Abstract
Fyn, a member of the Src-family protein-tyrosine kinase (PTK), is implicated in learning and memory that involves N-methyl-d-aspartate (NMDA) receptor function. In this study, we examined how Fyn participates in synaptic plasticity by analyzing the physical and functional interaction between Fyn and NMDA receptors. Results showed that tyrosine phosphorylation of NR2A, one of the NMDA receptor subunits, was reduced in fyn-mutant mice. NR2A was tyrosine-phosphorylated in 293T cells when coexpressed with Fyn. Therefore, NR2A would be a substrate for Fyn in vivo. Results also showed that PSD-95, which directly binds to and coclusters with NMDA receptors, promotes Fyn-mediated tyrosine phosphorylation of NR2A. Different regions of PSD-95 associated with NR2A and Fyn, respectively, and so PSD-95 could mediate complex formation of Fyn with NR2A. PSD-95 also associated with other Src-family PTKs, Src, Yes, and Lyn. These results suggest that PSD-95 is critical for regulation of NMDA receptor activity by Fyn and other Src-family PTKs, serving as a molecular scaffold for anchoring these PTKs to NR2A.
The N-methyl-d-aspartate (NMDA) receptor channel is essential for multiple functions of glutamate neurotransmission including those involved in learning, memory, and brain development (1, 2). NMDA receptors consist of two distinct types of subunits: NMDAR1 (NR1) and NMDAR2A–2D (NR2A–2D) (1, 2). NMDA channel activity is dynamically regulated by modulation of both intracellular and extracellular sites (3). Protein–tyrosine phosphorylation also regulates NMDA receptor activity (3–6), like those of other ion channels (7). Namely, NMDA receptor-mediated currents are potentiated by tyrosine kinases and suppressed by tyrosine phosphatases. One to two percent of the receptor subunits NR2A and NR2B are tyrosine-phosphorylated in the brain (8, 9). Moreover, tyrosine phosphorylation of NMDA receptor subunits is increased during long-term potentiation (LTP) and taste learning (10–12). These findings suggest that tyrosine phosphorylation of NMDA receptors is involved in modulation of neuronal functions. However, the molecular mechanisms by which NMDA receptors are tyrosine-phosphorylated are unknown.
Fyn is a member of the Src family of nonreceptor protein–tyrosine kinases (PTKs). The physiological importance of Fyn in the nervous system has been suggested by analyses of fyn-mutant mice. These mutant mice showed various neural defects including defective LTP, impaired spatial memory, uncoordinated hippocampal structure, increased fearfulness, impaired myelination, and ethanol sensitivity (13–18). These multiple effects of Fyn deficiency in the brain indicate that Fyn is involved in several neuronal signaling pathways (17, 19, 20). Because Src is activated during hippocampal LTP induction (6), involvement of other Src-family PTKs in synaptic plasticity is also suggested. However, little is understood about the molecular mechanisms by which these PTKs participate in synaptic plasticity.
Recently, the PSD-95 (postsynaptic density-95, also named SAP90, synapse-associated protein-90) subfamily of the membrane-associated guanylate kinase proteins has been implicated in clustering and targeting of NMDA receptors (21, 22). There are four members of the PSD-95 family in mammals: PSD-95, SAP97/hDlg, chapsyn-110/PSD-93, and SAP102 (21, 22). PSD-95 consists of three PDZ (PSD-95/Dlg/ZO-1) domains, an SH3 (Src Homology 3) domain, and a carboxy-terminal guanylate kinase domain (21). Direct binding of PSD-95 to NR2 subunits requires the two amino-terminal PDZ domains of PSD-95 and the conserved motif (-ESXV) at the carboxy terminus of NR2 subunits (23, 24). NMDA receptors and PSD-95 cocluster in cotransfected cells (25). Furthermore, both proteins are colocalized in primary neurons (23). The molecules involved in regulation of the receptor function appear to be recruited to the proximity of the receptor at synapses.
In this report, we show that Fyn is involved in tyrosine phosphorylation of the NMDA receptor subunit NR2A and that PSD-95 is important for the tyrosine phosphorylation event.
MATERIALS AND METHODS
Cells, Antibodies, and Reagents.
Human embryonic kidney 293T cells were maintained in DMEM/10% fetal bovine serum. Cell culture was performed at 37°C under 5% CO2. Anti-NR2A mAb was raised against the carboxy-terminal cytoplasmic region of NR2A. This antibody recognized a 180-kDa protein present in NR2A-expressing 293T cells, but not in parental cells, and was used as otherwise indicated. Anti-Src mAb was a gift from Y. Fukui (University of Tokyo). Anti-Fyn, anti-Lyn, and anti-Yes mAbs were purchased from Wako Biochemicals (Osaka) (17). Antiinfluenza hemagglutinin (HA) mAb (12CA5) was from Boehringer Mannheim. Rabbit anti-Fyn and goat anti-NR2A polyclonal antibodies were from Santa Cruz Biotechnology. Rabbit anti-NR1 and anti-NR2B polyclonal antibody were from Chemicon. Biotinylated antiphosphotyrosine mAb 4G10 was from Upstate Biotechnology (Lake Placid, NY). Rabbit anti-PSD-95 polyclonal antibody was produced and purified as described (26). Pefabloc SC, a serine protease inhibitor, was from Merck. (+)-MK-801, an NMDA receptor antagonist, was from Research Biochemicals.
