Abstract
Sustained activation of the Ras/Raf/MEK/extracellular signal-regulated kinase (ERK) pathway can lead to cell cycle arrest in many cell types. We have found, with human medullary thyroid cancer (MTC) cells, that activated Ras or c-Raf-1 can induce growth arrest by producing and secreting an autocrine-paracrine factor. This protein was purified from cell culture medium conditioned by Raf-activated MTC cells and was identified by mass spectrometry as leukemia inhibitory factor (LIF). LIF expression upon Raf activation and subsequent activation of JAK-STAT3 was also observed in small cell lung carcinoma cells, suggesting that this autocrine-paracrine signaling may be a common response to Ras/Raf activation. LIF was sufficient to induce growth arrest and differentiation of MTC cells. This effect was mediated through the gp130/JAK/STAT3 pathway, since anti-gp130 blocking antibody or dominant-negative STAT3 blocked the effects of LIF. Thus, LIF expression provides a novel mechanism allowing Ras/Raf signaling to activate the JAK-STAT3 pathway. In addition to this cell-extrinsic growth inhibitory pathway, we find that the Ras/Raf/MEK/ERK pathway induces an intracellular growth inhibitory signal, independent of the LIF/JAK/STAT3 pathway. Therefore, activation of the Ras/Raf/MEK/ERK pathway can lead to growth arrest and differentiation via at least two different signaling pathways. This use of multiple pathways may be important for “fail-safe” induction and maintenance of cell cycle arrest.
For decades, the Ras and Raf families of oncogenes have been known as transforming genes. However, in many normal cell types in culture, sustained expression of activated Ras or its downstream effector, Raf, can elicit cell cycle arrest or senescence (11, 12, 15, 23, 26, 32, 36, 41, 42, 46, 47, 60, 62). This Ras/Raf-mediated growth arrest has been proposed as a defense mechanism against inappropriate activation of the Ras/Raf signal transduction pathway (58). According to this hypothesis, spontaneous mutations in Ras genes, which may occur stochastically in all cell types, would be innocuous for the organism, since they would lead to growth arrest or senescence. Hence, for carcinogenesis to occur in response to Ras/Raf activation, the growth arrest response must be inactivated. Cell transformation may involve additional events, such as inactivation of a tumor suppressor gene and induction of telomerase (16, 36).
The growth arrest response to Ras/Raf activation is not limited to normal cells. Several tumor cell lines also undergo the growth arrest response, usually accompanied by differentiation or senescence (9, 11, 38-40, 49, 59). These cell lines are derived from several tumor types, including pheochromocytoma, medullary thyroid carcinoma, small cell lung carcinoma, and glioma. In most of these cell types, Ras mutation or elevated Raf signaling is rarely detected, suggesting that Ras/Raf signaling does not provide a growth advantage for these tumor types. These findings indicate that some tumors retain a capability for growth arrest in response to Ras/Raf activation. Understanding such growth inhibition pathways could provide insight into the process of tumor development, with potential therapeutic implications.
The mechanism by which Ras or Raf activation can induce growth arrest is not completely understood. As has been found for other inducers of cell cycle arrest, Ras/Raf-mediated growth arrest is accompanied by induction of cyclin-dependent kinase inhibitors (CDKIs), such as p16INK4a, p21WAF1/CIP1, or p27KIP1, tumor suppressors p53 or p19ARF, and by downregulation of phosphorylated Rb or the E2F family (9, 11, 15, 23, 26, 36, 38-40, 42, 46, 47, 59, 60, 62; reviewed in reference 29). The specific components involved appear to vary according to the cell type. However, the signal transduction steps between Ras/Raf/MEK/extracellular signal-regulated kinase (ERK) and the cell cycle regulatory machinery remain to be fully elucidated.
The Ras/Raf/MEK/ERK pathway can exploit autocrine or paracrine signaling mechanisms. For example, activation of the Ras/Raf/MEK/ERK pathway has been shown to upregulate transforming growth factor beta in the MDCK cell line (22) or in the prostate cancer cell line TSU-pr1 (37), heparin-binding epidermal growth factor or interleukin 1 (IL-1) in NIH 3T3 cells (27, 54), granulocyte-macrophage colony-stimulating factor in the murine lymphoid hematopoietic cell line FL5.12 or in human TF-1 cells (4, 5), and epidermal growth factor in the human breast epithelial cell line MCF-10A (45). In these cell lines, expression of these soluble factors was shown to be associated with Ras/Raf-mediated proliferation or tumorigenic phenotypes. Therefore, we examined whether Ras/Raf-induced growth inhibition could also be mediated through autocrine-paracrine signaling.
We have shown that in human medullary thyroid carcinoma (MTC) cells, Ras or Raf activation results in differentiation and growth arrest (9, 10, 31). In the present study, we report that Ras or Raf activation induces expression and secretion of a protein that can mediate differentiation and G1 cell cycle arrest. By protein purification and mass spectrometry, we identify this protein as leukemia inhibitory factor (LIF). STAT3 activation is necessary for LIF-mediated growth arrest and differentiation in MTC cells. In addition, the Ras/Raf/MEK/ERK pathway can also mediate growth arrest and differentiation by a second mechanism, independent of LIF/JAK/STAT3. This novel autocrine-paracrine mechanism, mediating cross talk between the Ras/Raf/MEK/ERK pathway and the JAK-STAT pathway, defines a novel mechanism of Ras/Raf-induced growth arrest.
MATERIALS AND METHODS
Cell culture and conditioned media treatment.
The human MTC cell line TT and the TTRaf cell line containing the activatable ΔRaf-1:ER construct, the catalytic domain of Raf-1 fused to the hormone binding domain of the human estrogen receptor, have been described previously (9). ΔRaf-1:ER was activated with 1 μM β-estradiol (Sigma, St. Louis, Mo.) as previously described (43). The control TTpLNCX cell line was produced by retroviral infection of the pLNCX vector into subconfluent TT cells. The TTSTAT3-DN cell line was produced by stably transfecting TT cells with a dominant-negative human STAT3Y705F (34), and the control cell line TTpcDNA3.1 was produced using the empty vector. TTpLNCX and TTRaf cells were maintained in phenol red-free RPMI 1640 (Life Technologies, Rockville, Md.) supplemented with 16% fetal bovine serum, 100 U of penicillin and 100 μg of streptomycin per ml, and 0.25 mg of Geneticin (Invitrogen, Carlsbad, Calif.) per ml for selection. The TTGAS3 cell line was produced by stably transfecting TT cells with the STAT3 reporter construct (GAS)3-Luc (21).
For the preparation of conditioned media, cells were incubated in serum-free RPMI1640 for 3 days before harvest. For treatment, conditioned media were mixed with fresh media at a ratio of 1:1 or 1:2. Recombinant LIF was produced from the HEK293LIFV5 cell line generated by stably expressing a V5 epitope-tagged LIF gene in HEK293 cells. Conditioned medium containing LIFV5 from HEK293 was mixed with fresh media at the ratio of 1:2 or 1:4 before use. Recombinant LIF was also purchased from Chemicon (Temecula, Calif.) and used at a concentration of 4,000 U/ml. 911 cells were maintained in Dulbecco's modified Eagle medium (Life Technologies) with 10% fetal bovine serum. For cell growth curves, cells were seeded in 24-well plates (Cellstar, Carrollton, Tex.) at a density of 4 × 104 cells per well, and cells were counted every 2 days using a hemocytometer.
