Abstract
The function of the p53 tumor suppressor protein must be highly regulated because p53 can cause cell death and prevent tumorigenesis. In cultured cells, the p90MDM2 protein blocks the transcriptional activation domain of p53 and also stimulates the degradation of p53. Here we provide the first conclusive demonstration that p90MDM2 constitutively regulates p53 activity in homeostatic tissues. Mice with a hypomorphic allele of mdm2 revealed a heretofore unknown role for mdm2 in lymphopoiesis and epithelial cell survival. Phenotypic analyses revealed that both the transcriptional activation and apoptotic functions of p53 were increased in these mice. However, the level of p53 protein was not coordinately increased, suggesting that p90MDM2 can inhibit the transcriptional activation and apoptotic functions of p53 in a manner independent of degradation. Cre-mediated deletion of mdm2 caused a greater accumulation of p53, demonstrating that p90MDM2 constitutively regulates both the activity and the level of p53 in homeostatic tissues. The observation that only a subset of tissues with activated p53 underwent apoptosis indicates that factors other than p90MDM2 determine the physiological consequences of p53 activation. Furthermore, reduction of mdm2 in vivo resulted in radiosensitivity, highlighting the importance of mdm2 as a potential target for adjuvant cancer therapies.
The mdm2 gene is essential for murine development unless the p53 tumor suppressor gene is inactivated (21, 43). mdm2 was discovered through its amplification on murine double-minute chromosomes (7) and encodes a negative regulator of p53, p90MDM2, which inhibits p53 in two genetically distinct ways in cultured cells (28). p90MDM2 binds directly to the transcriptional activation domain of p53 and blocks p53-dependent transcription (42, 46, 54). In addition, p90MDM2 ubiquitinates p53, stimulating its export from the nucleus (8, 50) as well as its degradation (15, 18, 27). It is not clear which of these inhibitory functions of p90MDM2 checks the function of p53 during embryogenesis; however, control of p53 is critical, because p53 can both arrest the cell cycle and induce apoptosis (23, 61).
p53 is critical for preventing tumors in humans and mice (6, 17, 20). However, since activation of p53 can be detrimental to cell survival, the mechanisms controlling the activity of p53 must be exquisitely balanced. In spite of their importance, little is known of the mechanisms that keep the activity of p53 low or undetectable in adult murine tissues (3, 10, 25, 34, 35, 37, 47). p53 protein is expressed in multiple murine tissues, and both its levels and activity are increased in response to whole-body ionizing radiation (40). Although some of the factors that activate p53 in response to DNA damage have been identified, none have been shown to influence the basal level or activity of p53.
In cultured cells, p53 constitutively stimulates expression of mdm2 (37), thereby determining the level of its own inhibitor, p90MDM2. This autoregulatory loop has given rise to a model in which p53 maintains its low basal level and activity by determining the level of p90MDM2 (1, 60). Although the p53/MDM2 autoregulatory loop model is appealing, it does not strictly apply to homeostatic tissues. The regulatory interactions between p53 and mdm2 clearly differ between cultured cells and intact tissues. Whereas p53 constitutively regulates mdm2 expression in cultured cells, it does not regulate the basal level of mdm2 expression in vivo (31, 37). However, p53 does stimulate mdm2 expression following whole-body ionizing radiation, suggesting that the p53/MDM2 autoregulatory loop may operate in vivo under specific conditions (37).
It is not known whether mdm2 is critical for regulating p53 constitutively in homeostatic tissues. Indirect evidence for a role for p90MDM2 in regulating p53 in adult tissues comes from studies of human tumors. In the subset of human tumors lacking inactivating mutations in p53, mdm2 is often overexpressed (30, 45), suggesting that high levels of p90MDM2 can substitute for mutation of p53 and allow tumorigenesis. Indeed, inhibition of p90MDM2 expression in cultured human tumor lines activates wild-type p53, indicating that p90MDM2 constitutively blocks the activity of p53 in these cells (4). Recently, the physical interaction between p53 and p90MDM2 has become the target of adjuvant chemotherapies designed to sensitize human tumors to cancer therapies that rely on activation of p53 for their efficacy (2, 32, 33, 41). The utility of such therapies depends in part on whether p90MDM2 is critical for inhibiting p53 in homeostatic tissues. If p90MDM2 is critical for maintaining low levels of p53 activity, inhibition of the p90MDM2/p53 interaction could induce widespread apoptosis that would be detrimental to the patient.
To test whether mdm2 was critical for regulating p53 under homeostatic conditions, we generated a conditionally null allele of mdm2. In doing so, we fortuitously generated a hypomorphic allele of mdm2 (mdm2puro) that expresses reduced amounts of mdm2 mRNA and p90MDM2. Mice carrying one mdm2puro allele and a known null allele of mdm2 are small, lymphopenic, and radiosensitive, with an increased frequency of apoptosis in both lymphocytes and epithelial cells. All of these phenotypes were dependent on p53, demonstrating that p90MDM2 does inhibit p53 constitutively in homeostatic tissues. However, although both the transcriptional activation and apoptotic functions of p53 are enhanced in mice expressing reduced levels of mdm2, the level of p53 protein is not coordinately increased. These results have important implications for rational approaches to activating p53 in human tumors.
MATERIALS AND METHODS
Generation of targeted ES cells.
Constructs containing genomic sequences of mdm2 from a murine 129/Sv genomic library were obtained from Stephen Jones, University of Massachusetts (21). To generate the targeting vector, a PGKpuro cassette flanked by loxP sites was inserted into an EcoRI site within mdm2 intron 6 in the same transcriptional orientation as mdm2. An 87-bp fragment containing a third loxP site was introduced into a HincII site within mdm2 intron 9. The herpes simplex virus thymidine kinase gene was cloned 1.7 kb upstream of mdm2 exon 7.
129/Sv-derived AB2.2 embryonic stem (ES) cells were electroporated with 30 μg of KpnI-linearized targeting vector DNA and selected in medium containing 3 μg of puromycin (Sigma) per ml and 2 μM ganciclovir 24 h after electroporation. Doubly-resistant ES clones were screened for homologous recombination at the 5′ end of the mdm2 allele by Southern analysis of BamHI-digested genomic DNA with a 0.7-kb BamHI-KpnI fragment of mdm2 intron 6 (probe a) external to the targeting vector. ES clones scoring positive were screened by Southern analysis of NdeI-digested DNA with a 750-bp fragment of mdm2 exon 12 (probe c) to detect homologous recombination at the 3′ end of the targeting vector. The presence of the loxP site 3′ to mdm2 exon 9 was confirmed by Southern blot analysis of BamHI-digested DNA with a 500-bp HincII fragment (probe b) spanning exon 9.
Generation of mice carrying mdm2puro, mdm2flox, and mdm2Δ7-9 alleles.
The University of Wisconsin Transgenic Animal Facility injected mdm2puro/+ ES cells into C57BL/6 blastocysts. Chimeric males from two ES cell lines (64 and 125) were used to produce mdm2+/puro mice on a mixed C57BL/6 × 129/Sv genetic background as well as an inbred 129/Sv background (Taconic). mdm2+/flox and mdm2+/Δ7-9 mice were created by mating mdm2+/puro mice to transgenic mice expressing Cre under control of the minimal rosa26 promoter (R26-Cre mice). The R26-Cre mice were kindly provided by Eric Sandgren, University of Wisconsin, and have been given the designation TgN(R26cre)15EPS (11). Mx-Cre mice were obtained from John Petrini with the kind permission of Klaus Rajewski (29).
Genotyping and maintenance of mice.
The mdm2puro and mdm2+ alleles were distinguished with primers 5′-CTGTGTGAGCTGAGGGAGATGTG-3′ and 5′-CCTGGATTTAATCTGCAGCACTC-3′, which yield a 397-bp (mdm2puro) and 310-bp (mdm2+) PCR product, respectively. The mdm2flox allele was detected with primers 5′-GTATTGGGCATGTGTTAGACTGG-3′ and 5′-CTTCAGATCACTCCCACCTTC-3′, which yield a 225-bp (mdm2flox) and 125-bp (mdm2+) PCR product, respectively. The mdm2Δ7-12 allele was detected with primers 5′-GGCAAAGGATGTGATACGTGGAAG-3′ and 5′-CCAGTTTCACTAATGACACAAACATG-3′ in the hprt minigene, which yield an 830-bp PCR product. The mdm2Δ7-9 allele was detected with primers 5′-GTATTGGGCATGTGTTAGACTGG-3′ and 5′-CCTGGATTTAATCTGCAGCACTC-3′, which yield a 240-bp PCR product.