DNA Constructs.
The cDNAs encoding NR2A (2) and PSD-95 (26) were subcloned into an SRα-driven mammalian expression vector, pME18S (27). For epitope-tagging of NR1, NR1 cDNA (1) was inserted in-frame with the oligonucleotides encoding an HA-epitope-containing sequence DYPYDVPDYASLV at HincII site that encoded amino acid residues 27 and 28. The resultant cDNA, HA-NR1, was subcloned to pME18S. Plasmids encoding FynR176K mutant and PSD-95 mutants, with carboxy-terminal or internal deletions, were generated by the method of Kunkel (28) and endonuclease digestion. These cDNAs were then cloned into pME18S or its derivative, pME18SM (27). For MBP (maltose-binding protein)-fusion protein production, the fyn cDNA fragments encoding SH3-SH2-linker (T82 to R268), SH3 (T82 to E148), SH2-linker (D142 to R268), SH2 (W149 to C246), and SH2 (W149 to C246) with R176K mutations were cloned in-frame into the pMAL-c plasmid (NEB, Beverly, MA) (29). The constructs were verified by DNA sequencing. The expression plasmids pME-Fyn, -FynY531F, -FynK299M, and -LynY508F have been described (27, 30).
Transient Cell Transfection.
293T cells (1.5 × 106) were transfected with combinations of expression plasmids (5 μg each) by the standard calcium phosphate method. The amount of DNA transfected was adjusted in each experiment by using a control expression vector, pME18SM. When HA-NR1 and NR2A were coexpressed, (+)-MK-801 (10 μM) was added to the media to prevent glutamate-induced cell death. Two days after transfection, cells were collected for protein extraction.
Preparation of Lysates and Immunoprecipitation.
For preparation of cell membrane fractions, cells were washed with PBS and then suspended in 1 ml of hypotonic buffer A (10 mM Hepes-NaOH, pH 7.4/10 mM NaCl/1.5 mM MgCl2/0.2 mM Na3VO4/0.5 mM DTT/0.1 mM Pefabloc SC with aprotinin at 50 units/ml). The cells were kept on ice for 15 min, then homogenized in a Dounce homogenizer with a tightly fitting pestle. Nuclei were separated by centrifugation at 400 × g for 5 min. The supernatant (800 μl) was mixed with one-tenth vol of buffer B (0.3 M Hepes-NaOH, pH 7.4/1.4 M NaCl/30 mM MgCl2), and centrifuged at 100,000 × g for 20 min. The resultant pellet (membrane fraction) was lysed in 1 ml of TNE buffer [1% (wt/vol) Nonidet P-40/50 mM Tris⋅HCl, pH 8.0/120 mM NaCl/5 mM EDTA/0.2 mM Na3VO4 with aprotinin at 50 units/ml]. Typically, 800 μl of lysates were used for immunoprecipitation. For preparation of whole-cell lysates of telencephalons, samples were homogenized in 0.2 vol. (ml/g tissue) of RIPA buffer [1% (wt/vol) Nonidet P-40/0.1% sodium deoxycholate/50 mM Tris⋅HCl, pH 8.0/120 mM NaCl/5 mM EDTA/0.2 mM Na3VO4 with aprotinin at 50 units/ml] containing 0.5% SDS. The lysates were boiled for 5 min and diluted with 4 vol of RIPA buffer. Synaptosomes of mouse brains, isolated essentially as described (31), were lysed in TNE buffer. For immunoprecipitation, lysates were cleared by centrifugation with an excess amount of Protein G Sepharose (Pharmacia) and then incubated with 2–5 μg of an appropriate antibody on ice for 1 hr. Immune complexes were collected on Protein G Sepharose (15 μl) and washed five times with lysis buffer. For experiments with MBP fusion protein, MBP fusion proteins were produced in Escherichia coli DH5α and purified basically as recommended in the manufacturer’s protocol (NEB). Parental or PSD-95-expressing 293T cells were lysed in TNE buffer, and the cleared lysates were incubated at 4°C for 4 hr with 5 μg of MBP-fusion protein immobilized on amylose resin. Bound proteins were separated by centrifugation and washed with TNE buffer.
Immunoblotting.
