid β-oxidation could occur in the absence of increased ALDRP expression (22).
The effect of 4PBA and TSA on VLCFA metabolism in vivo in ALD mice is to reduce VLCFA levels (Fig. 5A), with no effect on LCFA or VLCFA β-oxidation activity after 8 weeks of treatment (Fig. 5B). Thus, after drug treatment, there is a decrease in VLCFA levels in the liver in the absence of a change in VLCFA β-oxidation, supporting the hypothesis that VLCFA levels in vivo are not determined by the rate of peroxisomal VLCFA β-oxidation. Neither (i) the increase in VLCFA levels that results from the absence of ALDP in ALD mice nor (ii) the decrease following exposure to 4PBA or TSA correlates with any alteration in the rate of peroxisomal VLCFA β-oxidation activity.
FIG. 5.
Effect of 4PBA on fatty acid metabolism in vivo. VLCFA accumulation (A) and β-oxidation (B) were determined in liver postnuclear supernatants from wild-type (WT) mice, ALD mice, and ALD mice treated with 4PBA (0.16 g/kg/day) or TSA (0.3 mg/kg/day) in water. Total lipids were extracted, fractionated, and analyzed by gas chromatography as previously described (20). Wild-type mouse C26:0/C22:0 ratios and fatty acid β-oxidation activity levels were set to 1, and these values were used to calculate changes in ratios and activity levels in untreated and treated ALD mice. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Effect of 4PBA treatment on fibroblast β-oxidation.
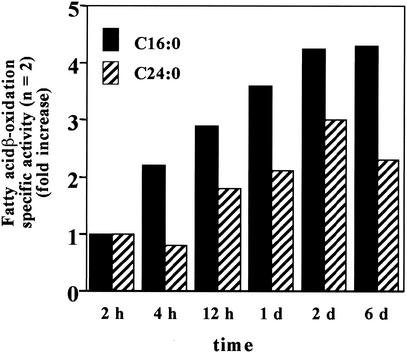
To evaluate the role of mitochondria in the effect of 4PBA treatment on fibroblasts β-oxidation, we examined the time course of the ALD mouse fibroblast in vitro β-oxidation response to 4PBA. As shown in Fig. 6, LCFA (C16:0) β-oxidation increased earlier (after 4 h of treatment) than peroxisomal VLCFA (C24:0) β-oxidation (after 12 h of treatment). This increase in β-oxidation appears to be independent of the increase in ALDRP expression, which was not seen until after 2 days of treatment (data not shown).
FIG. 6.
Time course of response to 4PBA. Fibroblasts from ALD mice were grown in medium containing 5 mM 4PBA for the times indicated. LCFA (C16:0) and VLCFA (C24:0) β-oxidation activities were measured, and values were expressed as fold increases relative to untreated-control values. 4PBA treatment (5 mM) increased LCFA (C16:0) before VLCFA (C24:0) β-oxidation in ALD mouse fibroblasts.
On the basis of the proposed interaction between peroxisomal β-oxidation and mitochondrial β-oxidation, we investigated the possibility that pharmacological agents affect VLCFA levels in ALD mice by inducing mitochondrial β-oxidation and have a secondary effect on peroxisomal β-oxidation.
Effect of 4PBA treatment on mitochondrial mutant fibroblast β-oxidation.
Human fibroblasts deficient in mitochondrial CPT 1 or (V)LCAD activity have reduced LCFA (C16:0) and VLCFA (C24:0) β-oxidation activity, as shown in Fig. 2. When these mutant fibroblasts were treated with 4PBA, there was no induction of either LCFA or VLCFA β-oxidation activity (Fig. 7). 4PBA treatment of human and mouse fibroblasts deficient in mitochondrial MCAD or SCAD, respectively, or mouse fibroblasts deficient in ALDP resulted in an increase in both LCFA and VLCFA β-oxidation activities. Therefore, the effect of 4PBA on peroxisomal VLCFA β-oxidation appears to be dependent on its effect on mitochondrial LCFA metabolism but not mitochondrial MCFA or SCFA metabolism. These data support the hypothesis that changes in the rate of mitochondrial LCFA β-oxidation profoundly affect the rate of peroxisomal VLCFA β-oxidation.
