Abstract
The γ-tubulin ring complex (γTuRC), consisting of multiple protein subunits, can nucleate microtubule assembly. Although many subunits of the γTuRC have been identified, a complete set remains to be defined in any organism. In addition, how the subunits interact with each other to assemble into γTuRC remains largely unknown. Here, we report the characterization of a novel γTuRC subunit, Drosophila gamma ring protein with WD repeats (Dgp71WD). With the exception of γ-tubulin, Dgp71WD is the only γTuRC component identified to date that does not contain the grip motifs, which are signature sequences conserved in γTuRC components. By performing immunoprecipitations after pair-wise coexpression in Sf9 cells, we show that Dgp71WD directly interacts with the grip motif–containing γTuRC subunits, Dgrips84, 91, 128, and 163, suggesting that Dgp71WD may play a scaffolding role in γTuRC organization. We also show that Dgrips128 and 163, like Dgrips84 and 91, can interact directly with γ-tubulin. Coexpression of any of these grip motif–containing proteins with γ-tubulin promotes γ-tubulin binding to guanine nucleotide. In contrast, in the same assay Dgp71WD interacts with γ-tubulin but does not facilitate nucleotide binding.
INTRODUCTION
γ-Tubulin is a ubiquitous and highly conserved centrosomal protein that is essential for microtubule (MT) function (Wiese and Zheng, 1999; Oakley, 2000). Early studies showed that disruption of γ-tubulin function resulted in various defects in MT assembly and lethality to the cell (Oakley and Oakley, 1989; Oakley et al., 1990; Horio et al., 1991; Joshi et al., 1992; Sobel and Synder, 1995; Sunkel et al., 1995). This led to the hypothesis that γ-tubulin is involved in microtubule nucleation at the centrosomes. Recent studies of γ-tubulin in Schizosaccharomyces pombe, Caenorhabditis elegans, and Aspergillus nidulans suggest that γ-tubulin is also involved in MT organization and spindle assembly (Paluh et al., 2000; Prigozhina et al., 2001; Strome et al., 2001). Therefore, γ-tubulin appears to have roles in MT organization in addition to regulating microtubule nucleation.
Higher eukaryotes contain centrosomal γ-tubulin and a significant pool of cytosolic γ-tubulin in the form of large protein complexes (Wiese and Zheng, 1999). The cytosolic γ-tubulin does not appear to exist as monomers in organisms examined so far, suggesting that it does not function alone. One γ-tubulin containing complex, the γ-tubulin ring complex (γTuRC), has been purified from Xenopus, Drosophila, and humans (Zheng et al., 1995; Oegema et al., 1999; Murphy et al., 2001). γTuRCs from these organisms share a similar structure and protein composition, and they can nucleate MTs in vitro (Zheng et al., 1995; Oegema et al., 1999; Murphy et al., 2001). Additional biochemical and structural studies reveal that γTuRC is recruited to the centrosome to mediate MT nucleation (Moritz et al., 1995a, 1995b; Martin et al., 1998; Schnackenberg et al., 1998).
In addition to the γTuRC, the cytosolic γ-tubulin in Drosophila embryos and Xenopus eggs exists in a smaller complex known as the γ-Tubulin Small Complex (γTuSC; Oegema et al., 1999; Zhang et al., 2000). The Drosophila γTuSC is a tetramer of ∼10 S composed of two γ-tubulin molecules and one each of Dgrips84 and 91 (Oegema et al., 1999). The γTuSC subunits are the most abundant proteins of the γTuRC and are thought to form the functional and structural core of the γTuRC (Oegema et al., 1999; Gunawardane et al., 2000). In addition, the γTuSC appears to be the most conserved unit of the γTuRC because its homologues show significant sequence conservation across species (Wiese and Zheng, 1999; Gunawardane et al., 2000). Indeed, the Tub4p complex, the cytosolic γ-tubulin complex found in Saccharomyces cerevisiae, is similar in size and composition to Drosophila γTuSC (Geissler et al., 1996; Knop and Schiebel, 1997; Vinh et al., 2002). Whereas the Tub4p of the Tub4p complex is a homolog of γ-tubulin, each of the other two subunits of the Tup4p complex, Spc97p and Spc98p, share significant homology to Dgrips84 and 91, respectively (Geissler et al., 1996; Knop and Schiebel, 1997; Oegema et al., 1999; Vinh et al., 2002).