Immunoprecipitates or lysates were resolved by SDS/7.5% PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad). Then the membranes were blocked and probed with appropriate antibodies (32). To test for phosphotyrosine, the membranes were probed with biotinylated 4G10 and then incubated with horseradish peroxidase-conjugated streptavidin (Amersham).
RESULTS
Reduced Tyrosine Phosphorylation of NR2A in fyn-Deficient Mice.
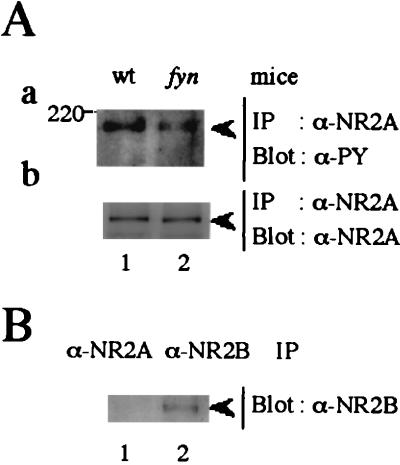
Recent studies have shown that Src-family PTKs potentiate both the NMDA channel in primary culture and the recombinant NR1/NR2A channel (4–6). Because fyn-mutant mice show defects in NMDA receptor-dependent LTP and spatial memory (13), we examined whether tyrosine phosphorylation of NR2A was affected in the mutant mice. NR2A was immunoprecipitated from lysates of the telencephalons of wild-type and fyn-mutant mice (14–18), which were boiled to dissociate the NMDA receptor subunits. Results showed that the level of tyrosine-phosphorylation of NR2A in fyn-mutant mice was significantly reduced, but not eliminated, compared with that of wild-type mice (Fig. 1Aa). The amounts of NR2A in these mice were similar (Fig. 1Ab). Because NR2B, another NMDA receptor subunit with a similar molecular weight to that of NR2A, is also tyrosine-phosphorylated in the brain (9), we examined whether it was coprecipitated in NR2A immunoprecipitates. As shown in Fig. 1B, little NR2B was present in NR2A immunoprecipitates from boiled brain lysates. These results showed that Fyn contributed significantly to tyrosine phosphorylation of NR2A. The residual tyrosine phosphorylation of NR2A in fyn-mutant mice would be caused by other members of the Src-family PTKs or other types of PTKs.
Figure 1.
Reduced tyrosine phosphorylation of NR2A in fyn-deficient mice. (A) Telencephalons from wild-type (wt) or fyn-mutant (fyn) mice were homogenized and boiled in RIPA/0.5% SDS buffer and then diluted with 4 vol of RIPA buffer. The lysates were immunoprecipitated (IP) with goat anti-NR2A antibody and the immunoprecipitates were subjected to immunoblotting (Blot) with the antiphosphotyrosine (PY) antibody 4G10 (a). The filter used in a was stripped, blocked, and reprobed with goat anti-NR2A (b). (B) Lysates of telencephalons from wild-type mice were immunoprecipitated with goat anti-NR2A and anti-NR2B. The immunoprecipitates were probed with anti-NR2B (b). All experiments were performed more than three times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A (180 kDa) and NR2B (180 kDa) are indicated by arrowheads.
Promotion of Fyn-Mediated Tyrosine Phosphorylation of NR2A by PSD-95.
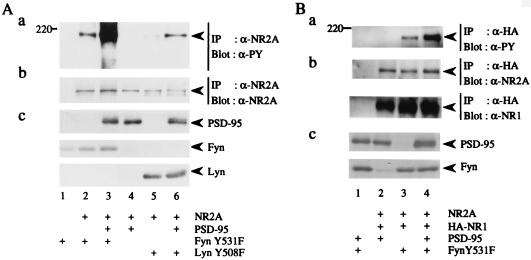
To show that Fyn is involved in tyrosine phosphorylation of NR2A, we transfected 293T cells with the expression plasmid of NR2A alone or together with that of FynY531F [an active form of Fyn (27)]. The anti-NR2A immunoprecipitates from lysates of the membrane fractions of the cells were probed with antiphosphotyrosine (PY) antibody. As shown in Fig. 2, tyrosine phosphorylation of NR2A was induced by the presence of Fyn. Because members of the PSD-95 family bind to and cluster with NMDA receptors (21), we examined the effect of PSD-95 on tyrosine phosphorylation of NR2A by Fyn. Membrane lysates were prepared from 293T cells transfected with various combinations of expression plasmids of NR2A, PSD-95, and FynY531F. Immunoblotting of NR2A immunoprecipitates from the lysates with anti-PY antibody revealed that tyrosine phosphorylation of NR2A was significantly increased in the presence of PSD-95 (Fig. 2A, lanes 2 and 3). Coexpression of PSD-95 also resulted in enhanced tyrosine phosphorylation of NR2A by LynY508F, an active form of another Src-family PTK Lyn (Fig. 2A, lanes 5 and 6). To elucidate whether PSD-95 also promotes tyrosine phosphorylation of NR2A in the functional NMDA receptors (NR1/NR2A), we coexpressed epitope HA-tagged NR1, NR2A, PSD-95, and FynY531F in 293T cells. From the lysates of the cells, NR2A that formed complex with NR1 was immunoprecipitated with anti-HA antibody. Immunoblotting of the HA immunoprecipitates with anti-PY antibody showed that PSD-95 promoted Fyn-mediated tyrosine phosphorylation of NR2A in the presence of NR1 (Fig. 2Ba). These data suggest that PSD-95 is important for tyrosine phosphorylation of functional NMDA receptors.
