Abstract
During mammalian testis development distinct generations of fetal and adult Leydig cells arise. Luteinising hormone (LH) is required for normal adult Leydig cell function and for the establishment of normal adult Leydig cell number but its role in the process of adult Leydig cell differentiation has remained uncertain. In this study we have examined adult Leydig cell differentiation in gonadotrophin-releasing hormone (GnRH)-null mice which are deficient in circulating gonadotrophins. Adult Leydig cell differentiation was assessed by measuring expression of mRNA species encoding four specific markers of adult Leydig cell differentiation in the mouse. Each of these markers (3β-hydroxysteroid dehydrogenase type VI (3βHSD VI), 17β-hydroxysteroid dehydrogenase type III (17βHSD III), prostaglandin D (PGD)-synthetase and oestrogen sulphotransferase (EST)) is expressed only in the adult Leydig cell lineage in the normal adult animal. Real-time PCR studies showed that all four markers are expressed in adult GnRH-null mice. Localisation of 3βHSD VI and PGD-synthetase expression by in situ hybridisation confirmed that these genes are expressed in the interstitial tissue of the GnRH-null mouse. Treatment of animals with human chorionic gonadotrophin increased expression of 3βHSD VI and 17βHSD III within 12 hours further indicating that differentiated, but unstimulated cells already exist in the GnRH-null mouse. Thus, while previous studies have shown that LH is required for adult Leydig cell proliferation and activity, results from the present study show that adult Leydig cell differentiation will take place in animals deficient in LH.
Keywords: testis GnRH-null, LH, adult Leydig cells
Background
In mice, as in most mammalian species, two generations of Leydig cells arise during normal testis development. A "fetal" population which appears shortly after testis differentiation in utero and an "adult" population which arises after birth around day 7 [1-3]. Morphological and functional maturation of the adult Leydig cells is critically dependent on luteinising hormone (LH) stimulation and this is clearly seen in mice lacking either circulating LH or the LH-receptor [4-7]. In these animals Leydig cell number fails to develop normally after birth and circulating androgen levels are low or undetectable [6-9]. In contrast, testicular androgen levels and Leydig cell number is normal during fetal development in animals lacking gonadotrophins [8,9] showing that the fetal population of Leydig cells does not require gonadotrophins during this period. Despite the clear role for LH in proliferation and maturation of adult Leydig cells in the postnatal testis, it is not known whether this hormone is essential for the onset of adult Leydig cell differentiation. Early studies suggested that LH is required for the differentiation process [10-12] while more recent, immunohistochemical studies have reported that specific steroidogenic enzymes are detectable in rat adult Leydig cell precursors before LH-receptors are detectable [13]. This evidence would suggest that Leydig cell differentiation begins in the absence of LH-stimulation although it remains possible that low, undetectable levels of receptor are present prior to differentiation. Certainly, Leydig cell precursor cells express the LH-receptor gene prior to apparent differentiation although it is not clear whether transcripts are translated or produce functional protein [14-16].
It has been shown previously that in the mouse mRNA species encoding 3β-hydroxysteroid dehydrogenase type VI (3βHSD VI), 17β-hydroxysteroid dehydrogenase type III (17βHSD III), prostaglandin (PG) D-synthetase, estrogen sulfotransferase (EST) and vascular cell adhesion molecule 1 (VCAM 1) are expressed only in the adult Leydig cell population and not in the fetal population [1,17-20]. It is possible, therefore, to use these markers to examine the role of LH in the initiation of adult Leydig cell differentiation using mice which lack gonadotrophin-stimulation. Expression of these markers in mice lacking gonadotrophins would be a clear demonstration that Leydig cell differentiation does not require LH stimulation. It would be anticipated, however, that even if adult Leydig cells exist in the absence of LH-stimulation the expression of these specific markers will be very low or non-existant since overall Leydig cell activity is determined by LH [21]. Treatment of gonadotrophin-deficient mice with hCG is likely, however, to lead to a rapid increase in expression of markers within 24 hours if adult Leydig cells already exist. If the cells have not differentiated then a more prolonged period of treatment is likely to be required to induce differentiation of Leydig cells and expression of markers. For these reasons the expression of adult Leydig cell markers has been examined in the gonadotrophin-deficient gonadotrophin-releasing hormone (GnRH)-null (hpg) mouse. This animal has an advantage over receptor-deficient models in that the gonads remain responsive to exogenous hormone treatment.