Fractionation of conditioned medium.
Five liters of serum-free TTRaf-E2-conditioned medium was concentrated by 30-kDa cutoff ultrafiltration (Millipore, Bedford, Mass.), desalted, and applied to an anionic exchanger unoQ6 column (Bio-Rad, Hercules, Calif.) connected with a heparin-Sepharose column (Amersham Pharmacia Biotech, Piscataway, N.J.) in 20 mM Tris-Cl (pH 7.9). Proteins bound to the heparin-Sepharose column were eluted by a linear gradient of NaCl. Fractions were analyzed for their ability to induce growth arrest and morphological change in TT cells. Active fractions, eluted at around 200 mM NaCl, were dialyzed in 50 mM Na phosphate (pH 7.2)-1 M ammonium sulfate and run on octyl- and butyl-Sepharose columns (Amersham Pharmacia Biotech) connected in tandem. Flow-through was collected, desalted in 50 mM HEPES (pH 8.1), and applied to a cationic exchanger unoS1 column (Bio-Rad). The active fractions eluted at around 150 mM NaCl were concentrated using 30-kDa cutoff ultrafiltration unit (Millipore) and resolved with a Superdex G200 gel filtration column (Amersham Pharmacia Biotech) in 50 mM sodium phosphate-100 mM NaCl (pH 7.2). Activity was recovered at about 35 kDa. The progress of purification was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining (Bio-Rad). The final purification fraction was concentrated by precipitating with 50% acetone, and the precipitates were resuspended in water before trypsinization and mass spectrometry.
Mass spectrometry.
Peptides were generated by tryptic digestion using 10 μg of trypsin/ml under N2 vapor and fractionated by reverse-phase high-performance liquid chromatography with a 0.8-mm Vydac C-18 column. Selected peak fractions were then analyzed by a matrix-assisted laser-desorption ionization-time-of-flight mass spectrometry workstation with delayed extraction technology (Voyager DE-STR; Applied Biosystems) and an electrospray ionization mass spectrometer with a time-of-flight analyzer (Qstar/Pulsar; Applied Biosystems/MDS Sciex). Tryptic peptide masses were compared with entries in both the nr (nonredundant) and dbEST databases from the National Center for Biotechnology Information using Mascot BLAST software (Matrix Science).
RT-PCR and Northern hybridization analysis.
Reverse transcription PCR (RT-PCR) of LIF was performed by reverse transcription of 0.25 μg of total RNA and 35 subsequent cycles of PCR using Pfx polymerase (Invitrogen) and the primers GGTTTCCTCTAGAGCCCTCTGAAGTGCAGC and ACCTCCTCGAGGAAGGCCTGGGCCAACACGGCGAT. The results were normalized for expression of glyceraldehyde-3-phosphate dehydrogenase, using the primers CAGCCGAGCCACATCG and TGAGGCTGTTGTCATACTTCTC.
Northern blot hybridization was done using 20 μg of total RNA isolated by using Trizol (Life Technologies) and transferred to HyBond-NX (Amersham Life Science). Blots were hybridized to probes specific for human calcitonin (pTT1062) and CGRP (pTT83) as previously described (31). These probes were labeled with [α-32P]dCTP (NEN, Boston, Mass.) by random primer labeling (Boehringer Mannheim). Hybridizations with radiolabeled probes (106 cpm/ml) were done at 42°C for 16 to 18 h, followed by washing with 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) and 1% SDS at 65°C.
Plasmids and recombinant adenoviruses.
Human LIF cDNA prepared by RT-PCR was ligated into the XbaI and XhoI sites of pcDNA3.1(−) containing a C-terminal V5 tag. The dominant-negative STAT3 adenovirus AdSTAT3-DN was made by using the AdEasy system (17). Briefly, dominant-negative human STAT3 was subcloned into HindIII restriction site of the pAdTrackCMV shuttle vector, and the resulting plasmid was recombined with the pAdEasy1 vector in BJ5183 bacterial cells. High-titer viral stocks were prepared from 911 cells. The control virus AdGFP was made using empty pAdTrackCMV that expresses green fluorescent protein alone. The viral titer was measured by plaque assay in low-passage HEK293 cells. The viral dose used for TT cells was 2.5 to 5 PFU per cell. Adenoviruses containing constitutively active Ras (V12) or Raf (BXB) are described elsewhere (20).
Cell cycle analysis.
Cells were washed with ice cold 0.2% bovine serum albumin in phosphate-buffered saline (PBS) and resuspended in 250 mM sucrose-40 mM citrate buffer (pH 7.6) containing 0.5% dimethyl sulfoxide. Nuclei were prepared, stained with propidium iodide (55), and analyzed with an LSR flow cytometer (Becton Dickinson, Franklin Lakes, N.J.) with a gate that selects single nuclei within a normal size range. The cell cycle parameters from 10,000 gated nuclei were determined by CellQuest software.
Immunoblot analysis.
Cells harvested at various times were lysed in 62.5 mM Tris (pH 7.5)-2% SDS-10% glycerol with aprotinin, leupeptin, pepstatin, and phenylmethylsulfonyl fluoride and briefly sonicated before determining the protein concentration using bicinchoninic acid reagents (Pierce, Rockford, Ill.). Fifty to one hundred micrograms of protein was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane filter (Millipore), and stained with Fast Green reagent (Fisher Scientific, Pittsburgh, Pa.). Membrane filters were then blocked in 0.1 M Tris (pH 7.5)-0.9% NaCl-0.05% Tween 20 with 5% nonfat dry milk and incubated with appropriate antibodies. Antibodies were diluted as follows: LIF, 1:1,000 (R&D Systems, Minneapolis, Minn.); RET, 1:1,000 (Santa Cruz Biotech, Santa Cruz, Calif.); p42/44 ERK, STAT3, pSTAT3 (Tyr 705), pSTAT3 (Ser 727), pSTAT1 (Tyr 701), pSTAT5 (Tyr 694), and pSTAT6 (Tyr 641), 1:1,000 (Cell Signaling, Beverly, Mass.); and glyceraldehyde-3-phosphate dehydrogenase, 1:5,000 (Trevigen, Gaithersburg, Md.). The Supersignal West Pico chemiluminescence kit (Pierce) was used for visualization of the signal.
Neutralization of LIF and gp130 receptor.
One milliliter of conditioned medium was incubated with 0.8 μg of anti-LIF neutralizing antibody (R&D Systems) at room temperature for 1 h prior to treating cells. To block gp130 receptor, cells were pretreated with 8 μg of anti-gp130 blocking antibody (R&D Systems) in six-well plates for 1 h.
DNA synthesis assay.
TT cells were plated in 24-well plates with 0.5 ml of culture medium. Cells (50 to 70% confluent) were treated with conditioned media and labeled with [3H]thymidine (NEN) at a concentration of 1 μCi/ml for 6 h. Cells were then washed once with 1 ml of PBS and twice with 1 ml of ice-cold 5% trichloroacetic acid followed by a second 1-ml rinse with PBS and solubilized in 250 μl of 0.25 N NaOH. Two-hundred-microliter aliquots were neutralized with 50 μl of 6 N HCl and measured by liquid scintillation counting.
STAT3 reporter assay.
