Abstract
Members of the cadherin family have been implicated as growth regulators in multiple tumor types. Based on recent studies from our laboratory implicating T-cadherin expression in mouse brain tumorigenesis, we examined the role of T-cadherin in astrocytoma growth regulation. In this report, we show that T-cadherin expression increased during primary astrocyte physiologic growth arrest in response to contact inhibition and serum starvation in vitro, suggesting a function for T-cadherin in astrocyte growth regulation. We further demonstrate that transient and stable reexpression of T-cadherin in deficient C6 glioma cell lines results in growth suppression. In addition, T-cadherin-expressing C6 cell lines demonstrated increased homophilic cell aggregation, increased cell attachment to fibronectin, and decreased cell motility. Cell cycle flow cytometry demonstrated that T-cadherin reexpression resulted in G2 phase arrest, which was confirmed by mitotic index analysis. This growth arrest was p53 independent, as T-cadherin could still mediate growth suppression in p53−/− mouse embryonic fibroblasts. T-cadherin-expressing C6 cell lines exhibited increased p21CIP1/WAF1, but not p27Kip1, expression. Lastly, T-cadherin-mediated growth arrest was dependent on p21CIP1/WAF1 expression and was eliminated in p21CIP1/WAF1-deficient fibroblasts. Collectively, these observations suggest a novel mechanism of growth regulation for T-cadherin involving p21CIP1/WAF1 expression and G2 arrest.
Astrocytomas are the most common primary malignant cancer affecting the nervous system, and despite aggressive therapy, the median survival of patients diagnosed with a high-grade astrocytoma (glioma) is only 9 to 12 months (35). These malignant glial tumors are hypothesized to develop as the result of the stepwise accumulation of specific genetic changes in astrocytes or astroglial precursors that promote astrocyte transformation and malignant progression (8). Genetic events important for astrocytoma formation and progression involve alterations in pathways involved in mitogenic signaling, cell cycle growth regulation, and environmental sensing. Previous studies have demonstrated that astrocytomas harbor changes in the epidermal and platelet-derived growth factor signaling pathways, involving amplification, mutation, or overexpression of these receptor tyrosine kinase molecules to result in increased mitogenic signaling. Similarly, astrocytomas harbor alterations in the p53 and retinoblastoma (Rb) cell cycle regulatory pathways. Inactivating mutations in the p53, p16, and Rb genes have been reported as well as overexpression of regulators of these pathways, including cyclin-dependent kinase 4 (cdk4) and MDM2.
In contrast, alterations in molecules involved in environmental sensing have not been explored in great detail in astrocytomas. Gene expression profiling experiments from our laboratory on a mouse astrocytoma model have implicated the novel cadherin molecule, T- or H-cadherin, in astrocytoma progression. Based on these initial observations, we initiated a detailed study of T-cadherin as a growth regulator for astrocytes.
Recent studies have suggested that cadherin family members play important roles in the malignant progression of multiple human cancers. In addition to their established functions in modulating cell-cell adhesion, cell polarity, and morphogenesis (1, 33, 36, 64), the classic cadherin molecules, E-cadherin and N-cadherin, have also been implicated in the molecular pathogenesis of breast, lung, gastric, and liver cancers (5, 6, 29, 40, 48). Loss of E-cadherin expression has been frequently reported in a diverse number of malignant tumors and the reintroduction of E-cadherin into highly invasive tumor cell lines results in suppression of both invasion and growth (70). E-cadherin and N-cadherin are cell surface glycoproteins containing definitive calcium binding extracellular domains and are anchored to the cell membrane through a transmembrane region. E-cadherin-induced growth regulation is mediated by the binding of catenins to the cadherin cytoplasmic tail and results in modulation of Wnt signaling (59), β-catenin/T-cell factor signaling (26), and p27Kip1 up-regulation (11). Similar mechanisms may underlie N-cadherin-mediated growth suppression (40).
In contrast, T-cadherin (for truncated-cadherin, also designated H-cadherin or cadherin-13) lacks the conventional transmembrane and cytoplasmic domains and is anchored to the membrane by a glycosylphosphatidylinositol anchor (1, 53, 69). T-cadherin mediates calcium-dependent cell adhesion and colocalizes with small trimeric G-proteins and SRC family kinases in lipid rafts, where it may be involved in modulating signal transduction pathways (16, 34, 50). The T-cadherin gene maps to human chromosome 16q24, and loss of T-cadherin expression has been reported in sporadic breast, prostate, liver, and lung cancers (38, 68, 74). Within the nervous system, T-cadherin is expressed in astrocytes in both the cerebral cortex and medulla. T-cadherin overexpression in neuroblastoma cells inhibits the mitogenic proliferative response to epidermal growth factor (65) and suppresses motor axon growth (21). The mechanism by which T-cadherin functions as a growth regulator has not been determined.
To gain insights into the potential function of T-cadherin in astrocytoma progression and growth regulation, we examined the effect of T-cadherin overexpression in C6 glioma cells. In this study, we report that T-cadherin expression increases during astrocyte physiological growth arrest in vitro, consistent with a role in growth regulation. In C6 glioma cell lines stably expressing T-cadherin, we observed reduced cell growth, increased cell attachment and homophilic adhesion, and decreased cell motility. The reduced C6 cell proliferation was associated with increased p21CIP1/WAF1, but not p27Kip1, expression and G2 phase growth arrest. Lastly, we demonstrate that T-cadherin growth regulation is dependent on p21CIP1/WAF1, but not p53, expression. Collectively, these results demonstrate that T-cadherin may function as a novel growth regulator in astrocytes relevant to astrocytoma progression by modulating p21CIP1/WAF1-mediated growth regulation.
MATERIALS AND METHODS
Antibodies and cell culture.
Anti-T-cadherin (H126), p21CIP1/WAF1 (C-19), and p27Kip1 (C-19) polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Monoclonal anti-α-tubulin (T9026; clone DM1A) antibody was obtained from Sigma (St. Louis, Mo.).
C6 rat glioma cells and fibroblast cells were passaged in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum and penicillin-streptomycin. p53+/− and p53−/− mouse embryonic fibroblast lines were kindly supplied by Jason Weber (Washington University, St. Louis, Mo.), while p21CIP1/WAF1-deficient 3T3 cells were a gift from Andrew Koff (Memorial Sloan Kettering Cancer Center, New York, N.Y.). Primary astrocyte cultures containing >97% glial fibrillary acidic protein-immunoreactive cells (astrocytes) were generated from day 2 postnatal pups as previously described (32).
T-cadherin construction, sequencing, and coupled transcription-translation assay.
Mouse T-cadherin sequence information was obtained from GenBank (accession no. NM_019707). RNA was extracted from normal mouse brain using TRIzol Reagent (Gibco-BRL Life Technologies), and 3 μg of RNA was subjected to first-strand synthesis using random polyhexamer primers and Superscript ΙΙ reverse transcriptase (Gibco-BRL Life Techologies) at 42°C. Five microliters of first-strand cDNA was PCR amplified in two separate fragments, with the first fragment containing nucleotides 108 (initiation codon) to 1139 (5′-ATGCAGCCGAGAACTCCGCTC and 5′-GTCAGTCCGACATCCAATCC-3′) and the second fragment containing nucleotides 909 to 2253 (stop codon) (5′-GACAGCGTTTGATGCAGATG-3′ and 5′-CTCACAGACCTGACAATAAGC-3′) for 39 cycles with a 62°C annealing step. After fragment 1 was digested with SalI and AvaI and fragment 2 was digested with AvaI and XhoI, the two fragments were ligated to XhoI-digested and calf-intestinal phosphatase-treated pcDNA3.HisC vector. Positive clones were confirmed by direct sequencing using the ABI PRISM dGTP BigDye Terminator ready reaction cycle sequencing kit (version 3.0; Applied Biosystems, Foster City, Calif.). The protein product of T-cadherin plasmid (105 kDa) was identified by coupled in vitro transcription/translation using the anti-T-cadherin (H-126) polyclonal antibody.
Colony formation assay, direct counting, and growth in soft agar.