FIG. 7.
Effect of 4PBA treatment (5 mM) on VLCFA (C24:0) β-oxidation specific activity in mitochondrial fatty acid metabolism mutants. Fibroblasts with mutations affecting CPT1, (V)LCAD, MCAD, and SCAD were exposed to 4PBA for 3 days. Cells lacking the ability to either transport LCFA across the mitochondrial membrane (CPT1) or metabolize LCFA [(V)LCAD] did not respond to 4PBA. However, MCAD- and SCAD-deficient cells, with normal LCFA metabolism, responded to 4PBA with increased LCFA (C16:0) and peroxisomal VLCFA (C24:0) β-oxidation activity. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Effect of 4PBA on mitochondrial mass.
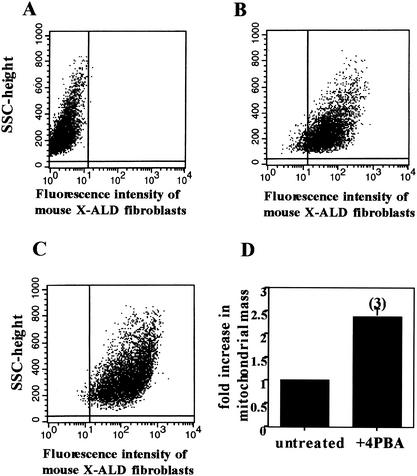
Treatment of mouse fibroblast cell lines with 4PBA resulted in a statistically significant 2.4-fold induction of mitochondrial mass in the three cell lines tested, as shown in Fig. 8. This increase reflects the approximately threefold increase in LCFA β-oxidation observed after drug treatment of mouse fibroblasts (22).
FIG. 8.
Effect of 4PBA (5 mM) on mitochondrial mass in human X-ALD fibroblasts. Mitotracker Green FM was used to measure mitochondrial mass in fibroblasts by flow cytometry before and after 4PBA treatment. The autofluorescence of unstained cells (A) was analyzed and subtracted from the analysis of fibroblasts stained with Mitotracker before (B) and after (C) 4PBA treatment. The 2.4-fold increase in mitochondrial mass after 4PBA treatment is shown in panel D. SSC, single scanned cell. Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
To test the hypothesis that ALDP might have an indirect effect on mitochondrial function, we examined the influence of peroxisomal ALDP on the function (auxotrophy experiment) and structure (ultrastructural analysis of adrenal glands) of mitochondria.
Auxotrophy.
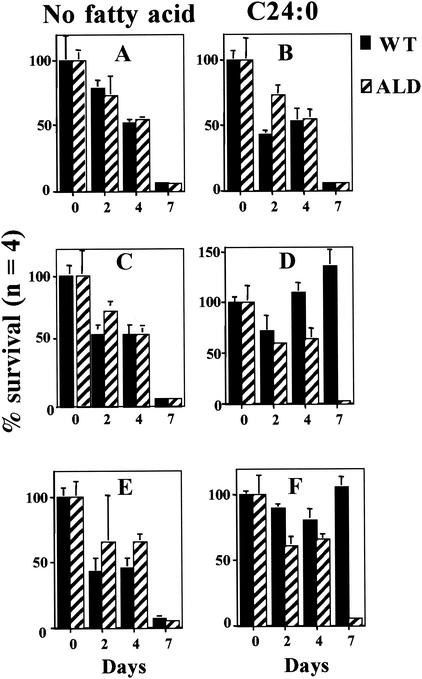
We tested the abilities of wild-type and ALD mouse fibroblasts to grow using a peroxisome-dependent substrate [VLCFA (C24:0)] as the energy source. As seen in Fig. 9A and B, both wild-type and ALD mouse fibroblasts died within 3 to 5 days when grown on delipidated, glucose-free medium with or without C24:0. When mitochondria were stimulated by exposure of cells to 4PBA (Fig. 9C and D) or TSA (Fig. 9E and F), wild-type, but not ALD, mouse fibroblasts were capable of growth with C24:0 as the source of energy. Since C24:0 degradation is uniquely peroxisomal, these observations support the existence of an interaction between peroxisomes and mitochondria that is mediated by ALDP and suggest that ALDP is required for functional interaction between peroxisomes and mitochondria.
