Abstract
The molecular mechanisms of clathrin-dependent internalization of epidermal growth factor receptor (EGFR) are not well understood and, in particular, the sequence motifs that mediate EGFR interactions with coated pits have not been mapped. We generated a panel of EGFR mutants and stably expressed these mutants in porcine aortic endothelial (PAE) cells. Interestingly, mutations of tyrosine phosphorylation sites 1068 and 1086 that interact with growth-factor-receptor-binding protein Grb2 completely abolished receptor internalization in PAE cells. Quantitative analysis of colocalization of EGF-rhodamine conjugate and coated pits labeled with yellow-fluorescent-protein–tagged β2 subunit of clathrin adaptor complex AP-2 revealed that EGFR mutants lacking Grb2 binding sites do not efficiently enter coated pits. The depletion of Grb2 from PAE as well as HeLa cells expressing endogenous EGFRs by RNA interference substantially reduced the rate of EGFR internalization through clathrin-dependent pathway, thus providing the direct evidence for the important role of Grb2 in this process. Overexpression of Grb2 mutants, in which the SH3 domains were either deleted or inactivated by point mutations, significantly inhibited EGFR internalization in both PAE and HeLa cells. These findings indicate that Grb2, in addition to its key function in signaling through Ras, has a major regulatory role at the initial steps of EGFR internalization through clathrin-coated pits. Furthermore, the EGFR mutant lacking Grb2 binding sites did not efficiently recruit c-Cbl and was not polyubiquitinated. The data are consistent with the model whereby Grb2 participates in EGFR internalization through the recruitment of Cbl to the receptor, thus allowing proper ubiquitylation of EGFR and/or associated proteins at the plasma membrane.
INTRODUCTION
Binding of epidermal growth factor (EGF) to its cell surface receptor (EGFR) initiates an array of signaling events emanating from activation of an intrinsic receptor tyrosine kinase that phosphorylates the receptor itself as well as other intracellular proteins (Schlessinger, 2000). These membrane-associated events ultimately lead to altered gene expression in the nucleus. The activation of EGFR by ligand also dramatically changes cellular localization of receptors due to rapid endocytosis of ligand-receptor complexes via clathrin-coated pits. By controlling receptor levels at the cell surface and in endosomes, endocytic trafficking serves as an important determinant of the intensity and duration of EGFR signaling. However, despite extensive studies over last two decades, the molecular mechanisms of clathrin-dependent endocytosis of EGFR are not well understood.
EGFR mutagenesis revealed that several regions of EGFR molecule can support rapid endocytosis of the receptor. Some of these regions contain well-defined internalization signal motifs (Chang et al., 1993; Sorkin et al., 1996). However, none of these motifs have been proven to be critical for endocytosis of the full-length EGFR. The functional role of other parts of the molecule may be related to the tyrosine phosphorylation of the receptor. Removal of major tyrosine phosphorylation sites in the full-length receptor partially reduced internalization, although the truncation of the entire carboxyl-terminal region containing these sites had no effect on endocytosis (Chen et al., 1989; Chang et al., 1991; Sorkin et al., 1991). These observations have led to the hypothesis that phosphotyrosines function in EGFR endocytosis by facilitating conformational changes in the EGFR molecules rather than by mediating interactions of the EGFR with Src homology (SH) 2 or phosphotyrosine-binding (PTB) domains. Thus, while SH2/PTB domain containing proteins, such as c-Cbl, Grb2, and Shc, have been proposed to participate in the EGFR endocytosis (Sakaguchi et al., 2001; Soubeyran et al., 2002; Wang and Moran, 1996; Waterman et al., 2002), it has been difficult to reconcile these views with the results of EGFR mutagenesis studies. Overall, these many previous studies suggest that multiple redundant mechanisms of EGFR internalization may exist. Because receptor tyrosine kinase activity is necessary for maximum rapid endocytosis of both full-length and truncated receptors, tyrosine phosphorylation events are likely to participate in more than one of these redundant pathways (Chen et al., 1989; Chang et al., 1993).
Another important, but often overlooked, feature of EGFR endocytosis is the saturability of the specific internalization pathway (Wiley, 1988; Lund et al., 1990). In cells heterologously expressing EGFRs or overexpressing endogenous EGFRs, activation of the large number of receptors results, nonetheless, in low apparent rates of internalization. Thus, it has been proposed that the capacity of the clathrin-dependent rapid internalization pathway is limited (Lund et al., 1990). As a result, the occupancy of the large number of EGFRs leads to their internalization through clathrin-independent pathway because the rapid pathway becomes saturated. The rapid saturable pathway is the major route of internalization of EGFR in cells expressing physiological levels of EGFRs and in the presence of low (physiological) concentrations of EGF. Therefore, the mechanisms regulating rapid saturable internalization are most important for our understanding of EGFR regulation in vivo. The practical implications of this internalization saturability are that low concentrations of the ligand as well as cell lines expressing moderate receptor levels must be used in order to compare clathrin-dependent internalization of different EGFR mutants under different experimental conditions.
In this study we decided to revisit the issue of multiplicity of internalization mechanisms of the EGFR. Although we think that multiple internalization mechanisms are possible, previous studies using EGFR truncations may be confounded by unmasking cryptic signals that are not functional in the full-length receptor. These cryptic signals may partially or fully substitute for the signals that normally operate in the native receptor. For instance, “kinase-dead” carboxyl-terminal truncation EGFR mutant is internalized at a relatively high rate compared with that of the kinase-dead full-length receptor (Chen et al., 1989). It is possible that although EGFR mutations and other experimental manipulations reveal a multiplicity of internalization mechanisms, endocytosis of native receptors under physiological conditions is mediated by only one or two mechanisms. To avoid these possible complications, here we took advantage of EGFR expression in porcine aortic endothelial (PAE) cells that appear to lack some of the redundant mechanisms supporting internalization of truncated EGFRs and focused our analysis on the carboxyl-terminal tail of the EGFR. Examination of EGFR endocytosis in a panel of cell lines expressing novel receptor mutants rejuvenated the hypothesis of the importance of Grb2 in EGFR trafficking previously proposed by Wang and Moran (1996). The data of EGFR mutagenesis paired with the analysis of the effects of Grb2 depletion by RNA interference and Grb2 mutants on endocytosis revealed a major role for EGFR–Grb2 interaction in the initial steps of clathrin-mediated endocytosis of the receptor.
MATERIALS AND METHODS
Antibodies
mAb to c-Cbl were from Transduction Laboratories (San Diego, CA); monoclonal antibodies 528 to EGFR from ATCC (Manassas, VA); mAb to GFP from Zymed Laboratories, Inc. (San Francisco, CA); rabbit polyclonal antibodies to GFP from Abcam Limited (Cambridge, UK); rabbit polyclonal anti-Grb2, anti-SOS1/2 and monoclonal antiubiquitin antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); polyclonal antibody to dynamin 2 from Affinity Bioreagents (Golden, CO). Polyclonal rabbit antibody to EGFR, Ab2913, was described previously (Beguinot et al., 1986).
Plasmid Construction
To generate EGFR truncation mutants C′1022, C′1063, C′1072, C′1090, and C′1107, a stop codon was created at the position corresponding to the amino acid residue 1023, 1064, 1073, 1091, 1108 in the full-length EGFR cloned into pEGFP-N1 (Clontech, Palo Alto, CA; Carter and Sorkin, 1998). Grb2-YFP, Grb2-CFP, and β2-YFP were described previously (Jiang and Sorkin, 2002; Sorkina et al., 2002). A cDNA fragment encoding the SH2 domain corresponding to residues 60–158 of human Grb2 was cloned into pEYFP-N1. Site-directed mutagenesis of EGFR and Grb2 was performed using QuickChange site-directed mutagenesis kit according to the manufacture protocol (Strategene Cloning Systems, La Jolla, CA).