To date five human and five Drosophila γTuRC subunits in addition to γ-tubulin have been identified (Murphy et al., 1998; Fava et al., 1999; Oegema et al., 1999; Gunawardane et al., 2000; Murphy et al., 2001). Sequence analysis of these γTuRC subunits revealed that they share regions of sequence conservation (Gunawardane et al., 2000; Murphy et al., 2001), which we named grip motifs 1 and 2 (Gunawardane et al., 2000). Electron microscopy (EM) analysis of the Drosophila γTuRC (Oegema et al., 1999; Moritz et al., 2000) showed that the complex consists of two major substructures: the γTuRC ring composed of repeating columnar units (Oegema et al., 1999; Moritz et al., 2000) and a cap structure that covers one face of the γTuRC ring (Moritz et al., 2000). Based on the EM analyses and biochemical characterization of the Drosophila γTuRC (Oegema et al., 1999), a structural model for the γTuRC was proposed (Moritz et al., 2000). According to this model, the modular subunits of the γTuRC ring correspond to multiple γTuSCs and the cap structure is composed of Dgrips75s, 128, and 163 (Oegema et al., 1999; Moritz et al., 2000). Indeed, Xgrip210, the Xenopus homolog of Dgrip163, is localized to the cap structure of the purified Xenopus γTuRC by immunogold EM (Keating and Borisy, 2000; Zhang et al., 2000).
Although the above γTuRC model is appealing, how the subunits interact with each other to assemble into γTuRC remains largely unknown. For example, it is not clear whether the ring of the γTuRC is made of the γTuSCs exclusively. It is also not clear why the subunits found in the cap structure such as Dgrip128 and Dgrip163 contain the conserved grip motifs. Because the two grip motif–containing subunits, Dgrip84 and Dgrip91, interact with γ-tubulin, an important question is whether γ-tubulin also interacts with the other subunits in the γTuRC that contain the grip motifs. The identification of a new γTuRC subunit, Dgp71WD, that does not contain grip motifs allowed us to address some of these important questions.
MATERIALS AND METHODS
Buffers and Reagents
H buffer: 50 mM HEPES, pH 7.4, 1 mM MgCl2, 1 mM EGTA, 1 mM β-mercaptoethanol (β-ME), 0.1 mM GTP, and protease inhibitor stock at 1:200 final dilution as previously described (Oegema et al., 1999; Gunawardane et al., 2000). H100, H150, and H500: H buffer plus 100 mM, 150 mM, or 500 mM NaCl, respectively. Protease inhibitor stock: 10 mM benzamidine-HCl and 0.1 mg/ml phenanthroline, and 1 mg/ml each of aprotinin, leupeptin, and pepstatin A in ethanol. Flag M2 mAb coupled to agarose beads was used to immunoprecipitate the γTuRC subunits that were tagged with Flag (Sigma, St. Louis, MO). Antibodies against γ-tubulin, Dgrips84, 91, 128, and 163 were described previously (Oegema et al., 1999; Gunawardane et al., 2000).
Cloning Dgp71WD
Database searches using internal peptides obtained by microsequencing allowed us to identify a partial EST clone that matched some of the peptide sequences. However, when sequenced, this EST clone did not contain a complete open reading frame (ORF). Using the EST sequence and the sequenced Drosophila genome, we identified a gene whose predicted protein sequence matched 10 of the peptides (KTVLSDFADLE, KTPEIQ, KQSLE, KSEYV, KEFSEL, DFVDQ, KAPLAVR, DEYIAAV, DAAVTRVAFVPVP, IAANLLS). By excising the intervening introns we obtained the full-length coding sequence for a putative γTuRC subunit whose sequence was further confirmed by GENSCAN (www.genes.mit.edu/GENSCAN.html). The full-length cDNA was then cloned using Drosophila embryonic mRNA by RT-PCR and subcloned into the pFASTBAC1 vector (Invitrogen, Carlsbad, CA) for baculovirus expression. Using the Network Protein Sequence Analysis (http://npsa-pbil.ibcp.fr/cgi-bin/secpred_consensus.pl), we found that this protein contained five WD repeats. We refer to this protein as Drosophila gamma ring protein of 71 kDa with WD repeats (Dgp71WD).