Colony formation (clonogenic) assays on C6 rat glioma cells and fibroblasts were performed by transfecting cells with equimolar amounts of pcDNA3.T-cadherin and pcDNA3 vector using Lipofectamine (Gibco-BRL Life Sciences). Five 100-mm-diameter plates for each transfection were grown for 14 to 21 days in the presence of G418 at 750 μg/ml. For the colony formation assays on p53−/− and p53+/− mouse embryonic fibroblasts or p21CIP1/WAF1-deficient 3T3 fibroblasts, cotransfections with equimolar amounts of pcDNA3.T-cadherin or pcDNA3 vector along with 10-fold less pBABE.puro plasmid were performed. At 48 h after transfection, cells were split into five 100-mm-diameter plates (105 cells for each dish) and grown for 14 to 21 days in the presence of puromycin (1 μg/ml). The number of colonies greater than 2 mm in diameter was counted after crystal violet staining and the mean and standard deviation (SD) were determined for each transfection. Each of the clonogenic assay experiments was performed at least three times with identical results.
Direct cell counting was conducted in 60-mm-diameter dishes. A total of 104 cells from each C6 clone were seeded in each of three 60-mm-diameter dishes and counted on days 3, 5, and 7. Cells in three dishes were counted for each C6 clone at each time point. The means and SDs were determined for each time point.
Soft agar growth assays were performed in quadruplicate on T-cadherin and vector transfected C6 clones. Briefly, 1,000 C6 cells were equally divided into four 24-well plate wells with medium containing 0.3% Noble agar and grown for 14 to 21 days. The number of colonies was determined by direct counting on an inverted microscope and the mean and SD determined for each clone.
Western blotting.
Western blotting was performed as previously described using ECL chemiluminescence detection (Amersham, Arlington Heights, Ill.) (28). The anti-T-cadherin antibody was used at a 1:1,000 dilution at 4°C overnight. The other antibodies were used at a 1:1,000 dilution at room temperature for 1 h.
Flow cytometry.
DNA content was determined by fluorescence-activated cell analysis of propidium iodide-stained cells on a FACS Calibur flow cytometer (Becton Dickinson). Each analysis was performed in triplicate with identical results. T-cadherin or vector-transfected C6 cells (105) were serum starved for 48 h. The cells were stimulated with DMEM containing 10% serum for 12, 24, and 48 h; harvested; washed with phosphate-buffered saline (PBS); and fixed with 70% ethanol. Immediately prior to the analysis, cells were incubated with fresh propidium iodide containing RNase A for 30 min at 37°C. A total of 104 cells were analyzed from each sample. The debris and doublets were removed, and singlets were analyzed using ModFitLT V2.0 software.
Mitotic index assay.
A total of 104 T-cadherin-expressing C6 and vector-transfected C6 clones were serum starved for 48 h and then stimulated with 10% serum for 0, 4, 8, 12, and 24 h. The cells were trypsinized, washed with ice-cold PBS, and resuspended in 6 ml of a 2% solution containing 3:1 (vol/vol) acetate acid-ethanol at 4°C overnight. Next, the cells were resuspended in 0.5 ml of a solution containing 3:1 (vol/vol) acetate-ethanol and 100 μl of each suspension were spread onto three slides. The slides were air dried for 30 min and stained with Giemsa (LabChem Inc., Pittsburgh, Pa.) for 30 min. The cells were analyzed at a magnification of ×400 to determine the mitotic status of the cells. Nuclei with broken envelopes, chromatin condensation, or typical mitotic figures were counted as mitotic. 200 to 300 cells were counted for T-cadherin-expressing and vector-transfected clones. The percentage of mitotic cells was calculated at each time point for T-cadherin-expressing and vector-transfected C6 clones. The means and SDs were determined for each time point.
Cell spreading.
Sterile glass coverslips were coated with laminin (10 mg/ml; Sigma) at 4°C overnight in 24-well plates and washed three times with complete medium. A total of 105 cells in 1 ml of complete medium was added to each well, and the plates were incubated at 37°C for 90 min. The wells were washed with warm 1% bovine serum albumin (BSA), fixed with 3.7% formaldehyde, and permeabilized with 0.1% Triton X-100 for 10 min. After incubation with 1% BSA for 30 min, the actin cytoskeleton was visualized with BODIPY-conjugated phalloidin (0.2 U in 50 μl; Molecular Probes, Eugene, Oreg.). Coverslips were then washed in PBS and mounted in one drop of Fluoromount G (EM sciences) and examined on a Zeiss Axiophot microscope and photographed.
Cell adhesion.
C6 cell adhesion was performed by precoating a 96-well plate with fibronectin (10 μg/ml; Sigma) in sterile PBS at 4°C overnight. The wells were washed twice with PBS and incubated with 2% heat-inactivated BSA for 2 h at 37°C. The cells were digested with 0.05% trypsin containing 0.5 mM CaCl2 and resuspended at 106 cells/ml in serum-free DMEM and incubated for 2 h at 37°C. Cells (100 μl) were added to each well and allowed to adhere for 1 or 4 h. At the end of the incubation period, cells were washed three times with PBS and stained with 0.5% crystal violet for 30 min at room temperature. The wells were washed three times, and 50 μl of 1% sodium dodecyl sulfate was added to each well overnight at room temperature. The number of adherent cells was quantitated by absorbance at 540 nm. Each experiment was repeated at least three times with identical results.
Cell aggregation assay.
Cells were washed with PBS (without Ca2+ and Mg2+) twice and digested with 0.05% trypsin containing 0.5 mM Ca2+. Ca2+ was used to protect T-cadherin from trypsin digestion. The cells were washed with HCMF (10 mM HEPES [pH 7.4], 0.137 M NaCl, 5.4 mM KCl, 0.34 mM Na2HPO4 · 12H2O, 0.1% glucose) to remove the Ca2+ and were then suspended in 1 ml of HCMF. The cells were resuspended at 105 per 0.5 ml with HCMF, and 0.5 ml of the cell suspension was seeded in each of quadruplicate wells of 24-well plates precoated with 1% BSA. Ca2+ was added to 1 mM final concentration for each well, while the plates were kept on ice. The plates were placed in a 37°C shaker and rotated at 80 rpm for 1 h, which was followed by fixation with 0.5 ml of 8% paraformaldehyde on ice for 15 min. The wells were then gently stirred. The number of aggregates and single cells were counted with a hemacytometer, and the aggregation index (Nt/N0) was calculated (Nt = total particle number, including aggregates and single cells; N0 = total cell number in cell suspension). The assay was repeated three times, and the means and SDs were determined.
Motility assay.
Cells were suspended at 5 × 105 cells/ml in serum-free medium. Two hundred microliters of the cell suspension (105 cells) was added in each of triplicate Transwells with an 8.0-μm-pore size (Fisher Scientific, St. Louis, Mo.). Six hundred microliters of DMEM containing 10% serum were added into each well of 24-well plate. Transwells were placed over the wells of 24-well plate. The plates were incubated at 37°C for 4 h. The membranes were fixed, stained, and removed. The number of cells on the lower surface of membranes was counted and the means and SDs determined. The assay was repeated three times with identical results.
Statistical methods.
Data are presented as the means ± SD and were analyzed with analysis of variance followed by the t test, with significance (P) set at <0.01.
RESULTS
T-cadherin overexpression results in C6 cell growth suppression in vitro.
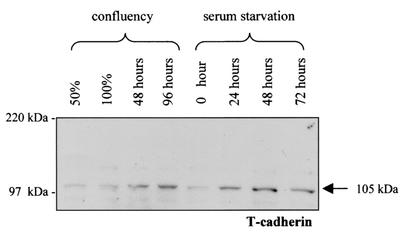
To explore the role of T-cadherin in astrocyte growth regulation, we first examined the expression of T-cadherin under different physiological growth arrest conditions. Primary neocortical astrocyte cultures have previously been shown to undergo growth arrest in response to confluence and serum starvation (27, 31). In these experiments, we observed increased expression of the mature form of T-cadherin (105 kDa) 48 to 96 h after the cells became confluent or within 24 to 48 h of serum starvation at 75% confluence (Fig. 1). This pattern of expression is consistent with T-cadherin expression increasing during physiological growth arrest in astrocytes and suggests that T-cadherin may be involved in the regulation of growth in astrocytes.
FIG. 1.
T-cadherin expression in primary mouse astrocytes under different physiological growth arrest conditions. Western blotting demonstrates that the mature form (105 kDa) of T-cadherin expression gradually increases in primary mouse astrocytes after the cells reached confluency (48 and 96 h after confluence). Similarly, T-cadherin expression gradually increased as a function of time after the cells were serum starved for 24, 48, and 72 h at 75% confluency. The arrow denotes the mature form of T-cadherin expression (105 kDa).