Human c-Cbl and 70Z-Cbl cDNAs were provided by Drs. Levkowitz and Yarden (Weizmann Institiute of Science, Rehovot, Israel). To generate YFP fusion proteins, full-length c-Cbl or 70Z-Cbl were amplified by PCR and ligated into pEYFP-N1 (Clontech; Palo Alto, CA) by using KpnI and BamHI restriction sites. To generate Grb2-Cbl chimeric proteins, P49L/G203R-Grb2 was first subcloned into pEYFP-N1 using XhoI and HindIII restriction sites and then c-Cbl or 70Z-Cbl were ligated into P49L/G203R-Grb2-YFP by using KpnI and BamHI sites, thus placing Cbl or 70Z-Cbl between Grb2 and YFP. All constructs were verified by dideoxynucleotide sequencing.
Cells and Transfections
Cell lines of PAE cells stably expressing various mutated receptors were established using standard single-cell cloning and G418 selection procedures. Several clones of each EGFR mutant expressing from 1 to 4 ×105 receptors/cell (most clones—in the range between 1 and 2 × 105) were typically used to measure internalization rates. PAE cell lines were grown in F12 medium containing 10% fetal bovine serum, antibiotics, and glutamine and supplemented with G418. HeLa cells were grown in DMEM containing 10% fetal bovine serum, antibiotics, and glutamine. Transient expressions were performed using Effectine (Qiagen; Valencia, CA) and the cells were used for experiments 36–48 h after transfection.
RNA Interference
Three pairs of 21 nucleotide sense and antisense RNA oligonucleotides protected by two 3′-overhanged (2′-deoxy) thymidines (dT) (siRNA) were synthesized by Dharmacon Research, Inc. (Lafayette, CO). These oligonucleotides are: siRNA1: sense, 5′ GAA GAA UGU GAU CAG AAC UTT 3′ antisense, 5′ AGU UCU GAU CAC AUU CUU CTT 3′; siRNA2: sense, 5′ UAU CAC AGA UCU ACA UCU GTT 3′; antisense, 5′ CAG AUG UAG AUC UGU GAU ATT 3′; siRNA3: sense, 5′ CAU GUU UCC CCG CAA UUA UTT 3′; antisense, 5′ AUA AUU GCG GGG AAA CAU GTT 3′, corresponding to human Grb2 coding nucleotides 86–106, 398–418, and 607–627, respectively. In additions, siRNAs targeted to human Eps15 (nucleotides 307–329, sense 5′ AGA ACC UGU GCC AAU GUC CTT 3′, antisense: 5′ GGA CAU UGG CAC AGG UUC UTT 3′) or targeted to human MEKK3 (provided by Dr. G. Johnson, University of Colorado) were used as controls to Grb2-targeted siRNAs.
Equal amount of sense and antisense RNA oligonucleotides were mixed and annealed according to the manufacture protocol to form RNA duplex before transfection. PAE or HeLa cells in 12-well plates were transfected twice with 3 μl of 20 μM siRNA duplex in 3 μl Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) at 24-h intervals. Cells were plated to normal culture medium 12 h before experiments.
Internalization of 125I-EGF and 125I-transferrin
Mouse receptor-grade EGF was obtained from Collaborative Research Inc. (Bedford, MA) and iodinated using a modified chloramine T method as described previously (Sorkin et al., 1991). Iron-saturated human transferrin was purchased from Sigma Chemical Co. (St. Louis, MO) and iodinated using Iodo-Beads (Pierce, Inc.; Rockford, IL). The specific activity of 125I-transferrin and 125I-EGF was 3.0 × 105 cpm/μg and 1.5–2.0 × 105 cpm/ng, respectively. Cells were incubated with 125I-EGF (0.5–1 ng/ml) or 125I-transferrin (2 μg/ml) in binding medium (DMEM, 0.1% bovine serum albumin) for 1–6 min at 37°C and the ratio of internalized and surface radioactivity was determined as described before (Nesterov et al., 1999). This ratio was plotted against time, and the specific rate constant for internalization ke was calculated as linear regression coefficient.
Immunoprecipitation and Western Blotting
The cells were lysed in Triton X-100/glycerol solubilization buffer as described previously (Jiang and Sorkin, 2002). In experiments where ubiquitylation of the EGFR was analyzed, 1% Na deoxycholate and 10 mM N-ethyl-maleimide were included in the lysis buffer to minimize coprecipitation of other proteins and inhibit deubiquitination enzymes, respectively. EGFR or Grb2-YFP was immunoprecipitated using Ab528 or rabbit anti-GFP, respectively, for 3 h at 4°C followed by a 1-h incubation with Protein A-Sepharose (Sigma). Immunoprecipitates and cell lysates were electrophoresed on SDS-PAGE and transferred to nitrocellulose membranes, and Western blotting was performed with several antibodies followed by detection using enhanced chemiluminescence system (Pierce).
Coated Pit Recruitment and Internalization of EGFR by Fluorescence Microscopy
Cells transiently transfected with β2-YFP, Grb2-CFP, Grb2-YFP, or YFP-c-Cbl were grown on coverslips and incubated with 1–2 ng/ml EGF-Rh (Molecular Probes, Eugene, OR) for 1 h at 4°C or at 37°C for 6 min. The cells were observed live or fixed with freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences; Fort Washington, PA) for 30 min at 4°C. The coverslips with live or fixed cells were mounted into microscopy chamber and directly visualized in phenol red-free medium or phosphate buffer, respectively. A deconvolution imaging workstation was described previously (Jiang and Sorkin, 2002; Sorkina et al., 2002). The detection of YFP and rhodamine fluorescence in the same sample was performed using FITC and Cy3 filter channels, respectively, and a 66100bs dichroic mirror, whereas the detection of YFP, CFP, and rhodamine was performed using YFP, CFP, and Cy3 filter channels, and a 86004BS dichroic mirror (all filters and mirrors are from Chroma, Inc.; Brattleboro, VA).
Typically, several two-dimensional images were obtained from the bottom and the top of the cell, so that the large flat areas of the cell containing coated pits could be clearly visualized. Binning 2 × 2 mode was used. Images were deconvoluted using a nearest-neighbor method. The calculation of the amount of EGF-Rh colocalized with β2-YFP was performed using SlideBook 3.0 “AND data w/mask” algorithm (Intelligent Imaging Innovation, Denver, CO; Sorkina et al., 2002). Rhodamine images were background-subtracted. The images were then segmented using a minimal intensity of YFP dots as a low threshold, and new images were generated from the segmented images, so that all pixels that did not overlap with YFP dots were assigned “0” fluorescence intensity. The integrated intensity of rhodamine fluorescence that overlapped with YFP dots in the segmented image was considered as EGF-Rh localized in coated pits. Finally, the extent of EGF-Rh localization in coated pits was calculated as a ratio of the total integrated fluorescence of the segmented image to that in the original image.
RESULTS
The Internalization of Truncated C′1022 EGFR Is Impaired in PAE Cells
Previous work in mouse fibroblast B82 (Chen et al., 1989) and NIH 3T3 cells (Sorkina et al., 2002) showed that EGFR deletion mutants lacking the last 164/165 amino acid residues are internalized at the same or even higher rates compared with wild-type receptor. These data suggested that internalization signal motifs are located in the carboxyl-terminus of the EGFR upstream of residue 1022. To map these motifs in more detail, several clones of PAE cells expressing C′1022 truncated receptor (Figure 1) and site-specific point mutants of C′1022 (Figure 2A) were generated. These mutants had alanine substitutions within putative internalization sequences that include Tyr974 (Sorkin et al., 1996), Phe999/Phe1000 (Chang et al., 1993), or Leu1010/Leu1011. We chose PAE cells for this analysis because they lack endogenous EGFRs and heterologously expressed human EGFRs traffic in these cells with the kinetics similar to that observed in cells expressing endogenous EGFRs (Carter and Sorkin, 1998). In addition, PAE cells are easy to transfect, select stable clonal lines, and image using fluorescence microscopy.
