Abstract
Tumor necrosis factor alpha (TNF-α) plays an important role in host containment of infection by Mycobacterium tuberculosis, one of the leading causes of death by an infectious agent globally. Using the pathogenic M. tuberculosis strain H37Rv, we present evidence that upon stimulation of monocytic cells by M. tuberculosis a unique TNF-α enhanceosome is formed, and it is distinct from the TNF-α enhanceosome that forms in T cells stimulated by antigen engagement or virus infection. A distinct set of activators including ATF-2, c-jun, Ets, Sp1, Egr-1 and the coactivator proteins CBP/p300 are recruited to the TNF-α promoter after stimulation with M. tuberculosis. Furthermore, the formation of this enhanceosome is dependent on inducer-specific helical phasing relationships between transcription factor binding sites. We also show that the transcriptional activity of CBP/p300 is potentiated by mycobacterial stimulation of monocytes. The identification of TNF-α regulatory elements and coactivators involved in M. tuberculosis-stimulated gene expression thus provides potential selective molecular targets in the modulation of TNF-α gene expression in the setting of mycobacterial infection.
Tumor necrosis factor-alpha (TNF-α) plays a key role in the inhibition of mycobacterial growth in vitro (2) and in granuloma formation in mice in vivo (8, 15). Furthermore, in TNF-α-deficient mice, structural deficiencies in granuloma formation appear to lead to heightened susceptibility to aerosol Mycobacterium tuberculosis infection (1). TNF-α may contribute to the immunopathology of tuberculosis by causing extensive tissue damage (13) and weight loss (22). Most significantly, reports of the development of active tuberculosis in latently infected individuals treated with a humanized antibody against TNF-α for Crohn's disease or rheumatoid arthritis highlight the critical role played by TNF-α in the control of latent tuberculosis (14).
The transcriptional initiation of the TNF-α gene serves as the primary control point of the regulation of TNF-α production (7, 9-11, 23, 26). Studies with multiple cell types using a variety of inducers have established that NFAT, ATF-2, Jun, Ets/Elk, and Sp-1 proteins and the CBP/p300 coactivator proteins are involved in the inducer and cell type-specific regulation of the human TNF-α gene and that distinct enhancer complexes are recruited to the promoter depending on cell type and stimulus (6, 7, 23, 25). In T cells, for example, different sets of activators are recruited to a shared set of transcription factor binding sites depending on the particular stimulus, virus, or antigen engagement (26). Furthermore, the precise arrangement of transcription factor binding sites in the promoter and the inducer-specific recruitment of the cognate activators is critical for transcription and these generate a unique network of inducer-specific protein-protein and protein-DNA interactions (enhanceosomes) (26).
Despite the critical role that TNF-α plays in the containment of infection with M. tuberculosis, the mechanisms involved in its regulation by M. tuberculosis remain poorly defined. Here, we characterize events involved in immediate early TNF-α gene induction following infection of monocytes by M. tuberculosis. We identify the TNF-α regulatory elements, activators and coactivators involved in the induction of the gene by the pathogenic M. tuberculosis strain H37Rv. We present evidence that a unique TNF-α enhanceosome is formed after stimulation of monocytic cells by M. tuberculosis and that this enhanceosome is distinct from the inducer-specific TNF-α enhanceosomes that form in T cells stimulated by antigen engagement or virus infection. Thus, these results establish a novel mechanism for inducer and cell type-specific gene expression.
MATERIALS AND METHODS
Cell culture, transfections, and analysis.