Figure 2.
Promotion of Fyn-mediated tyrosine phosphorylation of NR2A by PSD-95. (A) 293T cells were transfected with combinations of expression plasmids of NR2A, PSD-95, FynY531F, and LynY508F. Membrane fractions of the cells were lysed in TNE buffer. NR2A was immunoprecipitated (IP) and subjected to immunoblotting (Blot) with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A. The doublet bands in the lane 5 and 6 may be caused by degradation of NR2A (b). The expression levels of PSD-95, FynY531F, and LynY508F were confirmed by immunoblotting (c). (B) 293T cells were transfected with combinations of expression plasmids of NR2A, HA-tagged NR1, PSD-95, and FynY531F. NR2A that formed complex with HA-NR1 was immunoprecipitated (IP) with anti-HA antibody and the HA immunoprecipitates were subjected to immunoblotting (Blot) with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A (b Upper) and anti-NR1 (b Lower) to confirm the amounts of the proteins. The expression levels of PSD-95 and FynY531F were confirmed by immunoblotting (c). All experiments were performed more than four times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A, HA-NR1 (120 kDa), PSD-95 (95 kDa), FynY531F (59 kDa), and LynY508F (56 kDa) are indicated by arrowheads.
PSD-95-Mediated Complex Formation Between Fyn and NR2A.
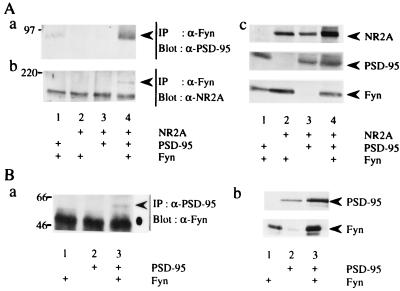
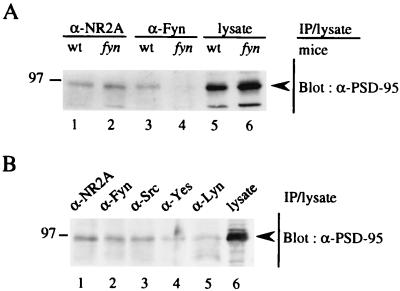
To determine how PSD-95 promotes Fyn-mediated tyrosine phosphorylation of NR2A, we first tested whether PSD-95 increases the kinase activity of Fyn. Fyn was immunoprecipitated from lysates of 293T cells expressing Fyn and/or PSD-95, and the immunoprecipitates were subjected to immune complex kinase assay with the exogenous substrate enolase (17). The activity of Fyn to autophosphorylate and phosphorylate enolase was not affected by the presence of PSD-95 (data not shown). Next, we examined whether PSD-95 promotes interaction of Fyn with NR2A. 293T cells were transfected with expression plasmids of NR2A, PSD-95, and/or Fyn, and then lysates were prepared from the membrane fractions of the cells. Fyn immunoprecipitates from the lysates were probed with anti-PSD-95 or anti-NR2A antibody. PSD-95 was coprecipitated with Fyn only when both proteins were expressed (Fig. 3Aa). Conversely, Fyn was present in PSD-95 immunoprecipitates (Fig. 3Ba), supporting the idea that PSD-95 associated with Fyn when the two are coexpressed in heterologous cells. Furthermore, NR2A was coimmunoprecipitated with Fyn only in the presence of PSD-95 (Fig. 3Ab, lanes 2 and 4). These results showed that PSD-95 induced formation of a ternary complex of NR2A, PSD-95, and Fyn. The finding also suggests that NR2A and Fyn respectively bind to distinct regions of PSD-95. In similar experiments, we found that PSD-95 could associate with Lyn but not with CAKβ/Pyk2, another type of nonreceptor PTKs (data not shown), suggesting that physical interaction of PSD-95 with the Src-family PTKs is important in promoting tyrosine phosphorylation of NR2A. To demonstrate that PSD-95 associates with Fyn in brain synapses, we prepared synaptosomes of wild-type and fyn-mutant mice for coimmunoprecipitation experiments. PSD-95 was coimmunoprecipitated with Fyn from the lysates of wild-type mice, but not from those of mutant mice (Fig. 4A). The amounts of PSD-95 in the lysates and in NR2A immunoprecipitates of wild-type mice and fyn-mutant mice were virtually the same. These results support physical interaction of PSD-95 with Fyn in the brain. As shown in Fig. 4B, PSD-95 also associated with other members of the Src-family PTKs (Src, Yes, and Lyn).