Mice were housed in an American Association for the Accreditation of Laboratory Animal Care-approved facility. The mdm2Δ7-12 allele (21) was backcrossed to C57BL/6 mice (Jackson Laboratory) for eight to ten generations and also maintained on a mixed 129/Sv × C57BL/6 background. mdm2flox/Δ7-12 mice were of mixed genetic background (FVB × 129/Sv × C57BL/6) and were compared with their littermates. 129/Sv and C57BL/6 mice carrying a deletion for p53 (20) were obtained from Paul Lambert (University of Wisconsin). All mice were analyzed at 5 to 6 weeks of age unless otherwise noted.
Conditional deletion of mdm2.
Upon injection of double-stranded RNA, expression of cre can be induced efficiently in the spleen, liver, and thymus of mice carrying the cre gene under the control of an interferon-responsive promoter, Mx-1 (29). mdm2flox/+ mice carrying one Mx-cre allele were bred to mdm2+/Δ7-12 mice to generate mdm2flox/Δ7-12 Mx-cre+ mice and controls. Cre expression was induced as described before (29), and 48 h after induction, tissues were isolated. Conversion of the mdm2flox allele to the mdm2Δ7-9 allele was assessed by PCR.
Flow cytometry.
Bone marrow cells were flushed from both femurs with phosphate-buffered saline supplemented with 3% fetal bovine serum (PF3). Single-cell suspensions from spleen and thymus were prepared by pressing tissues between frosted-glass microscope slides and dispersing cells in PF3. Contaminating mature erythrocytes were lysed in Gey's solution. Aliquots of 5 × 105 cells were stained with the indicated antibody and appropriate isotype controls. Hematopoietic lineages were identified with fluorescein isothiocyanate-conjugated anti-CD4 (clone GK1.5), phycoerythrin-conjugated anti-CD8 (clone 53-6.7), fluorescein isothiocyanate-conjugated anti-CD45R/B220 (clone RA3-6B2), phycoerythrin-conjugated anti-immunoglobulin M (clone R6-60.2), and phycoerythrin-conjugated anti-CD43 (clone S7) (BD-Pharmingen). Following a 20-min incubation on ice, cells were washed and resuspended in PF3.
To assess apoptosis, 106 cells were stained either for CD8, CD4, or B220 as described above. An aliquot of 2 × 105 cells was removed, washed in annexin binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4), and incubated with annexin V (633 conjugate; Molecular Probes) for 15 min at room temperature. The cells were then diluted in cold annexin binding buffer and incubated with propidium iodide. Early apoptotic (annexin positive, propidium iodide negative) cells were counted on a FACSCalibur flow cytometer, and the data were analyzed with Cell Quest software (Becton Dickinson).
Peripheral blood analysis.
Peripheral blood was collected from the retroorbital sinus of anesthetized mice and transferred immediately into Microtainer tubes containing dipotassium EDTA (Becton Dickinson). Complete blood counts were performed by the Clinical Pathology Laboratory of the Veterinary Medical Teaching Hospital at the University of Wisconsin School of Veterinary Medicine with an Advia 120 automated hematology analyzer (Bayer). Differential counts were performed manually.
Histology and cytology.
Tissues were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin blocks. Apoptosis was assayed by detecting terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL)-positive cells with an ApopTag Fluorescein Plus in situ apoptosis detection kit (Intergen), with the following modifications of the recommended protocol. Following incubation with terminal deoxynucleotidyltransferase enzyme, slides were incubated in stop-wash buffer for 30 min at 37°C. After antidigoxigenin conjugate was applied, slides were incubated for 1 h at room temperature. Slides were mounted under glass coverslips with Vectashield antifade mounting medium (Vector) containing 0.5 μg of propidium iodide per ml and visualized on a Bio-Rad MRC 1024 laser scanning confocal microscope at the Keck Biological Imaging Laboratory (University of Wisconsin). For bone marrow differential counts, cells were placed onto glass slides with fine brushes, air dried, and stained with Wright stain.
In vivo radiation sensitivity.
At 5 to 6 weeks of age, mice were irradiated with γ rays from a 137cesium source at a dose rate of 3.1 gray (Gy)/min and monitored daily.
Northern analyses.
Northern blots were prepared and probed for the glyceraldehyde-3-phosphate dehydrogenase (gapdh) and mdm2 genes as described previously (38). The p21 probe was a 750-bp EcoRI fragment from pCMW35 (a gift from Bert Vogelstein, Johns Hopkins University).
Western analyses.
Whole tissues were lysed in radioimmunoprecipitation assay (RIPA) buffer (16) supplemented with the protease inhibitors phenylmethylsulfonyl fluoride, aprotinin, and leupeptin. For the analysis of p90MDM2 expression, p90MDM2 was immunoprecipitated from 1.5 mg of lysate and detected by Western analysis as described previously (48). For analysis of p53, TATA-binding protein (TBP), and p21 levels, 100 μg of lysates of whole thymuses or spleens were loaded in each lane. Individual thymocytes were isolated as described above, and 2 × 106 cell equivalents were loaded in each lane. p53 was detected with the CM5 polyclonal antiserum (Novocastra), TBP was detected with a monoclonal antibody from Richard Burgess (University of Wisconsin), p21 was detected with monoclonal antibody F-5 (Santa Cruz), and BAX was detected with polyclonal antibody N-20 (Santa Cruz). Secondary antibodies conjugated to horseradish peroxidase were anti-mouse immunoglobulin G (American Qualex) and anti-rabbit immunoglobulin G (Sigma). Signals were detected by enhanced chemiluminescence (Amersham).
Cell cycle analysis of MEFs.
Mouse embryo fibroblasts (MEFs) with various mdm2 genotypes were isolated from 14-day-old embryos and plated in triplicate at a density of 106 cells per 10-cm dish at passage 2 (55). Every 3 days, the cells were trypsinized, counted, and replated at the starting density. To determine sensitivity to ionizing irradiation, MEFs were plated at a density of 5 × 105 cells per 6-cm dish and irradiated with either 2 or 8 Gy 24 h after plating. At 16 h following treatment, irradiated and untreated cells were labeled with 10 μM bromodeoxyuridine for 4 h (24). Cells were fixed with 70% ethanol, and nuclei were prepared for staining by established methods (52). The nuclei were stained for 1 h with fluorescein isothiocyanate-conjugated antibromodeoxyuridine antibody (Becton Dickinson), incubated overnight with 0.3 mg of RNase A per ml, and then stained with 50 μg of propidium iodide per ml. Propidium iodide and bromodeoxyuridine incorporation were quantitated on a FACSCalibur flow cytometer.
RESULTS
mdm2 Gene Targeting.
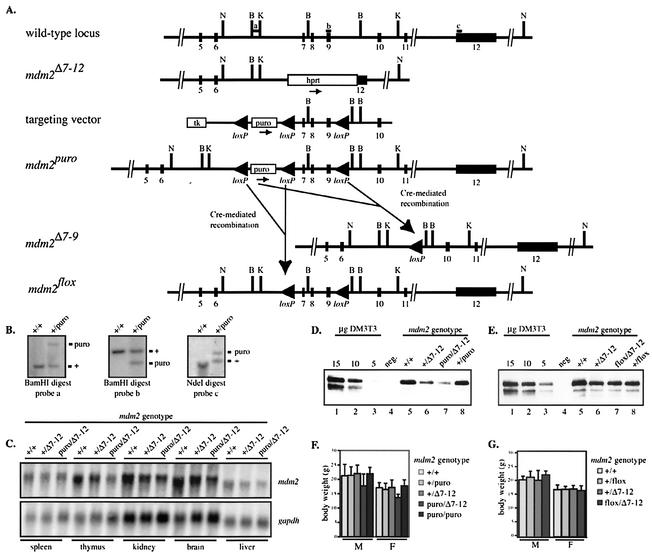
To generate a conditional allele of mdm2 (mdm2puro), we flanked mdm2 exons 7 through 9 with Cre recombination (loxP) sites (Fig. 1A) (51) so that Cre-mediated recombination would remove three critical coding exons of mdm2 (mdm2Δ7-9). A third loxP site was placed 5′ of the puromycin resistance (puro) cassette to allow its removal after targeting. ES cells were electroporated with the targeting vector, and ES cells surviving selection were screened by Southern analysis (Fig. 1B). Lines of mice from two independently derived mdm2+/puro ES cell clones were established on both a mixed C57BL/6 × 129/Sv and an inbred 129/Sv background, all of which demonstrated the phenotypes reported here.
FIG. 1.
