Abstract
Several in vivo studies have reported the presence of immunoreactive transforming growth factor-β's (TGF-β's) in testicular cells at defined stages of their differentiation. The most pronounced changes in TGF-β1 and TGF-β2 immunoreactivity occurred during spermatogenesis. In the present study we have investigated whether germ cells and Sertoli cells are able to secrete bioactive TGF-β's in vitro, using the CCl64 mink lung epithelial cell line as bioassay for the measurement of TGF-β. In cellular lysates, TGF-β bioactivity was only observed following heat-treatment, indicating that within these cells TGF-β is present in a latent form. To our surprise, active TGF-β could be detected in the culture supernatant of germ cells and Sertoli cells without prior heat-treatment. This suggests that these cells not only produce and release TGF-β in a latent form, but that they also release a factor which can convert latent TGF-β into its active form. Following heat-activation of these culture supernatant's, total TGF-β bioactivity increased 6- to 9-fold. Spermatocytes are the cell type that releases most bioactive TGF-β during a 24 h culture period, although round and elongated spermatids and Sertoli cells also secrete significant amounts of TGF-β. The biological activity of TGF-β could be inhibited by neutralizing antibodies against TGF-β1 (spermatocytes and round spermatids) and TGF-β2 (round and elongating spermatids). TGF-β activity in the Sertoli cell culture supernatant was inhibited slightly by either the TGF-β1 and TGF-β2 neutralizing antibody.
These in vitro data suggest that germ cells and Sertoli cells release latent TGF-β's. Following secretion, the TGF-β's are converted to a biological active form that can interact with specific TGF-β receptors. These results strengthen the hypothesis that TGF-β's may play a physiological role in germ cell proliferation/differentiation and Sertoli cell function.
Keywords: TGF-β release, spermatocytes, round and elongating spermatids, Sertoli cells
Background
The normal physiological functions of the testis are regulated by the gonadotrophins luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, locally derived paracrine factors are also postulated to play an important role in maintaining cellular function, growth and differentiation in the testis. A number of peptide growth factors that affect the growth and metabolic activities of testicular cell types have been identified in the testis, including the transforming growth factor-β's (TGF-β's) [1].
The TGF-β's are polypeptide growth factors that are multifunctional regulators of both growth and development in many different tissues. To date three different forms of TGF-β have been identified in the testis. Sertoli cells and Leydig cells in the porcine testis express TGF-β1 mRNA [2,3]. In the adult mouse TGF-β1 and TGF-β3 mRNA's have been shown to be expressed in the somatic cell compartment of the germ cell depleted testis, while TGF-β1 mRNA expression has also been detected in spermatogenic cells [4]. In the rat testis TGF-β1, TGF-β2 and TGF-β3 mRNAs are expressed by Sertoli cells and peritubular/myoid cells. The expression pattern of these mRNAs has been shown to undergo clear changes during testicular development [5,6].
We have expanded these findings to the protein level and have shown that immunoreactive TGF-β1 and TGF-β2 are present in vivo in testicular cells at defined stages of their differentiation [7]. TGF-β1 predominated in spermatocytes and early round spermatids, but as the spermatids elongated around stages VIII-IX of the cycle of the seminiferous epithelium, the TGF-β1 immunoreactivity declined. TGF-β2 was undetectable in spermatocytes and early round spermatids, but as spermiogenesis progressed, around stages V-VI, spermatids rapidly became positive for TGF-β2 and remained positive as the spermatids elongated. TGF-β1 immunoreactivity was present in Sertoli cells throughout testicular development, while TGF-β2 immunoexpression rapidly declined after birth [7].
Although the observation of immunoreactive TGF-β1 and TGF-β2 in germ cells at defined stages of their differentiation suggests that these growth factors may play a physiological role in germ cell differentiation, there is no evidence that these germ cells and Sertoli cells also secrete TGF-β's. Hence, in the present study we have investigated whether Sertoli cells, spermatocytes, round and elongated spermatids release TGF-β's in vitro, using the CCl64 mink lung epithelial cell line for the measurement of TGF-β bioactivity. Culture media we added to the bioassay before and after heat-activation, in order to determine whether these cell types secrete a factor that can activate the secreted latent TGF-β1 as well.