Materials and Methods
Animals and treatments
Normal and GnRH-null mice were maintained at the University of Glasgow and at the University of Oxford under UK Home Office regulations as previously described [8]. Adult animals (greater than 2 months) (four per group) were used without treatment or after sub-cutaneous injections of recombinant hCG (4IU/injection in saline) (Serono Pharmaceutials Ltd, Feltham, Middlesex, UK). The injections were given every 12 hours and animals were killed 2 hour after the final injection. The testes were frozen in liquid N2 or fixed overnight in Bouin's fluid before being stored in 70% ethanol.
Reverse transcription and Real-time PCR
For quantification of the testicular content of specific mRNA species a real-time PCR approach was used which utilised the TaqMan PCR method following reverse transcription of the isolated RNA [22]. To allow specific mRNA levels to be expressed per testis and to control for the efficiency of RNA extraction, RNA degradation and the reverse transcription step an external standard was used [18]. The external standard was luciferase mRNA (Promega UK, Southampton, UK) and 5 ng was added to each testis at the start of the RNA extraction procedure. Testis RNA was extracted using Trizol 9 Life Technologies, Paisley, UK) and residual genomic DNA was removed by DNAse treatment (DNA-free, Ambion Inc, supplied by AMS biotechnology, UK). The RNA was reverse transcribed using random hexamers and Moloney murine leukemia virus reverse transcriptase (Superscript II, Life Technologies, UK) as described previously [23,24].
Primers and probes for use in the TaqMan method have been described previously [19]. The real-time PCRs were carried out in a 25 μl volume using a 96-well plate format. Components for real-time PCR were purchased from Applied Biosystems (Warrington, UK) apart from primers and probes which came from MWG Biotech (Milton Keynes, UK). Each PCR well contained reaction buffer, 5 mM MgCl2, 200 μM dNTPs (including dUTP), 300 nM each primer, 200 nM probe and 0.02 U/μl enzyme (Amplitaq Gold). Reactions were carried out and fluorescence detected on a GeneAmp 5700 system (Applied Biosystems, Warrington, Cheshire, UK). For each sample a replicate was run omitting the reverse transcription step and a template negative control was run for each primer/probe combination. The quantity of each measured cDNA was then expressed relative to the internal standard luciferase cDNA in the same sample. This method allows direct comparison of expression levels per testis between different samples [18].
In situ hybridisation and histology
Testes from adult GnRH-null mice and GnRH-null mice treated with hCG were fixed in Bouins and prepared for in situ hybridisation as described previously [1]. In situ probes for 3βHSD type VI and PGD-synthetase were produced as described previously [1,18]. Sense and antisense cRNA probes were prepared using T3 and T7 polymerase and were labelled with [35S]dUTP. Tissue sections (7 μm and at least four sections per animal) were hybridised overnight at 60°C with probe (100,000 c.p.m./μL) in hybridisation buffer (50% deionized formamide, 10% dextran sulphate, 50 mM dithiothreitol, 500 μg/mL DNA from calf thymus, 1 × Denhardt's solution, 20 mM Tris/HCl, pH8.0, 5 mM EDTA, 10 mM sodium phosphate, pH 6.8). Following hybridisation, slides were washed, dehydrated and allowed to air dry. Autoradiography was carried out using Ilford K5 emulsion (Ilford, Cheshire, UK) and the slides were stained with Mayer's haematoxylin and eosin (Merck Ltd, Leicestershire, UK).
To generate semi-thin (1 μm) sections for histology testes were fixed in 1% glutaraldehyde-4% paraformaldehyde, in phosphate buffer (0.1 M), pH 7.2 for 24 h at 4°C and embedded in araldite. Cut sections were stained with toluidine blue.
Statistical analysis
To examine the effects of hCG injection on Leydig cell gene expression data from two experiments were normalised and combined. Differences between groups was analysed by single factor analysis of variance of log-transformed data. Differences beween means was determined using the Newman-Keul post-test.