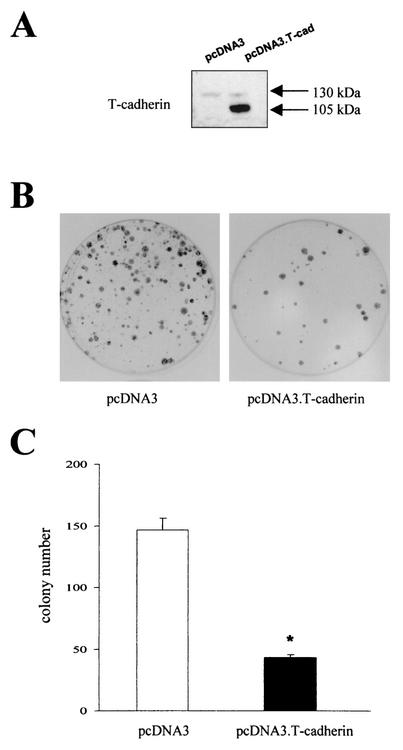
Since normal cultured astrocytes express T-cadherin, we chose to determine the effect of T-cadherin expression on cell growth in C6 rat glioma cells. C6 cells express minimal levels of T-cadherin under all growth conditions compared to normal cultured human fetal or mouse neocortical astrocytes (data not shown). In these experiments, equimolar amounts of pcDNA3.T-cadherin and pcDNA3 were transfected into C6 cells and the colonies greater than or equal to 2 mm were determined after selection in G418 (750 μg/ml) for 14 days. Western blotting demonstrated T-cadherin expression in the pooled cells from the pcDNA3.T-cadherin transfected dishes compared with the pooled cells from the pcDNA3 vector-transfected dishes (Fig. 2A). A 70% decrease in colony number was observed in the pcDNA3.T-cadherin-transfected dishes (Fig. 2B and C), supporting a role for T-cadherin in astroglial growth regulation.
FIG. 2.
Transfection of T-cadherin into C6 glioma cells results in growth suppression. (A) Western blot demonstrates overexpression of the mature (105-kDa) and uncleaved precursor (130-kDa) forms of T-cadherin in C6 cells transfected with pcDNA3.T-cadherin compared to those transfected with pcDNA3 vector. (B) Equimolar amounts of pcDNA3.T-cadherin and pcDNA3 were transfected into C6 cells. Representative plates are shown after 14 days of selection and quantitated in panel C. (C) The single asterisk denotes a statistically significant decrease in colony number in each of the three dishes transfected with pcDNA3. T-cadherin compared to those transfected with pcDNA3 vector (P < 0.01). Error bars, SDs.
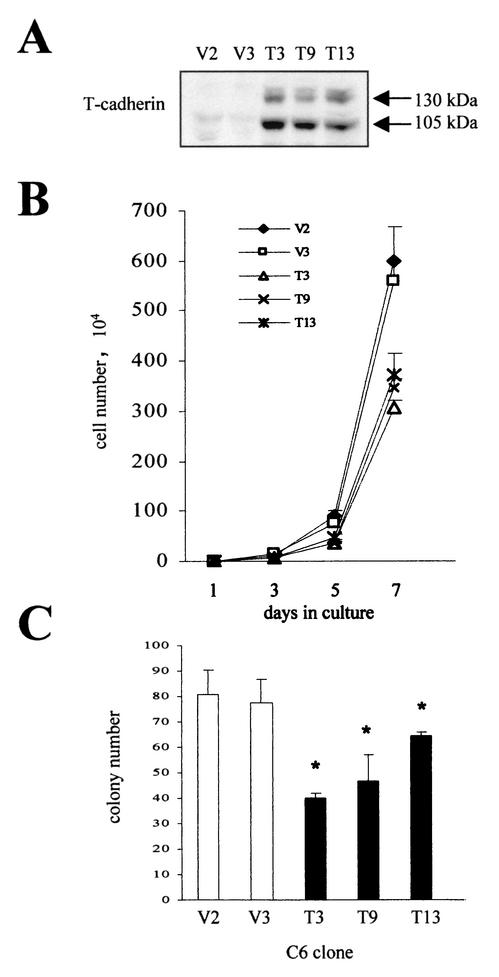
To further study T-cadherin growth suppression, we established constitutively expressing T-cadherin C6 clones. Three T-cadherin-overexpressing C6 cell lines and two vector-transfected C6 lines were selected. T-cadherin clones T3, T9, and T13 express various amounts of T-cadherin compared with vector clones V2 and V3 (Fig. 3A). The same number of T-cadherin-expressing and vector-transfected C6 cells (104) were seeded in 60-mm-diameter dishes and the number of cells counted on days 3, 5 and 7 (prior to confluence). We observed a 33 to 49% decrease in cell number in the T-cadherin-expressing clones compared to vector clones on day 7 (Fig. 3B). Similar results were obtained in soft agar growth assays, where we observed an average 36.3% decrease in colony number in T-cadherin clones compared to vector clones (Fig. 3C). In addition, there is a strong correlation between the level of T-cadherin overexpression in these clones and the magnitude of the growth suppression observed.
FIG. 3.
C6 glioma cell lines constitutively overexpressing T-cadherin exhibit decreased cell proliferation. (A) Western blot demonstrates overexpression of the mature (105-kDa) and precursor (130-kDa) forms of T-cadherin in stably transfected C6 cell clones T3, T9, and T13. (B) Direct cell counting demonstrates that T-cadherin-expressing C6 cell clones T3, T9, and T13 have decreased cell proliferation compared to vector-transfected clones V2 and V3 on day 7 of culture. The means and SDs are shown for each time point. Error bars, SDs. (C) T-cadherin-overexpressing C6 cell clones (T3, T9, and T13) formed fewer colonies in soft agar than vector-transfected C6 cell clones (V2 and V3). The single asterisk denotes a statistically significant decrease in colony number of T-cadherin clones compared to vector clones. Error bars, SDs.
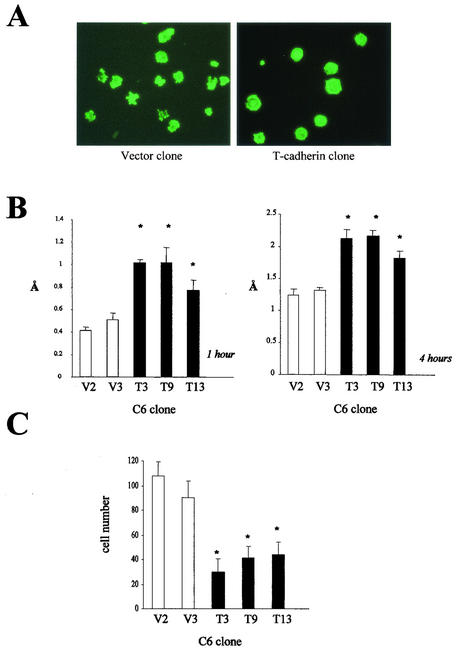
T-cadherin overexpression enhances C6 cell spreading and adhesion, but suppresses cell motility.
Cell spreading, adhesion and motility are important properties of tumor cells, which impact on their ability to invade normal tissue. Cadherin molecules have been shown to modulate cell motility and adhesion. In an effort to determine whether T-cadherin overexpression in C6 glioma cells resulted in similar effects on these tumorigenic features, we analyzed cell spreading, attachment, homophilic adhesion, and motility. In all three T-cadherin-overexpressing C6 cell lines, we observed increased cell spreading, as determined by phalloidin-BODIPY reorganization of the actin cytoskeleton. More than 90% of T-cadherin-expressing cells exhibited more extensive actin cytoskeleton reorganization at 60 to 90 min after initial plating compared with vector-transfected controls (Fig. 4A). To determine whether this increase in cell spreading resulted in increased attachment to fibronectin-coated surfaces, we examined cell attachment at 1 and 4 h after plating. T-cadherin-expressing clones T3, T9, and T13 exhibited significant increases in cell attachment at both time points compared to vector controls (P < 0.01) (Fig. 4B).
FIG. 4.
T-cadherin overexpression enhances cell spreading and attachment, but decreases cell motility. (A) T-cadherin-overexpressing C6 cells exhibit more spreading than vector-transfected cells on laminin-coated coverslips at 90 min, as assessed by actin cytoskeleton reorganization. (B) T-cadherin-overexpressing C6 cell clones (T3, T9, and T13) exhibit enhanced attachment at 1 h (left panel) and 4 h (right panel) compared with vector-transfected C6 clones (V2 and V3). The single asterisk denotes a statistically significant increase in absorbance at 540 nm. Error bars, SDs. (C) T-cadherin-overexpressing C6 cell clones (T3, T9, and T13) demonstrated reduced motility in a Boyden chamber motility assay after 4 h compared to vector-transfected C6 clones (V2 and V3). The single asterisk denotes a statistically significant decrease in motility. Error bars, SDs.