J774 (24), U937 (10), and MonoMac-6 cells (30) were maintained as previously described. M. tuberculosis H37Rv whole sonicate was prepared by resuspending whole irradiated M. tuberculosis H37Rv cell paste in sterile phosphate-buffered saline at 5 mg/ml. The suspension of bacteria was then sonicated on ice at full power for 5 min by use of a Branson Sonifier 250 (Branson Ultrasonics Corp., Danbury, Conn.). Sonicates were aliquoted and stored at −80°C. Transfections in J774 cells were performed by using FuGene6 (Boehringer-Mannheim) according to the manufacturer's protocol. After 8 h of transfection, cells were treated with H37Rv whole sonicate (10 μg/ml) or lipopolysaccharide (LPS) (from Escherichia coli O111:B4; Sigma) at 1 μg/ml and harvested as described in the figure legends. The sonicate and the plasmids were tested for endotoxin contamination by using a kinetic turbidimetric Limulus amoebocyte lysate assay (Endosafe; Charles River). The levels of endotoxin were <1 pg/ml in all experiments. Luciferase assays were performed according to the manufacturer's instructions (Dual Luciferase Reporter Assay System; Promega) by using a Dynex luminometer, with Renilla luciferase (pRL-TK) as a control.
In vitro infection with virulent M. tuberculosis.
All procedures carried out with virulent M. tuberculosis were carried out at biohazard level 3. A 100-ml liquid culture of M. tuberculosis H37Rv was grown in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose-catalase and grown to mid-log phase for 1 week at 37°C. The culture was then transferred to 50-ml tubes and centrifuged at 3,000 rpm in a Beckman centrifuge for 10 min in a tabletop centrifuge. The bacterial pellet was resuspended in 5 ml of RPMI medium containing 10% fetal calf serum, 2% human serum, and 0.05% Tween 80 and centrifuged at 3,000 rpm for 10 min. The bacterial pellet was resuspended in 5 ml of RPMI culture media and passed through a 5-μm-pore-size syringe filter (Millipore, Bedford, Mass.), and bacteria were counted under an inverted microscope by using a Petroff-Houser chamber. Bacteria were then added to the monocyte culture at a multiplicity of infection (MOI) of 5 to 1, and the plates were centrifuged for 1 min at 700 rpm and then placed in 5% CO2 for 1 h to allow bacteria to be internalized. The cells were then washed twice with warm RPMI media to remove free bacteria, and 2 ml of warm RPMI media was added to each well. The plates were then incubated for 1, 2, and 5 h. The efficiency of infection was determined by preparing a cytospin of formalin-fixed infected cells and staining for acid-fast bacteria. RNA was prepared, and an RNase protection assay was performed as previously described (9).
ELISA.
Fifty thousand J774 cells were seeded in triplicate for each time point onto 24-well plates and cultured overnight. The cells were stimulated with H37Rv whole sonicate (10 μg/ml) for the times indicated, and TNF-α protein levels were measured by a Quantikine murine TNF-α enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn.).
Plasmids.
The −200 TNF-α Luc reporter, point mutations, phasing mutants, the G5E1b-Luc reporter, and the Gal4-p300, Gal4-CBP, and E1A vectors have been previously described (7, 26, 29). All plasmids were isolated by use of EndoFree plasmid kits (Qiagen).
Formaldehyde cross-linking and chromatin immunoprecipitation.
J774 cells (6 × 107 cells per treatment) were treated with 10 μg of H37Rv whole sonicate per ml for 1 h. Then, the cells were treated with formaldehyde (1% final concentration) for 30 min at 4°C. Cells were harvested, and fixed chromatin was sonicated, extracted, and purified as described previously (27), followed by immunoprecipitation with anti-Sp1, anti-Egr-1, and anti-Ets-1 antibodies (Santa Cruz Biotechnology). Immunoprecipitated DNA was then amplified by PCR with primers specific to the TNF-α promoter as previously described (7). Titrations of PCR cycles were performed to ensure that experiments were performed in the linear range of amplification.
RESULTS AND DISCUSSION
H37Rv induces TNF-α transcription and protein expression.