Results
Leydig cell morphology in adult GnRH-null mice
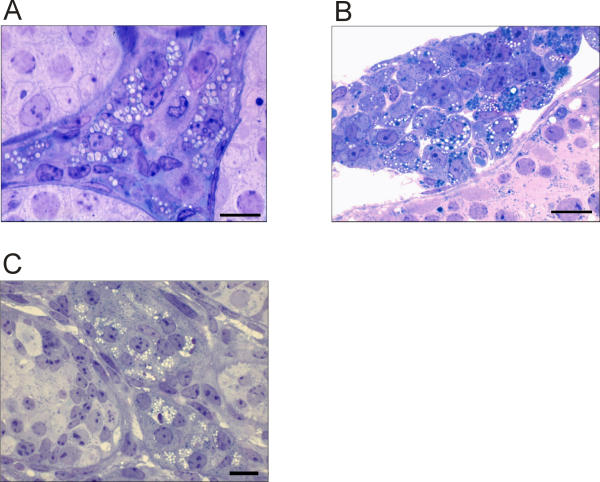
Leydig cells in adult GnRH-null mice were characterised by the presence of numerous large lipid droplets dispersed through the cell (Fig 1A). In normal adult mice the Leydig cells also frequently contained lipid droplets although these were generally smaller than in the GnRH-null mice (Fig 1B). In normal neonatal testes which contain only fetal-type Leydig cells there was more variation in Leydig cell morphology with lipid droplets present in many cells in characteristics clusters (Fig 1C). Some Leydig cells in the neonatal testis did not appear to contain lipid droplets although using semi-thin sections it is possible that they are present in the cell but not in the section shown.
Figure 1.
Light micrographs showing Leydig cell morphology in adult hpg testes (A), adult normal testes (B) and neonatal (day 7) testes (C). On micrographs A and C the bar represents 10 μm while on B it represent 20 μm.
Expression of adult Leydig cell markers in GnRH-null mice
A comparison of the expression of Leydig cell-specific mRNA species in GnRH-null mice and normal mice is shown in Table 1. Results are expressed relative to an external standard (luciferase) which was added to all testes at the RNA extraction phase and results are, therefore, comparable on a per testis basis [18]. The number of Leydig cells in the adult GnRH-null mouse testis is 10% of adult normal [9] and in the final column of Table 1 levels of mRNA are expressed relative to adult normal and corrected for cell number. Data from neonatal normal animals is included for control purposes since they contain only fetal-type Leydig cells [1,2]. The results show that, of those mRNA species tested, two (glutathione-S-transferase (GST) 5–5 and 3βHSD type I) are expressed at adult normal levels in the GnRH-null testis while the rest show a markedly reduced level of expression. There is, however, considerable variation with cytochrome P450 side chain cleavage (P450scc), 3βHSD VI and PGD-synthetase present at less than 1% of normal while epoxide hydrolase (EH) is nearly 20% of normal. The four markers of adult Leydig cell differentiation which were measured in this study (3βHSD VI, PGD-synthetase, EST and 17βHSD III) were all detectable in the adult GnRH-null testis although expressions levels were amongst the lowest for all mRNA species measured. In most cases expression levels of mRNA species in adult GnRH-null animals was similar to, or higher than, that in neonatal normal animals. The exceptions were P450scc and renin. As expected 3βHSD VI and EST levels were undetectable in neonatal normal animals while the relatively high levels of PGD-synthetase and 17βHSD III in these animals is due expression in the seminiferous tubules [17,25].
Table 1.
| Gene | Expression per testis (relative to Luciferase × 103) | GnRH-null relative to adult normal (corrected for cell number)2 | ||
| Adult GnRH-null | Adult normal | Neonatal normal1 | ||
| P450scc | 0.23 ± 0.04 | 242 ± 35 | 2.2 ± 0.5 | 0.95% |
| P450c17 | 15.3 ± 1.8 | 3453 ± 1042 | 13.5 ± 3.5 | 4.4% |
| GST5-5 | 60.6 ± 5.6 | 677 ± 91 | 76 ± 15 | 89.5% |
| RLF | 19.2 ± 2.1 | 2490 ± 611 | 5.3 ± 1.3 | 7.7% |
| 3βHSD I | 7.5 ± 1.7 | 51 ± 10 | 4.6 ± 1.8 | 147.0% |
| Renin | 0.027 ± 0.012 | 21.4 ± 3.5 | 5.8 ± 0.9 | 1.3% |
| EH | 18.5 ± 1.6 | 996 ± 112 | 8.0 ± 1.6 | 18.5% |
| StAR | 2.08 ± 0.2 | 177 ± 30 | 4.0 ± 1.1 | 11.7% |
| 3βHSD VI* | 0.014 ± 0.005 | 124 ± 15 | ND3 | 0.11% |
| PGD-synthetase* | 0.26 ± 0.06 | 843 ± 43 | 2.5 ± 1.1 | 0.31% |
| EST* | 0.014 ± 0.009 | 9.0 ± 2.4 | ND3 | 1.5% |
| 17βHSD III* | 2.39 ± 0.21 | 1644 ± 192 | 140 ± 7 | 1.45% |
* Genes expressed in adult Leydig cells but not fetal Leydig cells 1Neonatal animals were 5 days old. 2 Expression in GnRH-null mice is expressed as a percentage of normal mice and corrected for reduced Leydig cell number in GnRH-null animals [4]. 3ND = not detectable.
