Abstract
Environmental pH changes have broad consequences for growth and differentiation. The best-understood eukaryotic pH response pathway acts through the zinc-finger transcription factor PacC of Aspergillus nidulans, which activates alkaline pH-induced genes directly. We show here that Saccharomyces cerevisiae Rim101p, the pH response regulator homologous to PacC, functions as a repressor in vivo. Chromatin immunoprecipitation assays show that Rim101p is associated in vivo with the promoters of seven Rim101p-repressed genes. A reporter gene containing deduced Rim101p binding sites is negatively regulated by Rim101p and is associated with Rim101p in vivo. Deletion mutations of the Rim101p repression targets NRG1 and SMP1 suppress rim101Δ mutant defects in ion tolerance, haploid invasive growth, and sporulation. Therefore, transcriptional repression is the main biological function of Rim101p. The Rim101p repression target Nrg1p is in turn required for repression of two alkaline pH-inducible genes, including the Na+ pump gene ENA1, which is required for ion tolerance. Thus, Nrg1p, a known transcriptional repressor, functions as an inhibitor of alkaline pH responses. Our findings stand in contrast to the well-characterized function of PacC as a direct activator of alkaline pH-induced genes yet explain many aspects of Rim101p and PacC function in other organisms.
One environmental feature with broad consequences for adaptation and differentiation is extracellular pH. In the yeast Saccharomyces cerevisiae, extracellular pH governs expression of genes specifying ion pumps and transporters that promote adaptation to changes in pH (4, 18, 26, 45). Extracellular pH also governs two differentiation programs, i.e., haploid invasive growth and sporulation; these are inhibited in acidic conditions and are favored in alkaline conditions (19, 28). Several of these responses depend upon a conserved regulatory pathway that acts through the transcription factor Rim101p (6, 26, 28, 38, 44). Our focus here is to determine the molecular mechanism by which Rim101p governs pH-dependent responses.
Rim101p, a C2H2 zinc-finger protein, was first identified through mutant analysis as a positive regulator of meiotic gene expression and sporulation (44). Epistasis analysis argued that Rim101p is part of a pathway or complex that also includes Rim8p, Rim9p, and Rim13p (44). The possibility that these gene products act in a pH response pathway came from the finding that the Aspergillus nidulans pH response regulator PacC is a homolog of Rim101p (46). PacC and, as subsequently found, Rim101p are activated by C-terminal proteolytic cleavage that is stimulated at alkaline pH (28, 36). Several gene products required for PacC and Rim101p cleavage are homologous to one another and include the S. cerevisiae calpain-like protease Rim13p (also called Cpl1p), the protease scaffold Rim20p, the putative transmembrane proteins Rim9p and Rim21p, and Rim8p, of unknown biochemical function (5, 15, 26, 52). Studies with Yarrowia lipolytica and Candida albicans have established that Rim101p and its processing pathway are conserved and that they are required for pH-dependent responses (5, 11, 16, 27, 39, 40, 47, 50). Homologs of Rim13p and Rim20p are found in metazoans, so aspects of the Rim101p processing reaction may occur in diverse eukaryotes.
Most phenotypes of S. cerevisiae rim101 mutants are consistent with the idea that Rim101p is a positive regulator of alkaline pH-induced responses. For example, rim101 mutants fail to undergo the alkaline pH-stimulated differentiation pathways—haploid invasive growth and sporulation (19, 28). In addition, rim101Δ mutants have reduced expression of several alkaline pH-induced genes (26). Finally, rim101 mutants grow poorly in alkaline media (15, 26). However, RIM101 has roles that may extend beyond pH-dependent response regulation. For example, S. cerevisiae rim101 mutants are sensitive to Na+ or Li+ ions and grow poorly at low temperatures (26, 44). C. albicans rim101 mutants are also sensitive to Li+ ions (D. A. Davis et al., submitted for publication), and Y. lipolytica rim101 mutants are defective in mating (27). These observations suggest that Rim101p has a broader role than simply to promote alkaline pH-inducible responses.
The paradigm for Rim101p functional activity comes from extensive studies of A. nidulans PacC (6, 13, 38). PacC is required to activate expression of alkaline pH-induced genes, such as ipnA, and to repress transcription of acidic pH-induced genes, such as gabA (13, 21, 46). PacC binds to TGCCARG-containing sequences (PacC sites) found in target promoter regions (14). Mutation of the PacC sites in the ipnA promoter blocks alkaline pH induction of ipnA, suggesting that PacC is a transcriptional activator (13). However, in the acidic pH-induced gabA promoter, the PacC sites overlap with sites for IntA, a transcriptional activator. At alkaline pH PacC is thought to compete with IntA for binding (12). In this promoter, PacC apparently does not function as an activator. Similarly, in Y. lipolytica, the promoter region of the alkaline pH-induced XPR2 gene contains PacC sites that do not provide upstream activation sequence (UAS) activity (31). Thus, PacC DNA binding properties are well understood, but the nature of PacC functional activity may be complex.
It is not know whether S. cerevisiae Rim101p functions as an activator or a repressor, since no direct targets have been defined. Formally, Rim101p is a positive regulator of the meiotic activator gene IME1 and of several alkaline pH-induced genes (26, 44). However, neither IME1 nor the RIM101-responsive alkaline pH-induced genes have PacC sites in their promoters, suggesting that they may be indirect targets. To elucidate the molecular and biological roles of Rim101p, we have identified and analyzed direct Rim101p target genes. Our results indicate that most Rim101p biological functions are exerted through transcriptional repression and that divergent target pathways separately control ion tolerance and cell differentiation.
MATERIALS AND METHODS
Yeast strains.
Yeast strains (Table 1) were derived from SK-1 (24) or YC11 (MATa, ura3-52 trp1Δ1 lys2-801 ade2-101 his3Δ200), which was a gift from C. Horak and M. Snyder. The functional RIM101-HA2 allele has been described elsewhere (28). The rim101Δ::His3MX6, rim13Δ::His3MX6, nrg1Δ::His3MX6, and smp1Δ::His3MX6 disruptions were generated by replacing each entire open reading frame with His3MX6 (29, 52). The tup1-269 mutant (strain AMP1293) was provided by Lenore Neigeborn; the mutation was derived from a selection for increased IME1 expression much as described earlier (34). The Tup1− phenotype segregated as a Mendelian trait was complemented by a TUP1 plasmid and was linked to the TUP1 locus in a genetic cross. The mutation is an A-to-T substitution at nucleotide 808 and causes a nonsense mutation (TAG) immediately after codon 269.
TABLE 1.