Materials & Methods
Cell isolation
Highly purified (> 99%) Sertoli cell preparations were obtained by isolating Sertoli cells from testes of 21-day-old Wistar rats (substrain R-1 Amsterdam) as has been described by Themmen et al. [8]. Sertoli cells were cultured in Eagle's minimal essential medium (MEM; Gibco, Grand Island, NY) with 0.1% BSA (fraction V; Sigma, St Louis, MO) and antibiotics at a density of 12 × 106 cells per 175 cm2 in 20 ml medium at 37°C in culture flasks [8]. After a culture period of 24 h the culture supernatant was collected and the cells were scraped from the bottom of the culture flask, resuspended and homogenized in 2 ml phosphate buffered saline (PBS) after which both culture supernatant and cell homogenate were frozen and stored at -20°C until further processing.
Spermatogenic cells were isolated from 40/50-day-old Wistar rats (substrain R-1 Amsterdam) using collagenase and trypsin treatment, and purified using sedimentation at unit gravity (StaPut procedure) followed by density gradient purification (Percoll gradients) [9]. The purity of the cell preparations isolated according to this method, was analysed using DNA-flow cytometry [10]: the preparations enriched in spermatocytes, round and elongated spermatids contained more than 90% of cells with a 4C or 1C amount of DNA per cell, respectively. Spermatocytes, round spermatids and elongated spermatids were cultured in PBS with 0.1% BSA supplemented with 2 mM sodium pyruvate, 6 mM DL-lactate and antibiotics according to the method described by Jutte et al (11). The cell densities were 17 × 106 cells and 80 × 106 cells, respectively for spermatocytes and round spermatids, in 35 ml PBS at 32°C in culture flasks (Gibco). Elongated spermatids were cultured at a density of 16 × 106 cells, in 18 ml PBS at 32°C in culture flasks (Gibco). Under these culture conditions the viability and capacity of protein synthesis and RNA synthesis and processing remains remarkably constant, as has been shown previously by our group (11–13). After a culture period of 24 h the spermatogenic cells were spun down, resuspended and homogenized in 2 ml PBS after which both supernatant and cell homogenates were frozen and stored at -20°C until further processing.
All experimental procedures involving the use of rats, were approved by the ethical committee for laboratory animal welfare of the Faculty of Medicine, Erasmus University, Rotterdam.
Bioassay for TGF-β
TGF-β activity in cell homogenates and cell culture supernatants was determined using a CCl64 mink lung epithelial cell biological assay [14]. The cells were collected during their logarithmic growth phase and suspended at a concentration of 8 × 104 cells/ml in DMEM (Gibco) containing 0.2% fetal calf serum (Gibco). Fifty μl of the suspension was added to flat bottom 96-well plates and incubated at 37°C for 5 h. Samples, either assayed directly or heat activated (5 min, 80°C), were then added to the wells at various dilutions with or without the addition of neutralizing rabbit anti-TGF-β1 or anti-TGF-β2 antibodies (gift from dr. AJM Van den Eijnden-Van Raaij, Hubrecht Laboratory, Utrecht, The Netherlands), as has been described before [15]. After 20 h the cells were pulsed with 1 μCi [3H]thymidine (Amersham, Amsterdam; specific activity 0.7–1.1 × 108MBq/mmol) for 4 h and the incorporated radioactivity was counted. The inhibition of the proliferation of the cells was related to a standard curve of recombinant human TGF-β1 (Genzyme, Cambridge, MA). All experiments were carried out in triplicate. Values are expressed as mean ± SD. For statistical analysis the two-tailed Student's t-test was used.
Results
In cell lysates of spermatocytes, round and elongating spermatids, and Sertoli cells cultured for 24 h, TGF-β bioactivity was only measurable after heat activation. Elongating spermatids contained the highest levels of TGF-β bioactivity (Table 1).
Table 1.
TGF-β activity in lysates of isolated testicular cells (Tissue) and in cell culture.
| TGF-β activity in pg × 10-6 cells/24 h | ||||
| Tissue | Supernatant | |||
| Testicular cell type | Not activated | Heat activated | Not activated | Heat activated |
| Spermatocytes | ND | 5.0 ± 0.6 | 693.0 ± 231.0 | 6405.0 ± 105.0 |
| Round spermatids | ND | 1.5 ± 0.2 | 361.4 ± 100.6 | 2537.5 ± 262.5 |
| Elongating spermatids | ND | 103.1 ± 9.4 | 596.3 ± 138.4 | 2981.3 ± 393.8 |
| Sertoli cells | ND | 16.7 ± 6.7 | 233.3 ± 66.7 | 1458.3 ± 208.3 |
Supernatant, using the CCl64 mink lung epithelial cell line as a bioassay for the measurement of TGF-β activity. Spermatocytes, round spermatids, elongated spermatids and Sertoli cells were cultured for 24 h. TGF-β activity in the different preparations was measured before and after heat-activation of the samples (for details see Materials & Methods). The incubations were carried out in triplicate (values are presented as means ± SD); the data being representative of at least three different experiments. ND: not detectable.