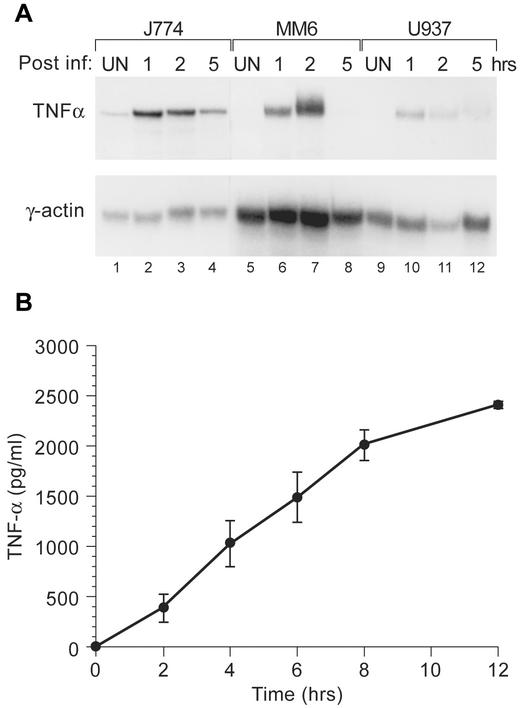
We first compared the induction of TNF-α gene expression by infection with the live pathogenic M. tuberculosis strain H37Rv in a murine (J774) cell line and in two human monocytic cell lines (Mono-Mac 6 and U937) to determine an appropriate system in which to study M. tuberculosis-induced TNF-α gene expression. After infection of the cell lines at an MOI of 5 to 1, we performed an RNase protection assay and demonstrated that TNF-α mRNA was inducible in all three cell lines (Fig. 1A). However, relatively higher levels of TNF-α mRNA were detected in the J774 cells (Fig. 1A). Furthermore, when a whole sonicate of irradiated H37Rv was used as the stimulus, TNF-α protein was also highly inducible in J774 cells (Fig. 1B).
FIG. 1.
H37Rv induces TNF-α transcription and protein expression. (A) An RNase protection assay was performed with three monocytic cell lines (murine J774, human MonoMac6, and human U937). Cells were infected with live M. tuberculosis H37Rv (MOI, 5:1) or mock infected. Cells were collected at 1, 2, and 5 h postinfection as indicated. Total RNA was extracted and hybridized to radiolabeled antisense RNA probes for TNF-α or actin (control) as described in Materials and Methods. (B) J774 cells were stimulated with H37Rv whole sonicate (10 μg/ml) for the times indicated, and supernatants were collected and assayed for TNF-α protein levels by using a Quantikine murine TNF-α ELISA kit. Unstimulated control cells did not produce detectable levels of TNF-α protein at any time point (data not shown). The H37Rv whole sonicate contained 0.075 pg of endotoxin/μg as determined by a kinetic, turbidimetric Limulus amoebocyte lysate assay.
The CRE, Sp1, Ets/Elk, and Egr sites are required for H37Rv induction of TNF-α gene expression.
We next transfected human TNF-α reporter constructs containing −982 or −200 nucleotides (nt) upstream of the transcription start site into J774 cells and demonstrated that −200 nt were sufficient for maximal activation of the TNF-α gene by H37Rv whole sonicate (Fig. 2A). To identify the specific promoter elements required for M. tuberculosis induction of the TNF-α gene, we transfected J774 cells with TNF-α luciferase reporter constructs bearing mutations in different regulatory elements within the context of the −200 TNF-α promoter fragment (Fig. 2B). As shown in Fig. 2C, mutations of the −180-Ets/NFAT, upstream Sp1 (upSp1M), Egr-1 (Egr1M), −117-Ets/NFAT (−117 M), CRE (C1M), κ3-NFAT (5′M and 3′M), −84-Ets (−84 M), −76-Ets/NFAT (−76 M), and Sp1 (SP1M) sites all significantly reduced M. tuberculosis induction of the gene. It should be noted that these same mutations also significantly reduced LPS induction of the TNF-α gene (reference 23 and data not shown). By contrast, induction of TNF-α gene expression by ionophore in T cells is not affected by mutation of the Sp1 sites or the −84-Ets site, whereas virus induction of the gene is significantly reduced by mutation of these sites (26). Thus, consistent with previous results (7, 23, 26), the promoter elements required for induction of TNF-α gene expression are stimulus specific.
FIG.2.