FIG. 9.
Effects of 4PBA and TSA on mouse fibroblast VLCFA (C24:0) auxotrophy. Wild-type (WT) and ALD mouse fibroblasts were grown in glucose-free, delipidated medium (minimal medium) (A, C, and E) or in minimal medium supplemented with C24:0 (1 nmol/ml of medium) (B, D, and F) either alone (A and B) or in the presence of 4PBA (5 mM) (C and D) or TSA (50 nM) (E and F). Results are shown as means ± the standard deviations with the number of experiments performed in parentheses.
Ultrastructural analysis of adrenal glands.
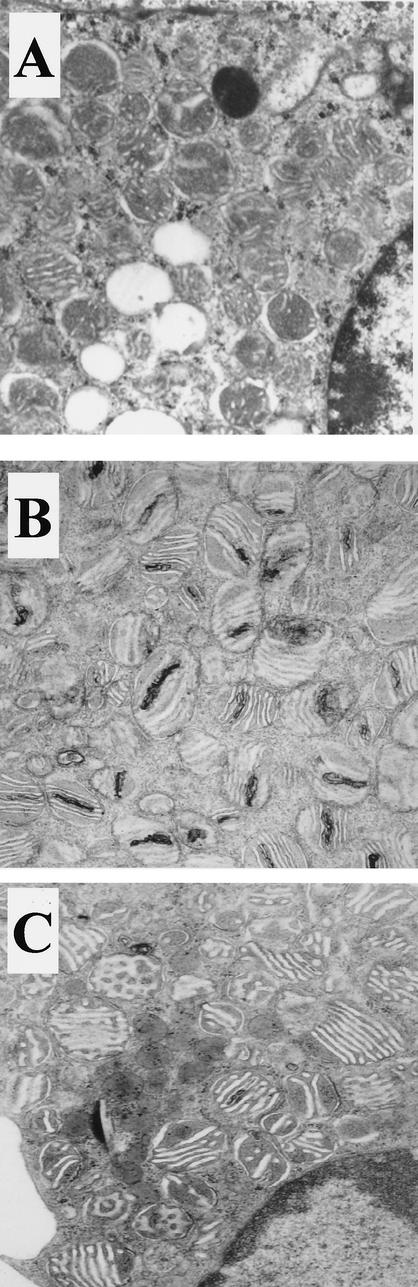
Electron microscopy of adrenocortical cells from older ALD mice (Fig. 10B) indicated some structural damage to mitochondria compared to those of wild-type controls (Fig. 10A). These changes consisted primarily of an electron-dense condensation of cristae but some myelinoid figures and mitochondrial dissolution in 12- to 13-month-old ALD mice. No difference was noted at 9 months of age. These abnormalities were reduced in adrenocortical cells from ALD mice treated with 4PBA for 1 month (Fig. 10C), indicating that 4PBA treatment affects mitochondrial structure, as well as function.
FIG. 10.
Effect of in vivo 4PBA treatment on mitochondrial ultrastructure in adrenocortical cells from ALD mice. Adrenal glands were harvested from wild-type (A), ALD (B), and 4PBA-treated ALD (C) mice after perfusion with 4% glutaraldehyde. They were postfixed in osmium tetroxide and embedded in epoxy resin. The embedded tissues were thin sectioned and stained with uranyl acetate-lead citrate and examined under an electron microscope (magnification, ×48,750).