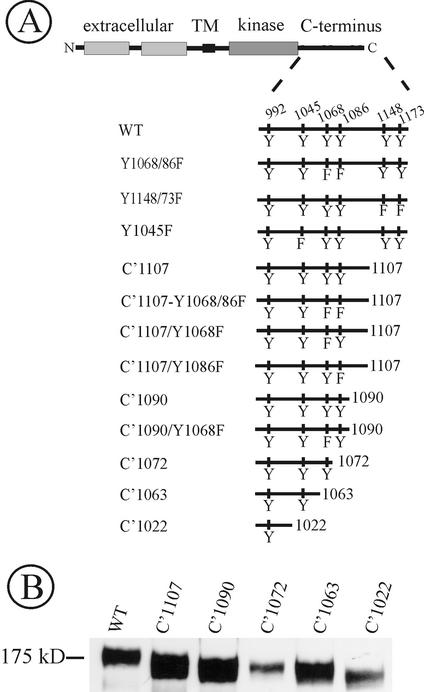
Figure 1.
Schematic representation of EGF receptor mutants. (A) Wild-type EGFR (WT) is drawn with the extracellular, transmembrane (TM) and intracellular domain (kinase and C terminus). Main tyrosine phosphorylation sites (residues 992, 1068, 1086, 1148, and 1173) and a Cbl binding site (Tyr1045) are indicated in WT receptor. Mutants have C-terminal truncation of 79 (C′1107), 96 (C′1090), 114 (C′1072), 123 (C′1063), and 164 amino acid residues (C′1022). Tyrosine substitutions by phenylalanines (F) are indicated. (B) To confirm the correct size of truncated EGFR mutants, PAE cells expressing WT, C′1107, C′1090, C′1072, C′1063, or C′1022 were lysed, and the EGFR was detected in lysates by immunoblotting with antibody 2913.
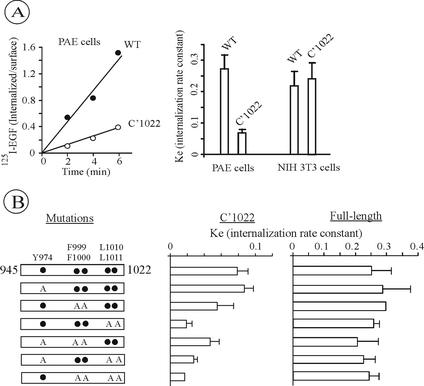
Figure 2.
125I-EGF Internalization by C′1022 truncated EGFR mutants. (A) The internalization of 1 ng/ml 125I-EGF was measured in PAE cells expressing wild-type (WT) or C′1022 mutant. The rate of internalization is expressed as ratio of internalized and surface 125I-EGF at each time point (left). The internalization rate constant (ke) values measured using 1 ng/ml 125I-EGF in PAE cells were compared with ke values measured under the same conditions (as shown on the left) in NIH 3T3 expressing wild-type or Dc165 mutant EGFR (corresponding to C′1021 truncation; Alvarez et al., 1995; right). The averaged values from multiple experiments with three independent clones of C′1022 PAE cells are presented. Error bars, SEs. (B) Schematic representation of single, double, triple, or quadruple point mutants of C′1022 or full-length EGFR (residues 945-1022 are shown). Tyrosine (Y974), phenylalanines (F999/1000), and leucines (L1010/1011) were substituted by alanines (A, left). The internalization rate constant (ke) of the site-point mutants of C′1022 (panel) and full-length (panel) EGFR were measured using 1 ng/ml 125I-EGF as in A. The bars represent averaged values from multiple experiments with 2–4 independent cell clones for each mutants and the error bars represent SEs.
The rate of endocytosis of C′1022 and other mutant receptors was measured using low concentrations of 125I-EGF to avoid saturation of the clathrin-mediated endocytic pathway. Several single-cell clones expressing moderate levels of mutant EGFRs were generated and analyzed for each receptor mutant. This was necessary in order to compare internalization kinetics of different receptor mutants through the rapid (presumably, clathrin-dependent) pathway using similar concentrations of 125I-EGF and to control for clonal variation. We have previously demonstrated that, when EGFR was transiently expressed, very low internalization rates were observed (Carter and Sorkin, 1998). These low rates are, probably, due to preferential binding of 125I-EGF to subpopulation of cells overexpressing EGFR, leading to saturation of the rapid internalization pathway.
Surprisingly, measurements of 125I-EGF uptake in several clones of PAE cells expressing C′1022 mutant yielded low internalization rates (ke ≈ 0.07/min; Figure 2A). In contrast, NIH3T3 cells expressing the same mutant displayed high internalization rates. The LL motif (1010/1011) was critical for the low-level internalization of C′1022 receptor observed in PAE cells, whereas the Y974RAL and GGQFF1000 motifs had minimal roles in internalization (Figure 2B). However, full-length receptors bearing these same mutations alone or in combination were internalized at high rates comparable to wild-type EGFR (ke ≥ 0.2/min; Figure 2C). Thus, truncation of last 164 residues appears to uncover cryptic internalization motifs that are not functional in the native receptor in PAE cells.
Tyrosines 1068 and 1086 Are Critical for EGFR Internalization
The data presented in Figure 2 suggested that in PAE cells the carboxyl-terminal 164 residues mediate EGFR endocytosis. We, therefore, next focused on defining internalization motifs within the region 1023–1186. Several new truncated EGFR mutants were prepared and constitutively expressed (Figure 1A). Analysis of 125I-EGF uptake by these mutants showed that the region between 1063 and 1090 residues is critical for EGFR internalization (Figure 3A). Interestingly, the internalization rate of C′1063 mutant was even lower than that of C′1022, suggesting that residues 1023–1063 may inhibit C′1022 internalization by sterically hindering LL or other cryptic motifs. The lower internalization rates of C′1063 or similar mutants compared with C′1022 mutant were also observed in other cell types (Chen et al., 1989; Sorkin et al., 1992).
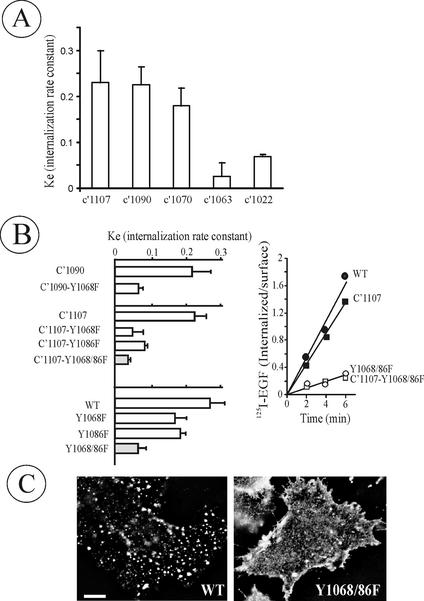
Figure 3.
Tyrosine 1068 and 1086 are critical for EGFR internalization. (A) The ke values were measured in PAE cells stably expressing C′1107, C′1090, C′1072, C′1063, or C′1022 truncated EGFRs as in Figure 2. (B) ke values were measured in cells expressing single or double Tyr1068/1086 mutants of full-length or truncated EGFRs (left) as in A. The bars in A and B represent averaged values from multiple experiments with 2–4 independent cell clones of each mutant and the error bars represent SEs. A representative 125I-EGF internalization experiment performed as in Figure 2A in cells expressing wild-type EGFR (WT), C′1107, C′1107-Y1068/86F, and Y1068/86F mutants is shown on the right. (C) WT and Y1068/86F-expressing PAE cells were incubated with 1 ng/ml EGF-Rh at 37°C for 6 min, and the rhodamine fluorescence images were acquired from living cells. Bar, 10 μm.