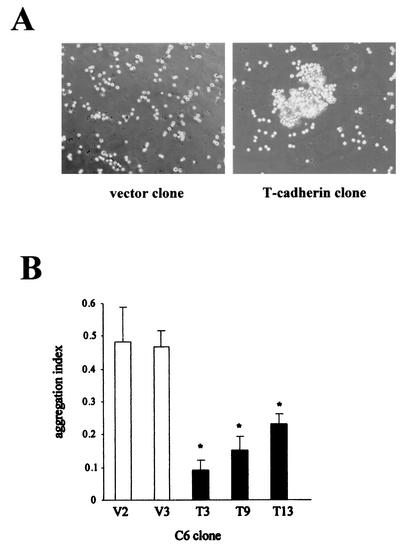
In contrast to the classical cadherins (45, 49), T-cadherin is not concentrated in regions of cell-cell contact (1), but it can induce calcium-dependent homophilic aggregation in CHO cells (69) and in HEK293 cells (54). Under standard in vitro culture conditions, T-cadherin-transfected C6 cells did not exhibit increased cell-cell interactions compared with vector-transfected C6 cells (data not shown). However, in the presence of 1 mM Ca2+, T-cadherin overexpression induced homophilic cell-cell aggregation (Fig. 5A). The aggregation indexes for T-cadherin clones T3, T9, and T13 were reduced by 80.6, 68.4, and 50.9% compared to those of vector clones (P < 0.01) (Fig. 5B), indicating that T-cadherin overexpression in C6 glioma cells results in increased cell-cell adhesion.
FIG. 5.
T-cadherin overexpression induces C6 cell homophilic aggregation. (A) T-cadherin-overexpressing C6 cell clones exhibit extensive cell-cell aggregations in the presence of 1 mM Ca2+. (B) The aggregation index was calculated for each T-cadherin-overexpressing C6 cell clone (T3, T9, and T13) and vector-transfected clone (V2 and V3). The single asterisk denotes a statistically significant decrease in aggregation index in T-cadherin-overexpressing clones. Error bars, SDs.
Lastly, we wished to determine whether these effects of T-cadherin on cell adhesion, spreading, and attachment also resulted in alterations in cell motility using a Boyden chamber assay (Fig. 4C). Compared to vector-transfected C6 cells, we observed a 55 to 70% reduction in cell motility in the T-cadherin-expressing clones (P < 0.01). The magnitude of the effect of T-cadherin overexpression on cell spreading, adhesion, and motility was directly related to the level of T-cadherin overexpression.
T-cadherin overexpression induces G2 arrest and increased aneuploidy in C6 cells.
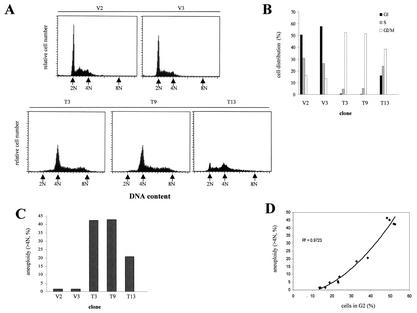
To explore the mechanism by which T-cadherin overexpression results in decreased cell growth, cell cycle analysis of T-cadherin-expressing and vector-transfected C6 clones was conducted. Compared to the vector clones V2 and V3, T-cadherin-expressing clones T3 and T9 demonstrated a significantly increased percentage of cells in G2/M phase (52.6 and 51.84% versus 16.17 and 13.47%) and a dramatically decreased percentage of cells in G1 (0.66 and 0.39% versus 50.6 and 57.9%) (Fig. 6B). Meanwhile, T3 and T9 showed aneuploidy (>4N) DNA histogram patterns with more than 42% of total cell population exhibiting more than 4N DNA content, with some containing 8N DNA content. In contrast, vector clones V2 and V3 showed no such effect, and only less than 1.5% of cells exhibiting aneuploidy (Fig. 6C). Consistent with the dose effect of T-cadherin overexpression on the other phenotypes examined, we observed a smaller increase in the percentage of cells in G2/M with the lowest expressing T-cadherin clone 13 (38.51%) and a decreased percentage of cells in G1 (16.03%) (Fig. 6B). Approximately 20.8% of the T13 cell population exhibited aneuploidy. The proportion of aneuploid cells positively correlates with the percentage of cells in G2 (R2 = 0.9723) (Fig. 6D). These observations demonstrate that the growth suppression associated with T-cadherin expression in C6 cells likely results in increased aneuploidy due to G2 arrest.
FIG. 6.
Cell cycle analysis demonstrates a dramatic increase in the percentage of T-cadherin-overexpressing cells in G2/M phase. (A) The T-cadherin-overexpressing clones T3, T9, and T13 and the vector-transfected clones V2 and V3 were synchronized by serum starvation for 48 h, and DNA content was measured by flow cytometry at 12 h after serum stimulation. The arrows depict the positions of 2N (G1), 4N (G2/M), and 8N. T3 and T9 showed aneuploid DNA histogram patterns, whereas V2 and V3 did not. (B) The cell distribution in G1, S, and G2/M phases is graphically represented for vector-transfected and T-cadherin-expressing C6 clones. T-cadherin-expressing clones T3 and T9 exhibit a high percentage of cells in G2/M phase and a decreased percentage of cells in G1 phase compared to the V2 and V3 clones. The T13 clone shows a slightly increased percentage of cells in G2/M phase and a modestly decreased percentage of cells in G1 phase. (C) T-cadherin-expressing C6 clones demonstrate increased numbers of aneuploid cells (>4N) compared to vector-transfected clones V2 and V3. (D) The proportion of the aneuploid cells directly correlates with the percentage of cells in G2 (R2 = 0.9723).
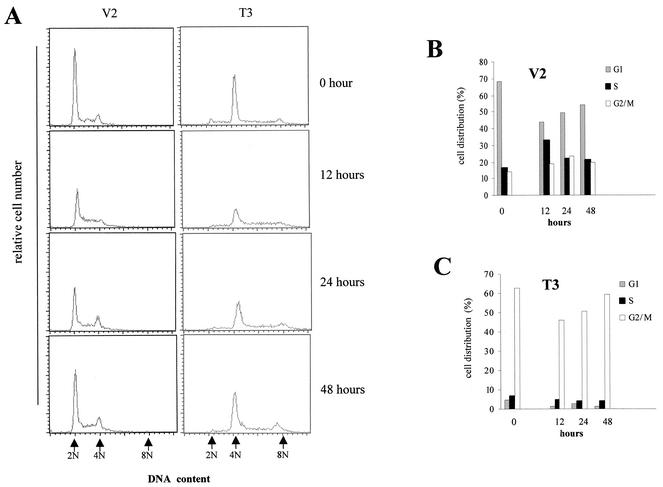
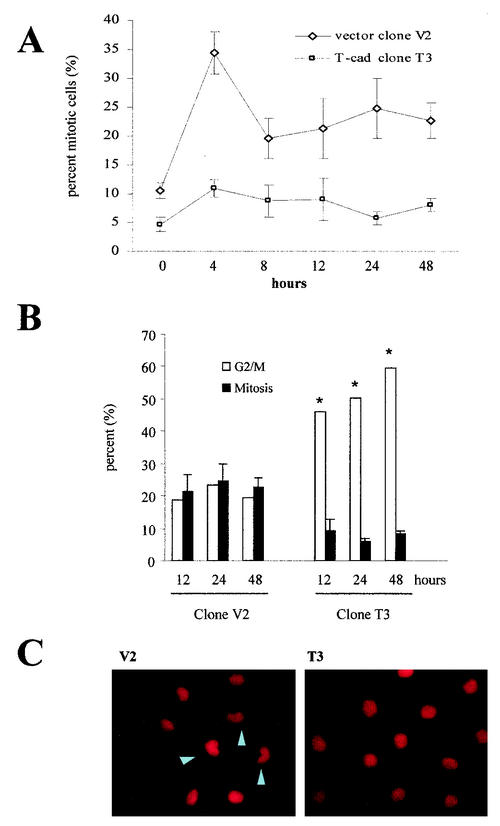
To confirm this effect, we performed additional analyses on the T-cadherin clone T3 and the vector clone V2. After synchronization by serum starvation for 48 h, T-cadherin clone T3 and vector clone V2 were stimulated with DMEM containing 10% serum for 0, 12, 24, and 48 h. Flow cytometry analysis demonstrated that the T3 T-cadherin clone exhibited significantly increased numbers of cells in G2/M phase at all times examined, even with serum starvation (Fig. 7A and C), compared to the V2 vector clone (Fig. 7A and B). Aneuploidy was also observed in the T3 clone at all time points. To provide additional support for a G2 arrest, mitotic index analysis was performed. In these experiments, the V2 clone had a high mitotic index at 12 (21.3% ± 5.2%), 24 (24.8% ± 5.2%), and 48 (22.7% ± 3.0%) h after serum stimulation compared with the T-cadherin-expressing clone T3 at the same time points (9.1 ± 3.7, 5.8 ± 1.1, and 8.1 ± 1.2%, respectively) (Fig. 8A). By comparing this mitotic index data with the flow cytometry data, we observed the same percentages of G2/M phase cells entering mitosis at the different time points for the V2 clone (18.7, 23.26, and 19.53% versus 21.3% ± 5.2%, 24.8%± 5.2% and 22.7% ± 3.0%). However, only 5.8% ± 1.1% to 9.1% ± 3.7% of the T3 cells enter mitosis despite the fact that 45.92 to 59.35% of the cells are in G2/M at 12, 24, and 48 h (Fig. 8B). These results support the notion that there is a G2 arrest associated with T-cadherin expression. Similarly, propidium iodide staining demonstrated that the nuclei of the T-cadherin-expressing C6 cells were rounder and larger than those of the vector-transfected C6 cells (Fig. 8C), which further supports a G2 phase arrest associated with T-cadherin overexpression.