Figure 3.
PSD-95-mediated complex formation between Fyn and NR2A. 293T cells were transfected with combinations of expression plasmids of NR2A, PSD-95, and Fyn. Membrane fractions of the cells were lysed in TNE buffer. (A) Fyn immunoprecipitates (IP) from the lysates were subjected to immunoblotting (Blot) with anti-PSD-95 (a), and anti-NR2A (b). The expression levels of NR2A, PSD-95, and Fyn were confirmed by immunoblotting (c). (B) PSD-95 immunoprecipitates were probed with anti-Fyn (a). The expression levels of PSD-95 and Fyn were confirmed by immunoblotting (b). All experiments were performed three times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A, PSD-95, and Fyn are indicated by arrowheads. The position of the heavy chain of anti-PSD-95 antibody is indicated by a dot.
Figure 4.
Association of Fyn with PSD-95 in mouse synaptosomes. Synaptosomes from wild-type (wt) or fyn-mutant (fyn) mice were lysed in TNE buffer. The lysates were immunoprecipitated with goat anti-NR2A, anti-Fyn, anti-Src, anti-Lyn, or anti-Yes antibody. These antibodies were not crossreactive (data not shown). The immunoprecipitates (IP) and lysates were subjected to immunoblotting with anti-PSD-95 antibody. All experiments were performed more than three times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of PSD-95 are indicated by arrowheads.
Association of NR2A and Fyn with Distinct Regions of PSD-95.
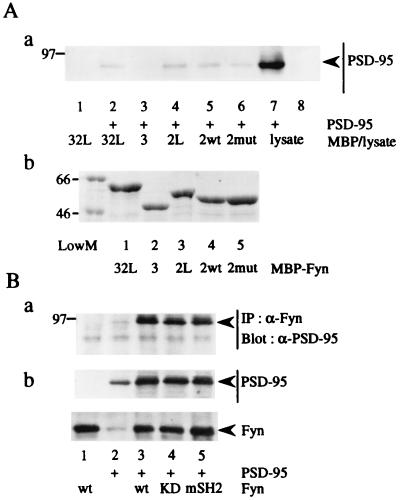
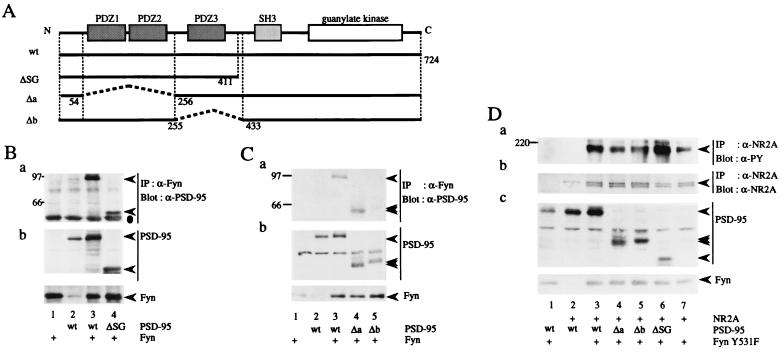
To identify the regions of Fyn that interact with PSD-95, various regions of Fyn were produced as bacterial fusion proteins with MBP. The MBP fusion protein immobilized on amylose resin was incubated with lysates of parental or PSD-95-expressing 293T cells, and then the amounts of PSD-95 bound to the fusion protein were examined (Fig. 5A). PSD-95 selectively bound to MBP/Fyn-SH2 but not to MBP/Fyn-SH3, showing that the SH2 domain of Fyn was involved in the interaction with PSD-95. Moreover, compared with MBP/Fyn-SH2, MBP/Fyn-SH3-SH2-linker and MBP/Fyn-SH2-linker comparably bound to PSD-95. Therefore, the SH3 and the linker region between the SH2 and kinase domain of Fyn were dispensable for the interaction with PSD-95. As the SH2 domain recognizes phosphotyrosine-containing peptide sequences (33), we examined whether association of PSD-95 with Fyn depends on tyrosine phosphorylation of PSD-95 by Fyn. As shown in Fig. 5B, a Fyn kinase-dead mutant FynK299M and a Fyn SH2 mutant FynR176K, in which the arginine residue critical for interaction with phosphotyrosine was mutated to lysine, could associate with PSD-95. Furthermore, the amount of PSD-95 bound to MBP/Fyn-mutant SH2 was comparable to that bound to MBP/Fyn-wild-type SH2 (Fig. 5A). Therefore, tyrosine phosphorylation of PSD-95 is not required for its interaction with Fyn. Then, to determine the region of PSD-95 that is involved in association with Fyn, we constructed a series of carboxy-terminal and internal deletion mutants of PSD-95 (Fig. 6A). These mutants were coexpressed with Fyn in 293T cells, and then their association was examined by coimmunoprecipitation experiments. The PSD-95 mutant ΔSG associated with Fyn (Fig. 6B). The Δa mutant also associated with Fyn (Fig. 6C, lane 4), but not with NR2A (data not shown). This finding is consistent with previous data obtained from in vitro and yeast two-hybrid studies (23, 24). However, the Δb mutant, which carries a deletion of 177 amino acids including the PDZ3 domain, did not associate with Fyn (Fig. 6C, lane 5). This mutant could bind to NR2A (data not shown), showing that NR2A and Fyn associated with distinct regions of PSD-95. Finally, we examined the effects of these PSD-95 mutations on Fyn-mediated tyrosine phosphorylation of NR2A. The Δa and Δb mutants, but not the ΔSG mutant, failed to promote tyrosine phosphorylation of NR2A (Fig. 6D). These observations strongly support the idea that PSD-95 promotes Fyn-mediated tyrosine phosphorylation of NR2A by inducing physical interaction between Fyn and NR2A.