Cells were seeded in triplicate in six-well plates and transfected the next day using Lipofectamine or Lipofectin reagents (Invitrogen). Cells were cotransfected with a STAT3 reporter construct (21), (GAS)3-Luc, and pRL-TK (Promega, Madison, Wis.) for normalization of data. Cells were then treated with recombinant LIF or Raf-E2-CM for 2 days. Cell lysates were prepared for luciferase activity assays per the manufacturer's instructions (Promega). To measure activation of STAT3 by Ras or Raf, TTGAS3 cells were infected for 2 days with adenoviral Ras V12 or Raf BXB, after which luciferase assays were performed.
RESULTS
Raf activates an autocrine-paracrine loop which can suppress MTC cell proliferation.
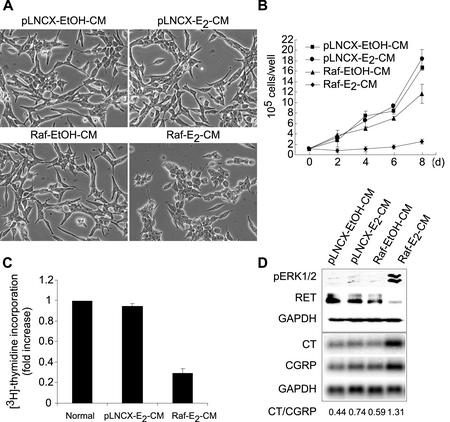
Activation of Raf in the human MTC cell line TT has been shown to result in decreased cellular growth, accompanied by a differentiation program characterized by morphological changes, changes in expression of the neuroendocrine differentiation markers calcitonin and calcitonin-gene-related peptide (CGRP), and downregulation of RET, the proto-oncogene responsible for MTC development in the MEN 2 syndromes (9, 10). In examining the mechanism of this Raf-mediated growth arrest and differentiation, we asked whether Raf could transmit its growth-inhibitory effect by an autocrine-paracrine mode. Therefore, we examined conditioned media from TT cells expressing either an estradiol-activatable allele (43) of c-Raf-1 (TTRaf) (9) or a vector control (TTpLNCX). When conditioned media harvested from TTRaf cells after treatment with estradiol (Raf-E2-CM) were applied to parental TT cells, cells exhibited cell rounding (Fig. 1A), retarded growth (Fig. 1B and C), and G1 cell cycle arrest (Table 1), similar to the changes caused by Raf activation (9, 10). This treatment also induced phosphorylation of ERK1/2, increased expression of the calcitonin/CGRP gene, and downregulation of RET (Fig. 1D), which had been shown to be regulated by Raf (9, 10). Treatment with the control conditioned media did not cause any change in phenotype or cell growth. Taken together, these data indicated that an autocrine-paracrine pathway is induced upon Raf activation, and the pathway is sufficient to mediate the growth-inhibitory effects of Raf in MTC cells.
FIG. 1.
The effects of conditioned media on MTC cells. TT cells were treated with the conditioned medium Raf-E2-CM and the control conditioned media pLNCX-EtOH-CM, pLNCX-E2-CM, and Raf-EtOH-CM. Cells were observed for changes in morphology (A), growth (B), incorporation of [3H]thymidine into DNA in 6 h (C), and expression of phosphorylated ERK1/2 and RET (Western blotting) or calcitonin genes (Northern hybridization) after 2 days (D). Data (mean ± standard error) are from a representative experiment performed in triplicate. P value is <0.001 for Raf-E2-CM compared to Raf-EtOH-CM on a cell growth curve (one-way analysis of variance). Experiments were repeated at least three times with similar results.
TABLE 1.
Cell cycle analysis of conditioned media-treated TT cellsa
| Treatment | % of cells in phase
|
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Normal | 75.0 ± 2.62 | 18.3 ± 0.85 | 4.95 ± 0.01 |
| LIF | 86.3 ± 0.94 | 8.86 ± 1.48 | 3.94 ± 0.26 |
| pLNCX-E2-CM | 77.3 ± 0.79 | 16.9 ± 0.29 | 5.21 ± 0.31 |
| Raf-E2-CM | 83.0 ± 2.23 | 9.30 ± 0.30 | 4.47 ± 0.50 |
| Raf-E2-CM + anti-LIF | 76.4 ± 2.06 | 17.9 ± 2.09b | 5.31 ± 0.34 |
| Raf-E2-CM + anti-gp130 | 78.3 ± 2.60 | 16.6 ± 2.29b | 4.52 ± 0.76 |
Data (means ± standard errors) are from a representative experiment performed in triplicate. Experiments were repeated at least three times with similar results.
P < 0.02 compared to Raf-E2-CM (one-way analysis of variance).
Purification and identification of the autocrine-paracrine factor.
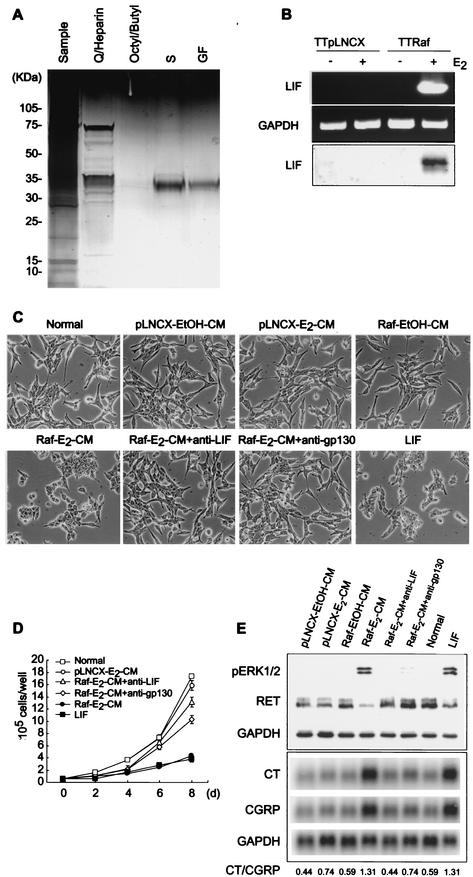
We sought to purify and identify the growth-inhibitory factor(s) in Raf-E2-CM. The conditioned medium was concentrated by ultrafiltration, dialyzed, and subjected to a series of protein purification columns as described in Materials and Methods. Column fractions were assayed for growth and differentiation activity on TT cells. Both the growth-inhibitory and differentiating activities copurified through these column chromatography steps, suggesting that they were mediated by the same protein. The final purification step, gel filtration, yielded a prominent 35-kDa protein detected by PAGE (Fig. 2A), which also appeared as a single spot with two-dimensional electrophoresis (data not shown). This purification product was digested by trypsin, fractionated by reverse-phase high-performance liquid chromatography, and subjected to matrix-assisted laser-desorption ionization-time of flight and electrospray ionization mass spectrometry. Analysis of mass spectrometry data identified it as human LIF.
FIG.2.