Identification of activator binding sites required for TNF-α gene regulation by M. tuberculosis. (A) J774 cells were transfected with 1 μg of a TNF-α luciferase reporter containing either 200 or 982 nt upstream of the transcription start site. Eight hours posttransfection, cells weretreated with H37Rv M. tuberculosis whole sonicate (10 μg/ml) for 16 h. All transfections included a control Renilla luciferase plasmid and were normalized to Renilla luciferase activity. Data are presented as means ± standard errors of the means (SEM) of three independent experiments. (B) The DNA sequence of the TNF-α promoter spanning nt −200 to −20 relative to the transcription start site is shown. Transcription factor binding sites are indicated, as are the locations of point mutations in the mutant constructs studied in panel C. (C) The CRE, Sp1, upstream Sp1, Egr-1, and Ets binding sites are required for M. tuberculosis induction of TNF-α. J774 cells were transfected with 1 μg of the −200 TNF-α luciferase reporter or with isogenic reporters containing the indicated mutations and treated with M. tuberculosis whole sonicate for 16 h as described above. We noted that the 3′M and −76 NFAT mutant abolished Ets binding to the adjacent −84-Ets/Elk site (reference 23 and data not shown). Data are presented as means ± SEM of three independent experiments. (D) Formaldehyde cross-linking and chromatin immunoprecipitation of J774 cells that were unstimulated (−) or treated with M. tuberculosis whole sonicate (+). Samples of sonicated and purified chromatin were immunoprecipitated with the indicated antibodies, and DNA isolated from immunoprecipitated material was amplified by PCR with primers specific for the TNF-α promoter. An increase in the relative amount of the TNF-α promoter-specific PCR product indicates binding of the protein to the endogenous amplified TNF-α promoter. Densitometry quantification of the induction ratios for the various transcription factors were 1.9 for ATF-2, 2.0 for c-Jun, 3.8 for Ets, 1.5 for Sp1, and 1.6 for Egr. Input DNA control lanes (lanes 12 and 13) and free primer (lane 1) are shown.
Elk-1, Ets, ATF-2/Jun, Egr-1, and Sp1 proteins interact with the endogenous TNF-α promoter upon H37Rv stimulation.
To establish which of the transcriptional activator proteins bind to the TNF-α promoter in J774 cells in vivo after stimulation with M. tuberculosis, we next performed chromatin immunoprecipitation assays with specific antibodies against the different activators. This technique has been used to detect binding of transcription factors to the TNF-α promoter following virus infection and LPS and ionophore stimulation (7, 23, 26). TNF-α promoter DNA was amplified by PCR of formaldehyde-fixed chromatin that had been immunoprecipitated by the antibodies shown in Fig. 2D. This method provides an indication of the amount of transcription factor binding to the promoter following stimulation by M. tuberculosis in vivo. Based on the site-directed mutagenesis of TNF-α promoter function in response to M. tuberculosis and based on previous quantitative DNaseI footprinting studies (6, 26), we used antibodies directed against proteins that recognize the CRE site (ATF-2 and c-jun), Ets binding sites (Ets-1/2, Elk-1), and the Sp1 and Egr-1 sites. As shown in Fig. 2D, M. tuberculosis stimulation of J774 cells results in the inducible binding of ATF-2, c-jun, Ets-1/2, Egr-1, and Sp1 to the TNF-α promoter in vivo. Taken together, the M. tuberculosis-inducible recruitment of ATF-2, c-jun, Ets, Egr-1, and Sp1 to the TNF-α promoter observed in the chromatin immunoprecipitation analysis strongly correlates with the critical functional roles that the Sp1, CRE, Ets, and Egr-1 TNF-α promoter sites play in the activation of the gene by M. tuberculosis.
CBP/p300 proteins are required for H37Rv stimulation of TNF-α and are transcriptionally activated by M. tuberculosis.