Effect of hCG injections on marker expression in GnRH-null mice
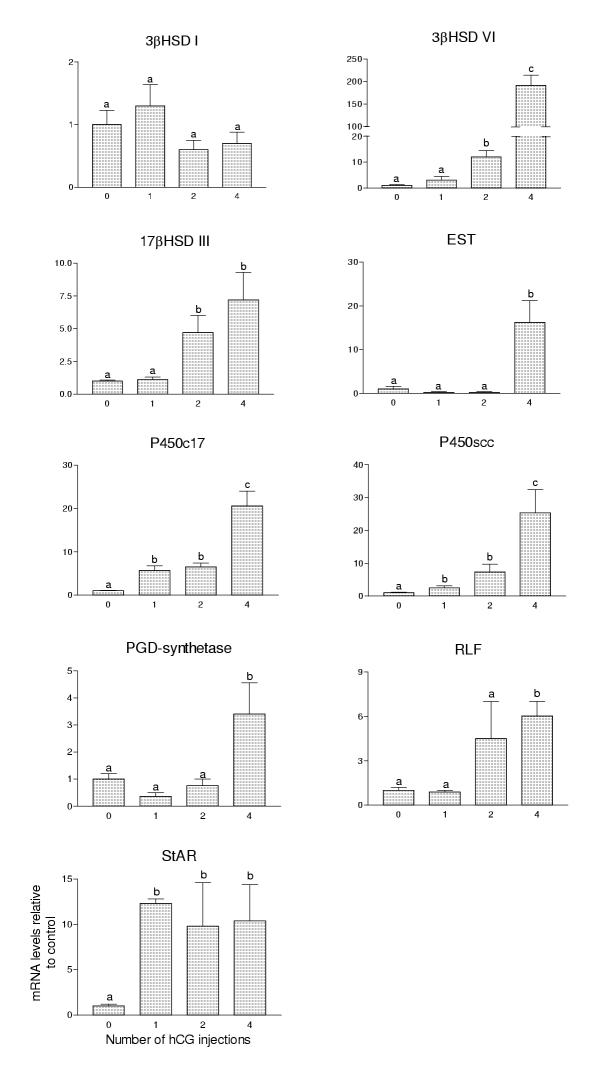
Injection of adult GnRH-null mice with hCG increased the levels of all mRNA species tested with the exception of 3βHSD I which showed no change under the injection regime used (Fig 1). A single injection of hCG was sufficient to increase significantly levels of mRNA encoding P450c17, P450scc and steroidogenic acute regulatory (StAR) protein while two injections, spaced over 12 hours, increased expression of 3βHSD VI and 17βHSD III. Levels of EST, PGD-synthetase and relaxin-like factor (RLF) did not increase significantly until after four injections. The biggest change in expression was seen in 3βHSD VI which showed a 191-fold increase over control after 4 injections.
In situ hybridisation
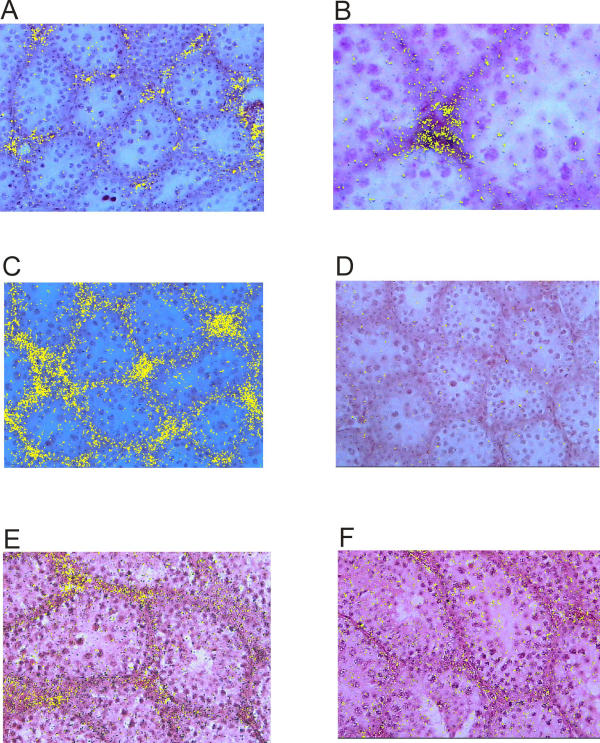
In situ hybridisation was used to localise expression of markers of adult Leydig cell differentiation in the GnRH-null testis. A probe for 3βHSD VI showed specific localisation to the interstitial tissue in untreated GnRH-null mice (Fig 2A, 2B) with a clear increase in expression after hCG treatment (Fig 2C). Hybridisation of the 3βHSD VI probe tended to be in the central interstitial tissue rather than in the peritubular region (Fig 2B). A probe for PGD-synthetase also showed localisation to the interstitial tissue in untreated GnRH-null mice (Fig 2E) although there was no clear change in expression after hCG treatment (not shown).