LIF is the autocrine growth inhibitor induced by Raf. (A) Conditioned medium was concentrated by ultrafiltration, dialyzed (sample), and subjected to a series of protein purification columns, consisting of the anion exchanger unoQ6 coupled with heparin-Sepharose (Q/Heparin), octyl-Sepharose coupled with butyl-Sepharose (Octyl/Butyl; flow through), the cation exchanger unoS1 (S), and Superdex G200 gel filtration (GF). Active fractions from each column were resolved by SDS-PAGE and visualized by silver staining. (B) Expression of LIF upon Raf activation was investigated by RT-PCR of total RNA from TTpLNCX or TTRaf cells treated with ethanol or estradiol (top and middle panels) and by Western blot detection of LIF in cell culture media (bottom panel). (C to E) TT cells were treated with the control conditioned media, Raf-E2-CM, LIF-neutralized Raf-E2-CM (Raf-E2-CM+anti-LIF), and LIF. Cells were also pretreated with anti-gp130 blocking antibody prior to Raf-E2-CM treatment (Raf-E2-CM+anti-gp130). Cells were then observed for changes in morphology (C), growth (D), and expression of phosphorylated ERK1/2, RET (Western hybridization), or calcitonin genes (Northern hybridization) after 2 days (E). Data (mean ± standard error) are from a representative experiment performed in triplicate. P value is <0.001 for Raf-E2-CM+anti-LIF and Raf-E2-CM+anti-gp130 compared to Raf-E2-CM on cell growth curve (one-way analysis of variance). Experiments were repeated at least three times with similar results.
It remained necessary to show that LIF was responsible for the growth inhibition and differentiation activities in Raf-E2-CM. Expression and secretion of LIF was markedly induced upon Raf activation, as detected by RT-PCR and Western blot analysis (Fig. 2B). Recombinant LIF reproduced all of the observed responses elicited by the purified Raf-E2-CM fraction, inducing morphological changes (Fig. 2C), growth inhibition (Fig. 2D) coupled with G1 arrest (Table 1), phosphorylated ERK, calcitonin and CGRP expression, and down-regulation of RET (Fig. 2E). In addition, neutralizing antibodies for LIF (anti-LIF) or for gp130 (anti-gp130), a component of the LIF receptor, could block the effects of Raf-E2-CM (Fig. 2C, D, and E and Table 1). Each of these neutralizing antibodies, by itself, did not cause any effect on the cells (data not shown). These results clearly indicated that LIF is the sole autocrine-paracrine factor, secreted upon Raf activation, responsible for the differentiation and growth inhibition of TT cells.
LIF production provides a signaling bridge between the Ras/Raf/MEK/ERK pathway and the JAK-STAT3 pathway.
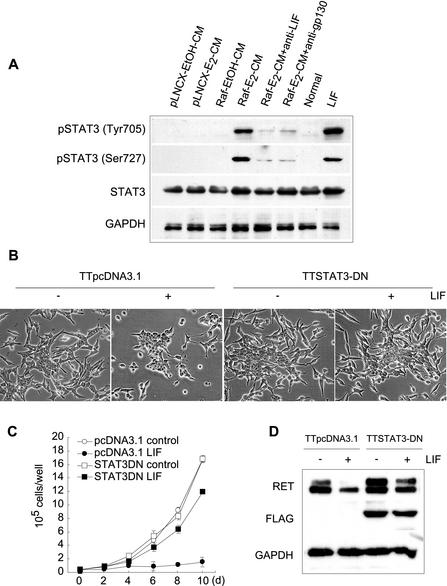
Binding of LIF to the LIFR-gp130 receptor is known to activate members of the JAK-STAT pathway in a cell-type-specific manner, most commonly utilizing JAK1 and STAT3 (3, 6). Upon treatment of TT cells with either Raf-E2-CM or LIF, STAT3 was significantly phosphorylated on tyrosine 705 and serine 727 (Fig. 3A); STAT3 is known to require phosphorylation on tyrosine 705 for activity and on serine 727 for maximal activation (7). We could not detect tyrosine-phosphorylated STAT1 (Tyr 701), STAT5 (Tyr 694), or STAT6 (Tyr 641) (data not shown). These data suggested that STAT3 may mediate LIF effects on differentiation and growth arrest in TT cells. We further examined the role of STAT3 in LIF-mediated growth inhibition and differentiation, using a TT cell line stably harboring a dominant-negative STAT3 construct (TTSTAT3-DN). Overexpression of dominant-negative STAT3 attenuated the effect of LIF on cell morphology (Fig. 3B), growth rate (Fig. 3C), and expression of RET (Fig. 3D). Similar results were obtained by adenovirus-mediated introduction of the dominant-negative STAT3 gene (see Fig. 5C for morphology and Table 2 for cell cycle analysis; data not shown for RET). Taken together, these experiments indicated that the JAK-STAT3 signal transduction pathway is essential for mediating the effects of LIF in TT cells.
FIG. 3.
STAT3 is essential for LIF action. (A) TT cells were treated with conditioned media or LIF for 2 days and examined by Western blot hybridization for STAT3 expression. (B to D) TTSTAT3-DN cells or the control TTpcDNA3.1 cells were treated for 2 days with LIF and observed for changes in morphology (B), growth (C), and downregulation of RET expression (D). The presence of STAT3-DN was detected by Western analysis of the C-terminal FLAG tag. Data (mean ± standard error) are from a representative experiment performed in triplicate. P value is <0.0001 for STAT3DN-LIF compared to pcDNA3.1-LIF on a cell growth curve (one-way analysis of variance). Experiments were repeated at least three times with similar results. Experiments were also done with other independent stably transfected clones with similar results (data not shown).
FIG. 5.
Raf has an intracellular growth-inhibitory pathway independent of LIF/JAK/STAT3. TTRaf cells were treated for 1 day with estradiol in the presence of the anti-LIF neutralizing antibody or anti-gp130 blocking antibody and observed for changes in expression of RET and phosphorylation of STAT3 by Western blot hybridization (A) and morphology (B). (C) TT or TTRaf cells infected for 2 days with AdSTAT3-DN were treated with LIF or estradiol, respectively, and observed for changes in morphology and growth. Cells were also infected with an equal dose of AdGFP control virus for comparison. Similar infection ratio was checked by green fluorescent protein expression. Similarity in levels of STAT3-DN expression in TT and TTRaf cells was confirmed by Western analysis of the C-terminal FLAG tag (data not shown). Experiments were repeated at least three times with similar results.
TABLE 2.
Effects of dominant-negative STAT3 expression on cell cycle arrest of MTC cells upon LIF treatment or Raf activation
| Cell type | Infection | Treatment
|
% of cells in phase
|
|||
|---|---|---|---|---|---|---|
| LIF | Estradiol | G0/G1 | S | G2/M | ||
| TT | Control | − | 80.4 ± 0.40 | 13.0 ± 0.56 | 6.54 ± 0.15 | |
| + | 89.0 ± 0.52 | 7.01 ± 0.10 | 4.00 ± 0.41 | |||
| STAT3DNb | − | 81.8 ± 0.07 | 12.0 ± 0.06 | 6.14 ± 0.01 | ||
| + | 84.0 ± 0.75 | 11.1 ± 0.62c | 4.87 ± 0.13 | |||
| TTRaf | Control | − | 79.6 ± 0.03 | 15.7 ± 0.13 | 4.74 ± 0.15 | |
| + | 85.7 ± 0.96 | 6.70 ± 0.19 | 7.60 ± 1.09 | |||
| STAT3DN | − | 80.0 ± 1.06 | 14.9 ± 0.58 | 5.01 ± 0.48 | ||
| + | 88.5 ± 0.02 | 5.09 ± 0.31 | 6.46 ± 0.32 | |||
TT or TTRaf cells infected with AdSTAT3-DN were treated for 2 days with LIF or estradiol. Cells were also infected with the control AdGFP for comparison. Data (means ± standard errors) are from a representative experiment performed in triplicate. Experiments were repeated at least three times with similar results.