Yeast strains
| Strain | Background | Genotype |
|---|---|---|
| AMP620 | SK-1a | MATatrp1-hisG met4 |
| AMP1293 | SK-1 | MATatrp1-hisG met4 tup1-269 |
| TLY869 | SK-1 | MATa |
| TLY870 | SK-1 | MATα |
| TLY907 | YC11b | MATaura3-52-URA3-CYC1PacC-lacZ RIM101::RIM101-HA2 |
| TLY909 | YC11 | MATaura3-52-URA3-CYC1PacC-lacZ |
| TLY912 | YC11 | MATaura3-52-URA3-CYC1PacC-lacZ RIM101::RIM101-HA2 rim13Δ::His3MX6 |
| TLY925 | YC11 | MATaura3-52-URA3 rim101 ΔHis3MX6 |
| TLY926 | YC11 | MATaura3-52-URA3-His3 |
| TLY928 | SK-1 | MATα smp1Δ::His3MX6 |
| TLY932 | SK-1 | MATarim101Δ::HIS3 smp1Δ::His3MX6 |
| TLY933 | SK-1 | MATα rim101Δ::His3 smp1Δ::His3MX6 |
| TLY936 | SK-1 | MATasmp1Δ::His3MX6 |
| TLY941 | SK-1 | MATaura3::His3MX6 |
| TLY942 | SK-1 | MATα nrg1Δ::His3MX6 |
| TLY944 | SK-1 | MATanrg1Δ::His3MX6 |
| TLY945 | SK-1 | MATα rim101Δ::His3MX6 nrg1Δ::His3MX6 |
| TLY947 | SK-1 | MATanrg1Δ::His3MX6 rim101::His3MX6 |
| WXY170 | SK-1 | MATatrp1-hisG met4 gal80::LEU2 RIM101-HA2 |
| WXY189 | SK-1 | MATatrp1-hisG met4 gal80::LEU2 RIM101-HA2 rim13Δ::His3MX6 |
| WXY222 | SK-1 | MATatrp1-hisG met4 gal80::LEU2 rim101Δ::His3MX6 |
| WXY278 | SK-1 | MATarim13Δ::His3MX6 |
| WXY281 | SK-1 | MATarim101Δ::His3MX6 |
| WXY289 | SK-1 | MATα rim101Δ::His3MX6 |
SK-1 strains all carry ura3 his3ΔSK leu2-hisG lys2 ho-LYS2 unless noted otherwise.
YC11 strains all carry trp1Δ1 lys2-801 ade2-101 his3Δ200.
We use the acronym ZPS1 (for zinc- and pH-regulated surface protein) to refer to yeast gene YOL154W (26, 30).
Growth conditions, β-galactosidase assays, and lacZ fusions.
Yeast growth media (yeast-peptone-dextrose [YPD], yeast-peptone-acetate, and synthetic complete) were of standard composition (23). Growth tests on LiCl- and NaCl-containing YPD plates (pH 9) have been described elsewhere (26). For sporulation assays, log-phase yeast-peptone-acetate cultures were shifted into sporulation medium (2% potassium acetate plus 20 mg each of uracil, leucine and lysine per liter) at an optical density at 600 nm of 0.5 and sporulation was counted after 18 h. β-Galactosidase assays were carried out as described earlier (23, 26) on yeast grown exponentially for at least two doublings in either synthetic complete-Ura selective medium (see Table 3) or in YPD of the appropriate pH (see Table 5). The reporter plasmid pAED39 was constructed by inserting the sequence TCGAGTGCCAAGATGCCAAGACTCGAGTCTTGGCATCTTGGCAC into the XhoI site of LGΔ312S (17). The ena1-lacZ and zps1-lacZ (previously called yol154w-lacZ) integrating reporters have been described elsewhere (26).
TABLE 3.
The effect of PacC sites on transcription in the wild type and in rim101Δ, rim13Δ, and tup1 mutants
| Strain | Relevant genotype | β-Galactosidase activitya for:
|
Repression (n-fold)b | |
|---|---|---|---|---|
| CYC1-lacZ | CYC1PacC-lacZ | |||
| TLY941 | RIM101 RIM13 | 1,542 | 7.3 | 211 |
| WXY281 | rim101Δ RIM13 | 1,759 | 829 | 2.1 |
| WXY278 | RIM101 rim13Δ | 1,020 | 322 | 3.2 |
| AMP620 | TUP1 | 990 | 2.3 | 430 |
| AMP1293 | tup1-269 | 940 | 322 | 2.9 |
Values are the mean of three or four determinations, and standard deviations were < 25% of the mean.
Values were calculated by dividing the β-galactosidase activity of CYC1-lacZ by that of CYC1PacC-lacZ.
TABLE 5.
The roles of RIM101, NRG1, and SMP1 in pH-responsive gene expression
| Strain | Relevant genotype for:
|
β-Galactosidase activitya for:
|
|||||
|---|---|---|---|---|---|---|---|
|
ena1-lacZ at:
|
zps1-lacZ at:
|
||||||
| RIM101 | NRG1 | SMP1 | pH 4 | pH 8 | pH 4 | pH 8 | |
| TLY869 | + | + | + | 0.60 | 134 | 5.2 | 33 |
| WXY281 | Δ | + | + | 0.02 | 32 | 1.1 | 9.4 |
| TLY944 | + | Δ | + | 5.80 | 163 | 19.9 | 167 |
| TLY947 | Δ | Δ | + | 2.58 | 125 | 19.3 | 376 |
| TLY936 | + | + | Δ | 0.53 | 121 | 3.7 | 46 |
| TLY932 | Δ | + | Δ | 0.03 | 36 | 2.6 | 11 |
Values are the mean of three or four determinations, and standard deviations were < 30% of the mean for ena1-lacZ and were < 22% of the mean for zps1-lacZ.
Gene expression analysis.
Poly(A)+-selected RNA was purified on an oligo(dT) cellulose column and was used as a template for cDNA synthesis with the T7-(dT)24 oligonucleotide as directed by Affymetrix. Biotin-labeled cRNA was generated using the Enzo-BioArray kit (Affymetrix). After fragmentation, the labeled cRNA was used to probe individual Affymetrix yeast DNA arrays, following the manufacturer's instructions. Hybridization signals for each array were normalized using all probe sets, and different arrays were compared with Microarray suite software (Affymetrix) by using statistical algorithms. We considered a twofold or greater change in expression significant and list those genes in Table 2.
TABLE 2.