TGF-β bioactivity was also measured in the supernatants of these cultures. Even without heat treatment considerable amounts of active TGF-β were detectable in the medium of all cell types. Following heat activation the amount of TGF-β increased 6- to 9-fold; the highest active TGF-β content was measured in the supernatant of the spermatocyte cultures (Table 1). TGF-β bioactivity in the culture supernatant's was higher than in the cell lysates, suggesting that most TGF-β did not accumulate within the cells but was released during culture.
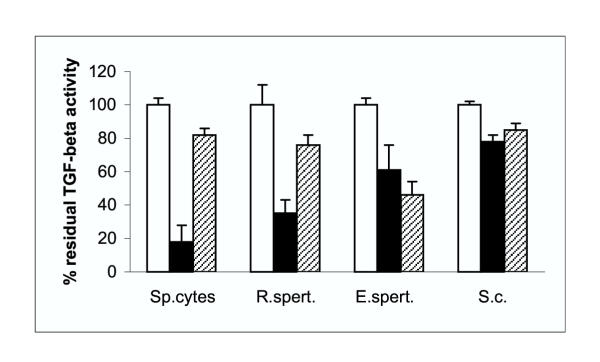
In order to confirm that it was indeed TGF-β that inhibited the growth of the CCl64 mink lung cells, and to investigate the types of TGF-β that were secreted by the testicular cells, neutralizing TGF-β1 and TGF-β2 antibodies were mixed with the culture supernatants 30 min before addition to the mink lung epithelial cell bioassay. As shown in Figure 1 TGF-β1 antiserum clearly reduced the growth inhibitory effects of the spermatocyte, round spermatid and Sertoli cell supernatants in CCL64 mink lung cells, while the effect of this antibody in the supernatant of elongated spermatids was less pronounced. The TGF-β2 antiserum reduced the growth inhibitory effects of round spermatid supernatant, while the effects in spermatocyte, round spermatid and Sertoli cell supernatant's was minimal (Fig. 1).
Figure 1.
Effect of anti-TGF-β1 (filled bars) and anti-TGF-β2 (hatched bars) antibodies on growth inhibition of mink lung CCl64 cells by heat treated culture supernatants of spermatocytes (sp.cytes), round spermatids (r. spert.), elongated spermatids (e.spert.) and Sertoli cells (S.c.). The dilutions of the culture supernatants that gave maximal growth inhibition were equivalent to 100% TGF-β activity (open bars). The final concentration of both antibodies used in this assay was 10 μg/ml. The values, which are expressed as percentage of residual TGF-β activity, represent means ± SD
Discussion
TGF-β has been purified from several nonneoplastic tissues, transformed cells and from conditioned media of several cell lines [16]. In the testis, Sertoli cells, peritubular/myoid cells and germ cells have been shown to contain mRNAs for different types of TGF-β's [2,4-6]. Secretion of TGF-β1 by Sertoli cell-germ cell co-cultures has been demonstrated by Western blotting [17]. The data of the present study showed that cell lysates of highly purified germ cells and Sertoli cells contained TGF-β, which became activated following heat-treatment. In addition, these cells released TGF-β in vitro during a 24 h culture period. The highest level of intracellular TGF-β bioactivity was found in elongating spermatids, while spermatocytes were the testicular cell type that released most TGF-β during the culture period, although round and elongated spermatids and Sertoli cells also secreted significant amounts of this growth factor.