DISCUSSION
Previously, our laboratory (20) and others (11, 18) have shown that there is residual peroxisomal VLCFA β-oxidation activity in the absence of ALDP in human and mouse X-ALD fibroblasts, demonstrating that ALDP is not necessary for peroxisomal VLCFA β-oxidation. In the present report, we show that ALD mouse tissues have normal levels of peroxisomal VLCFA β-oxidation in the absence of ALDP and yet have elevated VLCFA levels. These data demonstrate conclusively that (i) ALDP does not control the rate of peroxisomal VLCFA β-oxidation and (ii) VLCFA levels are not determined by the rate of VLCFA β-oxidation. Given the capacity for normal rates of VLCFA β-oxidation in the absence of ALDP, the question to be answered is why VLCFA β-oxidation activity is reduced in some cells (fibroblasts from X-ALD patients and mice).
Since we have recently shown that pharmacological agents capable of inducing peroxisomal VLCFA β-oxidation in X-ALD fibroblasts also induce mitochondrial LCFA β-oxidation (22), we investigated the relationship between mitochondrial fatty acid β-oxidation and peroxisomal VLCFA β-oxidation and the possible influence of mitochondria on peroxisomal β-oxidation in X-ALD. The effect of ALDP on peroxisomal β-oxidation has been shown to be dependent on the level of mitochondrial fatty acid β-oxidation activity and, more specifically, on the level of mitochondrial LCFA β-oxidation. (V)LCAD and CPT1 mutant fibroblasts were found to have significantly decreased LCFA β-oxidation activity, as expected, but also decreased VLCFA β-oxidation activity. Previously, Wanders et al. reported decreased VLCFA (C26:0) β-oxidation in fibroblasts from patients deficient in CPT1 and carnitine-acylcarnitine transferase (also involved in LCFA import into mitochondria). The decrease in VLCFA β-oxidation activity in X-ALD fibroblasts is significantly greater than in (V)LCAD or CPT1 mutants. Mutations in either MCAD or SCAD had no effect on peroxisomal VLCFA β-oxidation, indicating that peroxisomal VLCFA β-oxidation is related to mitochondrial LCFA, and not MCFA or SCFA, β-oxidation. We hypothesize that saturation of peroxisomal VLCS with excess LCFA could result in the decrease in VLCFA β-oxidation observed in X-ALD since the rate of peroxisomal VLCS activation of LCFA is 10- to 20-fold higher than the rate of activation of VLCFA (37).
There is no doubt that ALDP is a peroxisomal membrane protein and thus must be involved in some peroxisomal function. A number of observations in the present study support the hypothesis that the role ALDP plays in peroxisomal metabolism involves either a substrate or a product in a pathway that affects mitochondrial function in the absence of ALDP, i.e., in X-ALD. These include the findings that (i) peroxisomal VLCFA β-oxidation was inhibited by the addition of LCFA or the mitochondrial inhibitor antimycin A, (ii) treatment of ALD mice with a pharmacological agent that increases mitochondrial function (4PBA or TSA) resulted in no change in peroxisomal VLCFA β-oxidation and a decrease in VLCFA levels, (iii) the effect of 4PBA on human X-ALD fibroblasts was first to increase mitochondrial LCFA β-oxidation and later to increase peroxisomal VLCFA β-oxidation, and (iv) when mitochondrial LCFA β-oxidation could not be stimulated [in a CPT1 or (V)LCAD mutant], there was no increase in peroxisomal VLCFA β-oxidation. The presence of abnormal mitochondria in X-ALD mouse adrenal cortex supports the hypothesis that mitochondria are involved in the pathogenesis of X-ALD.