Within the 1063–1090 region are two tyrosine phosphorylation sites, Tyr1068 and Tyr1086, known to be responsible for EGFR interaction with Grb2 adaptor protein (Okutani et al., 1994). To test whether these tyrosines are important for receptor internalization, single and double phenylalanine substitutions were made in both truncated and full-length EGFRs (Figure 1A). As shown in Figure 3B, single mutation of either Tyr1068 or Tyr1086 reduced internalization rate in both backgrounds. Double substitution Y1068/1086F produced mutants (Y1068/86F and C′1107-Y1068/86F) that were internalized at minimal rates comparable to the rate of basal constitutive endocytosis (∼20% compared with the internalization rate of the wild-type receptor). Importantly, similarly slow internalization was observed in four independent clones of Y1068/86F-expressing cells (Figure 3). Western blot analysis of cell lysates using phosphotyrosine antibody revealed a number of proteins phosphorylated to the same extent by the wild-type and Y1068/1086F mutant EGFR, suggesting that the kinase activity of the mutant was not affected (unpublished data).
The results of 125I-EGF uptake experiments were confirmed by fluorescence microscopy. Cells expressing wild-type or Y1068/86F mutant receptors were incubated with EGF conjugated to rhodamine (EGF-Rh; 1 ng/ml) for 6 min at 37°C. In cells expressing wild-type receptors, EGF-Rh was concentrated in vesicular structures that are presumably endosomes. However, very few endosomes containing EGF-Rh were seen in Y1068/86F-mutant–expressing cells (Figure 3C). Similar differences in EGF-Rh endocytosis for C′1107 and C′1107-Y1068/86F mutants were also observed (unpublished data). Altogether, the data in Figure 3 show that the Grb2 binding sites have an important role in EGFR internalization in PAE cells.
Grb2 Binding Sites Are Important for EGFR Recruitment into Coated Pits
To determine which stages of endocytosis are inhibited by the Y1068/86F mutations, we focused on the initial step of endocytosis, where EGF-activated receptors are recruited into clathrin-coated pits. To this end, cells expressing wild-type or Y1068/86F mutant were transiently transfected with yellow fluorescent protein (YFP)-tagged β2 subunit of clathrin adaptor complex AP-2 to mark clathrin coats. We have previously observed the highest extent of EGFR clustering in coated pits at 4°C (Sorkina et al., 2002). Cells were incubated with EGF-Rh (1–2 ng/ml) at 4°C for 1 h to allow EGF-Rh binding and activation of the receptors in the absence of endocytosis, and the percent of EGF-Rh colocalized with coated pits (β2-YFP dots) of the total cell-associated EGF-Rh was measured. This technique enabled us to measure the extent of colocalization of the direct fluorescence signals without the problems of membrane disruption, loss of EGF-Rh and nonlinearity of detection, all associated with cell permeabilization and antibody staining. This assay is sufficiently sensitive to image the localization of low quantities of EGF-Rh (on average seven EGF-Rh molecules per coated pit), which is essential to study the clathrin-dependent pathway.
As seen in Figure 4, wild-type receptors occupied by EGF-Rh were localized in numerous punctate structures where they often colocalized with β2-YFP dots. In contrast, in cells expressing the Y1068/86F mutant, EGF-Rh was diffusely distributed in the plasma membrane and rarely overlapped with β2-YFP in punctate structures. Coated pits located on the upper membrane of the cells contained EGF-Rh more frequently than coated pits located at the basal membrane. The average fluorescence intensity and distribution of dots labeled by β2-YFP were essentially similar in wild-type and mutant EGFR expressing cells. The patterns of localization of fluorescent proteins were similar in both fixed and living cells.
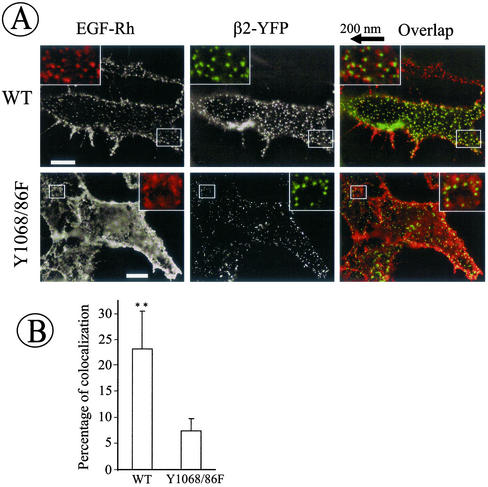
Figure 4.
Coated pit recruitment of wild-type but not Y1068/86F EGFR. (A) β2-YFP was transiently expressed in wild-type EGFR (WT) and Y1068/86F-expressing PAE cells. The cells grown on coverslips were incubated with 2 ng/ml EGF-Rh at 4°C for 1 h. Cells were then fixed, and the images were acquired through Cy3 (red) and FITC (green) filter channels. Insets: High magnification images of the small regions of the cell shown by white rectangles. In inset overlaps, rhodamine images were shifted approximately by 200 nm to the left relative to YFP images to clearly assess the colocalization of EGF-Rh and coated pits. Bars, 10 μm. (B) The images obtained in experiments described in A were used to quantitate the percent of rhodamine/YFP colocalization. The bars represent the average values from six cells and the error bars represent SDs (**p < 0.01).
Quantitation of the extent of rhodamine and YFP colocalization was performed on different z-axes optical sections in several flattened regions of the cells. This quantitation confirmed that recruitment of the mutant receptor into coated pits is significantly inhibited (Figure 4B). Thus, Grb2 binding sites, Tyr1068/1086, are essential for efficient coated-pit recruitment of the EGFR.
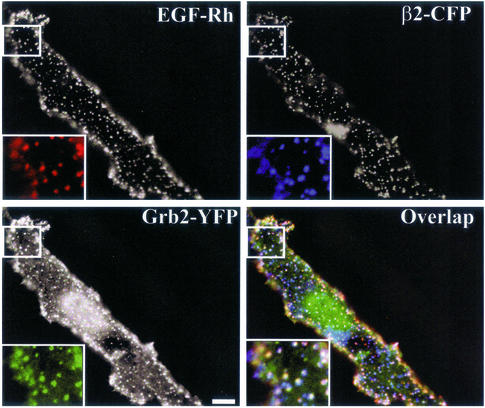
The importance of Grb2 binding sites for EGFR clustering in coated pits prompted us to examine whether Grb2 is located in coated pits together with the receptor. PAE cells expressing wild-type EGFR were transiently transfected with Grb2-YFP and β2 tagged with cyan fluorescent protein (CFP). When the cells were treated with 2 ng/ml EGF-Rh at 4°C, significant colocalization of EGF-Rh, Grb2-YFP, and β2-CFP in the punctuate structures was observed (Figure 5). This result indicates that Grb2 is bound to the activated EGFR in coated pits.
Figure 5.
Grb2-YFP is colocalized with EGFR in coated pits. Wild-type EGFR-expressing PAE cells were transiently transfected with β2-CFP and Grb2-YFP. The cells were incubated with 2 ng/ml EGF-Rh for 1 h at 4°C and fixed. The images were acquired from cells that express low levels of Grb2-YFP through Cy3, YFP and CFP filter channels. Insets: High magnification images of the small regions of the cell shown by white rectangles. The “white” indicates the overlap of rhodamine, YFP, and CFP fluorescence. Bars, 5 μm.
Depletion of Grb2 by RNA Interference
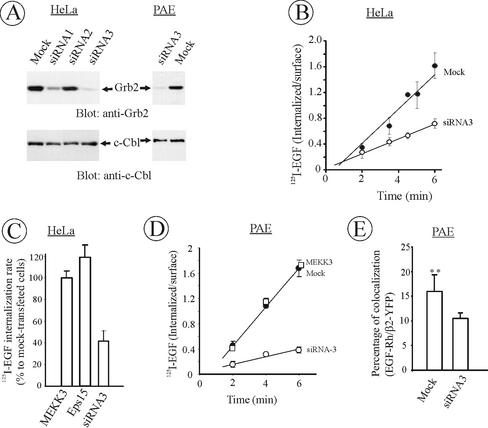
The data in Figures 3–5 implicate Grb2 binding to the receptor in the regulation of EGFR internalization in PAE cells that heterologously express EGFRs and apparently lack some redundant mechanisms of internalization. To directly test for the role of Grb2 in cells expressing endogenous EGFRs, Grb2 protein was depleted from HeLa cells using small interfering RNAs (siRNA; Elbashir et al., 2001). HeLa cells possess ∼1.5 × 105 EGFR per cell and internalize EGFRs very rapidly through clathrin-dependent pathway. As shown in Figure 6, it became possible to deplete endogenous Grb2 by 95% using siRNA duplex homologous to nucleotides 607–627 of the human Grb2 coding sequence. Under these conditions, levels of other proteins were unaffected (see, for example, c-Cbl, Figure 6). As expected, given the importance of Grb2 in growth factor signaling, a slight growth reduction in Grb2-depleted cultures (10–20%) was observed during a 48-h time course of siRNA transfection.