Generation and characterization of a hypomorphic allele of mdm2. (A) Partial structure of murine mdm2 locus, known null allele (mdm2Δ7-12), targeting vector, hypomorphic conditional allele (mdm2puro), recombined null allele (mdm2Δ7-9), and recombined conditional allele (mdm2flox). Abbreviations: loxP, Cre recombination site; hprt, hypoxanthine phosphoribosyl transferase minigene cassette; puro, puromycin resistance cassette; tk, herpes simplex virus thymidine kinase cassette. N, NdeI; B, BamHI; K, KpnI. Probes a, b, and c were used for the experiment shown in panel B. (B) Representative Southern analyses of targeted ES cells. Genomic DNA was digested with BamHI or NdeI and probed as indicated. Approximate sizes of fragments from wild-type and targeted alleles were 2.6 and 4.7 kb, respectively (probe a), 1.7 and 1.2 kb, respectively (probe b), and 13.0 and 15.1 kb, respectively (probe c). (C) Northern analysis of mdm2 mRNA in multiple tissues. gapdh mRNA was used as an internal control. (D and E) Immunoprecipitation followed by Western analysis of p90MDM2 in testes from (D) mdm2puro/Δ7-12 and (E) mdm2flox/Δ7-12 mice and respective control mice. Lanes 1 to 3, decreasing amounts of lysate from DM3T3 cells were used as standards. Lane 4, preimmune serum was incubated with lysate from a wild-type testis as a negative control. Lanes 5 to 8, lysates from the testis of mice of the indicated genotypes were incubated with antiserum to MDM2. (F and G) Average 5-week body weights of (F) mdm2puro/Δ7-12 and (G) mdm2flox/Δ7-12 mice and control mice. The average body weights of 5-week-old mdm2puro/puro mice are included in panel F.
The functionality of the mdm2puro allele prior to Cre-mediated recombination was assessed by its ability to rescue the embryonic lethality of mice lacking mdm2. mdm2+/puro mice were bred to mice carrying one wild-type allele and one known null allele of mdm2 (mdm2+/Δ7-12) (21). Among the progeny of such matings were compound heterozygotes of the genotype mdm2puro/Δ7-12, demonstrating that mdm2puro is a functional mdm2 allele (see below). With a similar genetic approach, we verified that a recombined mdm2 allele lacking exons 7 to 9 (mdm2Δ7-9) was functionally inactive.
By breeding mdm2+/puro mice to R26-Cre mice (11), we obtained mice with germ line mdm2 alleles that had undergone complete recombination (mdm2Δ7-9) or had selectively lost the puromycin cassette (mdm2flox) (Fig. 1A). Of 137 progeny from matings between mdm2+/Δ7-9 and mdm2+/Δ7-12 mice, none were of the genotype mdm2Δ7-9/Δ7-12, indicating that the mdm2Δ7-9 allele cannot rescue the mdm2 null phenotype (P < 0.001). Furthermore, when mdm2+/Δ7-9 heterozygotes were interbred, no mice of the genotype mdm2Δ7-9/Δ7-9 were born (0 of 160 offspring; P < 0.001). These tests indicate that the mdm2Δ7-9 allele is nonfunctional. Further testing of the mdm2Δ7-9 allele (see below) demonstrated that it was functionally equivalent to the known null allele mdm2Δ7-12.
mdm2puro is a hypomorphic allele of mdm2.
The percentage of mdm2puro/Δ7-12 mice obtained from matings between mdm2+/puro and mdm2+/Δ7-12 mice was slightly lower than the expected Mendelian frequency of 25%, suggesting that a subset of these mice was dying in utero. This underrepresentation was clearest in female progeny. On mixed and F1 C57BL/6 × 129/Sv genetic backgrounds, the percentage of female mdm2puro/Δ7-12 mice was only 15 and 16%, respectively (P < 0.05). The presence of the puromycin resistance cassette accounts for the decreased viability of female mdm2puro/Δ7-12 mice, since female mdm2flox/Δ7-12 mice, which differ from mdm2puro/Δ7-12 mice only by the absence of the puromycin cassette, were born at the expected frequency.
We reasoned that the partially penetrant embryonic lethality observed in mdm2puro/Δ7-12 mice might result from interference of the puromycin cassette with mdm2 expression. Northern analysis revealed that, in all tissues examined, expression of mdm2 was reduced approximately 50% in mdm2+/Δ7-12 mice relative to wild-type mice (Fig. 1C) consistent with a 50% reduction in mdm2 gene dosage. The level of mdm2 mRNA in tissues from mdm2puro/Δ7-12 mice was further reduced to, on average, 30% of the level in wild-type tissues. Detection of p90MDM2 by immunoprecipitation followed by Western analysis demonstrated that mdm2puro/Δ7-12 mice also expressed less p90MDM2 than wild-type, mdm2+/puro, or mdm2+/Δ7-12 mice in the testis (Fig. 1D), thymus, spleen, and brain (data not shown). Decreased expression of p90MDM2 in mdm2puro/Δ7-12 tissues was dependent on the puromycin cassette, as the levels of p90MDM2 in tissues from mdm2flox/Δ7-12 mice and mdm2+/Δ7-12 mice were equivalent (Fig. 1E). Thus, the puromycin cassette reduces expression of mdm2 and p90MDM2 in multiple adult tissues.
Together, these data demonstrate that the mdm2puro allele is hypomorphic, whereas the mdm2flox allele is functionally wild type. The low level of mdm2 expression in mdm2puro/Δ7-12 mice allowed us to perform a comprehensive analysis of the physiological functions of mdm2 because it eliminated the need to select tissues for Cre expression. In characterizing the mdm2puro/Δ7-12 mice, we determined that mdm2 regulates the activity of p53 in homeostatic tissues.
Decreased body weight in mdm2puro/Δ7-12 mice.
Viable mdm2puro/Δ7-12 mice developed normally, with no increase in lethality upon weaning. At five weeks of age, a 15 to 20% reduction in body weight was evident in both male and female mdm2puro/Δ7-12 mice compared with wild-type mice (Fig. 1F). In contrast, age-matched mdm2puro/puro mice (Fig. 1F) and mdm2flox/Δ7-12 mice (Fig. 1G) were comparable in weight to wild-type mice, demonstrating that normal body weight is dependent on the level of mdm2 expression. All organs examined were smaller in both male and female mdm2puro/Δ7-12 mice than in wild-type mice, with the most dramatic reductions in the lymphoid organs (Table 1). Histological analyses of the liver, kidney, and intestines failed to reveal any obvious defects that could account for the reduction in body weight. The decrease in both body weight and organ size were also seen in mdm2puro/Δ7-9 mice, indicating that our new deleted allele is functionally similar to the known mdm2Δ7-12 allele.
TABLE 1.
Comparison of wet weights of tissues from age-matched wild-type and mdm2puro/Δ7-12 micea
| Tissue | Age (wk) | Ratio, mdm2puro/Δ7-12/mdm2+/+
|
|
|---|---|---|---|
| Males | Females | ||
| Kidney | 10 | 0.8 ± 0.2 (0.01) | 0.8 ± 0.1 (0.06) |
| Liver | 10 | 0.9 ± 0.2 (0.34) | 0.6 ± 0.2 (0.02) |
| Spleen | 10 | 0.7 ± 0.3 (0.02) | 0.4 ± 0.1 (0.02) |
| Thymus | 5 | 0.4 ± 0.1 (<0.001) | 0.4 ± 0.1 (0.001) |
Presented as the ratio of the average weight ± standard deviation (P).
mdm2puro/Δ7-12 mice exhibit defects in multiple hematopoietic lineages.
Complete blood count analysis of peripheral blood revealed a mild anemia in mdm2puro/Δ7-12 mice, which had a red blood cell count that was 82% of that of wild-type mice (Table 2). The white blood cell count in mdm2puro/Δ7-12 mice was only 30% of the wild-type count. This dramatic reduction in white blood cells was paralleled by a decrease in the concentration of lymphocytes to 37% of that of wild-type mice. In contrast, the concentration of neutrophils was 60% of the wild-type value, suggesting that the deficiency in white blood cells is largely due to a decrease in lymphocytes.
TABLE 2.
Hematological profiles of age-matched wild-type and mdm2puro/Δ7-12 micea
| mdm2 genotype | No. | RBC (106/μl) | WBC (103/μl) | Neutrophils (103/μl) | Lymphocytes (103/μl) |
|---|---|---|---|---|---|
| +/+ | 6 | 8.2 (7.2-9.5) | 5.4 (3.5-7.3) | 0.5 (0.1-1.0) | 3.0 (2.7-3.4) |
| puro/Δ7-12 | 5 | 6.7 (6.3-7.5) | 1.6 (1.1-1.8) | 0.3 (0.1-0.4) | 1.1 (0.8-1.3) |
Total red blood cell (RBC) counts and total white blood cell (WBC) counts were determined with an Advia 120 automated hematology analyzer (Bayer). Total neutrophil and lymphocyte counts were calculated from the percentage of these leukocytes (manual differential count) and the total WBC count and are expressed as the average concentration (range).