CBP/p300 proteins play a critical role in the induction of TNF-α transcription by virus, T-cell receptor ligands, and by LPS (6, 23). CBP/p300 proteins function as coactivators for multiple transcription factors (reviewed in reference 19). We thus next examined the potential role of these proteins in TNF-α gene expression in response to M. tuberculosis. Using the adenovirus E1A 12S protein, which specifically inhibits CBP/p300 function (5), we performed cotransfection studies with the TNF-α luciferase reporter gene in J774 cells. As a control, we used a mutant form of the E1A 12S protein (E1A 12S Δ2-36), which lacks the CBP/p300 interaction domain and fails to inhibit CBP/p300 activity (16). As shown in Fig. 3A, activation of the TNF-α reporter upon stimulation of J774 cells with M. tuberculosis whole sonicate was inhibited by E1A 12S but not by E1A 12S Δ2-36, indicating a specific role for CBP/p300 coactivators in M. tuberculosis-mediated TNF-α transcription.
FIG. 3.
CBP/p300 proteins are required for M. tuberculosis induction of TNF-α. (A) Inhibition of CBP/p300 impairs TNF-α gene induction by M. tuberculosis. J774 cells were cotransfected with 1 μg of the −200 TNF-α luciferase reporter and with 2 μg of the vectors expressing wild-type E1A 12S or mutant (Δ2-36) forms of E1A 12S as indicated. Following transfection, cells were treated with M. tuberculosis whole sonicate for 16 h. Data are presented as means ± standard errors of the means (SEM) of three independent experiments. (B) M. tuberculosis potentiates the transcriptional activity of CBP and p300. J774 cells were cotransfected with a Gal4-dependent luciferase reporter (Gal4x5-luc, 1 μg) and increasing amounts of vectors expressing full-length CBP or full-length p300 fused to the Gal4 DNA-binding domain (0.02, 0.07, 0.2, 0.7, or 2 μg) or the Gal4 DNA-binding domain alone (2 μg). Transfected cells were then treated with M. tuberculosis whole sonicate for 16 h (Mtb) or mock stimulation (UN). Data are presented as means ± SEM of three independent experiments. DBD, DNA-binding domain.
CBP and p300 contain transcriptional activation domains (4, 17). Thus, our results raised the possibility that the transactivation of CBP/p300 proteins might be potentiated by M. tuberculosis. To examine this, we used CBP or p300 proteins fused to the DNA binding domain of Gal4 to determine the effect of M. tuberculosis stimulation upon Gal4 binding site-dependent transcription. Strikingly, both Gal4-CBP and Gal4-p300 were activated in response to stimulation by M. tuberculosis (Fig. 3B). Taken together, these data demonstrate that M. tuberculosis-stimulated assembly of the TNF-α enhancer complex is CBP/p300 coactivator dependent and in turn these coactivators are themselves activated by M. tuberculosis.
An M. tuberculosis-specific enhanceosome is recruited to the TNF-α gene enhancer.
Enhanceosomes are multicomponent transcription enhancer complexes whose assembly is highly cooperative and that promote high levels of transcriptional synergy. Enhanceosome formation takes place only at particular gene promoters and requires precise spatial interactions between the binding sites and the bound activators and coactivators involved in their regulation (3). Thus, the three-dimensional arrangement of the activators along the DNA double helix plays a critical role in the transcriptional initiation of genes that form enhanceosomes such as beta interferon (18), the T-cell receptor alpha enhancer (12), and TNF-α (26). Of these three examples of genes, only TNF-α has been shown to form inducer-specific enhanceosomes depending on the stimulus in T cells (26).
We were thus interested in determining whether precise helical phasing between the activators bound to their recognition elements within the TNF-α promoter was also essential for M. tuberculosis-induced enhancer function and if the enhanceosome formed in the monocytic lineage might be distinct from the enhanceosomes formed in T cells by virus or antigen engagement.
J774 cells were thus transfected with the wild-type −200 TNF-α Luc reporter gene or with constructs in which half- or full-helical turns (5 to 6 or 10 base pairs [bp], respectively) were inserted between individual transcription factor binding sites (26). The half-helical turn disrupts the phasing of the activators along the helix, while the full turn restores the phasing (18, 20). While M. tuberculosis induction of the wild-type −200 TNF-α Luc reporter resulted in a >5-fold increase in luciferase activity, the insertion of a half-helical turn between the −50 Sp1 and −55 NFAT-binding sites inhibited M. tuberculosis induction (Fig. 4, lane 1). Remarkably, the insertion of 10 bp, which reestablished the relative positions of binding sites on the face of the DNA helix, fully restored the activity of the promoter in response to M. tuberculosis (Fig. 4, compare constructs in lane 1).