Figure 6.
Depletion of Grb2 inhibits EGFR internalization. (A) HeLa and PAE cells were transfected with three different siRNA targeted to human Grb2 or mock-transfected as described in MATERIALS AND METHODS. After 2 d, equal amounts of cells were lysed, lysates were electrophoresed, and the amount of Grb2 and c-Cbl (control) in lysates was determined by Western blotting. (B) HeLa cells were depleted of Grb2 using siRNA3 as in A, and the rate of 125I-EGF (1 ng/ml) internalization was measured as in Figure 2. The data are averaged from four independent experiments and the error bars represent SEs. (C) Mean internalization 125I-EGF rates were measured in cells that were transfected with siRNA3 as in B or siRNAs targeted to MEKK3 or Eps15. The rates are expressed as percent to the internalization rate in mock-transfected cells. The data are averaged from three to four independent experiments and the error bars represent SEs. (D) PAE/EGFR cells were depleted of Grb2 using siRNA3 or transfected with MEKK3-targeted siRNA, and the rate of 125I-EGF (1 ng/ml) internalization was measured as in B. The data represent the averaged values from three independent experiments and the error bars represent SEs. The average reduction of endocytosis rate constant ke by siRNA3 in these experiments was 75% ± 5%. (E) β2-YFP was transiently expressed in PAE/EGFR cells depleted or not depleted of Grb2. The cells were incubated with 2 ng/ml EGF-Rh at 4°C for 1 h. The percent of cellular EGF-Rh colocalized with coated pits was calculated as in Figure 4. The bars represent the average values from seven cells and the error bars represent SDs of the mean (**p < 0.01).
Most important, the rate of 125I-EGF endocytosis in HeLa cells transfected with Grb2 siRNA was decreased by 60%, indicating that Grb2 has the major role in EGFR internalization (Figure 6, B and C). In our hands, the rate of 125I-EGF internalization was inhibited by 70% in HeLa cells expressing K44A dynamin mutant in tetracycline-dependent manner, conditions shutting down clathrin-dependent endocytosis (Damke et al., 1995). Thus, the inhibitory effects of Grb2 depletion are considered to be significant. The effect of Grb2-depletion was specific because the endocytosis of 125I-transferrin, a cargo that is constitutively internalized through coated pits, was not affected (unpublished data). siRNAs targeted to other proteins, Eps15 and MEKK3, had no effects on 125I-EGF internalization (Figure 6C).
Although siRNAs were designed based on human Grb2 sequence, it was found that siRNA3 efficiently depletes Grb2 in porcine cells (Figure 6A). Moreover, the rate of 125I-EGF endocytosis was inhibited by 75% in Grb2-depleted PAE cells (Figure 6D). The residual internalization rate was comparable to the rate of basal constitutive endocytosis. Measurements of the extent of colocalization of EGF-Rh and β2-YFP were performed in PAE cells transfected with Grb2-targeted siRNA as described in the analysis of EGFR mutants (Figure 4). These experiments showed that the EGFR recruitment efficiency into coated pits is reduced in Grb2-depleted PAE cells (Figure 6E). Overall, the data in Figure 6 represent the first direct evidence for the importance of Grb2 in EGFR internalization process.
Overexpression of Grb2 Mutants Inhibits EGFR Endocytosis
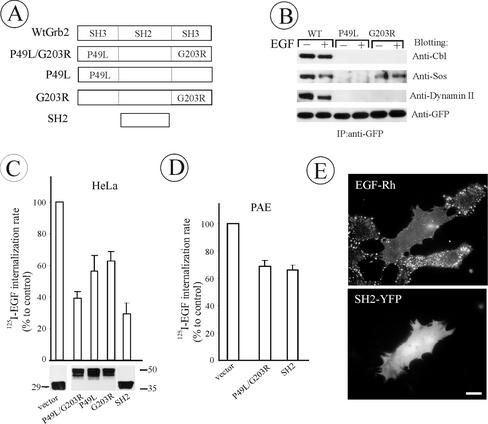
Analysis of EGFR mutants and RNA interference experiments implicate EGFR-Grb2 interaction in the regulation of clathrin-mediated internalization of the receptor. To further assess the role of Grb2 in endocytosis, several mutants of Grb2-YFP were generated in which SH3 domains were either deleted or poly-proline binding inactivated by point mutations (Figure 7A). Grb2 mutants were transiently expressed in HeLa cells, allowing high efficiency expression in most of the cells in the population. Immunoprecipitation experiments showed that inactivation of both SH3 domains results in the inability of Grb2-YFP to bind c-Cbl, dynamin 2, and son-of-sevenless, SOS (unpublished data). The amino-terminal SH3 domain was more important for SOS binding, whereas effective interaction of Grb2 with c-Cbl or dynamin required both SH3 domains (Figure 7B). Coimmunoprecipitation of putative SH3-binding proteins, Eps15 and REPS/POB1, with Grb2-YFP was not detected (unpublished data).
Figure 7.
The effect of Grb2 mutant overexpression on EGFR internalization in HeLa and PAE cells. (A) Schematic representation of wild-type and mutant Grb2 fusion proteins. YFP or CFP was attached to the carboxyl-terminus of Grb2 mutants (unpublished data). (B) HeLa cells were transiently transfected with WtGrb2-YFP, P49L-Grb2-YFP, or G203R-Grb2-YFP. After 36–48 h transfection, cells were either left untreated or stimulated with 1 ng/ml EGF for 5 min at 37°C. YFP-fusion proteins were immunoprecipitated from cell lysates with anti-GFP and immunoblotted with antibodies to c-Cbl, Sos-1/2, dynamin 2, and GFP. (C) HeLa cells transiently transfected with Grb2 mutants were incubated with 1 ng/ml 125I-EGF at 37°C, and the ke values were measured and expressed as percent of the value obtained for the vector-transfected cells. The level of expression (bottom) and transfection efficiency (unpublished data) were monitored, respectively, by Western blotting of cell lysates with anti-GFP and by imaging the YFP fluorescence of confluent cells before internalization assays and were similar for different Grb2 mutants. (D) 125I-EGF internalization rates in PAE cells tran-siently expressing Grb2 mutants were measured and expressed as in C. (F) PAE cells expressing wild-type EGFR were transfected with SH2-YFP. Two days later, the cells were incubated with EGF-Rh (1 ng/ml) for 1 h at 4°C, washed, and further incubated for 6 min at 37°C. The images were acquired from living cells through Cy3 filters. Bar, 10 μm. All data in the figure represent the mean values obtained from at least three independent experiments, and the error bars represent SE of the mean.
As shown in Figure 7C, SH2-YFP and P49L/G203R-Grb2-YFP exhibited the strongest inhibitory effects on 125I-EGF endocytosis. Transfection efficiency (typically ∼60–80% as calculated based of YFP fluorescence) and expression levels were similar across experiments. Surprisingly, expression of wild-type Grb2-YFP at levels comparable to those of Grb2 mutants resulted in slow proliferation and substantial detachment of cells form the substrate. Therefore, the effects of Grb2-YFP overexpression on EGFR endocytosis could not be determined. This Grb2 toxicity can be explained by the observation that overexpression of Grb2-YFP trapped Grb2-SH3 binding proteins, such as SOS and c-Cbl in the cytosol, which would lead to reduced recruitment of these proteins to the membrane. For example, Grb2-YFP overexpression decreased EGF-dependent activity of Ras and ERK1/2 (unpublished data). Importantly, none of the Grb2 fusion proteins inhibited endocytosis of 125I-transferrin (unpublished data). Therefore, the disruption of Grb2-mediated interactions of EGFR with SH3-binding proteins leads specifically to inhibition of the internalization of endogenous EGFR in HeLa cells.