The activity of TGF-β in the culture supernatants could be inhibited considerably by neutralizing antibodies raised against TGF-β1 (spermatocytes, round spermatids) and TGF-β2 (elongating spermatids). The TGF-β1 antibody had a less pronounced inhibitory effect on the bioactivity in elongated spermatid conditioned medium, while the same was the case for the TGF-β2 antibody in conditioned medium of spermatocytes and round spermatids. However, one has to keep in mind that the ED50's for growth inhibition of CCl64 mink lung epithelial cells by the different isoforms of TGF-β are not the same. Therefore, it is not possible to extrapolate the growth inhibitory effects to amounts of TGF-β1 and TGF-β2 released by spermatocytes, and round and elongating spermatids. The data of the present study fit very well with our immunohistochemical observations, where we found intense immunostaining for TGF-β1 in spermatocytes and round spermatids. TGF-β2 immuno-reactivity was low or absent in these cell types. In contrast, TGF-β2 immunoreactivity was high in elongating spermatids, while in these cells TGF-β1 immunostaining was negligible [7].
TGF-β activity in the Sertoli cell culture supernatant was slightly inhibited following the addition of the TGF-β1 or TGF-β2 neutralizing antibody. These results further extend previous observations by Avallet et al [17] who could not detect TGF-β1 secretion by Sertoli cells in vitro by Western blotting. Based on studies by Mullaney & Skinner [6] who showed that in Sertoli cells from 21-day-old rats TGF-β3 mRNA is the type of TGF-β that is predominantly expressed and secreted, we presume that remaining bioactivity in the Sertoli cell supernatant is probably due to the presence of TGF-β3.
Whereas many cell types have the potential to produce TGF-β in vitro, they have been reported to secrete TGF-β in an inactive (latent) form which is unable to bind to its receptors [18,19]. The CCl64 mink lung epithelial cell line which we used as a bioassay for the detection of TGF-β activity, does not detect latent TGF-β. Latent TGF-β can, however, be activated by physiochemical treatment [18,20]. In the present study TGF-β was measured in culture supernatants before and after activiation by heat treatment. Surprisingly, not only latent, but active TGF-β was also present in the culture supernatants of the diverse cell types. In contrast, in cell homogenate's bioactive TGF-β could only be measured after heat-activation. These results indicate that primary cell cultures of germ cells and Sertoli cells release TGF-β in a latent form and that this latent protein is converted into a bioactive form in the culture medium, presumably by the concomitant release of a factor that can convert this growth factor into its active form. The nature of this "converting" factor is unknown. Since the cell cultures used in the experiments described were highly purified, it is not likely that contaminating cells are responsible for the secretion of this factor. Only a few other studies have reported the presence of an activated (bioactive) form of TGF-β in culture supernatant's of primary cells, transformed and non-transformed cell lines was observed [18,21-23].
In an immunohistochemical study we have shown that there exists a marked transition from TGF-β1 to TGF-β2 during the cycle of the seminiferous epithelium [7]. The present investigation expands these findings with the observation that germ cells and Sertoli cells release these different types of TGF-β's in vitro as well. The physiological relevance of TGF-βs as growth- and differentiation-regulatory factors appears to rest on the regulation of its activation [15]. Once activated, these TGF-β's have extremely short half-lives and are transported only within a short range [24], suggesting that these transforming growth factors presumably exert their action in a paracrine fashion within the seminiferous tubules.
So far actions of TGF-β on testicular cells have only been demonstrated in vitro. Morera et al. [25] and Benahmed and colleagues [26] have for instance shown that TGF-β can inhibit the stimulatory actions of FSH on Sertoli cell aromatase activity by attenuating cAMP levels in vitro. Other groups have reported that TGF-β increases inhibin " mRNA levels [27], lactate production [28] and proteoglycan synthesis [29]. TGF-β3 has been implicated to perturb the inter-Sertoli tight junction permiability barrier possibly by affecting occludin, zonula occludens-1 and claudin-11, genes expressed in junctional complexes (30). These observationd indicated that Sertoli cells presumably possess binding sites for TGF-β. Reports of direct effects of TGF-β on germ cells are limited. Hakovinta and colleagues [31] have demonstrated that TGF-β1 increased DNA synthesis in preleptotene spermatocytes in seminiferous tubules, suggesting the presence of functional receptors on these cells. Indeed, in more recent studies it has been shown that Sertoli cells, spermatocytes and spermatids express TGF-β receptor types I and/or II [27,32]. Taken together, these observations further strengthen the hypothesis that TGF-β's are important paracrine/autocrine factors within the seminiferous tubules and suggest the existence of stage dependent interaction and communication between neighboring Sertoli and germ cells.
In summary, the present study demonstrates the presence of several types of latent TGF-β's within spermatogenic cells and Sertoli cells which are released by these cells as well. Following release they are converted to a biological active form that can interact with specific TGF-β receptors. The presence of active TGF-β within the seminiferous tubules may have important implications for the role of TGF-β's in the function of Sertoli cells, and germ cell development.