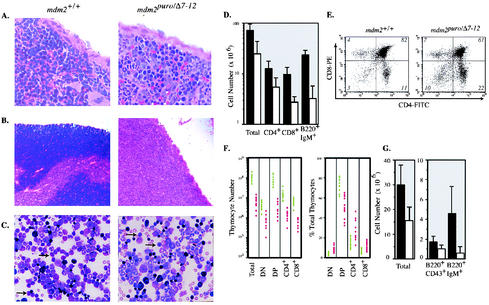
The spleen, thymus, and bone marrow of mdm2puro/Δ7-12 mice also showed decreases in the number of lymphoid cells (Fig. 2A to C). The spleens of mdm2puro/Δ7-12 mice contained, on average, 34% of the number of cells in spleens from wild-type mice (Fig. 2D). Flow cytometric analysis indicated the number of T cells in mdm2puro/Δ7-12 mice was reduced to 40% of that in the wild type, whereas the B cells were reduced to 15% (Fig. 2D). Thus, decreased splenic cellularity of mdm2puro/Δ7-12 mice is attributed to deficits in both T and B lymphocytes stemming from a subphysiological level of mdm2.
FIG. 2.
Lymphopoietic defects in mdm2puro/Δ7-12 mice. (A and B) Hematoxylin- and eosin-stained 5-μm sections of (A) spleen and (B) thymus from wild-type and mdm2puro/Δ7-12 mice. (C) Wright-stained bone marrow from mdm2 wild-type and mdm2puro/Δ7-12 mice. Lymphocytes are indicated by arrows. (D) Number of total cells, CD4+ and CD8+ T cells, and B220+ IgM+ B cells in the spleen. Black bars, wild type; white bars, mdm2puro/Δ7-12. (E) Representative flow cytometric analysis of thymocytes isolated from mdm2+/+ and mdm2puro/Δ7-12 mice. (F) Number and percentage of thymocytes in each maturation stage. Each circle represents an individual animal. Green, wild type; red, mdm2puro/Δ7-12. (G) Average number of total, B220+ CD43+, and B220+ IgM+ bone marrow cells per two femurs. Black bars, wild type (n = 12); white bars, mdm2puro/Δ7-12 (n = 12).
Consistent with the dramatic reduction in thymus weight, mdm2 was also determined to be critical for thymocyte development. The thymi of mdm2puro/Δ7-12 mice were hypocellular (Fig. 2B), containing on average 5% of the number of thymocytes present in wild-type mice (Fig. 2F). During T-cell development, thymocytes progress from a CD4− CD8− double-negative stage to a CD4+ CD8+ double-positive stage and then to either a CD4+ or a CD8+ single-positive stage (19). Flow cytometric analysis with CD4 and CD8 markers revealed a significant reduction in the absolute number of thymocytes in all differentiation stages in mdm2puro/Δ7-12 mice (Fig. 2E and F). The relative proportion of double-positive thymocytes was decreased 20 to 40% compared with wild-type mice (Fig. 2F), suggesting either a partial block in thymocyte maturation from the double-negative to double-positive stage or decreased survival of double-positive cells. However, the decreased cellularity of mdm2puro/Δ7-12 thymuses cannot be attributed solely to a block in thymocyte development because the absolute number of the least mature, double-negative thymocytes was reduced by greater than 80% from the number in wild-type animals. Together, these results suggest that mdm2 may play a critical role in T-cell development at multiple stages.
Given that both early T-cell precursors and B cells originate in the bone marrow, the decrease in lymphocytes in the thymuses and spleens of mdm2puro/Δ7-12 mice could arise from a deficiency in bone marrow lymphocytes. mdm2puro/Δ7-12 mice contained only half as many bone marrow cells as wild-type mice (Fig. 2G), with a sevenfold decrease in the number of lymphocytes (Fig. 2C). As in the blood, this profound decrease in the number of lymphocytes was accompanied by smaller decreases in other hematopoietic lineages. Both a twofold decrease in the number of erythroid cells, consistent with the mild anemia identified by complete blood count analysis, and a 1.2-fold decrease in the number of granulocytic cells support the conclusion that the hematopoietic defects in mdm2puro/Δ7-12 mice are most severe in the lymphoid lineage.
Next we assessed whether the deficiency of B lymphocytes could be due to a defect in maturation. Immature progenitor B cells express the CD43 and B220 (CD45R) surface markers, whereas, after the transition from pro-B to pre-B, B cells downregulate CD43 (53, 59). Following the synthesis of μ light chains, B cells express surface IgM and higher levels of B220 (53). Flow cytometric analysis revealed a 40% reduction in the number of B220+ CD43+ immature B cells in the bone marrow of mdm2puro/Δ7-12 mice compared with wild-type mice, with a further reduction (87%) in the number of more mature, B220+ IgM+ B cells (Fig. 2G). Therefore, as with thymocytes, loss of mdm2 may interfere with B-cell development at multiple stages, with the reduction in the number of B220+ IgM+ B cells stemming from both a decrease in the number of progenitor cells and a defect in maturation.
Increased p53-dependent apoptosis in mdm2puro/Δ7-12 lymphocytes.
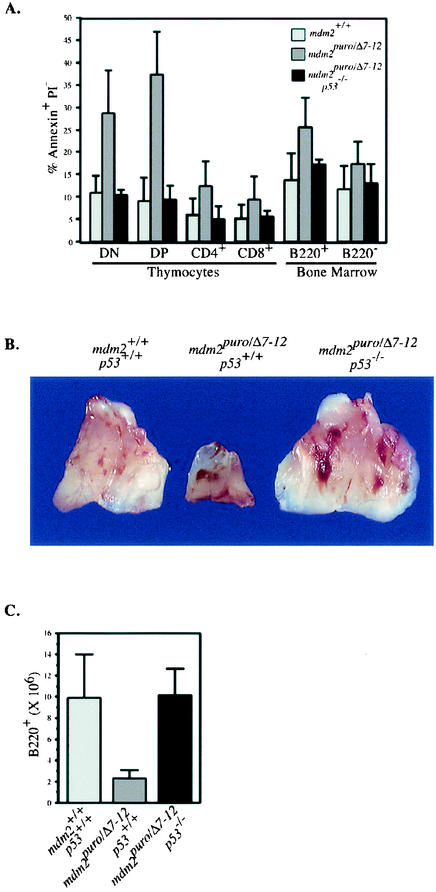
Because p90MDM2 can inhibit the apoptotic function of p53 in cultured cells (14), we predicted that the lymphoid deficiencies in mdm2puro/Δ7-12 mice were due to an increase in the apoptotic activity of p53. We therefore determined the number of apoptotic T and B cells in the thymus and bone marrow, respectively. Costaining for T-cell markers and annexin V, a marker of early stages of apoptosis, revealed a 4.1-fold increase in the percentage of apoptotic double-positive thymocytes, which likely explains the relative paucity of the double-positive population. Increased apoptosis did not strictly coincide with T-cell development, however, as thymocytes of all maturation stages, including the least mature, double-negative cells, showed at least a twofold-greater proportion of annexin V-positive cells (Fig. 3A).
FIG. 3.
p53 deficiency rescues lymphopoietic defects in mdm2puro/Δ7-12 mice. (A) Percentage of apoptotic cells in the lymphoid compartment of wild-type, mdm2puro/Δ7-12, and mdm2puro/Δ7-12 p53−/− mice. Freshly isolated bone marrow and thymocytes were stained for the indicated B- and T-cell markers, respectively, incubated with annexin V and propidium iodide (PI), and analyzed by flow cytometry. DN, double negative; DP, double positive. (B) Thymuses from wild-type, mdm2puro/Δ7-12, and mdm2puro/Δ7-12 p53−/− mice. (C) Average number of B220+ bone marrow cells per two femurs from wild-type, mdm2puro/Δ7-12, and mdm2puro/Δ7-12 p53−/− mice.
Analysis of the number of apoptotic thymocytes in mdm2puro/Δ7-12 mice that were homozygous for a null allele of p53 (20) showed wild-type levels of apoptosis (Fig. 3A), demonstrating that decreased expression of mdm2 can result in p53-dependent apoptosis. Increased p53 function appears to account for the thymic defects, since mdm2puro/Δ7-12 mice lacking p53 had thymuses of normal size (Fig. 3B).
Costaining for B220 and annexin V also revealed a 1.9-fold increase in the percentage of apoptotic B cells in the bone marrow of mdm2puro/Δ7-12 mice relative to wild-type bone marrow (Fig. 3A). B cells from mdm2puro/Δ7-12 mice lacking p53 showed wild-type levels of apoptosis, and the number of B220+ B cells in the bone marrow of mdm2puro/Δ7-12 mice was rescued by loss of p53 (Fig. 3C). Together, these results demonstrate that mdm2 expression is critical for the inhibition of p53-mediated apoptosis during lymphopoiesis.
Increased transactivation function of p53 correlates with decreased mdm2 expression.