FIG. 7.
T-cadherin overexpression after serum starvation and serum stimulation results in a consistent increase in the percentage of cells in G2/M phase. (A) The T3 T-cadherin-overexpressing clone and the V2 vector-transfected clone were synchronized by serum starvation for 48 h and then stimulated with DMEM containing 10% serum for 0, 12, 24, and 48 h. The DNA content was measured at each time point. The arrows depict the positions of 2N (G1), 4N (G2/M), and 8N. The distribution of cells in G1, S and G2/M phases is graphically represented for vector-transfected V2 (B) and T-cadherin-expressing T3 cells (C) after serum starvation and at each time point after serum stimulation. At each time point, there are significantly more cells in G2/M in the T-cadherin-expressing cells than vector controls.
FIG. 8.
Mitotic index analysis demonstrates a G2 arrest associated with T-cadherin-overexpression. (A) The V2 vector clone and the T3 T-cadherin-overexpressing clone cells were serum starved and then stimulated with DMEM containing 10% serum. Mitotic index analysis was performed as described in the Materials and Methods section at 0, 4, 8, 12, 24, and 48 h after serum stimulation. The means and SDs (error bars) were determined for each time point. T3 demonstrates a lower mitotic index at every time point compared with V2. The mitotic index data and the cell cycle analysis results are graphically represented in panel B.(B) For clone V2, the percentage of cells in G2/M phase was comparable to the observed mitotic index at 12, 24 and 48 h after serum stimulation. In contrast, the percentage of T3 cells in G2/M phase was significantly higher than the mitotic index at 12, 24, and 48 h after serum stimulation. The single asterisk denotes a statistically significant increase in percentage of G2/M phase cells (P < 0.01). Error bars, SDs. (C) Propidium iodide staining demonstrates that T-cadherin-overexpressing cells have rounder and larger nuclei compared to vector-transfected C6 cells (arrowheads denote cells undergoing mitosis).
Growth inhibition mediated by T-cadherin is dependent on p21CIP1/WAF1 up-regulation, but is independent of p53 expression.
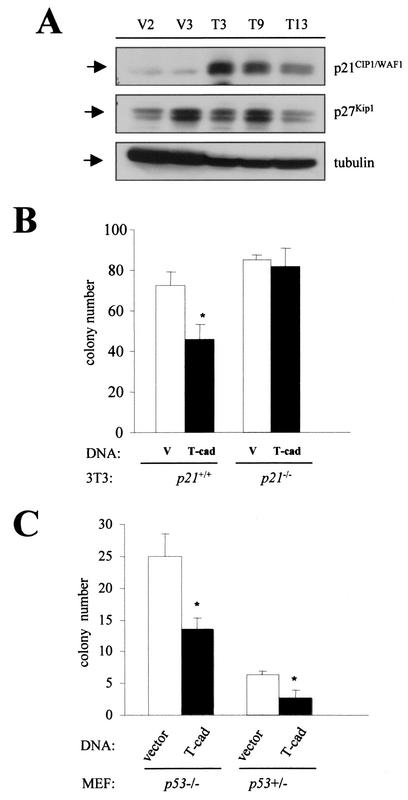
Cadherin-mediated growth suppression has been previously associated with alterations in p27Kip1 expression. But unlike other cadherins, such as N-cadherin (40) and E-cadherin (11), we found no consistent change in p27Kip1 expression associated with T-cadherin overexpression (Fig. 9A). However, T-cadherin-expressing cells exhibited increased p21CIP1/WAF1 expression. The increase in p21CIP1/WAF1 expression observed positively correlated with the level of T-cadherin overexpression in the individual clones (Fig. 9A). Tubulin was included as a loading control in these experiments. Induction of p21CIP1/WAF1 expression was also observed following transient transfection of C6 and NIH3T3 cells with T-cadherin (data not shown).
FIG. 9.
T-cadherin-mediated growth suppression requires p21CIP1/WAF1 expression, but is p53 independent. (A) Western blot demonstrates that p21CIP1/WAF1 expression is elevated in T-cadherin-overexpressing C6 cell clones (T3, T9, and T13) compared with vector-transfected C6 cell clones (V2 and V3). The amount of increased p21CIP1/WAF1 positively correlates with the level of T-cadherin overexpression. There was no consistent effect of T-cadherin overexpression on p27Kip1 levels. Tubulin was used as a loading control. (B) Clonogenic assays were used to demonstrate that T-cadherin could not suppress cell growth in p21CIP1/WAF1-deficient 3T3 cells. The single asterisk denotes a statistically significant decrease in colony number in the p21CIP1/WAF1-expressing 3T3 cells transfected with pcDNA3.T-cadherin (P < 0.01). Error bars, SDs.(C) Clonogenic assays were used to demonstrate that T-cadherin could suppress fibroblast cell growth in the absence of p53 expression. The single asterisk denotes a statistically significant decrease in colony number in the p53−/− and p53± MEFs transfected with T-cadherin (P < 0.01). Error bars, SDs.
Since T-cadherin overexpression was associated with increased p21CIP1/WAF1 expression, we next wished to determine whether T-cadherin growth suppression required p21CIP1/WAF1 expression. In fibroblasts expressing p21CIP1/WAF1, we observed suppression of cell growth upon T-cadherin overexpression (Fig. 9B). In contrast, T-cadherin-mediated growth suppression was not observed in p21CIP1/WAF1 deficient fibroblasts, suggesting that T-cadherin growth suppression requires p21CIP1/WAF1 expression.
Some studies have shown that p21CIP1/WAF1 expression can be regulated at the transcriptional level by both p53-dependent and -independent mechanisms (23). p21CIP1/WAF1 is transcriptionally regulated by wild-type p53 under a wide range of stress stimuli (18, 25). Based on these results, we wished to evaluate whether the T-cadherin effect on cell growth required p53 expression. Using matched p53−/− and p53+/− mouse embryonic fibroblasts (MEFs), we observed similar levels of growth suppression in response to T-cadherin overexpression (Fig. 9C). The number of colonies observed was greater in the p53−/− MEFs than in the p53+/− MEFs, which was likely due to the increased cell proliferation and survival of p53-deficient cells. Moreover, we did not observe a statistically significant difference in T-cadherin growth suppression related to p53 gene dosage (data not shown). Collectively, these results suggest that the growth suppression mediated by T-cadherin requires p21CIP1/WAF1 expression, but is independent of p53.
DISCUSSION
Studies of astrocyte growth regulation relevant to astrocytoma formation and progression provide a tremendous opportunity to identify key molecules which may serve as additional targets for future drug design in the treatment of these deadly nervous system tumors. Molecular genetic analyses of gliomas have revealed a number of important proteins involved in astrocytoma tumorigenesis. In addition to genes involved in cell cycle regulation (e.g., p53, p16, CDK4, and MDM2) and mitogenic signaling (e.g., epidermal and platelet-derived growth factor receptors), a select number of other pathways have been implicated in glioma formation and progression. In the brain, astrocytes have intricate and highly choreographed relationships with neurons and oligodendrocytes during development and differentiation, which is in part mediated by cell surface molecules involved in adhesion and attachment. Cadherins represent one such receptor system that mediates neuronal and oligodendrocyte interactions with astrocytes (67). N-cadherin promotes chick retinal neurite outgrowth (46) as well as oligodendrocyte migration and adhesion to astrocytes (60). Similarly, E-cadherin expression in WC5 rat astrocyte-like cells results in increased cell adhesion and decreased cell motility (10). In gliomas, the relationship between cadherin molecule expression and tumorigenesis has not been resolved. All gliomas, regardless of grade, appear to lack E-cadherin expression, whereas N-cadherin expression is reduced in some, but not all, studies of high-grade recurrent gliomas (2, 3, 62). Recently, expression of another cadherin protein, cadherin-11, was shown to be decreased in gliomas (75).