RIM101 responsive gene expression
| rim101Δ/wt ratioa,b | rim13Δ/wt ratioc | tup1Δ/wt ratiod | Open reading frame or gene | Description | No. of PacC sitese | Rim101p bindingf |
|---|---|---|---|---|---|---|
| 42.2 | 52.0 | 7.7 | YPL277C | Similar to YOR389W | 1 | Yes |
| 36.8 | 27.9 | 4.9 | YJR061W | Similar to mannosylphosphate transfer protein, Mnn4p | 1 | Yes |
| 22.6 | 22.6 | 7.0 | YOR389W | Similar to YPL277C | 1 | Yes |
| 9.8 | 7.0 | 2.8 | RIM8 | Required for Rim101p processing | 2 | Yes |
| 7.0 | 9.2 | 17.7 | YMR322C | Similar to YDR533C | 0 | NDi |
| 5.7 | 4.0 | 4.8 | SMP1 | Putative transcription factor, similar to RLM1 | 1 | Yes |
| 4.9 | 5.3 | 73.6 | FDH1 | Similar to formate dehydrogenases | 0 | ND |
| 4.0 | 5.7 | 3.4 | YNL274C | Similar to glycerate and formate dehydrogenases | 1 | ND |
| 3.7 | 5.7 | 3.3 | ARN1 | Ferrichrome iron transporter | 0 | ND |
| 3.7 | 3.7 | 1.8 | KTR5 | Putative mannosyltransferase | 1 | ND |
| 3.5 | 3.2 | 11.2 | YDL038C | Similar to mucin proteins | 0 | No |
| 3.5 | 3.0 | 0.3 | YIL121W | Similar to antibiotic resistance proteins | 0 | ND |
| 3.0g | 3.0 | 1.4 | CTS1 | Endochitinase | 0 | No |
| 2.8 | 3.0 | 8.7 | NRG1 | Transcriptional repressor in glucose response pathway | 1 | Yes |
| 2.8 | 2.1 | 2.6 | PRB1 | Vacuolar protease B | 1 | Yes |
| 2.8 | 3.2 | 2.8 | YNL208W | Similar to N starvation-induced protein | 1 | ND |
| 2.3 | 2.1 | 1.6 | YPL088W | Similar to aryl-alcohol dehydrogenases | 1 | ND |
| 0.50 | 0.56 | 0.27 | UTR2 | Putative cell wall hydrolase | 0 | ND |
| 0.50 | 0.36 | 0.91 | YGR035C | Hypothetical protein | 0 | ND |
| 0.50 | 0.77 | 2.4 | WSC4 | Putative integral membrane protein | 0 | ND |
| 0.50 | 0.77 | 1.3 | YPL014W | Hypothetical protein | 0 | ND |
| 0.48 | 0.48 | 3.2 | FET4 | Low-affinity Fe(II) transporter | 0 | ND |
| 0.43 | 0.43 | 1.0 | MFA1 | A-factor mating pheromone precursor | 0 | ND |
| 0.43 | 0.43 | 1.4 | AGA2 | Adhesion subunit of a-agglutinin | 0 | ND |
| 0.43 | 0.40 | 0.45 | BAR1 | Extracellular protease that inactivates α-factor | 0 | No |
| 0.40 | 0.38 | 3.2 | YRO2 | Similar to HSP30 heat shock protein, Yro1p | 0 | ND |
| 0.40 | 0.48 | 0.90 | ARN4 | Siderophore iron transporter | 0 | No |
| 0.29h | 1.1 | 34.4 | SHC1 | Sporulation-specific homolog of SKT5 | 0 | ND |
| 0.22 | 0.43 | 4.7 | YOR049C | Similar to YER185W, RTA1 | 0 | ND |
| 0.20 | 0.13 | 0.90 | COS8 | Subtelomeric protein | 0 | ND |
| 0.17 | 1.1 | 1.2 | RIM101 | Zn finger transcriptional regulator | 0 | ND |
| 0.15 | 0.23 | 2.0 | YDR133C | Questionable open reading frame | 0 | ND |
| 0.13 | 0.19 | 1.6 | CWP1 | Cell wall mannoprotein | 0 | ND |
| 0.094 | 0.051 | 3.2 | YDL241W | Hypothetical protein | 0 | ND |
| 0.031g | 0.036 | 5.2 | FLO10 | Flocculation protein | 0 | ND |
In all experiments, ratios above 2 indicate up-regulation in the mutant and ratios below 0.5 indicate up-regulation in the wild type (wt). The table is sorted in descending order of the rim101Δ/wild-type ratios.
Ratio is calculated as the rim101Δ (WXY281) signal divided by the wild-type (TLY941) signal.
Ratio is calculated as the rim13Δ (WXY278) signal divided by wild-type (TLY941) signal.
Values reported in reference 20.
Number of TGCCAAG sites within 600 bp upstream of start site.
Indicates promoter enrichment in Rim101-HA2p chromatin IP (Fig. 2).
Regulated only in SK-1 strains.
Regulated only in YC11 strains (ratio compares TLY925 and TLY926 strains).
ND, not determined.
Probes for Northern blot analysis were generated by the PCR using AMP108 (wild-type SK-1) genomic DNA as a template with the following oligonucleotide pairs (listed 5′ to 3′): SMP1 (F-CTGCTAAATGGGTAGAAGAA and R-CTGGAGAGTTTGTCGAACTCG), NRG1 (F-GATTGTTCCTCTCGACCAGC and R-AACACGGGTATACCGTCAAT), PRB1 (F-CTGCATGCCTGCACCCGACAGATCAGG and R-CAAACGATAGTGAAGAGGGA), RIM8 (F-ATGGCCATGGAGGCCCCGGGTATGTCGTTACTGAGACTGTGG and R-GAGAAGCTTGGATCCTTAATAGTCATCACAAGGGG), YDL038C (F-CAAGTGTTGCTGGTATGTATCG and R-GACTAGATGATACTGTTTGGG), YJR061W (F-ATGCATGCGTAGTGGAGAGGATTACCTGA and R-CCGAAGGATAAGGGAACGTTT), and CTS1 (F-GACGGAAGTATTTGGCTTCAT and R-AAGGCAGGGTACCTTGACGA). The ENO1 oligonucleotides and Northern analysis methods have been described elsewhere (26).
Chromatin IP.
Log-phase cultures were fixed with formaldehyde and lysed with glass beads, and extracts were prepared as described earlier (22). Extracts were sonicated so that the average DNA length was roughly 500 to 1,000 bp, and equal amounts of extract were incubated with antihemagglutinin (anti-HA) antibodies at 4°C overnight. Protein A-Sepharose beads were used to pull down the HA antibody conjugates, and then the beads were washed several times and eluted (22). DNA isolated from these samples is referred to as anti-HA immunoprecipitate. DNA that was in the starting material before the immunoprecipitation (IP) is referred to as whole-cell extract. PCRs were carried out to detect promoters using the following oligonucleotide pairs: CYC1 (F-TCCGTGTGAGACGACATCGT and R-AATATTTAGAGAAAAGAAG), CYC1PacC (F-GCAGGCTGGGAAGCATATTTG and R-AATATTTAGAGAAAAGAAG), ACT1 (F-ATAAACCGTTTTGAAACCAAACTCG and R-TCTAAAAGCTGATGTAGTAGAAGATCC), CTS1 (F-GACGGAAGTATTTGGCTTCAT and R-TGATGTAAAGGAGTGACATTCT), NRG1 (F-CCGCATGCCTGTGGCAGATAAGCCTTTC and R-AGCCTGCAGCCAGACTGTAGA), PRB1 (F-CTGCATGCCTGCACCCGACAGATCAGG and R-TTGGTACCACTTCATCTTTGCTTGTTAG), RIM8 (F-TAAGTTTCTTCTCTTCTATTC and R-TGTTTGGTCAATGCTACCG), SMP1 (F-TACCTGTACCGTTCCCGATGA and R-CGGGTACCTTCTTCTACCCATTTAGCAG), YDL038C (F-GGCTGCAGTGTAACCAGTTCAACCATTC and R-CCGAATTCTCTTGTACGATACATAGCCG), YJR061W (F-ATGCATGCGTAGTGGAGAGGATTACCTGA and R-AAGGTACCGCGCAGTGATAACATCATTGG), YOR389W (F-ATGCATGCAACCACTTGAACAAGGGGAG and R-TCGGTACCTTGACGGTGGAATCTCATTATT), and YPL277C (F-CTGCATGCTCAAGCGTGCACCTTCAACTT and R-ATGGTACCTTGACGATGGAATCGCATTCTC).
RESULTS
Gene expression analysis.
To identify possible Rim101p target genes, we performed genomewide expression analysis. Wild-type and rim101Δ strains were grown logarithmically in rich YPD medium, and labeled samples were used to probe yeast DNA arrays. We carried out three independent comparisons of rim101Δ with wild-type strains in two different strain backgrounds. We found 17 genes that were up-regulated twofold or more in rim101Δ mutant strains and 18 genes that were down-regulated twofold or more in rim101Δ mutant strains (Table 2). Several of these genes have known or predicted functions in the cell wall (YJR061W, KTR5, YDL038C, CTS1, UTR2, AGA2, SHC1, CWP1, and FLO10), some have a role in iron uptake (ARN1, FET4, and ARN4), some are potential membrane proteins (YPL277C, YOR389W, YIL121W, and WSC4), and two are transcriptional regulators (SMP1 and NRG1). Notably, three genes involved in the mating response—AGA2, BAR1, and MFA1—were down-regulated in rim101Δ strains; down-regulation of the homologous genes may cause the mating defect of Y. lipolytica rim101Δ mutants.