Antibody Production and Purification
Three peptides, EKDGKTPEIQRV (aa 59–70), DWETLNRKPOPYETANRQS (aa 399–417), and ENEMLKAKLKFYQEQEQTES (aa 624–643) of Dgp71WD were synthesized and used for antibody production (Zymed, San Francisco, CA). Only one peptide, DWETLNRKPOPYETANRQS (aa 399–417), induced an immune response in rabbits. The antibodies were affinity-purified against the peptide coupled to Sulfo-Link resin (Pierce, Rockford, IL). Antibody against the C-terminal peptide of γ-tubulin (DrosC) was previously described (Oegema et al., 1999; Gunawardane et al., 2000). Antibody against the full-length maternal form of Drosophila γ-tubulin (Dγ2) was raised in rabbit and affinity-purified.
Immunofluorescence
One- to 2-h Drosophila embryos were fixed in methanol and examined by immunofluorescence (Theurkauf, 1992). Double labeling was performed with a mouse mAb against human γ-tubulin (Sigma) and rabbit antibody against Drosophila Dgp71WD, followed by Alexa Red or Green secondary antibodies (Molecular Probes, Eugene, OR). Images were obtained using a cooled CCD camera (Princeton Scientific Instruments, Inc., Monmouth Junction, NJ) on a Nikon E800 microscope and processed using Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Expression and Immunoprecipitation of Proteins
Baculoviruses containing Flag-tagged or untagged γ-tubulin, Dgrips84, 91, 128, 163, and Dgp71WD were used to infect 100 ml cultures of 2 × 106/ml Sf9 cells in different combinations. Each virus was infected at an MOI of ∼3 and the cells were harvested 48–72 h postinfection. Soluble cell lysates were prepared in H150 by sonication (Gunawardane et al., 2001) and centrifugation at 50,000 rpm for 10 min in a TLA120 rotor (Beckman, Palo Alto, CA). The lysates were used for immunoprecipitation with either antibodies against each protein or with Flag-M2 agarose beads (Sigma). For immunoprecipitations, antibodies against the γTuRC subunits were first bound to protein A Affiprep beads (Bio-Rad) for 1 h at room temperature. BSA, 1–2 mg/ml, was used to block the antibody bound beads as well as Flag-M2 beads for at least 2 h at 4°C. After blocking, the beads were washed with H150 and then added to the cell lysates. The lysates were rotated for 1–2 h at 4°C to allow binding of the antibodies to proteins. After incubation, the beads were washed three times each with H150, followed by H500 and then by H150. The proteins that bound to the beads were separated by SDS-PAGE and analyzed by either Coomassie Blue staining or by Western blotting.
GTP Cross-linking
The GTP binding properties of γ-tubulin were assayed as described (Oegema et al., 1999; Gunawardane et al., 2000). Flag-tagged γ-tubulin was expressed alone or coexpressed pair-wise with untagged Dgrips84, 91, 128, 163, or Dgp71WD in 2 × 108 Sf9 cells. The proteins in the soluble cell lysates were immunoprecipitated with 50 μl of settled Flag-M2 agarose beads, washed with buffer containing no GTP, and resuspended in 128 μl BRB80 (80 mM K-PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA. 1 mM PMSF). Sixteen microliters of resuspended beads was incubated with or without 10 μCi of α-32P-GTP (Amersham Pharmacia Biotech Inc.) for 90 min on ice. The samples were then UV cross-linked using a Stratalinker UV (Stratagene, La Jolla, CA) for 5 min, separated by 10% SDS-PAGE, and analyzed by autoradiography.
Sucrose Gradient Sedimentation Analysis of Proteins
Linear gradients of 5–40% sucrose were prepared in H100 with 0.1 mM GTP and 1:200 protease inhibitor stock as previously described (Gunawardane et al., 2001). Zero- to 2-h fly embryo extracts were fractionated on the gradients as described (Oegema et al., 1999).