FIG. 4.
M. tuberculosis enhanceosome formation is dependent upon helical phasing. J774 macrophages were transfected with the wild-type −200 TNF-α luciferase reporter gene or constructs with an insertion of 5 or 10 nt as indicated. These insertions add one-half of a helical turn (5 nt) and a full helical turn (10 nt) and disrupt or restore the precise helical phasing of DNA-bound transcription factors. The cells were then stimulated with M. tuberculosis H37Rv whole sonicate and the increase in inducibility (n-fold) was calculated. All transfections included a control Renilla luciferase plasmid and were normalized to Renilla luciferase activity. Data are presented as means ± standard errors of the means of four independent experiments.
Moreover, insertion of a half-helical turn between the −84-Ets site and the κ3/CRE composite site compromised transcriptional activity by approximately 50% and restoration of helical phasing augmented gene activation ≈10-fold compared to the ≈6-fold wild-type increase (lane 3). These results suggested that after stimulation by M. tuberculosis the activators bound to the TNF-α promoter upstream of the −50 Sp1 site and the −84 Ets site likely contact the basal transcription complex directly or indirectly through protein-protein interactions with each other or via the coactivator proteins p300/CBP. Flipping all the necessary activators out of the correct helical surface strongly inhibits the formation of the functional enhancer complex on the TNF-α promoter following stimulation by M. tuberculosis.
The insertion of nucleotides disrupting the phasing and the spacing between other sites also has inducer-specific effects, but none of the effects can be rescued by restoring helical phasing. For example, the introduction of 5 or 10 bp between CRE and κ3 sites or between CRE and −117 Ets sites decreases the inducibility of the promoter by M. tuberculosis (Fig. 4, constructs in lanes 4 and 5). These results are consistent with the CRE-κ3-Ets site functioning as a composite element where ATF-2, c-jun, and Ets proteins must directly contact each other or a coactivator protein for gene activation to occur. We note that when LPS is used as the stimulus with the phasing mutant constructs results virtually identical to those obtained when using M. tuberculosis as the stimulus are obtained (data not shown).
T cells transfected with the same helical-phasing mutants stimulated with ionophore or Sendai virus gave us surprisingly different results. Disruption of the DNA phasing at the −50 Sp1 site with one-half of a helical turn of the DNA reduced promoter activity by ionophore in a fashion similar to what was observed with M. tuberculosis, but when virus was used as the stimulus, activity was reduced by only approximately 50% (26), and this activity was not restored by correct helical phasing, indicating that in the case of virus-stimulated TNF-α gene expression, proteins bound to the Sp1 site must directly interact with proteins bound to adjacent sites. By contrast, one-half of a DNA turn between the −84 Ets binding site and the κ3/CRE composite site decreases the TNF-α promoter response to virus slightly compared to the response of the wild-type construct, but introduction of a 10-bp spacer between the same sites completely restores the activity of the promoter to wild-type levels and has little effect upon ionophore induction (26).
Taken together, these results suggest that a unique TNF-α enhanceosome is formed after M. tuberculosis and LPS stimulation of monocytes. Furthermore, consistent with our mutagenesis data (Fig. 2B), these results suggest that for induction of TNF-α by M. tuberculosis, the transcription factors Sp1 and Ets interact with proteins bound to abutting sequences and must be in phase with other activators in order to interact with the basal transcription complex. We speculate that this occurs through protein-protein interactions with the coactivator proteins p300/CBP, which appear to act as an enhanceosome surface (20), allowing the activators to correctly line up on the helix for enhanceosome assembly (Fig. 5).
FIG. 5.