Grb2 mutants also inhibited EGFR endocytosis when transiently expressed in PAE cells (Figure 7D). The inhibitory effects on endocytosis were less pronounced in these cells, because the transfection efficiency and expression levels of Grb2 fusions were lower in PAE than in HeLa cells. However, single-cell analysis of EGF-Rh endocytosis in PAE cells showed that in cells that express large amounts of SH2-YFP, EGF-Rh was diffusely distributed and not efficiently endocytosed, whereas in untransfected cells EGF-Rh was located mainly in endosomes (Figure 7E).
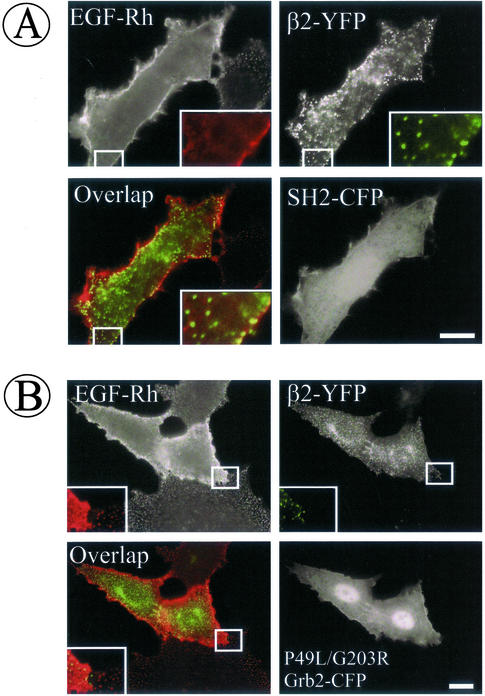
Because SH2 and P49L/G203R-Grb2 mutants produced maximal inhibition of endocytosis, these dominant-interfering constructs were used to test whether the initial stage of endocytosis is affected when Grb2 binding sites of EGFR are blocked. These experiments were performed in PAE cells because they are large, flattened, and have low background fluorescence, thus allowing clear visualization of coated pits and low quantities of EGF-Rh. Cells were transiently transfected with CFP-tagged Grb2 mutants and β2-YFP, and the recruitment of EGF-Rh (2 ng/ml) into coated pits was examined at 4°C as described in Figure 4. In cells overexpressing SH2-CFP or P49L/G203R-Grb2-CFP, EGF-Rh was diffusely distributed on the cell surface (Figure 8). Very few clusters of EGF-Rh overlapped with β2-YFP in punctate structures. In contrast, EGF-Rh displayed typical punctate appearance in untransfected cells or cells that express moderate levels of Grb2 mutants, reminiscent of the pattern of EGF-Rh localization in coated pits shown in Figures 4 and 5. Altogether, the results of Grb2 overexpression experiments support the data of EGFR mutagenesis in that Grb2 binding sites of the EGFR are important during early stages of clathrin-dependent endocytosis of EGFR.
Figure 8.
Grb2 mutants inhibit EGFR recruitment into coated pits. Wild-type EGFR-expressing PAE cells were transiently transfected with β2-YFP and SH2-CFP (A) or P49L/G203R-Grb2-CFP (B). Two days after transfection, the cells were incubated with 2 ng/ml EGF-Rh at 4°C for 1 h and fixed, and the images were acquired through Cy3, YFP, and CFP filter channels. Insets: High magnification images of the small regions of the cell shown by white rectangles. Bars, 10 μm.
Grb2 Binding Sites Are Essential for Binding of Cbl to EGFR-containing Organelles and Polyubiquitylation of the Receptor
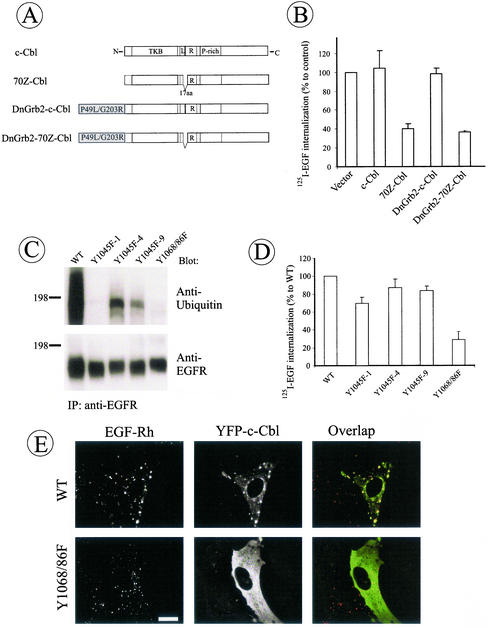
Several lines of evidence point to the specific role of Grb2 in EGFR internalization. Grb2 binds to several proteins through its SH3 domains (Figure 7B). One of these proteins, Cbl is an E3 ubiquitin ligase responsible for ubiquitylation of the EGFR and implicated in the regulation of EGFR trafficking (Levkowitz et al., 1999). The importance of Cbl for EGFR internalization through rapid pathway is demonstrated in Figure 9. Overexpression of 70Z deletion mutant of c-Cbl, YFP-70Z-Cbl, which is incapable of promoting ubiquitylation of the EGFR (Levkowitz et al., 1999), significantly inhibited 125I-EGF internalization in HeLa cells (Figure 9B).
Figure 9.
Grb2 binding sites are important for EGFR ubiquitylation and recruitment of c-Cbl. (A) Schematic representation of wild-type c-Cbl and 70Z c-Cbl mutant (70Z-Cbl) fusion proteins and their hybrid proteins with P49L/G203R Grb2 mutant. YFP was attached to the carboxyl-terminus of proteins (unpublished data). Tyrosine kinase binding domain (TKB), linker (L), RING finger domain (R), and proline-rich (P-rich) domain are indicated in c-Cbl. A part of the linker and the first amino acid residue of the RING domain (17 a.a.) are deleted in 70Z-Cbl. (B) HeLa cells were transiently transfected with YFP-tagged c-Cbl, 70Z-Cbl, DnGrb2-c-Cbl, or DnGrb2–70Z-Cbl, and the internalization of 125I-EGF was measured as in Figures 2 and 7. (C) Wild-type EGFR (WT), Y1045F or Y1068/86F mutant–expressing PAE cells were incubated with EGF (20 ng/ml) for 2 min at 37°C (conditions of maximal ubiquitylation of EGFR) and lysed, and EGFRs were precipitated using antibody 528. The immunoprecipitates were resolved by electrophoresis and then probed by Western blotting with ubiquitin antibody and anti-EGFR (antibody 2319). (D) The internalization rates of 125I-EGF in three single-cell clones of Y1045F EGFR mutant expressed in PAE cells were compared with these rates of cells expressing wild-type EGFR and Y1068/1086F mutant. The internalization was measured as in Figure 2. (E) Wild-type and Y1068/86F cells were transiently transfected with YFP-c-Cbl; after 2 d of expression, the cells were incubated with EGF-Rh (1 ng/ml, WT; 20 ng/ml, Y1068/86F) for 6 min at 37°C. After fixation of the cells, rhodamine and YFP images were acquired as described in Figure 4. Bar, 10 μm.
If Cbl physically or functionally links EGFR-Grb2 complex to the endocytic machinery, direct recruitment of Cbl to the EGFR should be sufficient for receptor internalization. Indeed, fusion of c-Cbl to the carboxyl-terminus of P49L/G203R-Grb2 (DnGrb2-c-Cbl-YFP chimera) reversed the dominant-negative effect of P49L/G203R-Grb2-YFP on EGFR internalization (Figure 9B). In contrast, the chimera of dominant-negative Grb2 and 70Z-Cbl (DnGrb2–70Z-Cbl) imposed a strong inhibitory effect on EGFR internalization. Similar blocking effects of 70Z-Cbl and its Grb2 chimera were observed in PAE cells. Although the data obtained with Cbl mutants and Grb2-Cbl chimeras may have several explanations, one possible interpretation is that the recruitment of c-Cbl to the EGFR through Grb2 SH2 domain is sufficient to support clathrin-dependent endocytosis of the receptor.