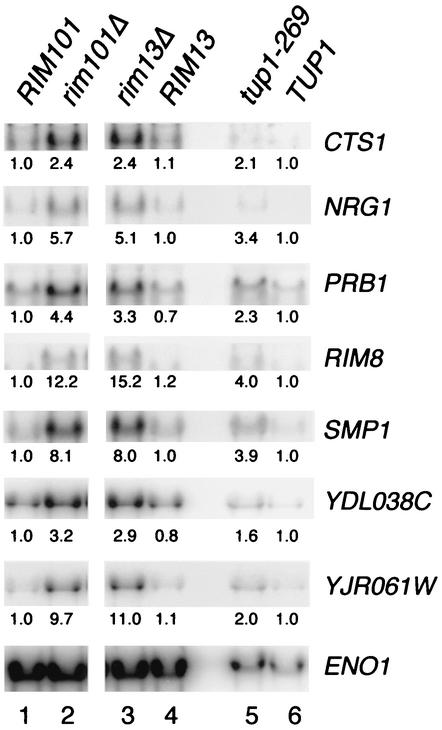
We used Northern analysis to confirm the array results for several genes. We focused on genes that were up-regulated in rim101Δ strains, as explained below. Transcripts of CTS1, NRG1, PRB1, RIM8, SMP1, YDL038C, and YJR061W were detected at higher levels in a rim101Δ strain than in an isogenic RIM101 strain (Fig. 1, lane 2 compared to lane 1). Levels of a control ENO1 transcript were similar in the two strains (Fig. 1). Thus, these genes are negatively regulated by Rim101p.
FIG. 1.
Northern blot analysis of Rim101p-repressed genes. RNA prepared from YPD cultures of strains TLY941 (wild type, lanes 1 and 4), WXY281 (rim101Δ, lane 2), WXY278 (rim13Δ, lane 3), AMP1293 (tup1-269, lane 5), and AMP620 (wild type, lane 6) was used to prepare Northern blots, which were probed for SMP1, NRG1, PRB1, RIM8, YDL038C, YJR061, CTS1, and ENO1 transcripts. Blots were visualized and quantitated with a phosphorimager. The number under each lane represents the probe signal, corrected for ENO1 expression and setting the wild-type signal (lanes 1 or 6) at 1.0. Lanes 1 to 4 show 10 μg of poly(A)+ RNA; lanes 5 and 6 show 20 μg of total RNA.
Based on the amino acid similarity within the zinc-finger region of Rim101p and PacC, Rim101p is predicted to bind to a PacC site (TGCCARG). Promoter region TGCCAAG sites occur in most of the genes that were up-regulated in rim101Δ strains but not in genes that were down-regulated (Table 2). Also, analysis of the complete expression data set with the algorithm for regulatory element detection using correlation with expression (3) revealed that the presence of the 7-nucleotide motif TGCCAAG in a promoter most strongly correlated (Δχ2 = 0.005064) with increased expression of the downstream gene in the rim101Δ mutant (data not shown). Thus, if Rim101p regulates transcription directly through PacC sites in S. cerevisiae, then Rim101p is predicted to function as a repressor.
The role of PacC sites in S. cerevisiae.
We used artificial reporter constructs to determine whether Rim101p acts through PacC sites and whether it functions as a repressor. Four PacC sites were inserted between the UAS and TATA region of a CYC1-lacZ fusion to create a reporter designated CYC1PacC-lacZ. The CYC1-lacZ construct lacking PacC sites was expressed at similar high levels in both RIM101 and rim101Δ strains (Table 3). CYC1PacC-lacZ expression was 211-fold lower than that of CYC1-lacZ in the RIM101 strain. Repression was almost entirely dependent on RIM101 because CYC1PacC-lacZ expression was only twofold lower than that of CYC1-lacZ in the rim101Δ strain (Table 3). In similar experiments, we found that placement of PacC sites in front of a promoter lacking other activation sequences did not stimulate lacZ reporter expression, regardless of the RIM101 allele (data not shown). Thus, in this artificial context, PacC sites do not have UAS activity; instead they direct Rim101p-dependent repression. These results are consistent with the model that Rim101p functions as a repressor.
Association of Rim101p with target promoters.
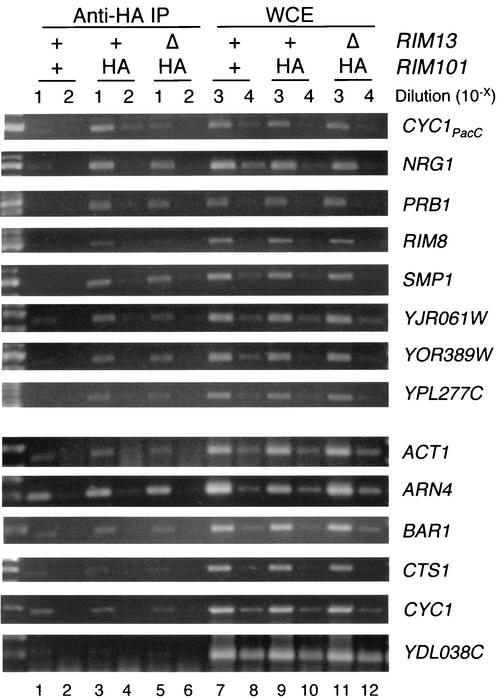
To determine whether Rim101p associates with target promoter regions in vivo, we carried out chromatin IP experiments (Fig. 2). We examined strains expressing wild-type Rim101p or a functional HA epitope-tagged derivative (Rim101-HAp), expressed from the RIM101 promoter. DNA isolated from anti-HA chromatin IPs was used in PCR assays to detect target promoters (Fig. 2, lanes 1 to 4). As a control, the whole-cell extracts were used in parallel PCR assays to ensure the equivalence of the IP starting material (Fig. 2, lanes 7 to 10). We observed that the Rim101p-repressed NRG1, PRB1, RIM8, SMP1, YJR061W, YOR389W, and YPL277C promoter regions were enriched in the anti-HA IPs of the Rim101-HAp strain (Fig. 2, lanes 3 and 4) compared to the untagged Rim101p strain (Fig. 2, lanes 1 and 2). As an internal positive control, the CYC1PacC-lacZ reporter had been integrated in the genome of each strain, and we observed that the CYC1PacC-lacZ promoter was also enriched in anti-HA IPs of the Rim101-HAp strain. In contrast, promoter sequences for two other Rim101p-repressed genes (CTS1 and YDL038C), two Rim101p-activated genes (ARN4 and BAR1), and a Rim101p-nonresponsive gene (ACT1) were present at similar levels in IPs of both strains (Fig. 2). Also, the native CYC1 promoter lacking PacC sites was present at similar levels (Fig. 2). Thus, Rim101p may act indirectly to repress CTS1 and YDL038C and to activate ARN4 and BAR1. However, our results indicate that Rim101p acts directly at the promoters of NRG1, PRB1, RIM8, SMP1, YJR061W, YOR389W, and YPL277C to cause repression.
FIG. 2.