STAT3 DN, dominant-negative STAT3.
P < 0.02 compared to TT-control-LIF (one-way analysis of variance).
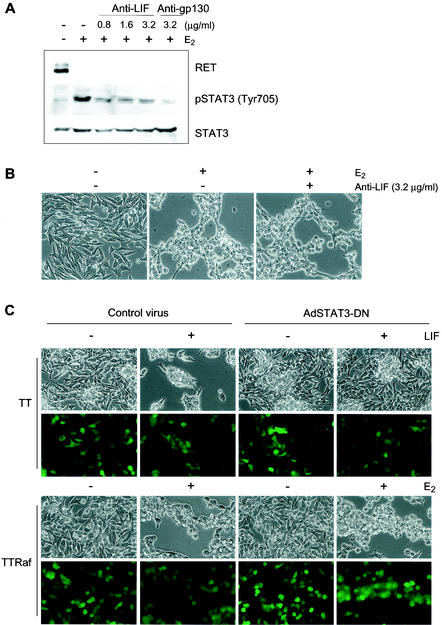
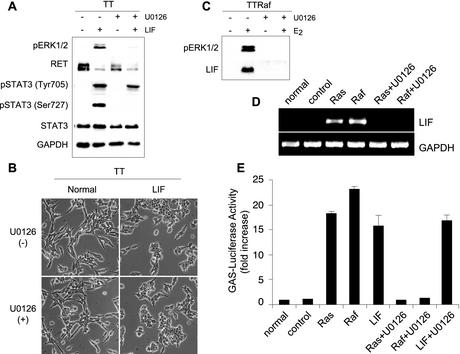
In addition to activation of the JAK-STAT pathway, LIF has been reported to activate mitogen-activated protein kinases and the PI3-kinase/Akt pathway (1, 35, 44, 52). LIF treatment induced phosphorylation of ERK1/2 in TT cells (Fig. 2E), and we have previously shown that activation of MEK/ERK is essential for Raf-mediated differentiation and growth arrest in TT cells (10). Nevertheless, the effects of LIF that we have observed in TT cells are independent of its activation of ERK. Pretreatment of TT cells with the MEK inhibitor U0126 prior to the addition of LIF or Raf-E2-CM blocked phosphorylation of ERK (Fig. 4A), but the cells still displayed the typical LIF-induced morphological changes (Fig. 4B) and downregulation of RET expression (Fig. 4A). U0126 treatment did not also affect LIF-induced phosphorylation of tyrosine 705 of STAT3 (Fig. 4A) and STAT3 transcriptional activity (Fig. 4E). However, phosphorylation of STAT3 on serine 727 was blocked by this inhibitor (Fig. 4A), indicating that LIF mediates serine 727 phosphorylation via the MEK/ERK cascade, but phosphorylation of STAT3 on serine 727 is not essential for LIF effects in these cells. In contrast, the Raf-induced production of LIF was dependent upon MEK activation, since Raf-mediated LIF expression was blocked by pretreatment with U0126 (Fig. 4C and D). Thus, activation of the MEK/ERK pathway is necessary for Raf-mediated production of LIF, but activation of MEK/ERK is not essential for the subsequent effects of LIF. LIF treatment did not induce phosphorylation of Akt, and pretreatment with the PI3-kinase inhibitor LY294002 did not affect LIF action on TT cells, indicating that the PI3-kinase/Akt pathway is not involved in the effects of LIF in these cells (data not shown).
FIG. 4.
MEK1/2 is essential for production of LIF but is not required for LIF action. (A and B) TT cells were treated for 2 days with LIF in the presence of the MEK1/2 inhibitor U0126 (10 μM) and observed for changes in expression of phosphorylated ERK1/2, RET, and phosphorylated STAT3 by Western blotting (A) and morphology (B). (C) TTRaf cells were treated for 2 days with estradiol in the presence of U0126 and observed for changes in expression of phosphorylated ERK1/2 and LIF by Western blotting. (D and E) TT cells were infected with adenoviruses containing constitutively active Ras V12 or Raf BXB in the presence of U0126. After 2 days, cells were observed for LIF expression by RT-PCR (D) and STAT3 activation (E). The data presented are fold increases of activity upon treatment. P values are <0.005 for Ras, Raf, LIF, and LIF+U0126 compared to the control (one-way analysis of variance). Experiments were repeated at least three times with similar results. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Since we had shown that Ras activation can cause growth arrest and differentiation in TT cells (31), we examined whether Ras also induced LIF expression and STAT3 activation in these cells. Induction of LIF expression and STAT3 activation was observed in TT cells upon infection with an adenovirus harboring constitutively active Ras, and this induction could be blocked by the MEK inhibitor U0126 (Fig. 4D and E). These results demonstrate that activation of Ras leads to activation of LIF/JAK/STAT signaling through the Raf/MEK/ERK pathway.
The Ras/Raf/MEK/ERK pathway can also mediate cell cycle arrest and differentiation through a LIF-independent intracellular mechanism.
In this study, we have shown that the Ras/Raf/MEK/ERK pathway can mediate differentiation and cell cycle arrest in MTC cells through LIF expression and consequent activation of the JAK-STAT3 pathway. We now show that activation of the Ras/Raf/MEK/ERK pathway can also mediate differentiation and growth arrest in TT cells through a second, LIF-independent mechanism. To show this, TTRaf cells were cultured in the presence of anti-LIF neutralizing antibody or anti-gp130 blocking antibody during Raf activation by estradiol treatment. These antibody treatments blocked Raf-mediated activation of STAT3, as demonstrated by Western blotting with an anti-phospho STAT3 antibody (Fig. 5A). Nevertheless, TTRaf cells underwent morphological changes (Fig. 5B) and downregulation of RET expression (Fig. 5A), indistinguishable from cells treated with estradiol alone. Similar results were obtained when cells were infected with an adenovirus encoding dominant-negative STAT3. While these cells were unable to respond to LIF treatment (Fig. 5C and Table 2), they still responded to Raf activation with G1 cell cycle arrest (Table 2), morphological changes (Fig. 5C), and downregulation of RET expression (data not shown). Taken together, these data indicate that the Ras/Raf/MEK/ERK pathway has a second, LIF/JAK/STAT-independent mechanism for inducing cell growth inhibition and differentiation.
Small cell lung cancer (SCLC) cells also produce LIF upon Raf activation.