Using a transgenic mouse model of astrocytoma (B8) in which an oncogenic H-RAS molecule was expressed in astrocytes, we identified T-cadherin as a protein associated with malignant transformation. B8 mice develop high-grade astrocytomas within 3 months of life and die around 4 to 6 months (14). We first noted that T-cadherin expression was increased in malignant astrocytoma cells from B8 mice compared with postnatal day 2 normal cultured astrocytes or nonneoplastic B8 astrocytes (28). Several T-cadherin molecular species were noted (50, 53-54), ranging from 95 to 130 kDa. Since the active form of the molecule is 105 kDa, it was unclear from these studies whether this increase in T-cadherin expression was associated with astrocyte tumorigenesis. Using additional antibodies that recognize T-cadherin, we examined T-cadherin expression in fresh surgical tumor specimens and failed to detect expression in many of the high-grade astrocytomas (N. Hedrick and D. H. Gutmann, unpublished observations, 2002). This is consistent with studies on breast and lung cancers, in which T-cadherin expression was reduced or absent in a high proportion of tumors (38, 39, 68, 74). In addition, we noted that the 105-kDa molecular species was decreased in human and rat glioma cell lines compared to normal human astrocytes. These results suggested that T-cadherin expression might be regulated by culture conditions in vitro. Previous studies have demonstrated that growth factors, confluence, and serum deprivation all modulate T-cadherin expression in other cell types (37). Similar to these results, we found that T-cadherin expression increases in primary astrocytes in vitro in response to conditions that promote growth arrest (serum starvation and contact inhibition). These observations are consistent with a role for T-cadherin in negatively regulating astrocyte cell growth and raise the possibility that T-cadherin downregulation may promote astrocytoma progression.
To explore the potential role of T-cadherin in astrocytoma growth regulation, we initially demonstrated using a clonogenic assay that forced overexpression of T-cadherin reduced C6 cell colony formation in vitro. We extended these findings by establishing several T-cadherin expressing glioma cell lines. Similarly, T-cadherin reduced astrocytoma cell growth under both anchorage-dependent and anchorage-independent conditions. Previous studies on T-cadherin in cells other than astrocytes have implicated T-cadherin in homophilic adhesion, cell proliferation, and motility. T-cadherin-expressing kidney, neuroblastoma, and breast cancer cell lines exhibited decreased cell growth, increased calcium-dependent adhesion, decreased tumor invasiveness, and reduced mitogenic signaling in response to epidermal growth factor (65, 66). In astrocytoma cells, we also observed that T-cadherin expression was associated with increased cell adhesion to fibronectin, increased homophilic cell aggregation, and decreased cell motility.
Surprisingly, T-cadherin expression in C6 glioma cells resulted in G2 phase arrest as determined by flow cytometry and mitotic index, and induced aneuploidy (>4N) in C6 cells. In the three independently derived T-cadherin expressing cell lines, we observed a strong positive correlation between the level of T-cadherin overexpression and the magnitude of the G2 arrest. In addition, the increased aneuploidy observed in the T-cadherin-expressing clones is likely the consequence of G2 arrest, resulting in endoreplication. G2 arrest in response to genotoxic agents (12) or constitutive c-myc activation (20, 22) has also been reported to result in aneuploidy.
G2 phase arrest is typically associated with DNA damage, such as radiation (71), UV exposure (7), and genotoxic chemical treatment (15), in which p38 kinase plays an important role. In contrast, the G2 arrest by T-cadherin overexpression was not induced by any genotoxic agents, was not associated with increased p38 activation, and could not be abolished by treatment with the p38 kinase inhibitor SB292190 (Z. Huang and D. H. Gutmann, unpublished observations). Caffeine is an inhibitor of G2 arrest induced by DNA damage (43, 52, 57) and induces CDC2 kinase activation (51, 58, 73). In astrocytoma cells, caffeine failed to abolish the G2 arrest induced by T-cadherin overexpression (Huang and Gutmann, unpublished observations, 2002). Finally, the T-cadherin growth reduction was not dependent on p53 expression, suggesting that G2 arrest induced by T-cadherin is mediated by a different mechanism from that induced by DNA damage and does not require p53 function.
Previous studies on E-cadherin and N-cadherin have implicated the cyclin-dependent kinase inhibitor, p27Kip1, in E- or N-cadherin-mediated growth suppression. In these experiments, E-cadherin-dependent growth suppression of EMT/6 mouse mammary carcinoma cells was associated with increased p27Kip1 expression and reduced cyclin E-cdk2 activity (11). Similarly, N-cadherin-mediated contact inhibition of CHO cell growth was associated with elevations in p27Kip1 expression (40). In our studies, we observed that the level of T-cadherin overexpression strongly correlated with the magnitude of the increase in p21CIP1/WAF1 and not p27Kip1 expression. In addition, T-cadherin suppressed cell growth in fibroblasts expressing p21CIP1/WAF1, but failed to suppress in cells lacking p21CIP1/WAF1. Collectively, these results suggest that the mechanism by which T-cadherin suppresses cell growth is distinct from that described for the classic cadherin molecules and likely involves p21CIP1/WAF1.
p21CIP1/WAF1 functions as a cell cycle negative regulator and the introduction of p21CIP1/WAF1 into normal (30) as well as tumor cells (18) results in G1 arrest and growth suppression. However, p21CIP1/WAF1 may also play a regulatory role during G2 cell cycle transition. p21CIP1/WAF1 accumulation, in addition to its role in negatively regulating G1/S transition, also contributes to regulation of the G2/M transition (47). p21CIP1/WAF1 mRNA expression in human fibroblasts exhibits bimodal periodicity with peaks in G1 and G2/M (41) and p21CIP1/WAF1 protein reaccumulates in the nucleus at the onset of mitosis (17). Inducible p21CIP1/WAF1 expression causes cell cycle arrest at both G1 and G2 (9, 24, 44) and G2 arrest when p21CIP1/WAF1 was induced at the beginning of the S phase (63). Similarly, calcitonin receptor-mediated growth suppression of HEK-293 cells is accompanied by induction of p21CIP1/WAF1 and G2/M arrest (19). Conversely, antisense blockade of p21CIP1/WAF1 decreases G2 arrest in esophageal squamous cell carcinoma in response to radiation (55). Lastly, by modulating p21CIP1/WAF1 expression, H2O2 induces a transient multiphase cell cycle arrest including G2 arrest in mouse fibroblasts (4).
p53-dependent accumulation of p21CIP1/WAF1, at least in part, has been shown to mediate G1 arrest caused by DNA damage or cellular senescence (61), but also affects G2-M phase progression if the p53-dependent accumulation of p21CIP1/WAF1 occurs in G2 phase (17). However, other mechanisms involving transcriptional and posttranscriptional events, independent of p53, have been found to contribute to the regulation of p21CIP1/WAF1 expression. Transforming growth factor-β causes a rapid transcriptional induction of p21CIP1/WAF1 in a cell line containing two mutant alleles of p53 (13). In addition, p21CIP1/WAF1 can be induced by activated Raf (72), activated mitogen-activated protein kinase (42), and AKT activation (56) in a p53-indepedent manner.
Although the precise mechanism by which T-cadherin results in p21CIP1/WAF1-mediated G2 arrest and aneuploidy has not been elucidated to date, these results identify a novel role for T-cadherin in growth regulation. Our results demonstrate that T-cadherin growth suppression is associated with G2 phase arrest and requires p21CIP1/WAF1 expression. Further studies on the relationship between T-cadherin, p21CIP1/WAF1, and G2 phase growth arrest will likely elucidate alternative pathways important in regulating astrocyte proliferation. In addition, given the emerging contribution of T-cadherin to the molecular pathogenesis of a number of diverse tumor types, future work may result in the development of novel targets for anticancer therapies.