Chromatin IPs to detect Rim101-HAp DNA binding in vivo. DNA from wild-type (TLY 909, lanes 1, 2, 7, and 8), RIM101-HA (TLY907, lanes 3, 4, 9, and 10), and rim13Δ RIM101-HA (TLY912, lanes 5, 6, 11, and 12) strains was purified from equal amounts of extract before (WCE, lanes 7 to 12) and after anti-HA chromatin IP (anti-HA IP, lanes 1 to 6). Purified DNA was diluted as indicated, and 1 μl was used as a template to detect several promoter regions in separate 50-μl PCRs. One-fifth of each reaction was separated on 1.2 to 2.0% agarose Tris-borate-EDTA gels and visualized with ethidium bromide. The NRG1, RIM8, and BAR1 promoters were detected with 30 cycles of amplification; the CYC1PacC, CYC1, ACT1, CTS1, and YDL038C promoters were detected with 35 cycles, and the other promoters were detected with 28 cycles.
Effect of Rim101p processing on repression and promoter association.
The activity of Rim101p depends on processing by the calpain-like protease Rim13p (15, 26). In keeping with this model, we observed that rim13Δ and rim101Δ mutations caused similar gene expression alterations (Table 2 and Fig. 1). Also, repression by Rim101p through PacC sites is dependent upon Rim13p function (Table 3). These data confirm that the main function of Rim13p under these growth conditions is to promote Rim101p activity. We considered the possibility that processing by Rim13p is required for Rim101p to bind DNA in vivo. If this were the case, then association of Rim101p with target promoters would depend upon RIM13. This seems to be true for the CYC1PacC-lacZ and RIM8 promoter regions: anti-HA IP enrichment of these regions was lost in the rim13Δ strain (Fig. 2, lanes 4 to 6). However, most of the natural Rim101p targets, including the NRG1, PRB1, SMP1, YJR061W, YOR389W, and YPL277C promoters, were similarly enriched in anti-HA IPs from the RIM13 and rim13Δ strains. Therefore, unprocessed Rim101p associates with many of these promoters in vivo, but repression is still dependent on Rim101p processing.
Requirement for Tup1p in Rim101p-dependent repression.
Many Rim101p-repressed genes are also negatively regulated by the corepressor subunits Tup1p and Ssn6p (summarized for Tup1p in Table 1), based upon genomewide expression surveys (7, 20). Northern analysis confirmed that several of these genes are expressed at elevated levels in a tup1 mutant (Fig. 1A, lanes 5 and 6). If repression by Rim101p depends upon Tup1p, then repression through PacC sites should be relieved in a tup1 mutant. A comparison of CYC1-lacZ and CYC1PacC-lacZ expression indicated that PacC sites direct only 2.9-fold repression in a tup1 mutant, compared to 430-fold repression in an isogenic wild-type strain (Table 3). These results indicate that repression through PacC sites depends upon Tup1p.
The role of NRG1 in Rim101p-dependent biological activity.
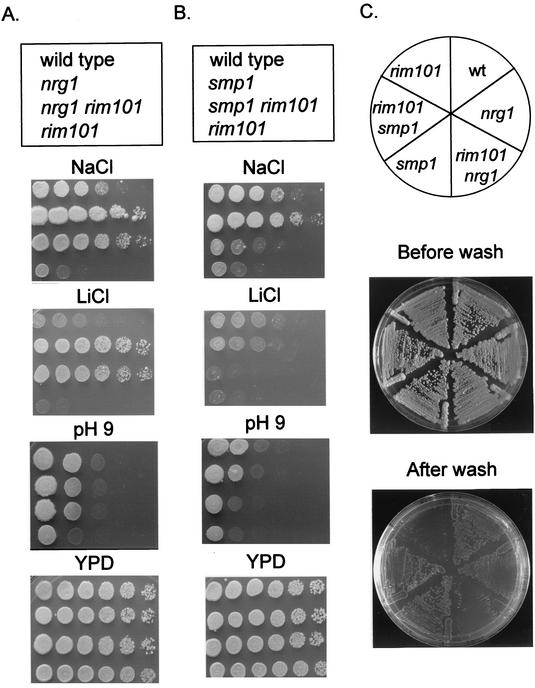
The direct Rim101p target NRG1 specifies a transcription factor. Nrg1p represses transcription of several glucose-repressed genes and, together with its close homolog Nrg2p, negatively regulates invasive growth (25, 37, 49, 53). Thus, it seemed possible that some rim101Δ mutant phenotypes might be due to increased expression of NRG1. If this hypothesis were true, then an nrg1Δ mutation would suppress some rim101Δ mutant phenotypes. The nrg1Δ mutation had no effect on the rim101Δ defects in invasive growth and sporulation (Fig. 3C and Table 4). However, the nrg1Δ mutation fully suppressed the rim101Δ defect in growth at pH 9 (Fig. 3A) and at 17°C (data not shown). In addition we observed that the nrg1Δ mutation confers resistance to Na+ and Li+ ions (Fig. 3A, compare the wild type and nrg1Δ) and found that Na+ and Li+ resistance cosegregated with nrg1Δ through meiosis (data not shown). In an nrg1Δ background, the rim101Δ mutation had no effect on Na+ and Li+ sensitivity. Therefore, increased expression of NRG1 can account for the rim101Δ mutant sensitivity to alkaline pH, low temperature, and Na+ and Li+ ions. In addition, our results reveal a new role for Nrg1p as a negative regulator of Na+ and Li+ tolerance.
FIG. 3.
Roles of SMP1 and NRG1 in Rim101p-dependent responses. (A) Fivefold serial dilutions of strains TLY941(RIM101 NRG1), WXY 281 (rim101Δ), TLY944 (nrg1Δ), and TLY947 (rim101Δ nrg1Δ) were spotted on a control YPD plate and on YPD with the following modifications: titrated to pH 9, containing 25 mM LiCl, or containing 0.4 M NaCl. (B) Fivefold serial dilutions of strains TLY941(RIM101 SMP1), WXY 281 (rim101Δ), TLY936 (smp1Δ), and TLY932 (rim101Δ smp1Δ) were spotted on plates as described above. (C) Invasive growth was determined by washing a YPD plate after 7 days of growth. wt, wild type.
TABLE 4.
The roles of RIM101, NRG1, and SMP1 in sporulation
| Diploid strain | Relevant genotype
|
% Sporulationa | ||
|---|---|---|---|---|
| RIM101 | NRG1 | SMP1 | ||
| TLY869 × TLY870 | +/+ | +/+ | +/+ | 92 ± 2 |
| WXY281 × WXY289 | Δ/Δ | +/+ | +/+ | 9 ± 3 |
| TLY945 × TLY946 | Δ/Δ | Δ/Δ | +/+ | 6 ± 3 |
| TLY942 × TLY944 | +/+ | Δ/Δ | +/+ | 94 ± 4 |
| TLY932 × TLY933 | Δ/Δ | +/+ | Δ/Δ | 33 ± 3 |
| TLY928 × TLY936 | +/+ | +/+ | Δ/Δ | 96 ± 2 |
Percent sporulation is the average value plus or minus standard deviation for four independent diploids.
One way that S. cerevisiae adapts to alkaline pH and excess Na+ and Li+ is by increased expression of the Na+ pump gene, ENA1 (18, 45). Expression of ENA1 partially depends on Rim101p (26). If Nrg1p acts downstream of Rim101p to govern alkaline pH, Na+, and Li+ sensitivity, then Nrg1p may function as a negative regulator of ENA1. We examined the pH response of ena1-lacZ to test this model (Table 5). At pH 4, the wild-type strain expressed ena1-lacZ at low uninduced levels, the rim101Δ strain expressed ena1-lacZ at 30-fold-lower levels, and the nrg1Δ mutant expressed ena1-lacZ at 10-fold-higher levels than did the wild-type strain. The rim101Δ nrg1Δ double mutant, like the nrg1Δ mutant, expressed ena1-lacZ at high levels. At pH 8, the wild-type strain expressed ena1-lacZ at induced levels, the rim101Δ mutant expressed ena1-lacZ at fourfold-lower levels, and the nrg1Δ and rim101Δ nrg1Δ strains expressed ena1-lacZ at the same high level as the wild type. Therefore, an nrg1Δ mutation is sufficient to increase ena1-lacZ expression at acidic pH and can suppress the rim101Δ mutant defect in alkaline pH-induced ena1-lacZ expression. These results support the idea that Nrg1p acts downstream of Rim101p to repress ENA1.