RESULTS
Dgp71WD Is a Novel γTuRC Subunit that Contains WD repeats
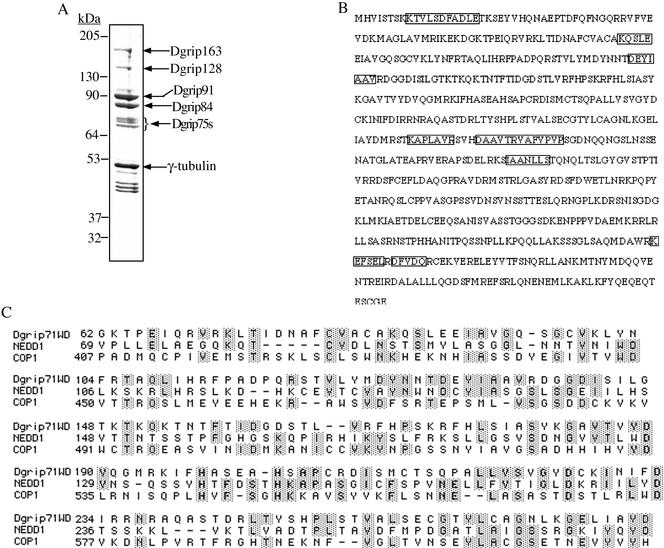
The Drosophila γTuRC consists of ∼8 polypeptides of which γ-tubulin and Dgrips75 (76p), 84, 91, 128, and 163 have been identified and characterized (Fava et al., 1999; Oegema et al., 1999; Gunawardane et al., 2000; also see Figure 1A). Interestingly, all of these Dgrips contain conserved grip motifs (Gunawardane et al., 2000). On the basis of the protein profile of the purified Drosophila γTuRC, we estimated that two to three additional Dgrips with apparent molecular masses of ∼75 kDa remained to be cloned and characterized (Figure 1A). Through database searches, we found that several peptide sequences that we obtained by microsequencing of the purified γTuRC matched a Drosophila partial EST (see MATERIALS AND METHODS). We cloned the full-length cDNA (Figure 1B) and found that it contained five WD repeats (Figure 1C). Therefore, we refer to this novel protein as Dgp71WD, which stands for Drosophila gamma ring protein of 71 kDa with WD repeats (Dgp71WD, accession number AF461267). Interestingly, Dgp71WD did not contain the grip motifs found in all of the other Dgrips identified thus far. Database searches revealed a large number of proteins from various organisms sharing sequence homology with the WD-repeat regions of Dgp71WD. The alignment of the five WD repeats in Dgp71WD with the WD repeats of the Arabidopsis photomorphogenesis repressor COP1 (accession number A44272; Holm et al., 2001) and the mouse Nedd1 (accession number P33215; Kumar et al., 1992) is shown (Figure 1C). Interestingly, the presently uncharacterized mouse Nedd1 is similar in size to Dgp71WD and both proteins have the WD repeats at their N-termini. Pair-wise alignment (GCG Lite at NIH) showed that the overall amino acid identity between Nedd1 and Dgp71WD is 20.8% and that the homology between the two proteins extends beyond the WD repeats. Further studies of Nedd1 will be needed to determine whether it is a mouse homolog of Dgp71WD.
Figure 1.
Dgp71WD contains five putative WD repeats. (A) Subunit composition of the Drosophila γTuRC. Drosophila γTuRC was immunoprecipitated from Drosophila embryo extracts with a peptide antibody against γ-tubulin cross-linked to protein A beads. The immunoprecipitates were separated on a 10% SDS gel and stained with Coomassie Blue (A). The group of proteins with apparent molecular masses of ∼75 kDa is referred to as Dgrip75s and is indicated with a bracket. (B) The amino acid sequence of Dgp71WD. The microsequenced peptides are shown in boxes. (C) Sequence alignment of the five putative WD repeats in Dgp71WD, Nedd1, and COP1 using Clustal W.
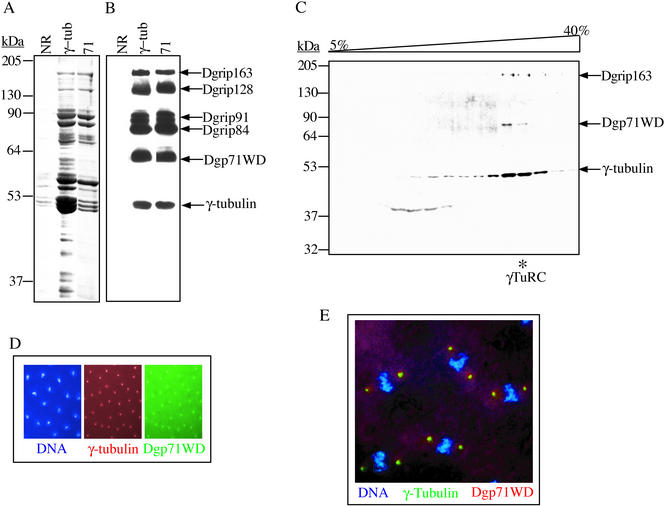
To determine if Dgp71WD is a γTuRC subunit, rabbit polyclonal antibodies against the C-terminal peptide of γ-tubulin (DrosC; Oegema et al., 1999) or against a peptide corresponding to amino acids 399–417 of Dgp71WD were used to immunoprecipitate Dgp71WD from Drosophila embryo extract. We found that anti-Dgp71WD antibody immunoprecipitated the same set of γTuRC subunits as did the anti–γ-tubulin antibody, suggesting that Dgp71WD was a subunit of the γTuRC. Furthermore, the antibody against Dgp71WD recognized a protein of ∼71 kDa in γTuRC immunoprecipitated with γ-tubulin antibodies (Figure 2, A and B). In addition, when Drosophila embryo extracts were subjected to sucrose gradient sedimentation, Dgp71WD comigrated with the other γTuRC subunits (Figure 2C). Because the previously characterized γTuRC subunits localize to the centrosome throughout the cell cycle, we tested if Dgp71WD showed a similar localization pattern. We found that Dgp71WD colocalized with γ-tubulin at centrosomes in Drosophila embryos during interphase and mitosis (Figure 2, D and E, respectively). Taken together, these results showed that Dgp71WD is a subunit of the Drosophila γTuRC.