Figure 5.
Involvement of the SH2 domain of Fyn in the interaction with PSD-95. (A) Lysates from parental and PSD-95-expressing (+) 293T cells were incubated with MBP/Fyn-SH3-SH2-linker (32L), MBP/Fyn-SH3 (3), MBP/Fyn-SH2-linker (2mut), MBP/Fyn wild-type-SH2 (2wt), and MBP/Fyn mutant-SH2 fusion protein immobilized on amylose resin. Linker indicates the linker region of the SH2 and kinase domain of Fyn. The proteins bound to the fusion proteins were subjected to immunoblotting (Blot) with anti-PSD-95 (a). The sizes and purities of the fusion proteins were confirmed by Coomassie brilliant blue R-250 staining. The amount of each standard protein was 1 μg (b). (B) 293T cells were transfected with combinations of expression plasmids of PSD-95, Fyn, and its mutants. Fyn immunoprecipitates (IP) were subjected to immunoblotting with anti-PSD-95 (a). The expression levels of PSD-95, Fyn, and its mutants were confirmed by immunoblotting. KD, kinase-dead. mSH2, SH2 mutant. All experiments were performed more than three times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of PSD-95, Fyn, and its mutants are indicated by arrowheads.
Figure 6.
Association of NR2A and Fyn with the distinct regions of PSD-95. (A) Schematic diagram of PSD-95 mutants. Residue numbers correspond to those in the amino acid sequence of PSD-95. The mutant ΔSG carried a deletion of 313 carboxy-terminal amino acids that included both the SH3 and guanylate kinase domains. The Δa mutant lacked 201 amino acids including the PDZ1 and PDZ2 domains. The Δb mutant carried a deletion of 177 amino acids including the PDZ3 domain. wt, wild-type. (B and C) 293T cells were transfected with combinations of expression plasmids of Fyn, PSD-95, or its mutants. Fyn immunoprecipitates (IP) were subjected to immunoblotting with anti-PSD-95 (a). The expression levels of PSD-95, Fyn, and their mutants were confirmed by immunoblotting (b). All experiments were performed more than three times. (D) 293T cells were transfected with combinations of expression plasmids of NR2A, FynY531F, PSD-95, and its mutants. NR2A immunoprecipitates were subjected to immunoblotting with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A. The doublet bands may be caused by degradation of NR2A (b). The expression levels of FynY531F, PSD-95 and its mutants were confirmed by immunoblotting (c). The experiments were performed more than six times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A, PSD-95, Fyn, and their mutants are indicated by arrowheads. The position of the heavy chain of anti-Fyn antibody is indicated by a dot in B.
DISCUSSION
In this report, we show that Fyn significantly contributes to tyrosine phosphorylation of NR2A and that PSD-95 promotes Fyn-mediated tyrosine phosphorylation of NR2A by mediating formation of the complex of Fyn and NR2A.