To determine whether Nrg1p governs expression of additional alkaline pH-induced genes, we also examined expression of a zps1(yol154w)-lacZ fusion. We verified that full levels of zps1-lacZ expression depend upon Rim101p (Table 5), as shown previously (26). Presence of an nrg1Δ mutation caused overexpression of zps1-lacZ at both pH 4 and pH 8 and rendered expression independent of Rim101p. Together, these results indicate that Rim101p promotes alkaline pH induction of ENA1 and ZPS1 by repressing the NRG1 repressor gene.
The role of SMP1 in Rim101p-dependent biological activity.
The direct Rim101p target gene, SMP1, also specifies a transcription factor. Smp1p (for second MEF2-like protein) is homologous to Rlm1p, a MADS box family transcription factor that activates transcription in response to the cell integrity-Mpk1p mitogen-activated protein kinase pathway (10). However, the function of Smp1p is not known. To determine whether some rim101Δ defects are the result of elevated Smp1p levels, we examined whether an smp1Δ mutation could suppress any rim101Δ mutant phenotypes. The rim101Δ mutant defects in alkaline pH and ion tolerance were largely unaffected by the smp1Δ mutation (Fig. 3B). We noted that the smp1Δ mutation conferred Na+ resistance but that a rim101Δ mutation caused Na+ sensitivity in both SMP1 and smp1Δ backgrounds (Fig. 3B). In keeping with these epistasis tests, the smp1Δ mutation had no effect on ENA1 expression at pH 4 or pH 8 (Table 5), thus suggesting that Smp1p and Rim101p govern Na+ tolerance through independent pathways. The smp1Δ mutation also had no effect on zps1-lacZ expression (Table 5). In contrast, the smp1Δ mutation fully suppressed the rim101Δ mutant defect in invasive growth (Fig. 3C) and partially suppressed the defect in sporulation (Table 4). The smp1Δ mutation also restored rough colony morphology to the otherwise smooth rim101Δ mutant (data not shown). These observations argue that elevated SMP1 expression in rim101Δ mutants inhibits invasive growth and sporulation and promotes smooth colony morphology.
DISCUSSION
Rim101p homologs are broadly distributed among fungi, where they are required for alkaline pH-induced gene expression and diverse differentiation pathways (6, 38). Here we show that S. cerevisiae Rim101p exerts its biological functions primarily as a repressor, based on three lines of evidence. First, Rim101p is associated in vivo with the promoters of several genes, and these genes are negatively regulated by Rim101p. Second, an artificial reporter gene containing deduced Rim101p binding sites is negatively regulated by Rim101p. Third, deletion mutations of two Rim101p repression targets, NRG1 and SMP1, each suppress a subset of rim101Δ mutant phenotypes. Our results show that Rim101p is associated with many target promoter regions regardless of whether it is processed or unprocessed; however, Rim101p processing is required for its activity as a repressor. As the corepressor complex Tup1p-Ssn6p also negatively regulates all direct Rim101p repression targets, it is possible that processing of Rim101p is required for functional Rim101p-Tup1p-Ssn6p interaction. Although our findings differ in several respects from the PacC paradigm, we argue that the biological and molecular repression functions of Rim101p and PacC may be conserved.
Rim101p DNA binding, processing, and repression.
We found that a functional epitope-tagged Rim101p associates with Rim101p-repressed promoter regions in vivo. Two observations indicate that Rim101p acts through the sequence TGCCAAG, a PacC site. First, the sequence appears in all Rim101p-associated promoter regions but in fewer than 10% of all S. cerevisiae promoters. Second, introduction of four copies of this sequence into the CYC1 promoter confers Rim101p-dependent repression. Our results are consistent with the idea that Rim101p binds directly to the sequence TGCCAAG because Rim101p is associated with the CYC1PacC-lacZ promoter but not with the CYC1 promoter. However, repression by Rim101p through a PacC site depends upon promoter context (W. Xu and A. P. Mitchell, unpublished results). Thus, a single PacC site may be necessary but not sufficient to direct Rim101p repression in vivo.
Rim101p-DNA association is processing independent at some promoters and processing dependent at others. DNA association by unprocessed Rim101p was unexpected since unprocessed A. nidulans PacC is largely cytoplasmic, whereas processed PacC is exclusively nuclear (33). Processing may influence Rim101p localization in S. cerevisiae as well, which would explain the processing-dependent association with the RIM8 promoter. The ability of unprocessed Rim101p to bind other target promoters could be due to the presence of higher-affinity sites. Several transcription factors are bound to target promoters in their inactive states (reviewed in reference 51). However, since repression of Rim101p target genes still depends on processing, the Rim101p C-terminal region must inhibit repression activity and cannot solely govern Rim101p DNA binding activity or intracellular localization. If Rim101p exerts repression through direct recruitment of Tup1p-Ssn6p, then a simple model is that the Rim101p C-terminal region blocks this recruitment.
Implications for the function of Rim101p/PacC homologs.
Rim101p/PacC homologs have been studied primarily as direct activators of alkaline pH-induced genes (6, 38). Our findings here differ from this paradigm in that S. cerevisiae Rim101p functions primarily as a repressor and that it promotes alkaline pH-induced genes indirectly through repression of Nrg1p. Perhaps the biochemical function of S. cerevisiae Rim101p has diverged substantially from its homologs, but several observations from other fungi are consistent with our findings. First, A. nidulans PacC functions as a repressor at the gabA promoter (12). Second, C. albicans Rim101p is formally a negative regulator of RIM8/PRR1 expression (40), as expected if direct repression of RIM8 by Rim101p is conserved. Third, C. albicans Rim101p is a positive regulator, while Nrg1p is a negative regulator, of hypha-specific genes and morphogenesis (2, 5, 11, 35, 40), as expected if Rim101p repression of Nrg1p is conserved. Thus, these previous findings can be understood if the repression function of Rim101p is conserved.
Although we argue that Rim101p/PacC homologs function as repressors, there is clear and compelling evidence that many also function as activators (reviewed in references 6 and 38). How might they function in both ways? One possibility is that Rim101p/PacC proteins function as repressors unless they associate with an activator. Indeed, in Y. lipolytica, PacC sites and an Abf1p activator site are required to create a pH-responsive UAS (31). A second possibility is that different forms of Rim101p/PacC homologs have opposite activities. PacC cleavage occurs in two steps to yield N-terminal fragments of ∼500 and ∼250 residues (9). The ∼500-residue form—the major form in S. cerevisiae—may be a repressor in all organisms, while the ∼250-residue form may be an activator.
The function of Rim101p target genes.