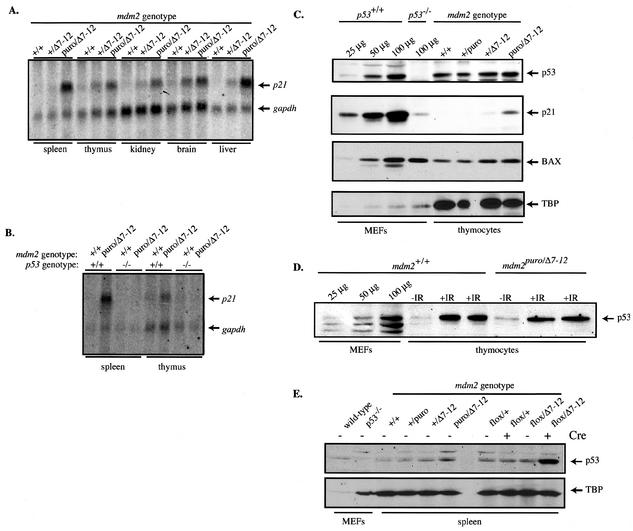
Based on our finding that a 70% decrease in mdm2 expression was sufficient to allow an increase in the apoptotic function of p53, we predicted that the transcriptional activation function of p53 would also be increased in mdm2puro/Δ7-12 mice. Northern analysis of the p53-responsive gene p21 revealed that, in both lymphoid and nonlymphoid tissues from mdm2puro/Δ7-12 mice, expression of p21 was increased relative to that of the control, gapdh (Fig. 4A). The greatest increase in p21 expression (an average of 10-fold) was found in the spleen, whereas only a 2-fold increase was seen in the thymus. An increase in p21 expression was also evident in all tissues examined from mdm2+/Δ7-12 mice, suggesting haploinsufficiency at the mdm2 locus. The increase in p21 mRNA was dependent on the presence of p53, since mdm2puro/Δ7-12 mice lacking p53 showed wild-type levels of p21 expression in both spleen and thymus (Fig. 4B). This is the first demonstration that mdm2 constitutively regulates the transactivation as well as the apoptotic function of p53 in homeostatic tissues.
FIG. 4.
p53 function is increased independently of p53 accumulation in mdm2puro/Δ7-12 thymocytes. (A) Northern analysis of p21 and gapdh mRNAs in multiple tissues. (B) Northern analysis of p21 and gapdh mRNAs in the spleen and thymus in the presence and absence of p53. (C) Western analysis of p53, p21, and BAX in isolated thymocytes. MEFs lacking p53 were used as the negative control. TBP was used as a loading control, and increasing amounts of lysate from wild-type MEFs were used as standards. (D) Western analysis of p53 prior to and following DNA damage. Mice were left unirradiated (−IR) or subjected in duplicate to 10 Gy of ionizing radiation (+IR). Four hours after irradiation, thymocytes were isolated. Increasing amounts of lysate from wild-type MEFs were used as standards. (E) Western analysis of p53 following deletion of exons 7 through 9 of mdm2 in spleens of mdm2flox/Δ7-12 Mx-cre+ mice.
Increased p53 activity in mdm2puro/Δ7-12 tissues occurs without a concomitant increase in the level of p53.
To determine whether the decreased level of p90MDM2 in mdm2puro/Δ7-12 mice allowed the level of p53 protein to increase, we performed Western analyses of p53. Instead of increasing, the level of p53 decreased in lysates from mdm2puro/Δ7-12 thymi compared to wild-type thymuses when equivalent amounts of total protein were analyzed (data not shown). The level of the loading control, TBP, was also decreased in lysates from mdm2puro/Δ7-12 mice, leading us to speculate that nuclear proteins were underrepresented in the samples. As the number of T cells was diminished 10-fold in mdm2puro/Δ7-12 thymuses, whereas the total thymus weight was decreased only 2.5-fold, a greater proportion of the total protein is likely to be stromal, causing a disproportionate representation of extracellular matrix proteins in lysates of whole thymuses. Thus, we tested whether the level of p53 was increased in preparations of mdm2puro/Δ7-12 thymocytes isolated identically to those used for the apoptosis assays that showed an increase in p53 function.
Although TBP levels were identical in isolated wild-type and mdm2puro/Δ7-12 thymocytes, there was no significant increase in the level of p53 protein (Fig. 4C). However, the same blot showed an increase in p21 protein (Fig. 4C) consistent with the p53-dependent increase in p21 mRNA shown in whole thymuses, suggesting that the specific activity of p53 was elevated in mdm2puro/Δ7-12 thymocytes. In contrast, there was no increase in the proapoptotic BAX protein, suggesting that expression of a subset of p53 target genes was increased (Fig. 4C). Indeed, Northern analysis showed no increase in bax mRNA in thymuses from mdm2puro/Δ7-12 mice (data not shown). The increase in p53 function does not appear to be due to a decrease in the shuttling function of p90MDM2, because there was no significant increase in the level of p53 in the nuclei of mdm2puro/Δ7-12 thymocytes (data not shown).
To verify that our detection method could detect an increase in the level of endogenous p53, we compared the level of p53 in thymocytes from untreated and irradiated wild-type and mdm2puro/Δ7-12 mice, because whole-body ionizing radiation is known to increase the level of p53 in this cell type (40). The levels of p53 were clearly increased in thymocytes from both mdm2puro/Δ7-12 and wild-type mice following irradiation (Fig. 4D). Together, these results suggest that, in homeostatic tissues, p90MDM2 selectively regulates the specific activity of p53 so that the function but not the stability of p53 is increased in mdm2puro/Δ7-12 tissues. In contrast to the thymi, the spleens of mdm2puro/Δ7-12 mice showed a slight increase in the level of p53 (Fig. 4E). However, the increase was much smaller than the 10-fold increase in the expression of p21 mRNA (Fig. 4B), again indicating that the specific activity of p53 is elevated in mdm2puro/Δ7-12 tissues.
The increase in the level of p53 was magnified in spleens from mdm2flox/Δ7-12 Mx-cre mice, from which mdm2 had been conditionally deleted (see Materials and Methods), suggesting that the level of p90MDM2 in spleens from mdm2puro/Δ7-12 mice may be sufficiently high to allow some ubiquitination of p53 (Fig. 4E). Together, these results indicate that although p90MDM2 can regulate both p53 function and stabilization in vivo, these two activities of p90MDM2 may be independently controlled, as in some tissues stabilization of p53 does not appear to be a prerequisite for increased function.
Spontaneous apoptosis in the small but not large intestine of mdm2puro/Δ7-12 mice.
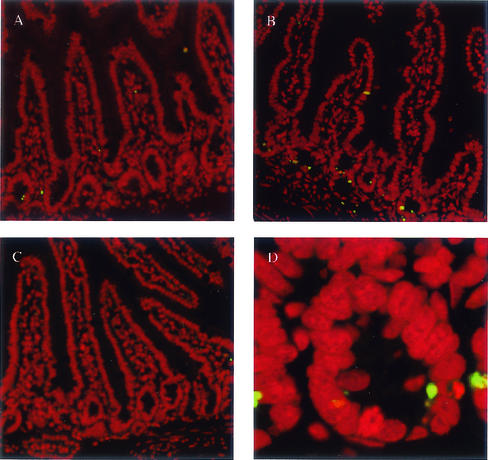
Given that the transcriptional activity of p53 was increased in all mdm2puro/Δ7-12 tissues examined, we next investigated whether cell types in addition to lymphocytes showed an increased frequency of p53-dependent apoptosis. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL), an assay for apoptotic cells (9), revealed no increase in apoptosis in the liver, kidney, or colon of mdm2puro/Δ7-12 mice (data not shown). In contrast, there was a pronounced, 17-fold increase in the frequency of spontaneous apoptosis in the small intestine (Fig. 5A and B). Most of the apoptotic cells in the small intestines of mdm2puro/Δ7-12 mice were in the crypts rather than the villi (Fig. 5D), in the same region as the proliferating transitional cells known to undergo p53-mediated apoptosis in response to ionizing radiation (39). The increased incidence of apoptosis in the small intestines of mdm2puro/Δ7-12 mice was dependent on p53, as loss of p53 abrogated the increased number of apoptotic cells (Fig. 5C). Thus, in addition to lymphocytes, the level of p90MDM2 is important for regulation of the apoptotic function of p53 in the epithelial cells of the small intestine.
FIG. 5.
Increased number of apoptotic cells in crypts of small intestines from mdm2puro/Δ7-12 mice. TUNEL staining (green) was performed on 5-μm sections of the small intestine. Propidium iodide (red) was used to stain all nuclei. (A) Wild type. (B) mdm2puro/Δ7-12. (C) mdm2puro/Δ7-12 p53−/−. (D) Close-up of crypt from mdm2puro/Δ7-12 mouse small intestine.