A model of the recruitment of inducer-specific TNF-α enhanceosomes in T lymphocytes and monocytic cells. Distinct enhanceosomes are formed on the TNF-α promoter region in T cells in response to ionophore (top panel) or virus stimulation (middle panel) and in monocytes in response to M. tuberculosis and LPS stimulation (bottom panel). The M. tuberculosis-LPS model shows simultaneous binding of Egr-1 and of Sp1 at the upstream Sp1 site; since their binding sites have some overlap. The phasing mutants (data not shown) and site-directed mutagenesis experiments previously reported (23) gave similar results when using LPS as the stimulus, indicating that the same enhanceosome (or a similar one) is assembled after LPS as that assembled after M. tuberculosis stimulation of monocytic cells.
Taken together, these results demonstrate that specific protein-protein and protein-DNA interactions are absolutely necessary for the assembly of a functional enhancer complex on the TNF-α promoter after stimulation by M. tuberculosis. The fact that precise helical phasing is absolutely required and that it is inducer specific to M. tuberculosis and LPS stimulation of monocytes is consistent with our observation that different sets of transcriptional activators bind to shared binding sites in the TNF-α promoter in response to virus, antigen engagement, and calcium flux in the same cell type, i.e., T cells (7).
M. tuberculosis-induced proinflammatory signaling in macrophages appears to be mediated by Toll-like receptor 2 (TLR 2), while TLR 4 is the primary LPS coreceptor of this family of proteins (21). Signaling of both TLR2 and TLR 4 appears to require the adapter protein MyD88, the kinase IRAK, and activation of TRAF 6, resulting in the nuclear translocation of NF-κB. While NF-κB binding motifs are present in upstream regions of the 5′ flanking region of the human TNF-α gene (9), deletion of these sequences have little effect on TNF-α gene activation by M. tuberculosis (Fig. 2A) or LPS (23), similar to other inducers tested (26). Intriguingly, in NF-κB p50-deficient mice, aerosol infection with M. tuberculosis results in reduced but not absent pulmonary mRNA levels of TNF-α during the first 3 weeks of infection; these levels normalize later in infection (28). Thus, TNF-α is not critically dependent upon NF-κB in these experiments and the reduction in levels observed at early time points may be due to the effects of NF-κB on required upstream signaling events rather than a direct effect upon gene regulation in these mice.
Finally, the data presented here lend insight both into the transcriptional mechanisms involved in M. tuberculosis-induced gene regulation and inducer and cell type-specific gene regulation in general and into the regulation of a gene important in tuberculosis-associated pathology specifically (Fig. 5). The identification of transcriptional targets and the signal transduction pathways upstream of these transcriptional events could provide the basis for the development of novel approaches aimed at modulating the expression of TNF-α, which plays a critical role in the switch from latent to active M. tuberculosis infection in humans (14).
Acknowledgments
This work was supported by a grant from the Heiser Program of the New York Community Trust to R.B., by a grant from the Arthritis National Research Foundation to A.V.T., and by grants from the American Heart Association and the NIH (GM56492 and HL59838) to A.E.G.
We thank Renate Hellmiss-Peralta for the artwork.
R.B., A.V.T., and A.K.B. made equal contributions to this study.