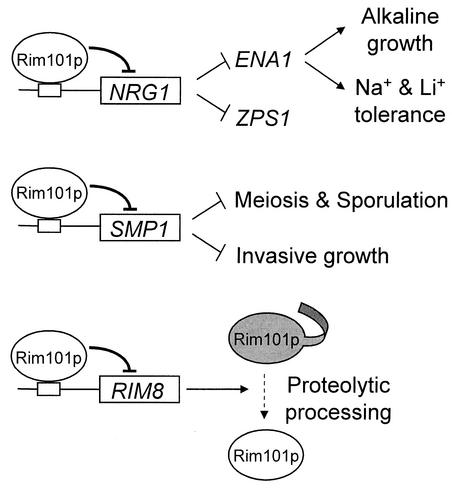
The Rim101p repression target RIM8 is required for Rim101p processing, a relationship that has properties of a negative feedback loop (Fig. 4). In C. albicans, a rim101Δ mutation also causes overexpression of RIM8/PRR1 (40), so this homeostatic circuit is conserved. Repression of RIM8 is functionally significant, because strains lacking functional Rim101p have elevated processing rates (Xu and Mitchell, unpublished). This mechanism may prevent either hyperaccumulation of processed Rim101p or hyperactivity of the Rim13p protease.
FIG. 4.
Relationship of Rim101p to repression targets and biological function. Rim101p associates with promoter regions of NRG1, SMP1, RIM8, and other genes to cause repression. Repression and, in some cases, promoter association depend upon processing of Rim101p by Rim13p. The repression target Nrg1p functions as a negative regulator of alkaline pH-induced genes ZPS1 and ENA1; Ena1p is required for alkaline growth and Na+ and Li+ tolerance. The repression target Smp1p functions as a negative regulator of invasive growth and sporulation, though other Rim101p targets may govern sporulation as well. The repression target Rim8p promotes Rim101p processing, and repression of RIM8 may prevent hyperactivity of Rim101p or of the protease Rim13p.
Our functional analysis here focused on two Rim101p repression targets, NRG1 and SMP1, because they specify transcription factors and thus seemed likely to mediate Rim101p-dependent functions. We have identified new functions for both of these gene products. Smp1p has properties of a negative regulator of haploid invasive growth, rough colony morphology, and sporulation (Fig. 4). Smp1p is a MADS box protein homologous to Rlm1p, a target of the protein kinase C/cell integrity mitogen-activated protein kinase pathway (10). A prospective Smp1p binding site (10) occurs upstream of CWP1, a mannoprotein gene that promotes cell wall integrity (8, 43, 48). CWP1 is down-regulated in the rim101Δ mutant, in which SMP1 expression is elevated, as expected if Smp1p were a transcriptional repressor. This hypothesis may permit identification of direct Smp1p targets that mediate Rim101p-dependent differentiation responses.
Nrg1p has a major role in pH-responsive gene regulation and ion tolerance (Fig. 4). One key role of Nrg1p is to negatively regulate ENA1, an Na+ efflux pump gene that is critical for growth in alkaline media and for Na+ and Li+ tolerance (18, 42, 45). Nrg1p is a repressor (37), and two possible Nrg1p binding sites (CCCCT and CCCTC) occur in the ENA1 5′ region at −650 and −725 in the ENA1 5′ region, so Nrg1p may repress ENA1 directly. Prior studies indicate that Nrg1p activity is inhibited by the protein kinase Snf1p, which mediates glucose repression (25, 49). Snf1p is known to promote ENA1 expression in part through inhibition of the repressor Mig1p (1), but it is possible that Snf1p also promotes ENA1 expression through inhibition of Nrg1p. Thus, Nrg1p may couple ENA1 expression and ion tolerance to both carbon and pH signaling pathways.
Nrg1p is a negative regulator of a second alkaline pH-induced gene, ZPS1. Zps1p function is uncertain, but both ZPS1 and its C. albicans homolog PRA1 are Rim101p-dependent alkaline pH-induced genes (5, 26, 41). ZPS1 has a prospective Nrg1p binding site within its promoter (at position −190), so it may be a direct target of Nrg1p repression. Therefore, S. cerevisiae Rim101p activates at least two alkaline pH-induced genes through a repression relay: Rim101p represses NRG1, and Nrg1p in turn negatively regulates alkaline pH-induced genes.
Because Nrg1p governs pH-responsive gene expression, it is possible that Nrg2p does so as well. Nrg1p and Nrg2p are close homologs that function together to repress FLO11, DOG2, pseudohyphal growth, and biofilm formation (25, 49). For FLO11 expression in particular, their roles seem redundant (25, 49). For several other genes, Nrg1p alone has a detectable role, though the role of Nrg2p in repression of many targets has not been examined (37, 49, 53). Our expectation is that Nrg1p has a more central role than Nrg2p during growth in acidic conditions, because NRG1 is up-regulated at acidic pH, while NRG2 is up-regulated at alkaline pH (4, 25). Thus, Nrg1p may function to repress alkaline pH-induced genes primarily in acidic growth conditions.
Our findings support the idea that S. cerevisiae pH-responsive gene expression involves the interplay of several regulatory pathways. While Rim101p and Nrg1p are important for adaptation to alkaline pH, ENA1 and ZPS1 are still induced at pH 8 in rim101Δ nrg1Δ double mutants. Induction of ENA1 by alkaline pH has been shown to depend on the calcineurin-activated transcription factor Crz1p (32). Similarly, we have observed that induction of ZPS1 by alkaline pH depends upon the zinc-responsive transcription factor Zap1p (T. M. Lamb and Mitchell, unpublished observations). Thus, Rim101p and Nrg1p control activity of the ENA1 and ZPS1 promoters in conjunction with other pH-responsive regulatory pathways. This interplay fits well with the finding that external pH changes have wide-ranging physiological impact, as reflected by the diverse groups of pH-responsive genes (4, 26).
Acknowledgments
We thank M. Kim for determining the tup1-269 allele sequence; H. Bussemaker for performing analysis via regulatory element detection using correlation with expression; D. A. Davis and W. Xu for communication of unpublished results; C. Horak and M. Snyder for strains and advice, V. Vyas and M. Carlson for strains and oligonucleotides; M. Shimizu for oligonucleotides and advice; V. Miljkovic and the Columbia University Gene Chip Facility for hybridizing and scanning DNA arrays; and M. Carlson, J. Erickson, and members of the Mitchell lab for critical reading of the manuscript.
This work was supported by grant GM39531 from the National Institutes of Health.