It is possible that the absence of ALDP in X-ALD is associated with abnormal mitochondrial function, resulting in an increase in LCFA in cells because of a deficiency in their β-oxidation. Such an increase in LCFA could affect peroxisomal VLCFA β-oxidation activity (as is seen in human and mouse X-ALD fibroblasts) since it is known that VLCS has a 10- to 20-fold greater affinity for LCFA than for VLCFA (37). Elevated VLCFA levels in X-ALD tissues, therefore, may be a secondary consequence of an effect on mitochondrial function. In ALD mouse tissues where the level of mitochondrial activity is higher than in ALD fibroblasts (data not shown), peroxisomal VLCFA β-oxidation is normal, presumably because ALD tissue mitochondria are capable of higher LCFA β-oxidation rates than ALD fibroblast mitochondria. The accumulation of VLCFA in ALD mouse tissues, however, remains unexplained but is probably dependent on the balance between the rate of VLCFA incorporation into complex lipids and the rate of complex lipid degradation, since the accumulated VLCFA in ALD mouse tissues are mostly in complex lipids (23). Therefore, the change in peroxisomal VLCFA β-oxidation in X-ALD appears to be dependent on a combination of the absence of ALDP and the level of mitochondrial activity in the cell type under investigation.
Treatment of X-ALD fibroblasts with 4PBA resulted in an increase in mitochondrial mass and an associated increase in mitochondrial LCFA β-oxidation rather than a specific increase in mitochondrial LCFA β-oxidation. Our findings implicate interruption of ALDP-mediated interactions between peroxisomes and mitochondria in VLCFA accumulation in X-ALD and add to a growing body of literature suggesting that mitochondria play a significant role in the pathogenesis of peroxisomal disorders. In addition to the mitochondrial abnormalities noted in a mouse model of Zellweger syndrome (2), the authors of the original study documenting a peroxisomal deficiency in human Zellweger syndrome also found ultrastructural abnormalities in liver and brain mitochondria and diminished oxygen consumption when using succinate and substrates reducing NAD; this suggested a defect in the electron transport system prior to the cytochromes (14). Ultrastructural abnormalities in skeletal muscle mitochondria, including lipidic inclusions (see below), have also been reported in Zellweger syndrome (32), evidence that mitochondrial function and/or structure are abnormal. Allen et al. (1) reported a dysfunction in the 11-hydroxylase system of the adrenal cortex, the mitochondrial phase of steroidogenesis, without a block in corticosteroid production in patients with X-ALD. Both histoenzymatic and ultrastructural abnormalities of mitochondria were found in biopsied X-ALD adrenal samples. Histoenzymatic decreases in the mitochondrial menadione-dependent α-glycerophosphate dehydrogenase and diphosphopyridine nucleotide diaphorase, and also succinate dehydrogenase and cytochrome oxidase, were observed in abnormal X-ALD adrenocortical cells compared to those of controls. The mitochondria in these same cells displayed a wide variation in size and electron density at the ultrastructural level, but the unavailability of suitable age-matched controls prevented a definitive interpretation of these findings. However, no paracrystalline, lipidic, or myelinoid alterations of these mitochondria were observed. Cytoplasmic lamellae, which are the hallmark and earliest ultrastructural lesion in X-ALD adrenocortical cells and have been proposed to represent bilayers of abnormal fatty acids, were occasionally noted to be in contact with mitochondria and the endoplasmic reticulum (30). Saturated long-chain (C16:0 to C18:0) fatty acids have been shown to be toxic to intestinal mitochondria (21). Most recently, lipidic intramitochondrial inclusions have been observed in atrophic neurons of dorsal root ganglia of patients with adrenomyeloneuropathy, the adult form of X-ALD (29). Lipidic inclusions may be derived from “disintegrating cristae caused by some noxious influence” (13) and have been noted in several mitochondrial disorders (reviewed in reference 29). The mitochondrial abnormalities in the adrenal cortexes of ALD mice (shown in Fig. 10) are similar to these previous observations reported in the literature. Recently, ultrastructural mitochondrial malformations were reported in X-linked dilated cardiomyopathy patients with mutations in the Xq28 gene, G4.5, which also had elevated levels of saturated fatty acids (5).