Acknowledgments
We gratefully appreciate the comments and the technical assistance of Amy L Boyet in The Flow Cytometry Core Facility at Washington University for processing the cell cycle analysis data. We thank Wen Li in our laboratory for technical assistance and Michaela L. Bajenaru, Anthony Apicelli, and Kathy Lee for helpful discussions.
This work was supported by funding from The National Institutes of Health (NS41097 to D.H.G.) and The American Cancer Society (D.H.G.).
REFERENCES
- 1.Angst, B. D., C. Marcozzi, and A. I. Magee. 2001. The cadherin superfamily: diversity in form and function. J. Cell Sci. 114:629-641. [DOI] [PubMed] [Google Scholar]
- 2.Asano, K., O. Kubo, Y. Tajika, K. Takakura, and S. Suzuki. 2000. Expression of cadherin and CSF dissemination in malignant astrocytic tumors. Neurosurg. Rev. 23:39-44. [DOI] [PubMed] [Google Scholar]
- 3.Asano, K., O. Kubo, Y. Tajika, M. C. Huang, K. Takakura, K. Ebina, and S. Suzuki. 1997. Expression and role of cadherins in astrocytic tumors. Brain Tumor Pathol. 14:27-33. [DOI] [PubMed] [Google Scholar]
- 4.Barnouin, K., M. L. Dubuisson, E. S. Child, S. F. de Mattos, J. Glassford, R. H. Medema, D. J. Mann, and E. W.-F. Lam. 2002. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip expression. J. Biol. Chem. 277:13761-13770. [DOI] [PubMed] [Google Scholar]
- 5.Berx, G., and F. Van Roy. 2001. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 3:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremnes, R. M., R. Veve, E. Gabrielson, F. R. Hirsch, A. Baron, L. Bemis, R. M. Gemmill, H. A. Drabkin, and W. A. Franklin. 2002. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J. Clin. Oncol. 20:2417-2428. [DOI] [PubMed] [Google Scholar]
- 7.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102-107. [DOI] [PubMed] [Google Scholar]
- 8.Caskey, L. S., G. N. Fuller, J. M. Bruner, W. K. Yung, R. E. Sawaya, E. C. Holland, and W. Zhang. 2000. Toward a molecular classification of the gliomas: histopathology, molecular genetics, and gene expression profiling. Histol. Histopathol. 15:971-981. [DOI] [PubMed] [Google Scholar]
- 9.Cayrol, C., M. Knibiehler, and B. Ducommun. 1998. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene 16:311-320. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., N. E. Paradies, M. Fedor-Chaiken, and R. Brackenbury. 1997. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J. Cell Sci. 110:345-356. [DOI] [PubMed] [Google Scholar]
- 11.Croix, B. S., C. Sheehan, J. W. Rak, V. A. Flørenes, J. M. Slingerland, and R. S. Kerbel.1998. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27KIP1. J. Cell Biol. 142:557-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damiens, E., B. Baratte, D. Marie, G. Eisenbrand, and L. Meijer. 2001. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene 20:3786-3797. [DOI] [PubMed] [Google Scholar]
- 13.Datto, M. B., Y. Li, J. F. Panus, D. J. Howe, Y. Xiong, and X.-F. Wang. 1995. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl. Acad. Sci. USA 92:5545-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding, H., L. Roncari, P. Shannon, X. Wu, N. Lau, J. Karaskova, D. H. Gutmann, J. A. Squire, A. Nagy, and A. Guha. 2001. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 61:3826-3836. [PubMed] [Google Scholar]
- 15.Dmitrieva, N. I., D. V. Bulavin, A. J. Fornace, Jr., and M. B. Burg. 2002. Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc. Natl. Acad. Sci. USA 99:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle, D. D., G. E. Goings, J. Upshaw-Earley, E. Page, B. Ranscht, and H. C. Palfrey. 1998. T-cadherin is a major glycophosphoinositol-anchored protein associated with noncaveolar detergent-insoluble domains of the cardiac sarcolemma. J. Biol. Chem. 273:6937-6943. [DOI] [PubMed] [Google Scholar]
- 17.Duliæ, V., G. H. Stein, D. F. Far, and S. I. Reed. 1998. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 19.Evdokiou, A., L.-J. Raggatt, G. J. Atkins, and D. M. Findlay. 1999. Calcitonin receptor-mediated growth suppression of HEK-293 cells is accompanied by induction of p21WAF1/CIP1 and G2/M arrest. Mol. Endocrinol. 13:1738-1750. [DOI] [PubMed] [Google Scholar]
- 20.Felsher, D. W., A. Zetterberg, J. Zhu, T. Tlsty, and J. M. Bishop. 2000. Overexpression of MYC causes p53-dependent G2 arrest of normal fibroblasts. Proc. Natl. Acad. Sci. USA 97:10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredette, B. J., J. Miller, and B. Ranscht. 1996. Inhibition of motor axon growth by T-cadherin substrata. Development 122:3163-3171. [DOI] [PubMed] [Google Scholar]
- 22.Gandarillas, A., D. Davies and J.-M. Blanchard. 2000. Normal and c-Myc-promoted human keratinocyte differentiation both occur via a novel cell cycle involving cellular growth and endoreplication. Oncogene 19:3278-3289. [DOI] [PubMed] [Google Scholar]
- 23.Gartel, A. L., and A. L. Tyner. 1999. Transcriptional regulation of the p21(WAF1/CIP1) gene. Exp. Cell Res. 246:280-289. [DOI] [PubMed] [Google Scholar]
- 24.Giannakakou, P., R. Robey, T. Fojo, and M. V. Blagosklonny. 2001. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 20:3806-3813. [DOI] [PubMed] [Google Scholar]
- 25.Gorospe, M., X. Wang, and N. J. Holbrook. 1999. Functional role of p21 during the cellular response to stress. Gene Expr. 7:377-385. [PMC free article] [PubMed] [Google Scholar]
- 26.Gottardi, C. J., and B. M. Gumbiner. 2001. Adhesion signaling: how beta-catenin interacts with its partners. Curr. Biol. 11:R792-R794. [DOI] [PubMed] [Google Scholar]
- 27.Gutmann, D. H., A. Loehr, Y. Zhang, J. Kim, M. Henkemeyer, and A. Cashen. 1999. Haploinsufficiency for the neurofibromatosis 1 (NF1) tumor suppressor results in increased astrocyte proliferation. Oncogene 18:4450-4459. [DOI] [PubMed] [Google Scholar]
- 28.Gutmann, D. H., Z.-Y. Huang, N. M. Hedrick, H. Ding, A. Guha, and M. A. Watson. 2002. Mouse glioma gene expression profiling identifies novel human glioma-associated genes. Ann. Neurol. 51:393-405. [DOI] [PubMed] [Google Scholar]
- 29.Handschuh, G., S. Candidus, B. Luber, U. Reich, C. Schott, S. Oswald, H. Becke, P. Hutzler, W. Birchmeier, H. Hofler, and K. F. Becker. 1999. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 18:4301-4312. [DOI] [PubMed] [Google Scholar]
- 30.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinase. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 31.Hewett, S. J., D. W. Choi, and D. H. Gutmann. 1995. Expression of the neurofibromatosis 1 (NF1) gene in reactive astrocytes in vitro. Neuroreport 6:1565-1568. [DOI] [PubMed] [Google Scholar]
- 32.Huang, Z.-Y., R. L. Baldwin, N. M. Hedrick, and D. H. Gutmann. 2002. Astrocyte-specific expression of CDK4 is not sufficient for tumor formation, but cooperates with p53 heterozygosity to provide a growth advantage for astrocytes in vivo. Oncogene 21:1325-1334. [DOI] [PubMed] [Google Scholar]
- 33.Kemler, R. 1992. Classical cadherins. Semin. Cell Biol. 3:149-155. [DOI] [PubMed] [Google Scholar]
- 34.Kinch, M. S., L. Petch, C. Zhong, and K. Burridge. 1997. E-cadherin engagement stimulates tyrosine phosphorylation. Cell Adhes. Commun. 4:425-437. [DOI] [PubMed] [Google Scholar]
- 35.Kleihues, P., and W. K. Cavenee. 2000. Tumours of the nervous system. IARC Press, Lyon, France.