Acknowledgments
Acknowledgements
The authors thank dr. van den Eijnden-van Raaij (Hubrecht laboratory, Utrecht, The Netherlands) for the gift of the TGF-β1 and TGF-β2 neutralizing polyclonal antibodies.
Contributor Information
Bart L Haagmans, Email: haagmans@viro.fgg.eur.nl.
Jos W Hoogerbrugge, Email: themmen@endov.fgg.eur.nl.
Axel PN Themmen, Email: themmen@endov.fgg.eur.nl.
Katja J Teerds, Email: katja.teerds@wur.nl.
References
- Bellvé AR, Zheng W. Growth factors as autocrine and paracrine modulators of male gonadale functions. J Reprod Fertil. 1989;85:771–793. doi: 10.1530/jrf.0.0850771. [DOI] [PubMed] [Google Scholar]
- Avallet O, Vigier M, Albaladejo V, de Cesaris P, Saez JM. Transforming growth factor β gene expression in cultured porcine Sertoli and Leydig cells: Effects of hormine and growth factors. Proceedings of the 6th European Workshop on Molecular and Cellular Endocrinology of the Testis. 1990;D13 [Google Scholar]
- Caussanel S, Tabone E, Hendrick JC, Dacheux F, Benahmed M. Cellular distribution of transforming growth factor betas 1, 2, and 3 and their types I and II receptors during postnatal development and spermatogenesis in the boar testis. Biol Reprod. 1997;56:357–367. doi: 10.1095/biolreprod56.2.357. [DOI] [PubMed] [Google Scholar]
- Watrin F, Scotto L, Associan RK, Wolgemuth DJ. Cell lineage specificity of expression of the murine transforming growth factor-β3 and transforming growth factor-β1 genes. Cell Growth & Differ. 1991;2:77–83. [PubMed] [Google Scholar]
- Skinner MK, Moses HL. Transforming growth factorβ gene expression and action in the seminiferous tubule: Peritubular cell-Sertoli cell interactions. Mol Endocrinol. 1989;3:625–634. doi: 10.1210/mend-3-4-625. [DOI] [PubMed] [Google Scholar]
- Mullaney BP, Skinner MK. Transforming growth factor-β (β1, β2, and β3) gene expression and action during pubertal development of the seminiferous tubule: Potential role at the onset of spermatogenesis. Mol Endocrinol. 1993;7:67–76. doi: 10.1210/mend.7.1.8446109. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Dorrington JH. Localization of transforming growth factor β1 and β2 during testicular development in the rat. Biol Reprod. 1993;48:40–45. doi: 10.1095/biolreprod48.1.40. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Blok LJ, Post M, Baarends WM, Hoogerbrugge JW, Parmentier M, Vassert G, Grootegoed JA. Follitropin receptor down-regulation involves a cAMP-dependent post-transcriptional decrease of receptor mRNA expression. Molec Cell Endocrinol. 1991;78:R7–R13. doi: 10.1016/0303-7207(91)90130-k. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Jansen R, van der Molen HJ. Effect of glucose on ATP dephosphorylation in rat spermatids. J Reprod Fertil. 1986;77:99–107. doi: 10.1530/jrf.0.0770099. [DOI] [PubMed] [Google Scholar]
- Toebosch AMW, Brussée R, Verkerk A, Grootegoed JA. Quantitative evaluation of the maintenance and development of spermatocytes and round spermatids in cultured tubule fragments from immature rat testis. Int J Androl. 1989;12:360–374. doi: 10.1111/j.1365-2605.1989.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Jutte NHPM, Jansen R, Grootegoed JA, Rommerts FFG, van der Molen HJ. Protein synthesis by isolated pachytene spermatocytes in the absence of Sertoli cells. J Exp Zool. 1985;233:285–290. doi: 10.1002/jez.1402330217. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Grollé-Hey AH, Rommerts FFG, van der Molen HJ. Ribonucleic acid synthesis in vitro in primary spermatocytes isolated from rat testis. Biochem J. 1977;168:23–31. doi: 10.1042/bj1680023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootegoed JA, Krüger-Sewnarain RC, Jutte NHPM, Rommerts FFG, van der Molen HJ. Fucosylation of glycoproteins in rat spermatocytes and spermatids. Gam Res. 1982;5:303–315. [Google Scholar]
- Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB. Immunodetection and quantitation of the two forms of transforming growth factor-β (TGF-β1 and TGF-β2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Kwakkenbos-Isbrucker L, Mummery CL, de Laat SW, van den Eijnden-van Raaij AJ, van der Saag PT, van den Burg B. The role of TGF-beta production in growth inhibition of breast-tumor cells by progestins. Int J Cancer. 1995;61:80–86. doi: 10.1002/ijc.2910610114. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Wakefield LM, De Crombrugge B. Some recent advances in the chemistry and biology of transforming growth factor-β. J Cell Biol. 1987;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallet O, Gomez E, Vigier M, Jégou B, Saez JM. Sertoli cell-germ cell interactions and TGFβ1 expression and secretion in vitro. Biochem Biophys Res Comm. 1997;238:905–909. doi: 10.1006/bbrc.1997.7275. [DOI] [PubMed] [Google Scholar]
- Takiuchi H, Tada T, Li XF, Ogata M, Ikeda T, Fujimoto S, Fujiwara H, Hamaoka T. Particular types of tumor cells have the capacity to convert transforming growth factor β from a latent to an active form. Cancer Res. 1992;52:5641–5646. [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Wakefield LM, Associan RK. Transforming growth factor β: Biological function and chemical structure. Science. 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Pircher R, Jullien P, Lawrence DA. β-Transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986;136:30–37. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB. Evidence that transforming growth factor β is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987;48:417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Associan RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Rains EW, Ross R, Sporn MB. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell JJ, Dorrington J. Estradiol-17β stimulates DNA synthsis in rat granulosa cells: Action mediated by transforming growth factor-β. Endocrinology. 1991;128:2663–2665. doi: 10.1210/endo-128-5-2663. [DOI] [PubMed] [Google Scholar]
- Wakefield L. Growth factors: An overview. In: Isidori A, Fabbri A, Dufau ML, editor. Hormonal Communicating Events in the Testis. 1990. pp. 181–190. [Google Scholar]
- Morera AM, Esposito G, Ghiglieri C, Chauvin MA, Hartmann DJ, Benahmed M. Transforming growth factor β1 inhibits gonadotropin action in cultured porcine Sertoli cells. Endocrinology. 1992;130:831–836. doi: 10.1210/endo.130.2.1370796. [DOI] [PubMed] [Google Scholar]
- Benahmed M, Cochet C, Keramides M, Chauvin MA, Morera AM. Evidence for a FSH dependent secretion of a receptor reactive transforming growth factor-β-like material by immature Sertoli cells in primary culture. Biochem Biophys Res Commun. 1988;154:1222–1231. doi: 10.1016/0006-291x(88)90270-7. [DOI] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B, Morera AM, Goddard I, Benahmed M. Expression of mRNAs for transforming growth factor-beta receptors in the rat testis. Endocrinology. 1995;136:2788–2791. doi: 10.1210/endo.136.6.7750505. [DOI] [PubMed] [Google Scholar]
- Espositi G, Keramidas M, Mauduit C, Feige JJ, Morera AM, Benahmed M. Direct regulating effects of transforming growth factor beta 1 on lactate production in cultured porcine Sertoli cells. Endocrinology. 1991;128:1441–1449. doi: 10.1210/endo-128-3-1441. [DOI] [PubMed] [Google Scholar]
- Panthou P, Barbey P, Thiebot B, Bocquet J. Effects of transforming growth factor-beta1, interleukin-1 alpha and interleukin-6 on rat Sertoli cell proteoglycan synthesis. Biochem Mol Biol Int. 1994;34:603–612. [PubMed] [Google Scholar]
- W-Lui Y, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli cell tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Harkovirta H, Kaipia A, Soder O, Parvinnen M. Effects of avidin-A, inhibin-A, and transforming growth factor α1 on stage-specific deoxyribonucleic acid synthesis during rat seminiferous epithelial cycle. Endocrinology. 1993;133:1664–1668. doi: 10.1210/endo.133.4.8404607. [DOI] [PubMed] [Google Scholar]
- Olaso R, Pairault C, Habert R. Expression of type I and II receptors for transforming growth factor β in the adult rat testis. Histochem Cell Biol. 1998;110:613–618. doi: 10.1007/s004180050324. [DOI] [PubMed] [Google Scholar]