Figure 2.
Dgp71WD is a γTuRC subunit. (A and B) A peptide antibody raised against Dgp71WD immunoprecipitates the γTuRC. Clarified Drosophila embryo extracts were immunoprecipitated with a control IgG from nonimmunized rabbits (NR) or specific antibodies against γ-tubulin (γ-tub) or Dgp71WD (71). The immunoprecipitates were separated by 10% SDS-PAGE and visualized by Coomassie Blue staining (A) or Western blotting (B), probing with antibodies against the γTuRC subunits as indicated. Because the heavy chain of the rabbit antibodies used for the immunoprecipitations migrated with a similar molecular mass as γ-tubulin, we used mouse mAb (Sigma) against human γ-tubulin (that cross reacts with Drosophila γ-tubulin) to specifically detect γ-tubulin in B. (C) Dgp71WD comigrates with the γTuRC on sucrose gradients. Clarified Drosophila embryo extract was sedimented on a 5–40% sucrose gradient in H100. The resulting fractions were separated by 10% SDS-PAGE and analyzed by Western blotting probing with peptide antibody against Dgp71WD. Antibodies against γ-tubulin and Dgrip163 were used to detect the γTuRC at ∼32 S (γTuRC peak indicated with an asterisk). (D and E) Dgp71WD colocalizes with γ-tubulin on interphase (D) and mitotic (E) centrosomes in Drosophila embryos. 4, 6-Diamino-2-phenylindole (DAPI) was used to visualize DNA, and peptide antibody against Dgp71WD and the mAb against γ-tubulin (Sigma) were used to detect the respective proteins.
Dgp71WD Directly Interacts with the Grip Motif-containing γTuRC Subunits Dgrips84, 91, 128, and 163
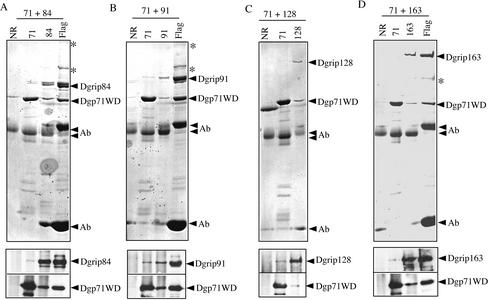
Because Dgp71WD contains WD repeats and no grip motifs, we reasoned that it might play a scaffolding role by binding to the grip motif–containing subunits. If this is true, Dgrips84, 91, 128, and 163 should directly interact with Dgp71WD. To test this possibility, we coexpressed Dgp71WD with each of the Flag-tagged Dgrips84, 91, 163, or untagged Dgrip128, in Sf9 cells. Specific antibodies that recognize Dgp71WD, Dgrips84, 91, 128, 163, or Flag antibody were used to immunoprecipitate each of the proteins. Nonimmunized rabbit IgG was used as a control for each set of immunoprecipitations. The immunoprecipitates were first separated by SDS-PAGE and the proteins were either stained by Coomassie Blue or probed by Western blotting with specific antibodies. We found that the Dgp71WD antibody immunoprecipitated Dgrips84, 91, 128, and 163 (Figure 3, A–D). Conversely, antibodies that immunoprecipitated each of the Dgrips also immunoprecipitated Dgp71WD (Figure 3, A–D). On the basis of these results, we conclude that Dgp71WD interacts with each of the four grip-motif–containing γTuRC subunits.