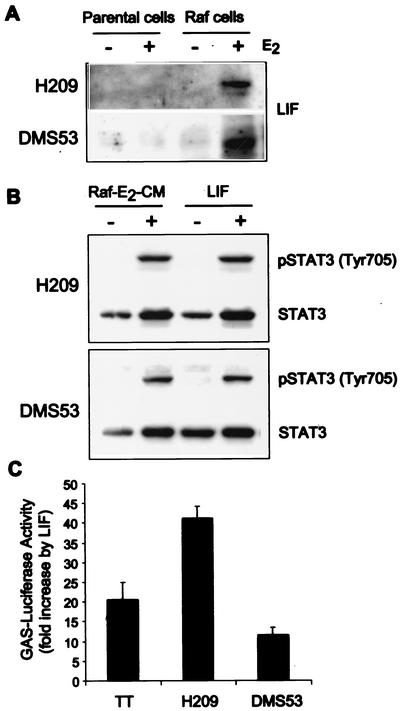
We have previously observed that, like MTC cells, SCLC cell lines undergo growth arrest in response to Raf activation (39, 40). We have explored whether Raf may mediate growth arrest in SCLC cells by the autocrine and intracellular mechanisms we have described for MTC cells. Indeed, we found that the SCLC cell lines NCI-H209 and DMS53 could produce LIF upon Raf activation (Fig. 6A) and that LIF could induce phosphorylation and activation of STAT3 in these cell lines (Fig. 6B and C). The Raf-E2-CM produced from these SCLC cell lines was active in producing growth arrest and morphological changes in TT cells, identical to the effects of the conditioned medium produced from TTRaf cells (data not shown). However, growth rates of the parental NCI-H209 or DMS53 cells were not affected by their own Raf-E2-CM or by recombinant LIF treatment (data not shown). These results suggest that the pathway of Raf-mediated LIF expression and consequent activation of STAT3 is maintained in SCLC cells, but the SCLC cells may be impeded in their ability to undergo LIF-mediated growth arrest at a step distal to STAT3 activation.
FIG. 6.
LIF/JAK/STAT3 signaling in small cell lung cancer cell lines. (A) Production and secretion of LIF was analyzed by Western hybridization of cell culture media from H209 and H209Raf cells or DMS53 and DMS53Raf cells treated with estradiol for 2 days. (B) H209 or DMS53 cells were treated for 2 days with LIF or Raf-E2-CM produced from TTRaf cells, and phosphorylation of tyrosine 705 residue of STAT3 was analyzed. (C) H209 or DMS53 cells were treated for 2 days with LIF, and activation of STAT3 was analyzed. The data presented are fold increases of activity upon LIF treatment. Data (means ± standard deviations) are from a representative experiment performed in triplicate. Experiments were repeated at least three times with similar results.
DISCUSSION
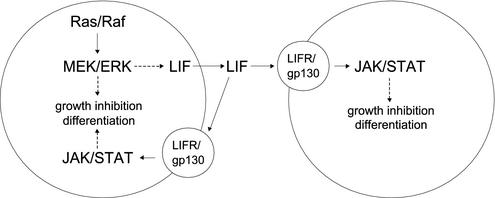
This study shows that activation of the Ras/Raf/MEK/ERK pathway can arrest cell growth by induction of a novel autocrine-paracrine loop involving LIF expression and JAK-STAT3 signaling in MTC cells. In addition, activation of the Ras/Raf/MEK/ERK pathway can also induce growth arrest in these cells by an intracellular pathway independent of LIF and STAT3 (Fig. 7). Our finding of LIF-mediated activation of the JAK-STAT pathway by Ras/Raf further expands the repertoire of signaling pathways known to be subject to activation by Ras/Raf. This autocrine-paracrine mechanism of Ras/Raf-mediated cell cycle arrest additionally suggests a novel means for amplification of a growth arrest stimulus from Ras/Raf, whereby activation of Ras in only a subset of a population of cells could result in the growth arrest of an entire cell population.
FIG. 7.
Signal transduction pathways for Ras/Raf-mediated growth inhibition and differentiation. The potential growth-inhibitory signaling by Ras/Raf is depicted. The autocrine-paracrine signaling diverges from the intracellular signaling distal to MEK/ERK. The extracellular pathway mediates its effect through LIF expression and activation of JAK-STAT3.
Activation of the STAT3 transcription factor can induce growth arrest or growth promotion, depending on the cell context or the stage of tumor progression (25). Similar to our results with MTC cells in this study, several other tumor cell lines have been shown to undergo differentiation and growth arrest upon treatment with LIF or other IL-6 family cytokines (14, 19, 25, 50, 51); indeed, one of the first functions attributed to LIF was its ability to inhibit the growth of the M1 leukemia cell line (14, 24, 61). However, in many cell types, STAT3 activation has been shown to stimulate cell growth. STAT3 activation results in the malignant transformation of fibroblasts (7), and many human cancers have constitutive activation of STAT signaling (13). One may suggest that depending on cell type, STAT3 can activate either of two gene expression programs, one for growth promotion and one for growth arrest. Alternatively, STAT3 may simultaneously activate parallel signals for growth arrest and growth promotion, with the signal for growth arrest dominant over that for growth promotion; such a model has been proposed to explain the disparate effects of Ras in various cell types (28). In cell types in which the growth arrest program is inactivated, STAT3 activation may result in growth promotion. In this model, we envision that the inability of SCLC cells to arrest growth in response to STAT3 activation may suggest that the putative STAT3-dependent growth arrest signal is inactive in these cells. Nevertheless, since we have shown that Raf activation can arrest growth in SCLC cells (39, 40), it appears that SCLC cells retain an intracellular Raf-dependent mechanism of cell arrest.
It is likely that Ras/Raf may also mediate activation of LIF/JAK/STAT signaling in cellular processes in vivo. For example, LIF expression has been shown to be an important component of inflammation, mediated by IL-1 in many tissues (2, 8, 30). IL-1 has been shown to activate c-Raf via a sphingomyelin/ceramide-mediated pathway (18), and in Schwann cells in culture, IL-1-mediated expression of LIF has been shown to be sensitive to inhibitors of both PKC and MEK (8, 30). These findings suggest that IL-1 may stimulate LIF expression via PKC/sphingomyelinase/ceramide-dependent activation of the Ras/Raf/MEK/ERK pathway.
In addition to the autocrine-paracrine activation of STAT3 via LIF, Ras/Raf activation in MTC cells appears to induce growth arrest by a cell-autonomous pathway independent of LIF and STAT3. This second mechanism appears to be intracellular, since immunodepletion of LIF fully abrogates the ability of conditioned media to arrest native TT cells (Fig. 2 and Table 1). Our characterization of the autocrine STAT3-dependent and intracellular STAT3-independent pathways suggests that they may converge on similar or identical effectors of cell cycle arrest. In cell cycle arrest by either pathway, the major changes we have observed in cell cycle-related proteins are depletion of Rb and E2F-1 proteins (data not shown). However, while increased G0/G1-phase and S-phase depletion is a common feature of growth arrest by Raf activation and LIF treatment, it should also be noted that Raf activation increased cells in G2/M but LIF decreased the population. This may indicate a subtle difference of growth arrest mechanisms for each pathway. In future studies, it will be important to elucidate how these proteins are downregulated by the LIF/JAK/STAT3-dependent and independent pathways and their roles in growth arrest of MTC cells.
Activation of multiple parallel pathways appears likely to be a common theme for growth arrest. For example, p53 activation induces cell cycle arrest by targeting numerous cell cycle effectors, including Cdc2, G1 and G2 cyclins, and cyclin-dependent kinases (53, 56). CDKI p21WAF1/CIP1, itself a transcriptional target of p53, can induce both G1 and G2 arrest, at least in part by inhibiting G1 and G2 cyclin-dependent kinases (33). The p19ARF tumor suppressor can induce cell cycle arrest by sequestering the p53 antagonist Mdm2 (48), but p19ARF also induces G1 arrest in murine fibroblasts lacking Mdm2, implying a second target for this tumor suppressor (57). Recently it has been shown that activation of c-Raf in human astrocytes results in growth arrest accompanied by induction of p16INK4a, but when p16INK4a function is disrupted, c-Raf still induces cell cycle arrest, accompanied by p21WAF1/CIP1 induction (11). It has also been shown that Raf activation in murine keratinocytes leads to p53-mediated growth arrest via a p19ARF-dependent or independent mechanism (42). These findings, together with our data, suggest that the use of multiple growth arrest pathways is important for “fail-safe” induction and maintenance of cell cycle arrest.