Figure 2.
Expression of Leydig cell-specific mRNA species in testes of GnRH-null mice following injection of hCG. Levels of mRNA were measured by real-time PCR. Results show expression of each mRNA species following 1, 2 or 4 injections of hCG spaced 12 hours apart. The results for each mRNA species are expressed relative to control (untreated) GnRH-null animals. In each group the mean ± SEM of four animals is shown. Groups with different letter superscripts are significantly (P < 0.05) different.
Discussion
The initial studies which identified fetal and adult Leydig cells as belonging to different cell populations were based on morphological differences between the two cell types [26,27]. These differences are not particularly suitable, however, for differentiating between fetal and adult cells under conditions of hormonal or growth-factor deprivation since the morphology itself will be affected by the state of deprivation. This is clearly seen in the present study, and in previous studies [4,9,28], which show that Leydig cells in GnRH-null mice contain numerous large lipid droplets which are unlike either normal fetal or adult Leydig cells but are similar to Leydig cells with significantly reduced levels of steroidogenesis [29]. It is worth noting, however, that lipid droplet distribution in the GnRH-null adult Leydig cell is similar to that of the normal adult Leydig cell although the lipid droplets are larger and more frequent. Lipid droplet distribution in adult GnRH-null mice is unlike the characteristic clusters seen in fetal Leydig cells.
While morphological markers are clearly not ideal for identifying fetal and adult Leydig cells other studies have now shown that there are fundamental differences in gene expression between the two Leydig cell populations which could be used to differentiate between the cell types. In initial studies it was shown that 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) is expressed in the adult Leydig cells of the rat but that there is no expression in fetal cells [30,31]. Our group and others have extended this to identify four mRNA species (3βHSD VI, EST, PGD-synthetase and 17βHSD III) which are expressed in the adult Leydig cells, but not the fetal Leydig cells, of the mouse [17-20]. So far, the only clearly identified differences in gene expression between the two populations are for genes expressed in the adult cell population but not in the fetal population [1,17-19]. Genes which are highly expressed in the fetal Leydig cell population, such as those encoding renin and thrombospondin 2, continue to be expressed in the adult testis, albeit at low levels [19,32,33]. This may be because adult Leydig cells express these genes at low levels or, more likely, because the fetal Leydig cell population persists in the adult testis [19,34-36]. Identification of genes specifically expressed in the adult Leydig cell population but not the fetal Leydig cell population does, however, allow us to determine whether adult Leydig cells develop in the absence of specific hormones or growth factors.
Normal differentiation of the adult Leydig cell population in the mouse begins around postnatal day 7 [1-3]. It has been shown previously that in the absence of LH there is a failure of normal proliferation of these cells and development of normal steroidogenic function does not occur [8,9]. Thus, there is no doubt that once differentiation has started adult Leydig cells require LH-stimulation but the role of LH at the onset of the initial differentiation phase has remained uncertain. Results from this study now show, however, that adult Leydig cells will differentiate in mice deficient in circulating LH. This conclusion is based primarily on the measured expression of specific adult Leydig cell markers in the testes of untreated GnRH-null mice. Expression of all four markers of adult Leydig cell differentiation was low but clearly detectable by real-time PCR in adult GnRH-null mice. Localisation, by in situ hybridisation, of two of these markers confirmed that expression was present in the untreated GnRH-null mouse testis and was localised to the interstitial tissue. For this data to be accepted as clear evidence that adult Leydig cells differentiate despite a deficiency of LH depends on the veracity of the markers chosen to indicate adult Leydig cell development. Evidence that these markers are specific to adult Leydig cells comes from previous studies which have shown that during development in normal mice mRNA species encoding 3βHSD VI and EST are undetectable by real-time PCR [19] [and confirmed by current studies], in situ hybridisation [1], immunohistochemistry [20] or Northern blotting [20] before adult Leydig cell differentiation occurs. In addition, while both PDG-synthetase and 17βHSD III are expressed in the fetal/neonatal testis, this expression is only in the seminiferous tubules and stops prior to puberty at which time expression appears in the adult Leydig cell population [17,18]. Expression of PGD-synthetase in the current experiments was confirmed by in situ hybridisation to be localised to the interstitial tissue of the adult GnRH-null mouse testis. Thus, as far as can be determined, the markers used are specific for the adult Leydig cell population and indicate that these cells are present in the GnRH-null testis.