Figure 3.
Dgp71WD interacts with Dgrips84, 91, 128, and 163. Dgp71WD was coexpressed with Flag-tagged Dgrip84 (A), Flag-tagged Dgrip91 (B), untagged Dgrip128 (C), or Flag-tagged Dgrip163 (D) in Sf9 cells. Antibodies against Dgp71WD (A–D), Flag (A, B, and D), and Dgrips84 (A), 91 (B), 128 (C), and 163 (D) were used to immunoprecipitate the respective proteins from the cell lysates. The immunoprecipitated proteins were fractionated by SDS-PAGE followed by either Coomassie Blue staining (A–D, top panels) or Western blotting (A–D, bottom panels). Antibody indicates the heavy and light chains of the antibody molecules on the Coomassie Blue–stained gels. The heavy chain of the Flag mAb migrated slower than the heavy chains of the rabbit polyclonal antibodies. Asterisks indicate contaminating proteins present in the immunoprecipitations.
Dgp71WD Interacts with γ-Tubulin
Next, we asked whether Dgp71WD also directly interacts with γ-tubulin. We coexpressed Dgp71WD and γ-tubulin in Sf9 cells and used antibodies against each of the proteins for immunoprecipitation. We found that although the antibody against γ-tubulin immunoprecipitated Dgp71WD, the antibody against Dgp71WD did not immunoprecipitate γ-tubulin (Figure 4, A and B). The lack of reciprocal immunoprecipitation could reflect a lack of interaction between γ-tubulin and Dgp71WD. Alternatively, it is possible that Dgp71WD and γ-tubulin do interact with each other, but the binding of this particular antibody to Dgp71WD disrupts this interaction. The disruption is possible if the Dgp71WD antibody bind to the region of Dgp71WD involved in its binding to γ-tubulin.
Figure 4.
Dgp71WD interacts with γ -tubulin. (A and B) Dgp71WD and γ-tubulin were coexpressed in Sf9 cells and then immunoprecipitated with antibodies against Dgp71WD (71), γ-tubulin (γ-tub), or nonimmunized rabbit IgG (NR). The immunoprecipitates were fractionated by 10% SDS-PAGE and then analyzed either by Coomassie Blue staining (A) or by Western blotting, probing with the antibody against Dgp71WD and the mAb against human γ-tubulin (Sigma). (C) Quantification of the expression levels of Dgp71WD and γ-tubulin. Dgp71WD and γ-tubulin were either expressed alone or together in Sf9 cells. The cell lysates were analyzed by Western blotting and probing with antibodies against Dgp71WD and γ-tubulin. (D) Two antibodies against γ-tubulin, DrosC, and Dγ2, the rabbit nonimmunized IgG (NR), and the antibody against Dgp71WD were used for immunoprecipitation of the lysates in C. The immunoprecipitates were analyzed by Western blotting and probing with antibodies against Dgp71WD and the mAb against human γ-tubulin.
We reasoned that if γ-tubulin and Dgp71WD do interact with each other, we should be able to show this interaction using different antibodies against γ-tubulin that do not interfere with this interaction. We expressed Dgp71WD and γ-tubulin either alone or together in Sf9 cells. Western blotting showed that similar amounts of Dgp71WD or γ-tubulin were expressed under either individual-expression or coexpression conditions (Figure 4C). We used the DrosC and Dγ2 antibodies raised against the C-terminal peptide of γ-tubulin and the full-length γ-tubulin, respectively, to immunoprecipitate γ-tubulin. As controls, nonimmunized rabbit IgG (NR) and the antibody against Dgp71WD were used. We found that NR did not immunoprecipitate γ-tubulin or Dgp71WD as expected (Figure 4D). Consistent with the earlier result (Figure 4, A and B), the Dgp71WD antibody did not immunoprecipitate γ-tubulin even when both Dgp71WD and γ-tubulin were coexpressed (Figure 4D). Importantly, the DrosC and Dγ2 antibodies did not immunoprecipitate Dgp71WD in the absence of γ-tubulin (Figure 4D). This result demonstrated that these antibodies against γ-tubulin did not nonspecifically bind to Dgp71WD. We found that when γ-tubulin and Dgp71WD were coexpressed, the two antibodies against γ-tubulin could immunoprecipitate both γ-tubulin and Dgp71WD (Figure 4D). On the basis of these results, we concluded that Dgp71WD and γ-tubulin directly interact with each other.