- 36.Koller, E., and B. Ransch. 1996. Differential targeting of T-and N-cadherin in polarized epithelial cells. J. Biol. Chem. 271:30061-30067. [DOI] [PubMed] [Google Scholar]
- 37.Kuzmenko, Y. S., F. Kern, V. N. Bochkov, V. A. Tkachuk, and T. J. Resink. 1998. Density- and proliferation status-dependent expression of T-cadherin, a novel lipoprotein-binding glycoprotein: a function in negative regulation of smooth muscle cell growth? FEBS Lett. 434:183-187. [DOI] [PubMed] [Google Scholar]
- 38.Lee, S. W. 1996. H-cadherin, a novel cadherin with growth inhibitory functions and diminished expression in human breast cancer. Nat. Med. 2:776-782. [DOI] [PubMed] [Google Scholar]
- 39.Lee, S. W., C. L. Reimer, D. B. Campbell, P. Cheresh, R. B. Duda, and O. Kocher. 1998. H-cadherin expression inhibits in vitro invasiveness and tumor formation in vivo. Carcinogenesis 19:1157-1159. [DOI] [PubMed] [Google Scholar]
- 40.Levenberg, S., A. Yarden, Z. Kam, and B. Geiger. 1999. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene 18:869-876. [DOI] [PubMed] [Google Scholar]
- 41.Li, Y., C. W. Jenkins, M. A. Nichols, and Y. Xiong. 1994. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 9:2261-2268. [PubMed] [Google Scholar]
- 42.Liu, Y., J. L. Martindale, M. Gorospe, and N. J. Holbrook. 1996. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 56:31-35. [PubMed] [Google Scholar]
- 43.Minemoto, Y., J. Gannon, M. Masutani, H. Nakagama, T. Sasagawa, M. Inoue, Y. Masamune, and K. Yamashita. 2001. Characterization of adriamycin-induced G2 arrest and its abrogation by caffeine in FL-amnion cells with or without p53. Exp. Cell Res. 262:37-48. [DOI] [PubMed] [Google Scholar]
- 44.Murai, T., Y. Nakagawa, H. Maeda, and K. Terada. 2001. Altered regulation of cell cycle machinery involved in interleukin-1-induced G1 and G2 phase growth arrest of A375S2 human melanoma cells. J. Biol. Chem. 276:6797-6806. [DOI] [PubMed] [Google Scholar]
- 45.Nagafuchi, A., and M. Takeichi. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 7:3679-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neugebauer, K. M., K. J. Tomaselli, J. Lilien, and L. F. Reichardt. 1988. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J. Cell Biol. 107:1177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niculescu, III, A. B., X. Chen, M. Smeets, L. Hengst, C. Prives, and S. I. Reed. 1998. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transition: pRb is a critical determinant in blocking DNA replication and in preventing endoduplication. Mol. Cell. Biol. 18:629-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nieman, M. T., R. S. Prudoff, K. R. Johnson, and M. J. Wheelock. 1999. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 147:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozawa, M., J. Engel, and R. Kemler. 1990. Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell 63:1033-1038. [DOI] [PubMed] [Google Scholar]
- 50.Philippova, M. P., V. N. Bochkov, D. V. Stambolsky, V. A. Tkachuk, and T. J. Resink. 1998. T-cadherin and signal-transducing molecules co-localize in caveolin-rich membrane domains of vascular smooth muscle cells. FEBS Lett. 429:207-210. [DOI] [PubMed] [Google Scholar]
- 51.Poon, R. Y., M. S. Chau, K. Yamashita, and T. Hunter. 1997. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 57:5168-5178. [PubMed] [Google Scholar]
- 52.Powell, S. N., J. S. DeFrank, P. Connell, M. Eogan, F. Preffer, D. Dombkowski, W. Tang, and S. Friend. 1995. Differential sensitivity of p53 (-) and p53 (+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 55:1643-1648. [PubMed] [Google Scholar]
- 53.Ranscht, B., and M. T. Dours-Zimmermann. 1991. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron 7:391-402. [DOI] [PubMed] [Google Scholar]
- 54.Resink, T. J., Y. S. Kuzmenko, F. Kern, D. Stambolsky, V. N. Bochkov, V. A. Tkachuk, P. Erne, and T. Niermann. 1999. LDL binds to surface-expressed human T-cadherin in transfected HEK293 cells and influences homophilic adhesive interactions. FEBS Lett. 463:29-34. [DOI] [PubMed] [Google Scholar]
- 55.Rigberg, D. A., T. A. Blinman, F. S. Kim, M. A. Cole, and D. W. McFadden. 1999. Antisense blockade of p21/WAF1 decreases radiation-induced G2 arrest in esophageal squamous cell carcinoma. J. Surg. Res. 81:6-10. [DOI] [PubMed] [Google Scholar]
- 56.Rössig, L., C. Badorff, Y. Holzmann, A. M. Zeiher, and S. Dimmeler. 2002. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J. Biol. Chem. 277:9684-9689. [DOI] [PubMed] [Google Scholar]
- 57.Russell, K. J., L. W. Wiens, G. W. Demers, D. A. Galloway, S. E. Plon, and M. Groudine. 1995. Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res. 55:1639-1642. [PubMed] [Google Scholar]
- 58.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 59.Sasaki, C. Y., H. Lin, P. J. Morin, and D. L. Longo. 2000. Truncation of the extracellular region abrogrates cell contact but retains the growth-suppressive activity of E-cadherin. Cancer Res. 60:7057-7065. [PubMed] [Google Scholar]
- 60.Schnadelbach, O., O. W. Blaschuk, M. Symonds, B. J. Gour, P. Doherty, and J. W. Fawcett. 2000. N-cadherin influences migration of oligodendrocytes on astrocyte monolayers. Mol. Cell. Neurosci. 15:288-302. [DOI] [PubMed] [Google Scholar]
- 61.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 62.Shinoura, N., N. E. Paradies, R. E. Warnick, H. Chen, J. J. Larson, J. J. Tew, M. Simon, R. A. Lynch, Y. Kanai, S. Hirohashi, J. J. Hemperly, A. G. Menon, and R. Brackenbury. 1995. Expression of N-cadherin and alpha-catenin in astrocytomas and glioblastomas. Br. J. Cancer 72:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smits, V. A., R. Klompmaker, T. Vallenius, G. Rijksen, T. P. Makela, and R. H. Medema. 2000. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J. Biol. Chem. 275:30638-30643. [DOI] [PubMed] [Google Scholar]
- 64.Takeichi, M. 1991. Cadherin cell adhesion receptor as a morphogenetic regulator. Science 251:1451-1455. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi, T., A. Misaki, S.-B. Liang, A. Tachibana, N. Hayashi, H. Sonobe, and Y. Ohtsuki. 2000. Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J. Neurochem. 74:1489-1497. [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi, T., and Y. Ohtsuki. 2001. Recent progress in T-cadherin (CDH13, H-cadherin) research. Histol. Histopathol. 16:1287-1293. [DOI] [PubMed] [Google Scholar]
- 67.Tomaselli, K. J., K. M. Neugebauer, J. L. Bixby, J. Lilien, L. F. Reichardt. 1988. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron 1:33-43. [DOI] [PubMed] [Google Scholar]
- 68.Toyooka, K. O., S. Toyooka, A. K. Virmani, U. G. Sathyanarayana, D. M. Euhus, M. Gilcrease, J. D. Minna, and A. F. Gazdar. 2001. Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung carcinomas. Cancer Res. 61:4556-4560. [PubMed] [Google Scholar]
- 69.Vestal, D. J., and B. Ranscht. 1992. Glycosyl phosphatidylinositol-anchored T-cadherin mediates calcium-dependent, homophilic cell adhesion. J. Cell Biol. 119:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vleminckx, K., L. Vakaet, Jr., M. Mareel, W. Fiers, and F. van Roy. 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107-119. [DOI] [PubMed] [Google Scholar]
- 71.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20:4543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woods, D., D. Parry, H. Cherwinski, E. Bosch, E. Lees, and M. McMahon. 1997. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21cip1. Mol. Cell. Biol. 17:5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao, S. L., A. J. Akhtra, K. A. McKenna, G. C. Bedi, D. Sidransky, M. Mabry, R. Ravi, M. I. Collector, R. J. Jones, S. J. Sharskis, E. J. Fuchs, and A. Bedi. 1996. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat. Med. 2:1140-1143. [DOI] [PubMed] [Google Scholar]
- 74.Zhong, Y., Y. Delgado, J. Gomez, S. W. Lee, and R. Perez-Soler. 2001. Loss of H-cadherin protein expression in human non-small cell lung cancer is associated with tumorigenicity. Clin. Cancer Res. 7:1683-1687. [PubMed] [Google Scholar]
- 75.Zhou, R., and O. Skalli. 2000. Identification of cadherin-11 down-regulation as a common response of astrocytoma cells to transforming growth factor-alpha. Differentiation 66:165-172. [DOI] [PubMed] [Google Scholar]