Comparison of expression patterns in adult GnRH-null testes with normal neonatal testes is also informative. If adult GnRH-null testes do not contain adult Leydig cells and have only fetal-type Leydig cells present it would be expected that the general pattern of mRNA expression would be that of a neonatal testis lacking gonadotrophin support. In fact, with the exceptions of P450scc and renin, expression levels are similar or higher in the adult GnRH-null testis. This is consistent, therefore, with differentiation of an adult population of cells in the adult GnRH-null testis.
The effects of hCG treatment on gene expression in the adult GnRH-null mouse testis also supports the hypothesis that adult Leydig cells are present in this animal. Expression of the markers 3βHSD VI and 17βHSD III was increased significantly after 2 injections of hCG within a 12 hour period. During normal testicular development there is a gap of at least four days between initial Leydig cell differentiation [around day 7 [2]] and first expression of 3βHSD VI mRNA (around day 11 [1]) and a longer gap before the first significant rise in 17βHSD III mRNA levels (after day 20 [19]). If only undifferentiated precursor cells were present in the GnRH-null testis it is likely that a more prolonged period of hCG treatment would be required before increased expression of 3βHSD VI and 17βHSD III would be seen. In contrast to 3βHSD VI and 17βHSD III expression of mRNA species encoding EST and PGD did not increase until after 48 hours of hCG treatment. This is consistent with previous data which has shown that these two genes exhibit a delayed pattern of expression during normal development [19].
In the GnRH-null mouse lack of pituitary stimulation by GnRH causes a marked reduction in pituitary synthesis and release of gonadotrophins [8]. This means that circulating levels of LH and FSH are very low but they are not, necessarily, abolished. Thus, the possibility cannot be excluded that there is low-level gonadotrophin-stimulation of Leydig cell precursors. It should be noted, however, that intratesticular testosterone levels in GnRH-null mice are undetectable and are lower than in LH-receptor-null mice [6,7]. Any circulating LH must, therefore, be at a minimal level and, as such, is unlikely to affect Leydig cell development.
If, as data described here would suggest, adult Leydig cells develop without LH-stimulation this begs the question as to what state of differentiation/development the cells reach. Morphological and functional development of the adult Leydig cell lineage has recently been reviewed by Mendis-Handagama & Ariyaratne [37]. Spindle-shaped, Leydig precursor cells are found in the peritubular region of the interstitial tissue and differentiate initially to progenitor cells in the same region. Further development to "newly formed" and "immature" adult Leydig cells is associated with rounding of the cells and movement to the central interstitial region [37]. The expression of adult Leydig cell genes in the unstimulated GnRH-null testis and the rapid response of some of these to hCG stimulation suggests that the cells have moved beyond initial steps of differentiation and may represent a form of unstimulated, immature adult cell. Further evidence that the cells have moved beyond the progenitor stage is provided by the 3βHSD VI in situ hybridisation studies which show that the hybridisation signal is localised primarily in the central interstitial tissue area rather than the peritubular region where the progenitor cells are located. It is also interesting to note that the total number of morphologically-recognisable Leydig cells (with no distinction drawn between fetal and adult cells) actually doubles over the pubertal period in the GnRH-null animal [9]. This would be consistent with morphological differentiation of adult Leydig cells during this period in GnRH-null mice and ties in with the functional data described above.
Despite apparent adult Leydig cell differentiation in GnRH-null mice the total number of Leydig cells in these animals remains markedly reduced at about 10% of control [9]. It is clear that development of the final number of adult Leydig cells is a combination of precursor differentiation and proliferation of the mature cell although the relative contribution of the two processes remains unclear [37]. If it is accepted, from the data presented here, that Leydig cell differentiation is largely an LH-independent event the marked reduction in Leydig cell number in the GnRH-null mouse would be consistent with a reduced role for cell differentiation and a more important role for proliferation in determining final cell number. A further implication of this model would be that while differentiation is an LH-independent event cell proliferation (including precursor/progenitor proliferation) is LH-dependent. This would be consistent with earlier studies showing that LH will induce Leydig cell proliferation in GnRH-null mice [28].