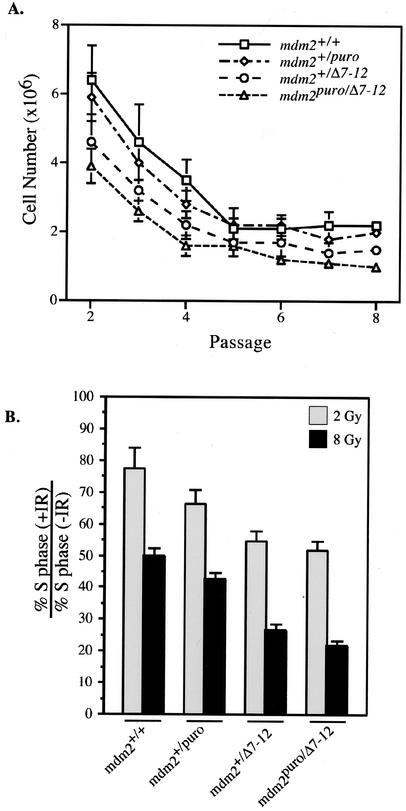
Growth arrest in mdm2puro/Δ7-12 mouse embryo fibroblasts.
In addition to stimulating apoptosis, p53 can mediate growth arrest in the G1 phase of the cell cycle (13, 23). To assess whether the reduction in mdm2 could contribute to a growth arrest, we measured the proliferation rates of mouse embryo fibroblasts (MEFs) from wild-type, mdm2+/puro, mdm2+/Δ7-12, and mdm2puro/Δ7-12 mice. The rate of proliferation correlated with the level of mdm2, with MEFs from mdm2puro/Δ7-12 mice being the slowest to proliferate (Fig. 6A). Moreover, the fraction of cells traversing S phase, as determined by bromodeoxyuridine incorporation, correlated directly with the level of mdm2 expression. For example, the S-phase fraction of mdm2puro/Δ7-12 MEFs was 72% ± 4% of that of wild-type MEFs (data not shown), suggesting that the low level of mdm2 leads to an increase in the G1 arrest function of p53. This result was consistent with the finding that ionizing radiation, which is known to enhance the G1 checkpoint function of p53 (24, 32), caused a decrease in the S-phase fraction that correlated inversely with the level of mdm2 (Fig. 6B). Thus, the level of mdm2 appears to regulate the G1 checkpoint function of p53.
FIG. 6.
Proliferative capacity of MEFs from mice of various mdm2 genotypes. (A) Growth curves for MEFs of the indicated genotypes. MEFs were seeded in triplicate at an initial density of 106 cells per plate and counted every 3 days, after which they were replated at the original density. Graphed is the average number of cells per plate at each passage. Consistent results were obtained for independently derived MEFs. (B) Ratio of the percentage of cells in S phase 16 h following treatment with 2 or 8 Gy of ionizing radiation (+IR) to the percentage in S phase in the absence of treatment (−IR). Bromodeoxyuridine incorporation was used to determine the S-phase fraction.
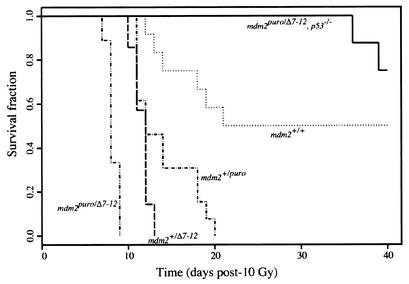
Level of mdm2 expression is a strong determinant of radiation sensitivity in vivo.
The increased sensitivity of mdm2puro/Δ7-12 MEFs to ionizing radiation suggested that the low level of mdm2 in mdm2puro/Δ7-12 mice may become critical in response to stress. Therefore, we asked whether mdm2puro/Δ7-12 mice were more sensitive than wild-type mice to whole-body ionizing radiation. At a dose of 10 Gy, 50% of wild-type mice died between days 12 and 22 posttreatment (Fig. 7), with the remainder surviving the course of the experiment (40 days). In contrast, 100% of mdm2puro/Δ7-12 mice died between 7 and 9 days posttreatment. Both mdm2+/puro and mdm2+/Δ7-12 mice were also more radiosensitive than wild-type mice, with 100% of mdm2+/puro and mdm2+/Δ7-12 mice dying by days 20 and 14, respectively, following treatment.
FIG. 7.
p53-dependent radiosensitivity in mdm2puro/Δ7-12 mice. Kaplan-Meier survival curves of age-matched wild-type (n = 12), mdm2puro/Δ7-12 (n = 13), mdm2+/Δ7-12 (n = 7), mdm2puro/Δ7-12 (n = 9), and mdm2puro/Δ7-12 p53−/− (n = 8) mice following exposure to 10 Gy of whole-body ionizing radiation.
Pairwise comparisons with the log-rank test indicate that the survival times of all genotype combinations except mdm2+/puro and mdm2+/Δ7-12 are significantly different (P < 0.005). The increased sensitivity of mdm2+/puro mice indicates that even a moderate, approximately 20% reduction in mdm2 is sufficient to sensitize mice to the lethal effects of ionizing radiation. Furthermore, since all the mdm2puro/Δ7-12 mice died before any mdm2+/Δ7-12 mice died, mdm2 expression and activity must be exquisitely controlled in vivo, as mdm2 expression differed by less than twofold between these genotypes. As p53 is a key mediator of radiation-induced death in vivo (58), the increased radiation sensitivity in mice with reduced mdm2 is likely due to aberrant p53 activity stemming from insufficient inhibition of p53 by p90MDM2. Indeed, the sensitivity of mdm2puro/Δ7-12 mice to whole-body irradiation was dependent on p53, as 100% of mdm2puro/Δ7-12 p53−/− mice survived for at least 35 days following irradiation (Fig. 7). Thus, mdm2 protects mice from the lethal effects of ionizing radiation. These studies identify mdm2 as a key determinant of radiation sensitivity in vivo.
DISCUSSION
mdm2 is a survival factor in the thymus, bone marrow, and small intestine.
Mice with decreased expression of mdm2 have defects in lymphopoiesis and in epithelial cell survival that can be attributed to an increased frequency of spontaneous p53-dependent apoptosis. While all of the phenotypes reported here are dependent on p53, demonstrating that the major role of mdm2 in adult tissues is to inhibit the activity of p53, our results do not exclude p53-independent functions for p90MDM2. Indeed, decreased expression of mdm2 alters the tumor spectrum in p53-null mice (36). However, to date, no p53-independent phenotypes have been observed in the mdm2puro/Δ7-12 mice.
Does p90MDM2 block a signal to p53?
Tissue-specific differences in susceptibility to p53-mediated apoptosis are not understood. Based on a strong correlation between tissues that accumulate high levels of p53 and those that undergo p53-mediated apoptosis following whole-body ionizing radiation, a model was proposed in which the induced level of p53 protein determines whether a cell type undergoes p53-mediated apoptosis (25, 26, 34). As we have demonstrated, a detectable increase in the level of p53 is not required to stimulate p53-mediated apoptosis in vivo, at least in thymocytes, which show an increased incidence of apoptosis in mdm2puro/Δ7-12 mice without an evident rise in the level of p53 protein. These results indicate that the tissue-specific differences in propensity to undergo p53-mediated apoptosis cannot be explained by differences in the accumulation of p53 protein.
Recent evidence suggests that specific covalent modifications alter the ability of p53 to induce apoptosis, and a two-step model has been put forth in which p53 is released from p90MDM2 to become stable and also covalently modified to become active (57). Our data indicate that accumulation of p53 is not critical for increased apoptosis. However, in mdm2puro/Δ7-12 mice, the decrease in mdm2 expression was sufficient to allow p53-dependent apoptosis in a subset of tissues, suggesting either that p53 is modified constitutively in lymphocytes and epithelial cells of the small intestine or that modification is not required for p53 to stimulate apoptosis. The mice described here will be valuable tools for deciphering which modifications of p53, if any, are required for its apoptotic function.
If modification of p53 is required for spontaneous apoptosis in mdm2puro/Δ7-12 tissues, it is not evident what signaling pathway mediates the modification. Although aberrant double-strand breaks are known to activate p53 in thymocytes, recombination is not required for thymocytes to undergo p53-mediated apoptosis (12, 44). Moreover, although double-strand breaks are likely to occur in unirradiated epithelial cells of the small intestine due to errors in replication (5), such breaks would also be expected in the actively dividing cells of the colon, which do not show an elevated frequency of apoptosis. Normal levels of p90MDM2 may nullify the effects of an unidentified signal intrinsic, for example, to epithelial cells in crypts of the small intestine but lacking in colonic epithelial cells. Alternatively, it may be that no signal is required to activate the apoptotic function of p53 other than one that inhibits p90MDM2. If the second explanation is correct, this implies that tissue-specific differences in p53-mediated apoptosis are mediated through events downstream of p53, since only a subset of tissues underwent p53-mediated apoptosis when levels of p90MDM2 were reduced, whereas all mdm2puro/Δ7-12 tissues tested showed an increase in the transcriptional activation function of p53.