In summary, these studies indicate that the rate of peroxisomal VLCFA β-oxidation in X-ALD is not determined solely by the presence or absence of ALDP and provide a strong suggestion of functional and structural alterations in mitochondria as a result of the loss of ALDP in X-ALD. The restoration of normal-appearing (Fig. 10) and normally functioning (Fig. 6) mitochondria after 4PBA treatment implies that the reduction of VLCFA levels caused by this agent may result from effects on mitochondria rather than peroxisomes. Since the role of ALDP in the pathogenesis of X-ALD is unclear, development of therapies for X-ALD should not be limited to those directed at normalization of VLCFA. Therapies directed toward increasing mitochondrial activity and/or mass might be beneficial in the treatment of X-ALD.
Acknowledgments
We thank Ann Moser, Richard Kelley, Michael Bennett, and Gerardo Jimenez-Sanchez for providing patient and mouse fibroblast cell lines and Richard Kelley for review of the manuscript.
This work was supported by Public Health service grants HD10981, HD 24061, and NS 37355 from the National Institutes of Health.
REFERENCES
- 1.Allen, J., T. Kepic, D. Garwicki, and M. Yunus. 1982. Adrenal defect in adrenomyelodystrophy. South. Med. J. 75:877-879. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart, E., I. Vanhorebeek, M. Grabenbauer, M. Borgers, P. E. Declercq, H. D. Fahimi, and M. Baes. 2001. Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse). Am. J. Pathol. 159:1477-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, J., S. Albet, M. Bentejac, N. Netik, A. Holzinger, A. A. Roscher, M. Bugaut, and S. Forss-Petter. 1999. The four murine peroxisomal ABC-transporter genes differ in constitutive, inducible and developmental expression. Eur. J. Biochem. 265:719-727. [DOI] [PubMed] [Google Scholar]
- 4.Bezman, L., A. Moser, G. Raymond, P. Rinaldo, P. Watkins, K. Smith, N. Kass, and H. Moser. 2001. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 49:512-517. [PubMed] [Google Scholar]
- 5.Bissler, J. J., M. Tsoras, H. H. H. Goring, P. Hug, G. Chuck, E. Tombragel, C. McGraw, J. Schlotman, M. A. Ralston, and G. Hug. 2002. Infantile dilated X-linked cardiomyopathy, G4.5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle. Lab. Investig. 82:335-344. [DOI] [PubMed] [Google Scholar]
- 6.Braiterman, L. T., S. Zheng, P. A. Watkins, M. T. Geraghty, G. Johnson, M. C. McGuinness, A. B. Moser, and K. D. Smith. 1998. Suppression of peroxisomal membrane protein defects by peroxisomal ATP binding cassette (ABC) proteins. Hum. Mol. Genet. 7:239-247. [DOI] [PubMed] [Google Scholar]
- 7.Dean, M., Y. Hamon, and G. Chimini. 2001. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42:1007-1017. [PubMed] [Google Scholar]
- 8.De Duve, C., and P. Baudhuin. 1966. Peroxisomes (microbodies and related particles). Physiol. Rev. 46:323-357. [DOI] [PubMed] [Google Scholar]
- 9.Dubois-Dalcq, M., V. Feigenbaum, and P. Aubourg. 1999. The neurobiology of X-linked adrenoleukodystrophy, a demyelinating peroxisomal disorder. Trends Neurosci. 22:4-12. [DOI] [PubMed] [Google Scholar]
- 10.Flavigny, E., A. Sanhaj, P. Aubourg, and N. Cartier. 1999. Retroviral-mediated adrenoleukodystrophy-related gene transfer corrects very long chain fatty acid metabolism in adrenoleukodystrophy fibroblasts: implications for therapy. FEBS Lett. 448:261-264. [DOI] [PubMed] [Google Scholar]
- 11.Forss-Petter, S., H. Werner, J. Berger, H. Lassmann, B. Molzer, M. H. Schwab, H. Bernheimer, F. Zimmermann, and K. A. Nave. 1997. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci Res. 50:829-843. [DOI] [PubMed] [Google Scholar]
- 12.Fouquet, F., J. M. Zhou, E. Ralston, K. Murray, F. Troalen, E. Magal, O. Robain, M. Dubois-Dalcq, and P. Aubourg. 1997. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 3:271-285. [DOI] [PubMed] [Google Scholar]
- 13.Ghadially, F. 1997. Intramitochondrial lipidic inclusions, p. 310-313. In F. Ghadially (ed.), Ultrastructural pathology of the cell and matrix. Butterworth-Heinemann, Boston, Mass.