Acknowledgments
We thank S. Baylin, J. Shaper, and members of our lab for helpful discussions, B. Vogelstein for use of chromatography apparatus, J. Darnell, Jr., and R. Arceci for STAT3 genes, N. Reich and R. Jove for STAT3 reporter constructs, L. Cheng for recombinant LIF protein, L. Parada for Ras V12 and Raf BXB adenoviruses, R. Cole from the Johns Hopkins Mass Spectrometry/Proteomics Facility for mass spectrometry analysis, and L. Meszler from the Cell Imaging Core Facility at Johns Hopkins for excellent technical assistance.
This work was supported by NCI R01-CA47480 and R01-CA85567 (to B.D.N.) and NCI R01-CA70244 (to D.W.B.).
REFERENCES
- 1.Aubert, J., S. Dessolin, N. Belmonte, M. Li, F. R. McKenzie, L. Staccini, P. Villageois, B. Barhanin, A. Vernallis, A. G. Smith, G. Ailhaud, and C. Dani. 1999. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J. Biol. Chem. 274:24965-24972. [DOI] [PubMed] [Google Scholar]
- 2.Auernhammer, C. J., V. Chesnokova, and S. Melmed. 1998. Leukemia inhibitory factor modulates interleukin-1Â-induced activation of the hypothalamo-pituitary-adrenal axis. Endocrinology 139:2201-2208. [DOI] [PubMed] [Google Scholar]
- 3.Auernhammer, C. J., and S. Melmed. 2000. Leukemia inhibitory factor-neuroimmune modulater of endocrine function. Endocr. Rev. 21:313-345. [DOI] [PubMed] [Google Scholar]
- 4.Blalock, W. L., P. W. Moye, F. Chang, M. Pearce, L. S. Steelman, M. McMahon, and J. A. McCubrey. 2000. Combined effects of aberrant MEK1 activity and BCL2 overexpression on relieving the cytokine dependency of human and murine hematopoietic cells. Leukemia 14:1080-1096. [DOI] [PubMed] [Google Scholar]
- 5.Blalock, W. L., M. Pearce, F. Chang, J. T. Lee, S. C. Pohnert, C. Burrows, L. S. Steelman, R. A. Franklin, M. McMahon, and J. A. McCubrey. 2001. Effects of inducible MEK1 activation on the cytokine dependency of lymphoid cells. Leukemia 15:794-807. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet, C., and S. Melmed. 1999. Critical role for STAT3 in murine pituitary adrenocorticotropin hormone leukemia inhibitory factor signaling. J. Biol. Chem. 274:10723-10730. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg, J. F., M. H. Wrzeszczynska, G. Devgan, Y. Zhao, R. G. Pestell, C. Albanese, and J. E. J. Darnell. 1999. Stat3 as an oncogene. Cell 98:295-303. [DOI] [PubMed] [Google Scholar]
- 8.Carlson, C. D., and R. P. Hart. 1996. Activation of acidic sphingomyelinase and protein kinase C zeta is required for IL-1 induction of LIF mRNA in a Schwann cell line. Glia 18:49-58. [DOI] [PubMed] [Google Scholar]
- 9.Carson, E. B., M. McMahon, S. B. Baylin, and B. D. Nelkin. 1995. Ret gene silencing is associated with Raf-1 induced medullary thyroid carcinoma cell differentiation. Cancer Res. 55:2048-2052. [PubMed] [Google Scholar]
- 10.Carson-Walter, E. B., D. P. Smith, B. A. Ponder, S. B. Baylin, and B. D. Nelkin. 1998. Post-transcriptional silencing of RET occurs, but is not required, during raf-1 mediated differentiation of medullary thyroid carcinoma cells. Oncogene 17:367-376. [DOI] [PubMed] [Google Scholar]
- 11.Fanton, C. P., M. McMahon, and R. O. Pieper. 2001. Dual growth arrest pathways in astrocytes and astrocytic tumors in response to Raf-1 activation. J. Biol. Chem. 276:18871-18877. [DOI] [PubMed] [Google Scholar]
- 12.Franza, B. R. J., K. Maruyama, J. I. Garrels, and H. E. Ruley. 1986. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell 44:409-418. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, R., C. L. Yu, A. Hudnall, R. Catlett, K. L. Nelson, T. Smithgall, D. J. Fujita, S. P. Ethier, and R. Jove. 1997. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell. Growth Differ. 8:1267-1276. [PubMed] [Google Scholar]
- 14.Gearing, D. P., N. M. Gough, J. A. King, D. J. Hilton, N. A. Nicola, R. J. Simpson, E. C. Nice, A. Kelso, and D. Metcalf. 1987. Molecular cloning and expression of cDNA encoding a murine myeloid leukemia inhibitory factor (LIF). EMBO J. 6:3995-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groth, A., J. D. Weber, B. M. Willumsen, C. J. Sherr, and M. F. Roussel. 2000. Oncogenic Ras induces p19ARF and growth arrest in mouse embryo fibroblasts lacking p21Cip1 and p27Kip1 without activating cyclin D-dependent kinases. J. Biol. Chem. 275:27473-27480. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 17.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huwiler, A., J. Brunner, R. Hummel, M. Vervoordeldonk, S. Stabel, H. van den Bosch, and J. Pfeilschifter. 1996. Ceramide-binding and activation defines protein kinase c-Raf as a ceramide-activated protein kinase. Proc. Natl. Acad. Sci. USA 93:6959-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamohara, H., K. Sakamoto, T. Ishiko, Y. Masuda, T. Abe, and M. Ogawa. 1997. Leukemia inhibitory factor induces apoptosis and proliferation of human carcinoma cells through different oncogene pathways. Int. J. Cancer 72:687-695. [DOI] [PubMed] [Google Scholar]
- 20.Klesse, L. J., K. A. Meyers, C. J. Marshall, and L. F. Parada. 1999. Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene 18:2055-2068. [DOI] [PubMed] [Google Scholar]
- 21.Kotanides, H., M. Moczygemba, M. F. White, and N. C. Reich. 1995. Characterization of the interleukin-4 nuclear activated factor/STAT and its activation independent of the insulin receptor substrate proteins. J. Biol. Chem. 270:19481-19486. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann, K., E. Janda, C. E. Pierreux, M. Rytomaa, A. Schulze, M. McMahon, C. S. Hill, H. Beug, and J. Downward. 2000. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14:2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd, A. C., F. Obermuller, S. Staddon, C. F. Barth, M. McMahon, and H. Land. 1997. Cooperating oncogenes converge to regulate cyclin/cdk complexes. Genes Dev. 11:663-677. [DOI] [PubMed] [Google Scholar]
- 24.Lotem, J., and L. Sachs. 1982. Mechanisms that uncouple growth and differentiation in myeloid leukemia cells: restoration of requirement for normal growth-inducing protein without restoring induction of differentiation-inducing protein. Proc. Natl. Acad. Sci. USA 79:4347-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, C., and R. S. Kerbel. 1993. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J. Cell Biol. 120:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malumbres, M., I. Perez De Castro, M. I. Hernandez, M. Jimenez, T. Corral, and A. Pellicer. 2000. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15(INK4b). Mol. Cell. Biol. 20:2915-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy, S. A., M. L. Samuels, C. A. Pritchard, J. A. Abraham, and M. McMahon. 1995. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 9:1953-1964. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, F. 1998. Signal transduction. Why Ras needs Rho. Nature 394:220-221. [DOI] [PubMed] [Google Scholar]