Regulation of mdm2 expression.
Our results demonstrate that mdm2 is critical for homeostasis, yet little is known about the mechanisms that regulate expression of mdm2 and the function of p90MDM2 in tissues. The p53-MDM2 autoregulatory loop model proposes that p53 regulates its own activity by determining the level of its inhibitor, p90MDM2 (1, 49, 60). However, our laboratory and others have shown that p53 does not regulate basal levels of mdm2 expression in tissues (31, 37). In fact, 80 to 90% of mdm2 mRNA in murine tissues is transcribed from an upstream promoter that is unresponsive to p53 (22, 37). The unidentified transcription factors that regulate this promoter may be critical for controlling p53, since the level of p90MDM2 correlates with the level of mdm2 mRNA transcribed from this promoter in murine testis, thymus, brain, and heart (7, 48). Although the level of p90MDM2 varies considerably among wild-type tissues (48), here we show that it is sufficient to keep the transcriptional activation and apoptotic functions of p53 low or undetectable in all tissues tested. In addition to the level of p90MDM2, posttranslational modifications that alter the activity of p90MDM2 may be critical for determining the activity of p53.
Specific activity of p53 is elevated in mdm2puro/Δ7-12 thymocytes.
In mdm2puro/Δ7-12 thymocytes, the percentage of apoptotic cells is increased approximately threefold in the absence of an apparent increase in the level of p53. The simplest interpretation of these data is a model in which the level of p90MDM2 in mdm2puro/Δ7-12 mice is sufficiently low to allow the transcriptional activation domain of p53 to interact more efficiently with the transcriptional machinery but high enough to ubiquitinate p53. The finding that p53 does not accumulate in the nuclei of mdm2puro/Δ7-12 thymocytes is consistent with a normal level of ubiquitination of p53, since nuclear export of p53 is enhanced by ubiquitination. In fact, our observation that whole-body irradiation does not result in a greater accumulation of p53 in mdm2puro/Δ7-12 thymocytes than in wild-type thymocytes suggests that the level of p90MDM2 is not rate-limiting for controlling the level of p53 in this cell type. In the mdm2puro/Δ7-12 spleen, the level of p53 was slightly elevated (less than twofold). However, p53 induced p21 expression 10-fold in this tissue, demonstrating that the specific activity of p53 is increased when mdm2 expression is reduced. Conditional deletion of mdm2 resulted in enhanced accumulation of p53 protein in the spleen, indicating that p90MDM2 does regulate the level of p53 in this homeostatic tissue.
mdm2 protects against the lethal effects of radiation.
The p90MDM2/p53 interaction is a potential target for adjuvant chemotherapy (2). This work provides in vivo evidence for the feasibility of such approaches. The activity of p53 is exquisitely balanced, as evidenced by the increased radiosensitivity of mdm2puro/+ mice, in which mdm2 expression is decreased approximately 20%. Prior to the work presented here, there was no evidence that partial inhibition of p90MDM2 would be sufficient to activate p53 in vivo. Moreover, the fact that a small decrease in p90MDM2 activates p53 and sensitizes cells to radiation suggests that efficient inhibition of p90MDM2 function would not be required in order for proposed adjuvant chemotherapies to be efficacious.
Our work has further implications for the design of adjuvant therapies to activate p53, as it suggests that the ubiquitin ligase function of p90MDM2 may not be the optimal target for inhibition. Drugs that target this function of p90MDM2 may need to be extremely efficient in order to increase the level of p53. However, targeting the physical interaction between p90MDM2 and p53 would appear likely to increase the activity of p53 and synergize with primary cancer therapies. Finally, the fact that few tissues undergo p53-mediated apoptosis in response to decreases in mdm2 implies that most nontumor tissues will be spared by adjuvant therapies that target the p53/p90MDM2 interaction. Based on a recent report suggesting that elevated levels of p53 activity accelerate aging (56), one might speculate that drugs inhibiting p90MDM2 would cause aging. However, no evidence for increased aging has been seen in mdm2puro/Δ7-12 mice up to 22 months of age. Thus, combined with primary therapies targeted to tumor sites, adjuvant therapies that inhibit p90MDM2 may in fact provide good tumor specificity.
Acknowledgments
We acknowledge the expert assistance of the Transgenic Animal Facility and the Flow Cytometry Facility of the Comprehensive Cancer Center of the University of Wisconsin. We are grateful to A. Vaccaro and D. Monson for expert technical assistance and to D. Austin for generously providing statistical expertise. S. Jones (University of Massachusetts) provided mice heterozygous for the mdm2Δ7-12 allele and mdm2 genomic plasmids. We are grateful to A. Messing, J. Petrini, T. Prolla (University of Wisconsin), and A. Bradley (Sanger Centre, Cambridge, United Kingdom) for help and advice in targeting embryonic stem cells. We thank A. Liem and P. Lambert for p53-null mice and E. Sandgren for R26-Cre mice. The expertise of pathologist H. Pitot is greatly appreciated.
This work was supported by funds from NIH grant CA-07175 to the McArdle Laboratory for Cancer Research, by NIH grant CA-14520 to the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Facility, and by NIH grant CA-70718 to M.E.P. S.M.M. and J.M. were supported by NIH Predoctoral Training grants CA-09135 and GM-07215, respectively.
REFERENCES
- 1.Barak, Y., T. Juven, R. Haffner, and M. Oren. 1993. mdm2 expression is induced by wild-type p53 activity. EMBO J. 12:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böttger, A., V. Böttger, A. Sparks, W.-L. Liu, S. F. Howard, and D. P. Lane. 1997. Design of a synthetic MDM2-binding mini protein that activates the p53 response in vivo. Curr. Biol. 7:860-869. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard, V., T. Zaitchouk, M. Vacher, A. Duthu, M. Canivet, C. Choisy-Rossi, M. Nieruchalski, and E. May. 2000. Tissue- and cell-specific expression of the p53 target genes bax, fas, mdm2 and waf1/p21 before and following ionizing irradiation in mice. Oncogene 19:649-660. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L., S. Agrawal, W. Zhou, R. Zhang, and J. Chen. 1998. Synergistic activation of p53 by inhibition of mdm2 expression and DNA damage. Proc. Natl. Acad. Sci. USA 95:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costanzo, V., K. Robertson, M. Bibikova, E. Kim, D. Grieco, M. Gottesman, D. Carroll, and J. Gutier. 2001. Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol. Cell 8:137-147. [DOI] [PubMed] [Google Scholar]
- 6.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 7.Fakharzadeh, S. S., S. P. Trusko, and D. L. George. 1991. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 10:1565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman, D. A., and A. J. Levine. 1998. Nuclear export is required for degradation of endogenous p53 by mdm2 and human papillomavirus E6. Mol. Cell. Biol. 18:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb, E., R. Haffner, A. King, G. Asher, P. Gruss, P. Lonai, and M. Oren. 1997. Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J. 16:1381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grippo, P. J., P. S. Nowlin, R. D. Cassaday, and E. Sandgren. 2002. Cell-specific transgene expression from a widely transcribed promoter with Cre/Lox in mice. Genesis 32:277-286. [DOI] [PubMed] [Google Scholar]
- 12.Guidos, C. J., C. J. Williams, I. Grandal, G. Knowles, M. T. F. Huang, and J. S. Danska. 1996. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 10:2038-2054. [DOI] [PubMed] [Google Scholar]
- 13.Harvey, M., A. T. Sands, R. S. Weiss, M. E. Hegi, R. W. Wiseman, P. Pantazis, B. C. Giovanella, M. A. Tainsky, A. Bradley, and L. A. Donehower. 1993. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8:2457-2467. [PubMed] [Google Scholar]
- 14.Haupt, Y., S. Rowan, E. Shaulian, K. Vousden, and M. Oren. 1995. Induction of apoptosis in HeLa cells by transactivation deficient p53. Genes Dev. 9:2170-2183. [DOI] [PubMed] [Google Scholar]
- 15.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. MDM2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 16.Hinds, P. W., C. A. Finlay, A. B. Frey, and A. J. Levine. 1987. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol. Cell. Biol. 7:2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollstein, M., M. Hergenhahn, Q. Yang, H. Bartsch, Z. Q. Wang, and P. Hainaut. 1999. New approaches to understanding p53 gene mutation spectra. Mutat. Res. 431:199-209. [DOI] [PubMed] [Google Scholar]