We found that NR2A as well as NR2B (unpublished results) are hypophosphorylated in fyn-mutant mice. The findings are apparently contradictory to the previous report (18). The contradiction might be explained by the differences in the experimental conditions or circumstances for raising mice. It should be noted that our observation is consistent with findings that Fyn phosphorylates NR2A and NR2B in vitro (34). Furthermore, we found that NR2A was tyrosine-phosphorylated in 293T cells when it was coexpressed with Fyn. These observations suggest that Fyn is a major PTK responsible for tyrosine phosphorylation of NMDA receptors. Tyrosine phosphorylation of NMDA receptors is enhanced during LTP or taste learning (10–12). Adult mice lacking NR2A show defective LTP and impaired spatial memory (35). Therefore, although the physiological roles of tyrosine phosphorylation of NMDA receptors need to be uncovered, reduced tyrosine phosphorylation of NR2A and NR2B may partly explain the defects in LTP and spatial learning in fyn-mutant mice (13). Because Src and Fyn increase glutamate-activated currents in NR1/NR2A, but not in NR1/NR2B channels (5), the roles of tyrosine phosphorylation of NR2A may be different from those of NR2B. It will be important to determine the tyrosine residues phosphorylated by Fyn and other Src-family PTKs on NR2A and NR2B and to characterize the effects of the phosphorylation events on the properties of NMDA receptors.
Although PSD-95 associates with Fyn as well as other members of the Src-family PTKs, Fyn significantly contributed to tyrosine phosphorylation of NMDA receptors. This observation is consistent with the finding that fyn-, but not src- or yes-, mutant mice show defects in NMDA receptor-dependent LTP (13). The phenotypical differences in LTP and spatial learning among these mutant mice would be primarily because of the differences of the expression and activators of each PTK.
The SH2 domain of Fyn was responsible for its association with PSD-95. SH2-dependent protein–protein interactions usually involve protein–tyrosine phosphorylation (33). However, interaction of PSD-95 with the SH2 domain of Fyn did not require tyrosine phosphorylation of PSD-95, as has been observed in several other SH2-dependent protein–protein interactions (36, 37). Indeed, tyrosine phosphorylation of PSD-95 was undetectable in the brain (data not shown), despite the fact that Fyn interacts with PSD-95 in the brain lysates. Other SH2-containing signaling molecules might also interact with PSD-95 in a phosphorylation-independent manner and be recruited to postsynaptic densities.
The PSD-95 family is a group of recently identified “molecular scaffold” proteins (38) that interact with several membrane and intracellular proteins including NMDA receptor subunits and guanylate kinase-associated protein (23, 24, 39–45). The physiological significance of the PSD-95 family has been demonstrated by genetic analyses of discs-large (dlg), a Drosophila homolog of this family (46, 47). The binding of Dlg to Shaker K+ channels was shown to be essential for their in vivo coclustering at larval neuromuscular junctions (46). Furthermore, in dlg mutant flies, the synaptic architecture was found to be abnormally arranged (47). As we showed here, association of Fyn with PSD-95 resulted in recruitment of Fyn to NR2A and in enhanced tyrosine phosphorylation of NR2A by Fyn. Therefore, PSD-95 (and possibly other family members) could also function as an anchoring protein for Fyn and other members of the Src-family in the synapse. Interestingly, the proline-rich region of hDlg binds to the SH3 domain of Lck in T cells, although the significance of this interaction is unknown (48). Previous studies supported the biological importance of kinase-anchoring proteins (49–51). For example, Ste5 was shown to link physically multiple kinases in the MAP kinase cascade required for mating in Saccharomyces cerevisiae (49). A-kinase-anchoring proteins function as scaffold proteins for protein kinase A and are essential for modulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid/kainate receptors by protein kinase A in hippocampal neurons (50). More recently, Drosophila InaD was shown to be a multivalent PDZ domain protein required for assembling different components of the phototransduction cascade (51). The biological role of PSD-95 as a kinase-anchoring protein requires further clarification by experiments on primary neurons.
Finally, the multiple neural defects in fyn-mutant mice have indicated critical roles of Fyn in several signaling pathways in the brain. Involvement of other Src-family PTKs in the brain function has been also reported (6). Our results suggest that proteins that bind to PSD-95 may be phosphorylated by Fyn and other Src-family PTKs. To understand further the role of Src-family PTKs in synaptic plasticity and brain functions, it is important to characterize activators and substrates of these PTKs at synapses.
Acknowledgments
We thank Y. Fukui for anti-Src mAb. We also thank S. Aizawa for fyn-deficient mice. This work was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan.
ABBREVIATIONS
- NMDA
N-methyl-d-aspartate
- NR1
NMDA receptor subunit 1
- NR2A–2D
NMDA receptor subunits 2A–2D
- LTP
long-term potentiation
- PTK
protein-tyrosine kinase
- PSD
postsynaptic density
- PDZ
PSD-95/Dlg/ZO-1
- SH2/3
Src homology 2/3
- HA
influenza hemagglutinin
- MBP
maltose-binding protein
- PY
antiphosphotyrosine
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A Commentary on this article begins on page 335.