REFERENCES
- 1.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 2.Bermudez, L. E., and L. S. Young. 1988. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140:3006-3013. [PubMed] [Google Scholar]
- 3.Carey, M. 1998. The enhanceosome and transcriptional synergy. Cell 92:5-8. [DOI] [PubMed] [Google Scholar]
- 4.Chrivia, J. C., R. P. Kwok, N. Lamb, M. Hagiwara, M. R. Montminy, and R. H. Goodman. 1993. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365:855-859. [DOI] [PubMed] [Google Scholar]
- 5.Eckner, R., M. E. Ewen, D. Newsome, M. Gerdes, J. A. DeCaprio, J. B. Lawrence, and D. M. Livingston. 1994. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8:869-884. [DOI] [PubMed] [Google Scholar]
- 6.Falvo, J. V., B. M. N. Brinkman, A. V. Tsytsykova, E. Y. Tsai, T.-P. Yao, A. L. Kung, and A. E. Goldfeld. 2000. A stimulus-specific role for CREB-binding protein (CBP) in T cell receptor-activated tumor necrosis factor α gene expression. Proc. Natl. Acad. Sci. USA 97:3925-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falvo, J. V., A. M. Uglialoro, B. M. Brinkman, M. Merika, B. S. Parekh, E. Y. Tsai, H. C. King, A. D. Morielli, E. G. Peralta, T. Maniatis, D. Thanos, and A. E. Goldfeld. 2000. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Mol. Cell. Biol. 20:2239-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 9.Goldfeld, A. E., C. Doyle, and T. Maniatis. 1990. Human tumor necrosis factor alpha gene regulation by virus and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 87:9769-9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfeld, A. E., and T. Maniatis. 1989. Coordinate viral induction of tumor necrosis factor α and interferon β in human B cells and monocytes. Proc. Natl. Acad. Sci. USA 86:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfeld, A. E., P. G. McCaffrey, J. L. Strominger, and A. Rao. 1993. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J. Exp. Med. 178:1365-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosschedl, R. 1995. Higher-order nucleoprotein complexes in transcription: analogies with site-specific recombination. Curr. Opin. Cell. Biol. 7:362-370. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann, S. H., and G. Kaplan. 1996. Immunity to intracellular bacteria. Res. Immunol. 147:487-489. [DOI] [PubMed] [Google Scholar]
- 14.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 15.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. S., K. M. Galvin, R. H. See, R. Eckner, D. Livingston, E. Moran, and Y. Shi. 1995. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 10:1188-1198. [DOI] [PubMed] [Google Scholar]
- 17.Lundblad, J. R., R. P. Kwok, M. E. Laurance, M. L. Harter, and R. H. Goodman. 1995. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature 374:85-88. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis, T., J. V. Falvo, T. H. Kim, T. K. Kim, C. H. Lin, B. S. Parekh, and M. G. Wathelet. 1998. Structure and function of the interferon-beta enhanceosome. Cold Spring Harbor Symp. Quant. Biol. 63:609-620. [DOI] [PubMed] [Google Scholar]
- 19.Shikama, N., J. Lyon, and N. B. La Thangue. 1997. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 7:230-236. [DOI] [PubMed] [Google Scholar]
- 20.Thanos, D., and T. Maniatis. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091-1100. [DOI] [PubMed] [Google Scholar]
- 21.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 22.Tramontana, J. M., U. Utaipat, A. Molloy, P. Akarasewi, M. Burroughs, S. Makonkawkeyoon, B. Johnson, J. D. Klausner, W. Rom, and G. Kaplan. 1995. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol. Med. 1:384-397. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai, E. Y., J. V. Falvo, A. V. Tsytsykova, A. K. Barczak, A. M. Reimold, L. H. Glimcher, M. J. Fenton, D. C. Gordon, I. F. Dunn, and A. E. Goldfeld. 2000. A lipopolysaccharide-specific enhancer complex involving ets, elk-1, sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol. Cell. Biol. 20:6084-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai, E. Y., J. Jain, P. A. Pesavento, A. Rao, and A. E. Goldfeld. 1996. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol. Cell. Biol. 16:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai, E. Y., J. Yie, D. Thanos, and A. E. Goldfeld. 1996. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol. Cell. Biol. 16:5232-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsytsykova, A. V., and A. E. Goldfeld. 2002. Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Mol. Cell. Biol. 22:2620-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-b enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 28.Yamada, H., S. Mizuno, M. Reza-Gholizadeh, and I. Sugawara. 2001. Relative importance of NF-kappaB p50 in mycobacterial infection. Infect. Immun. 69:7100-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan, W., G. Condorelli, M. Caruso, A. Felsani, and A. Giordano. 1996. Human p300 protein is a coactivator for the transcription factor MyoD. J. Biol. Chem. 271:9009-9013. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock, H. W., T. Sternsdorf, J. Liese, B. Belohradsky, C. Weber, A. Wedel, R. Schreck, P. Bauerle, and M. Strobel. 1993. Pyrrolidine dithiocarbamate inhibits NF-kB mobilization and TNF production in human monocytes. J. Immunol. 151:6986-6993. [PubMed] [Google Scholar]