γ-Tubulin Interacts Directly with the Grip Motif–containing Subunits Dgrip128 and 163
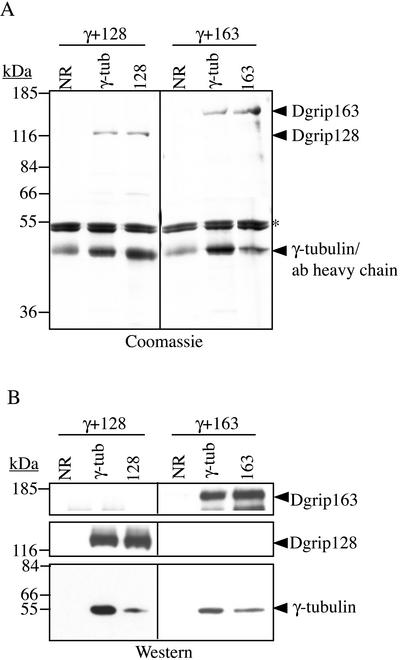
We hoped to determine whether γ-tubulin interacted with Dgrips128 and 163, two grip motif–containing subunits thought to be part of the cap structure. γ-Tubulin was coexpressed with either Dgrip128 or Dgrip163. Antibodies against each of the Dgrips and γ-tubulin were used to do reciprocal immunoprecipitations as described above. The immunoprecipitates were analyzed by SDS-PAGE followed by Coomassie Blue staining as well as by Western blotting. We found that the antibody against γ-tubulin immunoprecipitated γ-tubulin, Dgrip128, and Dgrip163, whereas the antibodies against Dgrip128 and Dgrip163 immunoprecipitated themselves and γ-tubulin (Figure 5, A and B). Therefore, γ-tubulin interacts directly with Dgrips128 and 163. Taken together, the above findings suggest that each of the four grip motif–containing subunits interact directly with Dgp71WD and γ-tubulin.
Figure 5.
γ-Tubulin interacts directly with Dgrips128 and 163. γ-Tubulin was coexpressed with either Dgrip128 or Dgrip163 in Sf9 cells and immunoprecipitated with control antibody (NR) or specific antibodies against γ-tubulin (γ-tub), Dgrip128 (128), or Dgrip163 (163). The immunoprecipitated proteins were analyzed by 10% SDS-PAGE followed by Coomassie Blue staining (A) or by Western blotting (B) probing with antibodies against the γTuRC subunits. Nonspecific proteins that immunoprecipitate with all three antibodies are marked with an asterisk. Mouse mAb (Sigma) against human γ-tubulin was used to detect γ-tubulin in B.
Dgp71WD, Unlike the Grip Motif–containing γTuRC Components, Does Not Facilitate γ-Tubulin Binding to GTP
We next hoped to develop an assay to compare the functional consequences of the binding of Dgp71WD to γ-tubulin with that of the grip motif–containing proteins. Because γ-tubulin in γTuRC and γTuSC can bind to guanine nucleotides (Oegema et al., 1999; Gunawardane et al., 2000), we reasoned that the nucleotide binding of γ-tubulin could be dependent on its interaction with other γTuRC subunits. Therefore, we asked whether γ-tubulin expressed alone or together with Dgrip84 or Dgrip91 could bind to GTP. Using the UV cross-linking assay developed previously (Oegema et al., 1999), we found that γ-tubulin when expressed alone and purified, did not bind to GTP (Figure 6, A–C). However, when γ-tubulin was coexpressed with either Dgrip84 or Dgrip91 in Sf9 cells and purified, it was able to bind to GTP in the cross-linking assay (Figure 6, A–C). As expected, γ-tubulin in the baculovirus reconstituted γTuSC also binds to GTP (Gunawardane et al., 2000 and this study, Figure 6, A–C). These findings suggest that the interactions between the Dgrip84 and Dgrip91 with γ-tubulin facilitate γ-tubulin binding to GTP.
Figure 6.