Interestingly, Western blot analysis revealed negligible levels of poly/multiubiquitylation of the Y1068/86F receptor mutant in PAE cells (Figure 9C), suggesting that binding of Cbl to this receptor could be impaired. We have not been able to detect specific coimmunoprecipitation of endogenous c-Cbl with EGFR activated with low concentrations of EGF, suggesting that the receptor-Cbl complexes are relatively unstable in detergent solutions under conditions of our experiments. Therefore, we used an indirect method to examine EGFR-Cbl interactions—visualization of Cbl recruitment to EGFR-containing endosomes. To this end, PAE cells expressing wild-type EGFR were transiently transfected with YFP-c-Cbl. YFP-c-Cbl was diffusely distributed in cells not treated with EGF. When cells were stimulated with EGF-Rh (1 ng/ml) for 30 min, YFP-c-Cbl accumulated in endosomes containing EGF-Rh (Figure 9E). To load endosomes with internalization-defective Y1068/86F mutant, the endocytosis through the slow clathrin-independent pathway was induced by high concentrations of EGF-Rh (20 ng/ml). However, no detectable association of YFP-c-Cbl with endosomes containing Y1068/86F mutant was observed (Figure 9C). Thus, Grb2 binding sites in the EGFR are important for the efficient recruitment of Cbl to the receptor-containing organelles and receptor polyubiquitylation.
The direct binding of Cbl to EGFR is thought to be mediated by Tyr1045 of the receptor (Levkowitz et al., 1999). In PAE cells, mutation of this residue (Y1045F) significantly affected ubiquitylation of the EGFR as observed in the case of Y1068/86F mutant (Figure 9C). However, Y1045F mutation had no visible effect on recruitment of YFP-c-Cbl to endosomes in EGF-stimulated cells (unpublished data) and only slightly reduced 125I-EGF internalization (Figure 9D). These experiments suggest that the maximal extent of receptor polyubiquitylation requires Cbl binding to both EGFR-associated Grb2 and receptor Tyr1045 and that the rate of EGFR internalization does not correlate with the extent of polyubiquitylation of the receptor. Altogether, data presented in Figure 9 suggest that the recruitment of Cbl to EGFR may be an important function of Grb2 in endocytosis.
DISCUSSION
The ligand-induced internalization of EGFR has been one of the most popular experimental systems for studying receptor-mediated endocytosis. Several mechanisms of EGFR internalization through clathrin-coated pits have been proposed and a number of protein-protein interactions have been implicated in the regulation of this process (Wang and Moran, 1996; Barbieri et al., 2000; Confalonieri et al., 2000; Sakaguchi et al., 2001; Soubeyran et al., 2002). Because many of these protein-receptor interactions involve receptor phosphotyrosines, the models proposed in above-mentioned studies contradicted to the previous observations that EGFR mutants lacking any detectable tyrosine phosphorylation are, nonetheless, internalized as efficiently as native EGFRs (Chen et al., 1989; Chang et al., 1991). The common explanation of these inconsistencies is that there are multiple redundant pathways of EGFR internalization through coated pits, some of which depend on receptor phosphorylation and others that do not.
To resolve this discrepancy, we took advantage of PAE cells that do not support internalization of a C′1022 mutant lacking major tyrosine phosphorylation sites (Figure 2). In contrast, C′1022 and similar mutants are rapidly internalized in mouse fibroblast NIH 3T3 and B82 cells (Chen et al., 1989; Chang et al., 1991; Sorkina et al., 2002), suggesting that an additional pathway mediates internalization in the absence of EGFR tyrosine phosphorylation in these cells. Unlike studies in other cells, analysis of EGFR mutants in PAE cells now clearly reveals the importance of Grb2 binding sites, Tyr1068 and Tyr1086, for rapid internalization of EGF-receptor complexes (Figure 3). The inherent problem in interpretation of mutagenesis data is the possibility of multiple effects of mutations, such as, conformational changes in parts of the molecule distant from the mutation sites. In our experiments, the very low internalization rates observed for the full-length receptors, bearing only two conservative substitutions of tyrosines by phenylalanines, imply that this defective internalization is unlikely to be caused by unexpected conformational changes in the EGFR molecule. This conclusion is supported by the observation that mutations of Tyr1068 and Tyr1086 also abolish internalization of various truncated receptor mutants. Another caveat of the interpretation of mutagenesis experiments is the possibility that proteins other than Grb2 bind to Tyr1068 and Tyr1086. Although such interactions have not been demonstrated in vivo in extensive studies of the EGFR over the years, this possibility cannot be formally ruled out.
Therefore, other experimental approaches, in which we tested the effects of Grb2 depletion and Grb2 mutant overexpression on EGFR endocytosis, were used to complement EGFR mutagenesis studies. The rationale for the experiments with Grb2 RNA interference and Grb2 mutants stems from the hypothesis that a Grb2-dependent mechanism operates in cells other than PAE, including cells with endogenous EGFRs, but that alternative mechanisms have made this Grb2-dependent pathway difficult to observe. In support of our hypothesis, low endocytic rates were observed in HeLa and PAE cells, where Grb2 protein was depleted by RNA interference (Figure 6).
The clathrin-mediated internalization and the transition of EGFR through coated pits were not completely abolished in cells with depleted Grb2, probably, because full depletion of Grb2 could not be achieved despite extensive optimization of the procedure and testing a number of different siRNAs. It is also possible that there is another pathway of EGFR endocytosis in HeLa cells that is independent of Grb2, analogous to the pathway operating in NIH 3T3 cells expressing truncated EGFRs. Nevertheless, RNA interference experiments provided the first direct evidence of the importance of Grb2 and, in fact, any protein in EGFR endocytosis. In contrast, depletion of Eps15 (Figure 6) or c-Cbl (unpublished data), both implicated in EGFR endocytosis, by RNA interference had no effect on the rate of 125I-EGF internalization, presumably, because of the presence of Cbl-b/Cbl-3 and Eps15R, sequence and functional homologues of c-Cbl and Eps15, respectively. Likewise, EGFR endocytosis was essentially unaffected in cells lacking Shc A or in cells expressing EGFR mutants lacking Shc binding sites Tyr1148 and Tyr1173 (Figure 3 and unpublished data).
The general role of Grb2 in EGFR endocytosis is supported by the observation of the inhibitory effects of Grb2-targeted siRNA and dominant-negative Grb2 mutants on EGF internalization not only in PAE cells but also in HeLa cells (Figure 7). Overexpression of Grb2 mutants reduced 125I-EGF internalization to a low level in HeLa cells. Considering that only a fraction of the total cell population transiently expressed these mutants at levels sufficiently high to inhibit endocytosis, it can be suggested that the Grb2-dependent pathway is the major route of endocytosis of the endogenous EGFR. This conclusion is further supported by single-cell analyses demonstrating substantial inhibition of EGF-Rh endocytosis in PAE cells overexpressing SH2 domain of Grb2 (Figure 7E).
What is the function of Grb2 in endocytosis? Because Grb2 binds to EGFR immediately upon receptor activation, it is logical to propose that Grb2 can participate in the early stages of internalization. To examine the localization of EGFR relative to coated pit proteins, we developed an assay allowing quantitative analysis of the distribution of EGF-Rh and β2-adaptin at the cell surface at 4°C, conditions permitting coated pit recruitment but restricting endocytosis of receptors (Sorkina et al., 2002). Colocalization experiments with EGFR mutants demonstrated that Tyr1068 and Tyr1086 are important for the efficient interaction of EGFRs with coated pits (Figure 4). This conclusion is supported by the observation that overexpression of dominant-negative Grb2 mutants that occupy these phosphotyrosine motifs significantly abolished colocalization of EGF-Rh and β2-labeled coated pits (Figure 8). Altogether, these experiments suggest that Grb2 binding sites participate in coated pit recruitment of EGFRs. Our experiments, however, cannot rule out the possibility that Grb2 is also involved in later stage(s) of internalization, such as, coat invagination or coated vesicle fission.