Results from this study suggest that adult Leydig cell differentiation does not require LH although the hormone is necessary for functional development of the cells and for the establishment of normal cell number. The initial stimulus for adult Leydig cell differentiation remains unknown although thyroid stimulating hormone appears to be required [30,38] along with the Sertoli cell products Dhh and PDGF-A [35,39]. Whatever other factor or factors are involved it appears likely that at least some will be of Sertoli cell origin since these cells are closely involved in regulation of the Leydig cells and are necessary for Leydig cell survival and function [40]
Figure 3.
In situ hybridization studies showing 3β-hydroxysteroid dehydrogenase (3βHSD) type VI and prostaglandin D (PGD)-synthetase expression in adult control GnRH-null mice and GnRH-null mice following injection with hCG. Hybridisation of 3βHSD VI anti-sense probe to control GnRH-null testis is shown in A) and at higher power in B). In C) hybridisation to testes from GnRH-null mice after 4 injections of hCG is shown. Hybridisation to a sense 3βHSD VI probe is shown in D). Hybridisation of an anti-sense PGD-synthetase probe to testis from control GnRH-null mice is shown in E) while hybridization of a sense probe is shown in F).
Acknowledgments
Acknowledgements
This study was supported by grants from the BBSRC and the Wellcome Trust. Technical assistance for this study came from Gary Jackson and Lynne Fleming.
Contributor Information
PJ Baker, Email: p.j.baker@vet.gla.ac.uk.
H Johnston, Email: h.johnston@vet.gla.ac.uk.
M Abel, Email: margaret.abel@human-anatomy.oxford.ac.uk.
HM Charlton, Email: harry.charlton@human-anatomy.oxford.ac.uk.
PJ O'Shaughnessy, Email: p.j.oshaughnessy@vet.gla.ac.uk.
References
- Baker PJ, Sha JA, McBride MW, Peng L, Payne AH, O'Shaughnessy PJ. Expression of 3β-hydroxysteriod dehydrogenase type I and VI isoforms in the mouse testis during development. Eur J Biochem. 1999;260:911–916. doi: 10.1046/j.1432-1327.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- Nef S, Shipman T, Parada LF. A molecular basis for estrogen-induced cryptorchidism. Dev Biol. 2000;224:354–361. doi: 10.1006/dbio.2000.9785. [DOI] [PubMed] [Google Scholar]
- Vergouwen RPFA, Jacobs SGPM, Huiskamp R, Davids JAG, Derooij Proliferative activity of gonocytes, sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadtrophin releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Gene Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- Zhang F-P, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotrophin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto A-M, Charlton HM, Huhtaniemi I. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology. 1998;139:1141–1146. doi: 10.1210/endo.139.3.5788. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Kelce WR, Klinefelter GR, Ewing LL. Differentiation of leydig cell precursors invitro – a role for androgen. Endocrinology. 1990;127:488–490. doi: 10.1210/endo-127-1-488. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Katikineni M, Catt KJ. Regulation of luteinizing-hormone-receptors and steroidogenesis in the neonatal rat testis. Endocrinology. 1981;109:588–595. doi: 10.1210/endo-109-2-588. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Closset J, Rommerts FF, de Rooij DG, Stocco DM, Colenbrander B, Wensing CJ, Hennen G. Effects of pure FSH and LH preparations on the number and function of Leydig cells in immature hypophysectomized rats. J Endocrinol. 1989;120:97–106. doi: 10.1677/joe.0.1200097. [DOI] [PubMed] [Google Scholar]
- Siril Ariyaratne HB, Mendis-Handagama SM, Hales DB, Mason JI. Studies of the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. doi: 10.1095/biolreprod63.1.165. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Zhang FP, Huhtaniemi I. Persistent expression of a truncated form of the luteinizing hormone receptor messenger ribonucleic acid in the rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology. 1994;135:1018–1024. doi: 10.1210/endo.135.3.8070344. [DOI] [PubMed] [Google Scholar]