References
- 1.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Nature (London) 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 2.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, et al. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 3.McBain C J, Mayer M L. Phys Rev. 1994;74(3):723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y T, Salter M W. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 5.Kohr G, Seeburg P H. J Physiol. 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X M, Roder J C, Davidow J, Salter M W. Science. 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- 7.Moss S J, Gorrie G H, Amato A, Smart T G. Nature (London) 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- 8.Moon I S, Apperson M L, Kennedy M B. Proc Natl Acad Sci USA. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau L F, Huganir R L. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- 10.Rostas J A, Brent V A, Voss K, Errington M L, Bliss T V, Gurd J W. Proc Natl Acad Sci USA. 1996;93:10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblum K, Dudai Y, Richter L G. Proc Natl Acad Sci USA. 1996;93:10457–10460. doi: 10.1073/pnas.93.19.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenblum K, Berman D E, Hazvi S, Lamprecht R, Dudai Y. J Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant S G, O’Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. Nature (London) 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- 15.Miyakawa T, Yagi T, Watanabe S, Niki H. Mol Brain Res. 1994;27:179–182. doi: 10.1016/0169-328x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 16.Miyakawa T, Yagi T, Tateishi K, Niki H. NeuroReport. 1996;7:2723–2726. doi: 10.1097/00001756-199611040-00063. [DOI] [PubMed] [Google Scholar]
- 17.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Nature (London) 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 18.Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tuboi K, Niki H. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- 19.Grant S G, Karl K A, Kiebler M A, Kandel E R. Genes Dev. 1995;9:1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- 20.Beggs H E, Baragona S C, Hemperly J J, Maness P F. J Biol Chem. 1997;272:8310–8319. doi: 10.1074/jbc.272.13.8310. [DOI] [PubMed] [Google Scholar]
- 21.Cho K O, Hunt C A, Kennedy M B. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 22.Craven S E, Bredt D S. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 23.Kornau H C, Schenker L T, Kennedy M B, Seeburg P H. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 24.Niethammer M, Kim E, Sheng M. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, Cho K O, Rothschild A, Sheng M. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 26.Satoh K, Yanai H, Senda T, Kohu K, Nakamura T, Okumura N, Matsumine A, Kobayashi S, Toyoshima K, Akiyama T. Genes Cells. 1997;2:415–424. doi: 10.1046/j.1365-2443.1997.1310329.x. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Kuramochi S, Fusaki N, Nada S, Kawamura T J, Matsuda S, Semba K, Toyoshima K, Okada M, Yamamoto T. J Biol Chem. 1993;268:27413–27419. [PubMed] [Google Scholar]
- 28.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semba K, Nishizawa M, Miyajima N, Yoshida M C, Sukegawa J, Yamanashi Y, Sasaki M, Yamamoto T, Toyoshima K. Proc Natl Acad Sci USA. 1986;83:5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fusaki N, Semba K, Katagiri T, Suzuki G, Matsuda S, Yamamoto T. Int Immunol. 1994;6:1245–1255. doi: 10.1093/intimm/6.8.1245. [DOI] [PubMed] [Google Scholar]
- 31.Carlin R K, Grab D J, Cohen R S, Siekevitz P. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tezuka T, Umemori H, Fusaki N, Yagi T, Takata M, Kurosaki T, Yamamoto T. J Exp Med. 1996;183:675–680. doi: 10.1084/jem.183.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Okumura N K. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 35.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Nature (London) 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 36.Cleghon V, Morrison D K. J Biol Chem. 1994;269:17749–17755. [PubMed] [Google Scholar]
- 37.Itoh T, Miura K, Miki H, Takenawa T. J Biol Chem. 1996;271:27931–27935. doi: 10.1074/jbc.271.44.27931. [DOI] [PubMed] [Google Scholar]
- 38.Faux M C, Scott J D. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim E, Niethammer M, Rothschild A, Jan Y N, Sheng M. Nature (London) 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 40.Brenman J E, Chao D S, Gee S H, McGee A W, Craven S E, Santillano D R, Wu Z, Huang F, Xia H, Peters M F, et al. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 41.Cohen N A, Brenman J E, Snyder S H, Bredt D S. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 42.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- 43.Kim E, Naisbitt S, Hsueh Y P, Rao A, Rothschild A, Craig A M, Sheng M. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg G H, Kawahara T, Kobayashi S, Okada M, Toyoshima K, et al. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 46.Tejedor F J, Bokhari A, Rogero O, Gorczyca M, Zhang J, Kim E, Sheng M, Budnik V. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lahey T, Gorczyca M, Jia X X, Budnik V. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanada T, Lin L H, Chandy K G, Oh S S, Chishti A H. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]
- 49.Choi K Y, Satterberg B, Lyons D M, Elion E A. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 50.Rosenmund C, Carr D W, Bergeson S E, Nilaver G, Scott J D, Westbrook G L. Nature (London) 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 51.Chevesich J, Kreuz A J, Montell C. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]