- 14.Goldfischer, S., C. L. Moore, A. B. Johnson, A. J. Spiro, M. P. Valsamis, H. K. Wisniewski, R. H. Ritch, W. T. Norton, I. Rapin, and L. M. Gartner. 1973. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 182:62-64. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman, M., and I. Pastan. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385-427. [DOI] [PubMed] [Google Scholar]
- 16.Higgins, C. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 17.Kemp, S., H.-M. Wei, J.-F. Lu, L. T. Braiterman, M. McGuinness, P. A. Watkins, A. B. Moser, and K. D. Smith. 1998. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat. Med. 4:1261-1268. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, T., N. Shinnoh, A. Kondo, and T. Yamada. 1997. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 232:631-636. [DOI] [PubMed] [Google Scholar]
- 19.Liu, L., K. Janvier, V. Berteaux-Lecellier, N. Cartier, R. Benarous, and P. Aubourg. 1999. Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J. Biol. Chem. 274:32728-32743. [DOI] [PubMed] [Google Scholar]
- 20.Lu, J.-F., A. M. Lawler, P. A. Watkins, J. M. Powers, A. B. Moser, H. W. Moser, and K. D. Smith. 1997. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 94:9366-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MaKay, D., H. Kauniyz, I. Csavossy, and R. Johnson. 1967. Electron microscope studies of the absorption of lipids. 3. Long chain saturated triglycerides. Metabolism 16:137-152. [DOI] [PubMed] [Google Scholar]
- 22.McGuinness, M. C., H.-P. Zhang, and K. D. Smith. 2001. Evaluation of pharmacological induction of fatty acid β-oxidation in X-linked adrenoleukodystrophy. Mol. Genet. Metab. 74:256-263. [DOI] [PubMed] [Google Scholar]
- 23.Moser, H., K. Smith, P. Watkins, J. Powers, and A. Moser. 2001. X-linked adrenoleukodystrophy, p. 3257-3301. In C. Scriver, A. Beaudet, W. Sly, and D. Valle (ed.), The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, N.Y.