- Veldhuizen-Tsoerkan MB, Ivell R, Teerds KJ. hCG-induced changes in LH/CG receptor mRNA transcript levels in the testis of adult hypophysectomized, ethane dimethyl sulphonate-treated rats. Mol Cell Endocrinol. 1994;105:37–44. doi: 10.1016/0303-7207(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Rannikko A, Kero J, Zhang FP, Huhtaniemi IT. Molecular mechanisms of reappearance of luteinizing hormone receptor expression and function in rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology. 1997;138:3340–3348. doi: 10.1210/endo.138.8.5325. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Heikkila M, Vainio S, McMahon AP. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis – androstenedione is the major androgen secreted by fetal/neonatal leydig cells. Endocrinology. 2000;141:2631–2637. doi: 10.1210/endo.141.7.7545. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ. Expression of prostaglandin D synthetase during development in the mouse testis. Reproduction. 2001;122:553–559. doi: 10.1530/rep.0.1220553. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- Song WC, Qian Y, Sun X, Negishi M. Cellular localization and regulation of expression of testicular estrogen sulfotransferase. Endocrinology. 1997;138:5006–5012. doi: 10.1210/endo.138.11.5512. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ. Steroidogenic enzyme-activity in the hypogonadal (hpg) mouse testis and effect of treatment with luteinizing-hormone. J Steroid Biochem Mol Biol. 1991;39:921–928. doi: 10.1016/0960-0760(91)90350-E. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Murphy L. Cytochrome P-450 17α-hydroxylase protein and mRNA in the testis of the testicular feminized (Tfm) mouse. J Mol Endocrinol. 1993;11:77–82. doi: 10.1677/jme.0.0110077. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Marsh P, Dudley K. Follicle-stimulating hormone receptor mRNA in the mouse ovary during post-natal development in the normal mouse and in the adult hypogonadal [hpg] mouse: structure of alternate transcripts. Mol Cell Endocrinol. 1994;101:197–201. doi: 10.1016/0303-7207(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ. Expression of prostaglandin D synthetase during development in the mouse testis. Reproduction. 2001;122:553–559. doi: 10.1530/rep.0.1220553. [DOI] [PubMed] [Google Scholar]
- Roosen-Runge EC, Anderson D. The development of the interstitial cells of the albino rat. Acta Anat. 1959;37:125–137. doi: 10.1159/000141460. [DOI] [PubMed] [Google Scholar]
- Lording DW, De Kretser DM. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil. 1972;29:261–269. doi: 10.1530/jrf.0.0290261. [DOI] [PubMed] [Google Scholar]
- Scott IS, Charlton HM, Cox BS, Grocock CA, Sheffield JW, O'Shaughnessy PJ. Effect of LH injections on testicular steroidogenesis, cholesterol side-chain cleavage P450 messenger RNA content and leydig cell morphology in hypogonadal mice. J Endocrinol. 1990;125:131–138. doi: 10.1677/joe.0.1250131. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB, Teunissen van Manen KR, Haupt RL. Differentiation of adult Leydig cells in the neonatal rat testis is arrested by hypothyroidism. Biol Reprod. 1998;59:351–357. doi: 10.1095/biolreprod59.2.351. [DOI] [PubMed] [Google Scholar]
- Phillips DM, Lakshmi V, Monder C. Corticosteroid 11 beta-dehydrogenase in rat testis. Endocrinology. 1989;125:209–216. doi: 10.1210/endo-125-1-209. [DOI] [PubMed] [Google Scholar]
- Perera EM, Martin H, Seeherunvong T, Kos L, Hughes IA, Hawkins JR, Berkovitz GD. Tescalcin, a novel gene encoding a putative EF-hand Ca[2+]-binding protein, Col9a3, and renin are expressed in the mouse testis during the early stages of gonadal differentiation. Endocrinology. 2001;142:455–463. doi: 10.1210/endo.142.1.7882. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Johnston H, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci. 2002;115:3491–3496. doi: 10.1242/jcs.115.17.3491. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Knell CM. The fate of fetal leydig-cells during the development of the fetal and postnatal rat testis. Development. 1988;103:535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Chamindrani Mendis-Handagama S. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol Reprod. 2000;62:680–690. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, de Jong FH, van Haaster LH. 1998 Development of the adult-type Leydig cell population in the rat is affected by neonatal thyroid hormone levels. Biol Reprod. 1998;59:344–350. doi: 10.1095/biolreprod59.2.344. [DOI] [PubMed] [Google Scholar]
- Gnessi L, Basciani S, Mariani S, Arizzi M, Spera G, Wang C, Bondjers C, Karlsson L, Betsholtz C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J Cell Biol. 2000;149:1019–1026. doi: 10.1083/jcb.149.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Warren J, Debeljuk L, Richardson LL, Mahar PL, Waymire KG, Amy SP, Ross AJ, MacGregor GR. Spermatogenesis in Bclw-deficient mice. Biol Reprod. 2001;65:318–332. doi: 10.1095/biolreprod65.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]