- 18.Honda, R., H. Tanaka, and H. Yasuda. 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420:25-27. [DOI] [PubMed] [Google Scholar]
- 19.Ikuta, K., N. Uchida, J. Friedman, and I. L. Weissman. 1992. Lymphocyte development from stem cells. Annu. Rev. Immunol. 10:739-783. [DOI] [PubMed] [Google Scholar]
- 20.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Jones, S. N., A. E. Roe, L. A. Donehower, and A. Bradley. 1995. Rescue of embryonic lethality in MDM2-deficient mice by absence of p53. Nature 378:206-208. [DOI] [PubMed] [Google Scholar]
- 22.Juven, T., Y. Barak, A. Zauberman, D. L. George, and M. Oren. 1993. Wild-type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene 8:3411-3416. [PubMed] [Google Scholar]
- 23.Kastan, M. B., O. Onyekwere, D. Sidransky, B. Vogelstein, and R. W. Craig. 1991. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51:6304-6311. [PubMed] [Google Scholar]
- 24.Kastan, M. B., Q. Zhan, W. S. El-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587-597. [DOI] [PubMed] [Google Scholar]
- 25.Komarova, E. A., M. V. Chernov, R. Franks, K. Wang, G. Armin, C. R. Zelnick, D. M. Chin, S. S. Bacus, G. R. Stark, and A. V. Gudkov. 1997. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 16:1391-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komarova, E. A., K. Christov, A. I. Faerman, and A. V. Gudkov. 2000. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene 19:3791-3798. [DOI] [PubMed] [Google Scholar]
- 27.Kubbutat, M. H. G., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by MDM2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 28.Kubbutat, M. H. G., R. L. Ludwig, A. J. Levine, and K. H. Vousden. 1999. Analysis of the degradation function of MDM2. Cell Growth Differ. 10:87-92. [PubMed] [Google Scholar]
- 29.Kuhn, R., F. Schwenk, M. Aguet, and K. Rajewsky. 1995. Inducible gene targeting in mice. Science 269:1427-1429. [DOI] [PubMed] [Google Scholar]
- 30.Leach, F. S., T. Tokino, P. Meltzer, M. Burrell, J. D. Oliner, S. Smith, D. E. Hill, D. Sidransky, K. Kinzler, and B. Vogelstein. 1993. p53 mutation and mdm2 amplification in human soft tissue sarcomas. Cancer Res. 53:2231-2234. [PubMed] [Google Scholar]
- 31.Leveillard, T., P. Gorry, K. Niederreither, and B. Wasylyk. 1998. mdm2 expression during mouse embryogenesis and the requirement of p53. Mech. Dev. 74:189-193. [DOI] [PubMed] [Google Scholar]
- 32.Lowe, S. W., H. E. Ruley, T. Jacks, and D. E. Housman. 1993. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957-967. [DOI] [PubMed] [Google Scholar]
- 33.Lowe, S. W., S. Bodis, A. McClatchey, L. Remington, H. E. Ruley, D. E. Fisher, D. E. Housman, and T. Jacks. 1994. p53 status and the efficacy of cancer therapy in vivo. Science 266:807-810. [DOI] [PubMed] [Google Scholar]
- 34.MacCallum, D. E., T. R. Hupp, C. A. Midgley, D. Stuart, S. J. Campbell, A. Harper, F. S. Walsh, E. G. Wright, A. Balmain, D. P. Lane, and P. A. Hall. 1996. The p53 response to ionizing radiation in adult and developing murine tissues. Oncogene 13:2575-2587. [PubMed] [Google Scholar]
- 35.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and -independent expression of p21 during cell growth, differentiation and DNA damage. Genes Dev. 9:935-944. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell, T. J., R. Montes de Oca Luna, S. Cho, L. Amelse, A. Chavez-Reyes, and G. Lozano. 1999. Loss of one but not two mdm2 null alleles alters the tumor spectrum in p53 null mice. J. Pathol. 188:322-328. [DOI] [PubMed] [Google Scholar]
- 37.Mendrysa, S. M., and M. E. Perry. 2000. The p53 tumor suppressor protein does not regulate expression of its own inhibitor, MDM2, except under conditions of stress. Mol. Cell. Biol. 20:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendrysa, S. M., M. K. McElwee, and M. E. Perry. 2001. Characterization of the 5′ and 3′ untranslated regions in murine mdm2 mRNAs. Gene 264:139-146. [DOI] [PubMed] [Google Scholar]
- 39.Merritt, A. J., C. S. Potten, C. J. Kemp, J. A. Hickman, A. Balmain, D. P. Lane, and P. A. Hall. 1994. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 54:614-617. [PubMed] [Google Scholar]
- 40.Midgley, C. A., B. Owens, C. V. Briscoe, D. B. Thomas, D. P. Lane, and P. A. Hall. 1995. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J. Cell Sci. 108:1843-1848. [DOI] [PubMed] [Google Scholar]
- 41.Midgley, C. A., J. M. P. Desterro, M. K. Saville, S. Howard, A. Sparks, R. T. Hay, and D. P. Lane. 2000. An N-terminal p14ARF peptide blocks MDM2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 19:2312-2323. [DOI] [PubMed] [Google Scholar]
- 42.Momand, J., G. P. Zambetti, D. C. Olson, D. George, and A. J. Levine. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69:1237-1245. [DOI] [PubMed] [Google Scholar]
- 43.Montes de Oca Luna, R., D. S. Wagner, and G. Lozano. 1995. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378:203-206. [DOI] [PubMed] [Google Scholar]
- 44.Nacht, M., and T. Jacks. 1996. V(D)J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Differ. 9:131-138. [PubMed] [Google Scholar]
- 45.Oliner, J. D., K. W. Kinzler, P. S. Meltzer, D. L. George, and B. Vogelstein. 1992. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358:80-83. [DOI] [PubMed] [Google Scholar]
- 46.Oliner, J. D., J. A. Pietenpol, S. Thiagalingam, J. Gyuris, K. W. Kinzler, and B. Vogelstein. 1993. Oncoprotein MDM2 conceals the activation domain of tumor suppressor p53. Nature 362:857-860. [DOI] [PubMed] [Google Scholar]
- 47.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. T. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 267:1024-1027. [DOI] [PubMed] [Google Scholar]
- 48.Perry, M. E., S. M. Mendrysa, L. J. Saucedo, P. Tannous, and M. Holubar. 2000. p76MDM2 inhibits the ability of p90MDM2 to destabilize p53. J. Biol. Chem. 275:5733-5738. [DOI] [PubMed] [Google Scholar]
- 49.Prives, C. 1998. Signaling to p53: breaking the MDM2-p53 circuit. Cell 95:5-8. [DOI] [PubMed] [Google Scholar]
- 50.Roth, J. M., M. Dobbelstein, D. A. Freedman, T. Shenk, and A. J. Levine. 1998. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17:554-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sauer, B., and N. Henderson. 1988. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 85:5166-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schutte, B., M. M. Reynders, C. L. van Assche, P. S. Hupperets, F. T. Bosman, and G. H. Blijham. 1987. An improved method for the immunocytochemical detection of bromodeoxyuridine labeled nuclei with flow cytometry. Cytometry 8:372-376. [DOI] [PubMed] [Google Scholar]
- 53.Shinkai, Y., G. Rathbun, K.-P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, and F. W. Alt. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 54.Thut, C. J., J. A. Goodrich, and R. Tjian. 1997. Repression of p53-mediated transcription by MDM2: a dual mechanism. Genes Dev. 11:1974-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todaro, G. J., and H. Green. 1963. Qualitative studies of the growth of mouse embryo cells in culture and their development into established cell lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyner, S. D., S. Venkatachalam, J. Choi, S. Jones, N. Ghebranious, H. Igelmann, X. Lu, G. Soron, B. Cooper, C. Brayton, S. H. Park, T. Thompson, G. Karsenty, A. Bradley, and L. A. Donehower. 2002. p53 mutant mice that display early ageing-associated phenotypes. Nature 415:45-53. [DOI] [PubMed] [Google Scholar]
- 57.Vogelstein, B., D. Lane, and A. J. Levine. 2000. Surfing the p53 network. Nature 408:307-310. [DOI] [PubMed] [Google Scholar]
- 58.Westphal, C. H., K. P. Hoyes, C. E. Canman, X. Huang, M. B. Kastan, J. H. Hendry, and P. Leder. 1998. Loss of atm radiosensitizes multiple p53 null tissues. Cancer Res. 58:5637-5639. [PubMed] [Google Scholar]
- 59.Willerford, D. M., W. Swat, and F. W. Alt. 1996. Developmental regulation of V(D)J recombination and lymphocyte differentiation. Curr. Opin. Genet. Dev. 6:603-609. [DOI] [PubMed] [Google Scholar]
- 60.Wu, X., J. H. Bayle, D. Olson, and A. J. Levine. 1993. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 7:1126-1132. [DOI] [PubMed] [Google Scholar]
- 61.Yonish-Rouach, E., D. Resnitzky, J. Lotem, L. Sachs, A. Kimchi, and M. Oren. 1991. Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature 352:345-347. [DOI] [PubMed] [Google Scholar]