- 24.Mosser, J., A.-M. Douar, C.-O. Sarde, P. Kioschis, R. Feil, H. Moser, A.-M. Poustka, J.-L. Mandel, and P. Aubourg. 1993. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361:726-730. [DOI] [PubMed] [Google Scholar]
- 25.Mosser, J., Y. Lutz, M. E. Stoeckel, C.-O. Sarde, C. Kretz, A. M. Douar, J. Lopez, P. Aubourg, and J.-L. Mandel. 1994. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum. Mol. Genet. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 26.Muralidharan, V. B., and Y. Kishimoto. 1984. Phytanic acid alpha-oxidation in rat liver: requirement of cytosolic factor. J. Biol. Chem. 259:13021-13026. [PubMed] [Google Scholar]
- 27.Netik, A., S. Forss-Peter, A. Holzinger, B. Molzer, G. Unterrainer, and J. Berger. 1999. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum. Mol. Genet. 8:907-913. [DOI] [PubMed] [Google Scholar]
- 28.Pollard, H., J. Moreau, and P. Aubourg. 1995. Localization of mRNAs for adrenoleukodystrophy and the 70 kDa peroxisomal (PMP70) proteins in the rat brain during post-natal development. J. Neurosci. Res. 42:433-437. [DOI] [PubMed] [Google Scholar]
- 29.Powers, J. M., D. P. DeCiero, C. Cox, E. K. Richfield, M. Ito, A. B. Moser, and H. W. Moser. 2001. The dorsal root ganglia in adrenomyeloneuropathy: neuronal atrophy and abnormal mitochondria. J. Neuropathol. Exp. Neurol. 60:493-501. [DOI] [PubMed] [Google Scholar]
- 30.Powers, J. M., H. H. Schaumburg, A. B. Johnson, and C. S. Raine. 1980. A correlative study of the adrenal cortex in adrenoleukodystrophy—evidence for a fatal intoxication with very long chain saturated fatty acids. Investig. Cell Pathol. 3:353-376. [PubMed] [Google Scholar]
- 31.Rieske, J. 1976. Composition, structure, and function of complex III of the respiratory chain. Biochim. Biophys. Acta 456:195-247. [DOI] [PubMed] [Google Scholar]
- 32.Sarnat, H., G. Machin, H. Darwish, and S. Rubin. 1983. Mitochondrial myopathy of cerebro-hepato-renal (Zellweger) syndrome. Can. J. Neurol. Sci. 10:170-177. [DOI] [PubMed] [Google Scholar]
- 33.Shani, N., and D. Valle. 1996. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. USA 93:11901-11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, K. D., S. Kemp, L. T. Braiterman, J.-F. Lu, H.-M. Wei, G. Stetten, J. S. Bergin, J. Pevsner, and P. Watkins. 1999. X-linked adrenoleukodystrophy: genes, mutations and phenotypes. Neurochem. Res. 24:511-525. [DOI] [PubMed] [Google Scholar]
- 35.Spies, T., and R. DeMars. 1991. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature 351:323-324. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg, S., S. Kemp, L. Braiterman, and P. Watkins. 1999. Role of very-long-chain acyl-coenzyme A synthetase in X-linked adrenoleukodystrophy. Ann. Neurol. 46:409-412. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg, S. J., S. J. Wang, D. G. Kim, S. J. Mihalik, and P. A. Watkins. 1999. Human very-long chain acyl-CoA synthetase: cloning, topography, and relevance to branched-chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 257:615-621. [DOI] [PubMed] [Google Scholar]
- 38.Subramani, S. 1998. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol. Rev. 78:171-188. [DOI] [PubMed] [Google Scholar]
- 39.Troffer-Charlier, N., N. Doerflinger, E. Metzger, F. Fouquet, J. L. Mandel, and P. Aubourg. 1998. Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur. J. Cell Biol. 75:254-264. [DOI] [PubMed] [Google Scholar]
- 40.Van den Bosch, H., R. B. Schutgens, R. J. Wanders, and J. M. Tager. 1992. Biochemistry of peroxisomes. Annu. Rev. Biochem. 61:157-197. [DOI] [PubMed] [Google Scholar]
- 41.Watkins, P. A. 1997. Fatty acid activation. Prog. Lipid Res. 36:55-83. [DOI] [PubMed] [Google Scholar]
- 42.Watkins, P. A., E. V. Ferrell, J. I. Pedersen, and G. Hoefler. 1991. Peroxisomal fatty acid β-oxidation in HepG2 cells. Arch. Biochem. Biophys. 289:329-336. [DOI] [PubMed] [Google Scholar]
- 43.Wei, H.-W., S. Kemp, M. C. McGuinness, A. B. Moser, and K. D. Smith. 1999. Pharmacologic induction of peroxisomes in peroxisome biogenesis disorders. Ann. Neurol. 47:286-296. [PubMed]