A role for Grb2 in EGFR endocytosis in MDCK cells has been previously proposed based on the observation of the inhibition of EGF uptake by microinjected Grb2 mutant proteins, although the stage of endocytosis regulated by Grb2 (internalization or recycling) was not determined (Wang and Moran, 1996). The latter study postulated that Grb2 binding to dynamin mediates endocytosis of EGFR. Recently, it has been proposed that Grb2 partially mediates binding of c-Cbl to the EGFR (Waterman et al., 2002). Our experiments showed that Cbl is indeed important for rapid internalization of EGFR and that Grb2 binding sites of the EGFR are essential for c-Cbl binding to the receptor and for receptor polyubiquitylation in vivo (Figure 9). The association of EGFR with c-Cbl is thought to promote receptor downregulation through either c-Cbl interaction with CIN85 or c-Cbl–mediated ubiquitylation of the EGFR (Soubeyran et al., 2002; Waterman et al., 2002). Although E3 ubiquitin ligase function of Cbl is important for EGFR internalization, high rates of endocytosis of the EGFR mutant Y1045F that was polyubiquitylated at a negligible extent suggest that receptor polyubiquitylation is not essential for clathrin-dependent endocytosis (Figure 9; Waterman et al., 2002). However, the presence of monoubiquitin moieties on the Y1045F receptor mutant cannot be ruled out due to the limited sensitivity of ubiquitin immunoblotting detection. Thus, it is possible that binding of Cbl proteins to EGFR through Grb2 and Tyr1045 allows monoubiquitylation of the EGFR and/or other associated proteins, which can mediate receptor interaction with coated pit proteins containing ubiquitin-interaction motifs.
The interaction of Grb2 with SOS, a key component in activation of Ras, may also play role in EGFR endocytosis. Activation of Rab5a by Ras was implicated in EGFR internalization, although the precise function of Rab5 in clathrin-mediated endocytosis remains unclear (Barbieri et al., 2000). It is possible that Ras may regulate EGFR endocytosis by activating Rab5a in close proximity to EGFR, thus facilitating formation of the receptor endocytic complexes.
The ability of Grb2 to couple receptor tyrosine kinases to several SH3-binding signal tranducers can explain multiple functions of Grb2 in various signal transduction pathways. For instance, the proposed role of Grb2 in clathrin-independent endocytosis of EGFR (Yamazaki et al., 2002) may be related to the ability of Grb2 to mediate EGFR signaling to actin cytoskeleton (She et al., 1997). The importance of Grb2 for essential cellular processes may be the reason why Grb2 concentrations are so tightly controlled in the cell. Targeted mutation of mouse Grb2 gene leads to early embryonic lethality that even precludes the generation of immortalized embryonic fibroblast cell lines from the knock-out animals (Cheng et al., 1998). To our knowledge, no constitutively expressing cell lines with exogenous wild-type Grb2 or its mutants have been developed, and all attempts to generate such cell lines have also failed in our own experiments.
To conclude, we propose here that Grb2 plays a major role in at least one of the mechanisms of EGFR internalization through coated pits. Because the down-stream event that mediates Grb2-dependent EGFR recruitment to coated pits has not been precisely characterized, there is still a possibility that this same event, for instance EGFR monoubiquitination, could be triggered through Grb2-independent mechanisms. Experiments are currently in progress to further analyze the role of Grb2-Cbl interaction in ubiquitylation of the EGFR and components of clathrin coat and to elucidate the role of this process in the recruitment of EGFR into coated pits and vesicle budding.
ACKNOWLEDGMENTS
We thank Drs. L Beguinot for Dc165 NIH 3T3 cells and antibody 2913; Levkowitz and Yarden for Cbl cDNA; and M. Dell'Acqua for critical reading of the manuscript. This work was supported by grants CA089151 from National Cancer Institute, RPG-00–247-01-CSM from the American Cancer Society, and BC995456 from the Department of Defense.
Abbreviations used:
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- CFP and YFP
cyan and yellow fluorescent protein
- EGF-Rh
EGF conjugated to rhodamine
- G418
geneticin
- SDS-PAGE
sodium-dodecyl-sulfate PAGE
Footnotes
DOI: 10.1091/mbc.E02–08–0532.
REFERENCES
- Alvarez CV, Shon K-J, Miloso M, Beguinot L. Structural requirements of the epidermal growth factor receptor for tyrosine phosphorylation of eps8 and eps15, substrates lacking Src SH2 homology domains. J Biol Chem. 1995;270:16271–16276. doi: 10.1074/jbc.270.27.16271. [DOI] [PubMed] [Google Scholar]
- Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:39–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguinot L, Werth D, Ito S, Richert N, Willingham MC, Pastan I. Functional studies on the EGF receptor with an antibody that recognizes the intracellular portion of the receptor. J Biol Chem. 1986;261:1801–1807. [PubMed] [Google Scholar]
- Carter RE, Sorkin A. Endocytosis of functional epidermal growth factor receptor- green fluorescent protein chimera. J Biol Chem. 1998;273:35000–35007. doi: 10.1074/jbc.273.52.35000. [DOI] [PubMed] [Google Scholar]
- Chang C-P, Kao JPY, Lazar CS, Walsh BJ, Wells A, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced internalization and increased cell calcium are Mediated via distinct structural elements in the carboxyl terminus of the epidermal growth factor receptor. J Biol Chem. 1991;266:23467–23470. [PubMed] [Google Scholar]
- Chang C-P, et al. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993;268:19312–19320. [PubMed] [Google Scholar]
- Chen WS, et al. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- Confalonieri S, Salcini AE, Puri C, Tacchetti C, Di Fiore PP. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J Cell Biol. 2000;150:905–912. doi: 10.1083/jcb.150.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Gossen M, Freundlieb S, Bujard H, Schmid SL. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–220. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Jiang X, Sorkin A. Coordinated trafficking of Grb2 and Ras during EGF receptor endocytosis visualized in living cells. Mol Biol Cell. 2002;13:1522–1535. doi: 10.1091/mbc.01-11-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1 [In Process Citation] Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Lund KA, Opresko LK, Strarbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–13723. [PubMed] [Google Scholar]
- Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutani T, Okabayashi Y, Kido Y, Sugimoto Y, Sakaguchi K, Matuoka K, Takenawa T, Kasuga M. Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J Biol Chem. 1994;269:31310–31314. [PubMed] [Google Scholar]
- Sakaguchi K, Okabayashi Y, Kasuga M. Shc mediates ligand-induced internalization of epidermal growth factor receptors. Biochem Biophys Res Commun. 2001;282:54–1160. doi: 10.1006/bbrc.2001.4680. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- She HY, Rockow S, Tang J, Nishimura R, Skolnik EY, Chen M, Margolis B, Li W. Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells. Mol Biol Cell. 1997;8:1709–1721. doi: 10.1091/mbc.8.9.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Helin K, Waters CM, Carpenter G, Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. J Biol Chem. 1992;267:8672–8678. [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM, Overholser KA, Carpenter G. Multiple autophosphorylation site mutations of the epidermal growth factor receptor. J Biol Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- Sorkina T, Huang F, Beguinot L, Sorkin A. Effect of tyrosine kinase inhibitors on clathrin coated pit recruitment and internalization of epidermal growth factor receptor. J Biol Chem. 2002;277:27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moran MF. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002;21:303–313. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS. Anomalous binding of epidermal growth factor to A431 cells Is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Zaal K, Hailey D, Presley J, Lippincott-Schwartz J, Samelson LE. Role of Grb2 in EGF-stimulated EGFR internalization. J Cell Sci. 2002;115:1791–1802. doi: 10.1242/jcs.115.9.1791. [DOI] [PubMed] [Google Scholar]