REFERENCES
- 1.Alepuz, P. M., K. W. Cunningham, and F. Estruch. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol. Microbiol. 26:91-98. [DOI] [PubMed] [Google Scholar]
- 2.Braun, B. R., D. Kadosh, and A. D. Johnson. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 20:4753-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bussemaker, H. J., H. Li, and E. D. Siggia. 2001. Regulatory element detection using correlation with expression. Nat. Genet. 27:167-171. [DOI] [PubMed] [Google Scholar]
- 4.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denison, S. H. 2000. pH regulation of gene expression in fungi. Fungal Genet. Biol. 29:61-71. [DOI] [PubMed] [Google Scholar]
- 7.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 8.Dielbandhoesing, S. K., H. Zhang, L. H. Caro, J. M. van der Vaart, F. M. Klis, C. T. Verrips, and S. Brul. 1998. Specific cell wall proteins confer resistance to nisin upon yeast cells. Appl. Environ. Microbiol. 64:4047-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diez, E., J. Alvaro, E. A. Espeso, L. Rainbow, T. Suarez, J. Tilburn, H. N. Arst, Jr., and M. A. Penalva. 2002. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 21:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodou, E., and R. Treisman. 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Barkani, A., O. Kurzai, W. A. Fonzi, A. Ramon, A. Porta, M. Frosch, and F. A. Mühlschlegel. 2000. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol. Cell. Biol. 20:4635-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espeso, E. A., and H. N. Arst, Jr. 2000. On the mechanism by which alkaline pH prevents expression of an acid-expressed gene. Mol. Cell. Biol. 20:3355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espeso, E. A., and M. A. Penalva. 1996. Three binding sites for the Aspergillus nidulans PacC zinc-finger transcription factor are necessary and sufficient for regulation by ambient pH of the isopenicillin N synthase gene promoter. J. Biol. Chem. 271:28825-28830. [DOI] [PubMed] [Google Scholar]
- 14.Espeso, E. A., J. Tilburn, L. Sanchez-Pulido, C. V. Brown, A. Valencia, H. N. Arst, Jr., and M. A. Penalva. 1997. Specific DNA recognition by the Aspergillus nidulans three zinc finger transcription factor PacC. J. Mol. Biol. 274:466-480. [DOI] [PubMed] [Google Scholar]
- 15.Futai, E., T. Maeda, H. Sorimachi, K. Kitamoto, S. Ishiura, and K. Suzuki. 1999. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 260:559-568. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Lopez, C. I., R. Szabo, S. Blanchin-Roland, and C. Gaillardin. 2002. Genetic control of extracellular protease synthesis in the yeast Yarrowia lipolytica. Genetics 160:417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarente, L., and T. Mason. 1983. Heme regulates transcription of the CYC1 gene of Saccharomyces cerevisiae via an upstream activation site. Cell 32:1279-1286. [DOI] [PubMed] [Google Scholar]
- 18.Haro, R., B. Garciadeblas, and A. Rodriguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, M., K. Ohkuni, and I. Yamashita. 1998. Control of division arrest and entry into meiosis by extracellular alkalisation in Saccharomyces cerevisiae. Yeast 14:905-913. [DOI] [PubMed] [Google Scholar]
- 20.Hughes, T. R., M. J. Marton, A. R. Jones, C. J. Roberts, R. Stoughton, C. D. Armour, H. A. Bennett, E. Coffey, H. Dai, Y. D. He, M. J. Kidd, A. M. King, M. R. Meyer, D. Slade, P. Y. Lum, S. B. Stepaniants, D. D. Shoemaker, D. Gachotte, K. Chakraburtty, J. Simon, M. Bard, and S. H. Friend. 2000. Functional discovery via a compendium of expression profiles. Cell 102:109-126. [DOI] [PubMed] [Google Scholar]
- 21.Hutchings, H., K. P. Stahmann, S. Roels, E. A. Espeso, W. E. Timberlake, H. N. Arst, Jr., and J. Tilburn. 1999. The multiply-regulated gabA gene encoding the GABA permease of Aspergillus nidulans: a score of exons. Mol. Microbiol. 32:557-568. [DOI] [PubMed] [Google Scholar]
- 22.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409:533-538. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Kane, S., and R. Roth. 1974. Carbohydrate metabolism during ascospore development in yeast. J. Bacteriol. 118:8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchin, S., V. K. Vyas, and M. Carlson. 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22:3994-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 27.Lambert, M., S. Blanchin-Roland, F. Le Louedec, A. Lépingle, and C. Gaillardin. 1997. Genetic analysis of regulatory mutants affecting synthesis of extracellular proteinases in the yeast Yarrowia lipolytica: identification of a RIM101/pacC homolog. Mol. Cell. Biol. 17:3966-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, W., and A. P. Mitchell. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 30.Lyons, T. J., A. P. Gasch, L. A. Gaither, D. Botstein, P. O. Brown, and D. J. Eide. 2000. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA 97:7957-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madzak, C., S. Blanchin-Roland, R. R. Cordero Otero, and C. Gaillardin. 1999. Functional analysis of upstream regulating regions from the Yarrowia lipolytica XPR2 promoter. Microbiology 145:75-87. [DOI] [PubMed] [Google Scholar]
- 32.Mendizabal, I., A. Pascual-Ahuir, R. Serrano, and I. F. de Larrinoa. 2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Gen. Genet. 265:801-811. [DOI] [PubMed] [Google Scholar]
- 33.Mingot, J. M., E. A. Espeso, E. Díez, and M. Á. Peñalva. 2001. Ambient pH signaling regulates nuclear localization of the Aspergillus nidulans PacC transcription factor. Mol. Cell. Biol. 21:1688-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno, T., N. Nakazawa, P. Remgsamrarn, T. Kunoh, Y. Oshima, and S. Harashima. 1998. The Tup1-Ssn6 general repressor is involved in repression of IME1 encoding a transcriptional activator of meiosis in Saccharomyces cerevisiae. Curr. Genet. 33:239-247. [DOI] [PubMed] [Google Scholar]
- 35.Murad, A. M., P. Leng, M. Straffon, J. Wishart, S. Macaskill, D. MacCallum, N. Schnell, D. Talibi, D. Marechal, F. Tekaia, C. d'Enfert, C. Gaillardin, F. C. Odds, and A. J. Brown. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orejas, M., E. A. Espeso, J. Tilburn, S. Sarkar, H. N. Arst, Jr., and M. A. Penalva. 1995. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 9:1622-1632. [DOI] [PubMed] [Google Scholar]
- 37.Park, S. H., S. S. Koh, J. H. Chun, H. J. Hwang, and H. S. Kang. 1999. Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:2044-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penalva, M. A., and H. N. Arst, Jr. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66:426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sentandreu, M., M. V. Elorza, R. Sentandreu, and W. A. Fonzi. 1998. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J. Bacteriol. 180:282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano, R. 1996. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int. Rev. Cytol. 165:1-52. [DOI] [PubMed] [Google Scholar]
- 43.Shimoi, H., Y. Iimura, and T. Obata. 1995. Molecular cloning of CWP1: a gene encoding a Saccharomyces cerevisiae cell wall protein solubilized with Rarobacter faecitabidus protease I. J. Biochem. (Tokyo) 118:302-311. [DOI] [PubMed] [Google Scholar]
- 44.Su, S. S., and A. P. Mitchell. 1993. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics 133:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenney, K. A., and C. V. Glover. 1999. Transcriptional regulation of the S. cerevisiae ENA1 gene by casein kinase II. Mol. Cell. Biochem. 191:161-167. [PubMed] [Google Scholar]
- 46.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treton, B., S. Blanchin-Roland, M. Lambert, A. Lepingle, and C. Gaillardin. 2000. Ambient pH signalling in ascomycetous yeasts involves homologues of the Aspergillus nidulans genes palF and paIH. Mol. Gen. Genet. 263:505-513. [DOI] [PubMed] [Google Scholar]
- 48.van der Vaart, J. M., L. H. Caro, J. W. Chapman, F. M. Klis, and C. T. Verrips. 1995. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J. Bacteriol. 177:3104-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vyas, V. K., S. Kuchin, and M. Carlson. 2001. Interaction of the repressors Nrg1 and Nrg2 with the Snf1 protein kinase in Saccharomyces cerevisiae. Genetics 158:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyrick, J. J., and R. A. Young. 2002. Deciphering gene expression regulatory networks. Curr. Opin. Genet. Dev. 12:130-136. [DOI] [PubMed] [Google Scholar]
- 52.Xu, W., and A. P. Mitchell. 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou, H., and F. Winston. 2001. NRG1 is required for glucose repression of the SUC2 and GAL genes of Saccharomyces cerevisiae. BMC Genet 2:5.. [DOI] [PMC free article] [PubMed] [Google Scholar]