- 29.McMahon, M., and D. Woods. 2001. Regulation of the p53 pathway by Ras, the plot thickens. Biochim. Biophys. Acta 1471:M63-M71. [DOI] [PubMed] [Google Scholar]
- 30.Nagamoto-Combs, K., S. A. Vaccariello, and R. E. Zigmond. 1999. The levels of leukemia inhibitory factor mRNA in a Schwann cell line are regulated by multiple second messenger pathways. J. Neurochem. 72:1871-1881. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa, T., M. Mabry, A. de Bustros, J. N. Ihle, B. D. Nelkin, and S. B. Baylin. 1987. Introduction of v-H-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc. Natl. Acad. Sci. USA 84:5923-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newbold, R. F., and R. W. Overell. 1983. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature 304:648-651. [DOI] [PubMed] [Google Scholar]
- 33.Niculescu, A. B., X. Chen, M. Smeets, L. Hengst, C. Prives, and S. I. Reed. 1998. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18:629-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning, Z. Q., J. Li, and R. J. Arceci. 2001. Signal transducer and activator of transcription 3 activation is required for Asp(816) mutant c-Kit-mediated cytokine-independent survival and proliferation in human leukemia cells. Blood 97:3559-3567. [DOI] [PubMed] [Google Scholar]
- 35.Oh, H., Y. Fujio, K. Kunisada, H. Hirota, H. Matsui, T. Kishimoto, and K. Yamauchi-Takihara. 1998. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J. Biol. Chem. 273:9703-9710. [DOI] [PubMed] [Google Scholar]
- 36.Olsen, C. L., B. Gardie, P. Yaswen, and M. R. Stampfer. 2002. Raf-1-induced growth arrest in human mammary epithelial cells is p16-independent and is overcome in immortal cells during conversion. Oncogene 21:6328-6339. [DOI] [PubMed] [Google Scholar]
- 37.Park, B. J., J. I. Park, D. S. Byun, J. H. Park, and S. G. Chi. 2000. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 60:3031-3038. [PubMed] [Google Scholar]
- 38.Ravi, R. K., M. McMahon, Z. Yangang, J. R. Williams, L. E. Dillehay, B. D. Nelkin, and M. Mabry. 1999. Raf-1-induced cell cycle arrest in LNCaP human prostate cancer cells. J. Cell Biochem. 72:458-469. [DOI] [PubMed] [Google Scholar]
- 39.Ravi, R. K., A. Thiagalingam, E. Weber, M. McMahon, B. D. Nelkin, and M. Mabry. 1999. Raf-1 causes growth suppression and alteration of neuroendocrine markers in DMS53 human small cell lung cancer cells. Am. J. Respir. Cell Mol. Biol. 20:543-549. [DOI] [PubMed] [Google Scholar]
- 40.Ravi, R. K., E. Weber, M. McMahon, J. R. Williams, S. Baylin, A. Mal, M. L. Harter, L. E. Dillehay, P. P. Claudio, A. Giordano, B. D. Nelkin, and M. Mabry. 1998. Activated Raf-1 causes growth arrest in human small cell lung cancer cells. J. Clin. Investig. 101:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridley, A. J., H. F. Paterson, M. Noble, and H. Land. 1988. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 7:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roper, E., W. Weinberg, F. M. Watt, and H. Land. 2001. p19ARF-independent induction of p53 and cell cycle arrest by Raf in murine keratinocytes. EMBO Rep. 2:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuels, M. L., M. J. Weber, J. M. Bishop, and M. McMahon. 1993. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 kinase. Mol. Cell. Biol. 13:6241-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiemann, W. P., and N. M. Nathanson. 1994. Involvement of protein kinase C during activation of the mitogen-activated protein kinase cascade by leukemia inhibitory factor. Evidence for participation of multiple signaling pathways. J. Biol. Chem. 269:6376-6382. [PubMed] [Google Scholar]
- 45.Schulze, A., K. Lehmann, H. B. Jefferies, M. McMahon, and J. Downward. 2001. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15:981-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 47.Sewing, A., B. Wiseman, A. C. Lloyd, and H. Land. 1997. High-intensity Raf signal causes cell cycle arrest mediated by p21cip1. Mol. Cell. Biol. 17:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherr, C. J. 2001. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell. Biol. 2:731-737. [DOI] [PubMed] [Google Scholar]
- 49.Shirasawa, S., M. Furuse, N. Yokoyama, and T. Sasazuki. 1993. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260:85-88. [DOI] [PubMed] [Google Scholar]
- 50.Spiotto, M. T., and T. D. Chung. 2000. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate 42:88-98. [DOI] [PubMed] [Google Scholar]
- 51.Spiotto, M. T., and T. D. Chung. 2000. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate 42:186-195. [DOI] [PubMed] [Google Scholar]
- 52.Stephens, J. M., S. J. Lumpkin, and J. B. Fishman. 1998. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J. Biol. Chem. 273:31408-31416. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, W. R., and G. R. Stark. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803-1815. [DOI] [PubMed] [Google Scholar]
- 54.Vale, T., T. T. Ngo, M. A. White, and P. E. Lipsky. 2001. Raf-induced transformation requires an interleukin 1 autocrine loop. Cancer Res. 61:602-607. [PubMed] [Google Scholar]
- 55.Vindelov, L. L., I. J. Christensen, and N. I. Nissen. 1983. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3:323-327. [DOI] [PubMed] [Google Scholar]
- 56.Wang, X. W., and C. C. Harris. 1997. p53 tumor-suppressor gene: clues to molecular carcinogenesis. J. Cell Physiol. 173:247-255. [DOI] [PubMed] [Google Scholar]
- 57.Weber, J. D., J. R. Jeffers, J. E. Rehg, D. H. Randle, G. Lozano, M. F. Roussel, C. J. Sherr, and G. P. Zambetti. 2000. p53-independent functions of the p19ARF tumor suppressor. Genes Dev. 14:2358-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinberg, R. A. 1997. The cat and mouse games that genes, viruses, and cells play. Cell 88:573-575. [DOI] [PubMed] [Google Scholar]
- 59.Wood, K. W., H. Qi, G. D'Arcangelo, R. C. Armstrong, T. M. Roberts, and S. Halegoua. 1993. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc. Natl. Acad. Sci. USA 90:5016-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods, D., D. Parry, H. Cherwinski, E. Bosch, E. Lees, and M. McMahon. 1997. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21cip1. Mol. Cell. Biol. 17:5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto-Yamaguchi, Y., M. Tomida, and M. Hozumi. 1992. Prolongation by differentiation-stimulating factor/leukemia inhibitory factor of the survival time of mice implanted with mouse myeloid leukemia cells. Leuk. Res. 16:1025-1029. [DOI] [PubMed] [Google Scholar]
- 62.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]