γ-Tubulin binds GTP when coexpressed with Dgrip84 or Dgrip91. Flag-tagged γ-tubulin expressed alone or coexpressed with Dgrip84 and/or Dgrip91 was immunoprecipitated with Flag antibody-bound agarose beads. The immunoprecipitates were incubated with α-32P-GTP, UV cross-linked, and separated by 10% SDS-PAGE. The gel was first stained with Coomassie Blue to visualize the proteins (A), dried, and then subjected to autoradiography to detect the α-32P-GTP (B). Asterisk in A indicates a contaminating protein present in Sf9 cells. The presence of γ-tubulin in the cross-linking reactions was further confirmed by Western blotting, probing with antibodies against γ-tubulin (C).
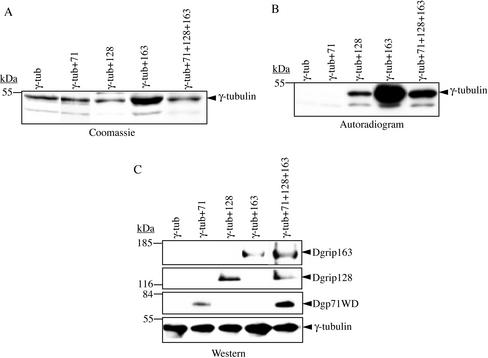
The above finding prompted us to ask whether Dgp71WD, Dgrip128, and Dgrip163 could also facilitate γ-tubulin to bind to GTP. γ-Tubulin was coexpressed with Dgp71WD, Dgrip128, or Dgrip163, isolated, and assayed for GTP binding using the UV cross-linking assay as above. The expression levels of Dgrip128 and Dgrip163 were about the same as that of Dgp71WD in these experiments (unpublished data). We found that γ-tubulin bound to GTP when coexpressed with either Dgrip128 or Dgrip163, but not when coexpressed with Dgp71WD (Figure 7, A–C). Furthermore, γ-tubulin could bind to GTP when coexpressed with all three subunits (Figure 7, A–C), suggesting that Dgp71WD did not inhibit GTP binding. These studies suggested that the interaction between γ-tubulin and the grip motif–containing subunits could facilitate γ-tubulin binding to GTP, but the interaction between γ-tubulin and Dgp71WD did not. From these studies, we conclude that the nature of the interaction of Dgp71WD with γ-tubulin differs from that of the grip motif–containing γTuRC subunits.
Figure 7.
Dgp71WD does not facilitate the binding of γ-tubulin to GTP, whereas Dgrips128 and 163 do. Flag-tagged γ-tubulin was expressed alone or coexpressed with one or all three of the untagged Dgrip128, Dgrip163, and Dgp71WD and immunoprecipitated with Flag-antibody bound agarose beads. After washing with buffer containing no GTP, the immunoprecipitates were incubated with α-32P-GTP, UV cross-linked, and separated by 10% SDS-PAGE. The gel was stained by Coomassie Blue to visualize γ-tubulin (A), dried, and subjected to autoradiography to detect α-32P-GTP (B). (C) Western blotting of the immunoprecipitates confirmed the presence of γ-tubulin, Dgrips128, Dgrip163, and Dgrip71WD in these experiments.
DISCUSSION
To understand the function of γTuRC, it is important to identify all of its subunits and study their interactions. We report here the identification of Dgp71WD, a new γTuRC subunit that does not have grip motifs. We found that both Dgp71WD and γ-tubulin interacted directly with the subunits containing grip motifs. These findings shed new light on the structural organization of the γTuRC.
Sequence analyses suggested that there are two additional Drosophila grip motif–containing proteins with predicted molecular masses of 79.8 kDa (AAF50536) and 223 kDa (AAF44968; Murphy et al., 2001). The putative 79.8-kDa protein, which we will refer to as Dgrip79 hereafter, may correspond to one of the uncharacterized γTuRC subunits in the ∼75-kDa molecular mass range (Figure 1A). However, the putative 223-kDa protein may not be an integral subunit of the Drosophila γTuRC, because all the Drosophila γTuRC subunits are smaller than 223 kDa. If Dgrip79 is a γTuRC subunit and if our estimation of a total of eight subunits in γTuRC is accurate, we may have identified a complete set of Drosophila γTuRC subunits.
Interestingly, six (Dgrips75, 79, 84, 91, 128, and 163) of eight γTuRC subunits contain the conserved grip motifs. This sequence conservation suggests that the Dgrips interact with common proteins either within or outside of the γTuRC. We showed that, consistent with this idea, Dgrips84, 91, 128, and 163 directly interacted with γ-tubulin and Dgp71WD, two γTuRC subunits with no grip motifs (Gunawardane et al., 2000 and this study). It will be important to determine whether the grip motifs in the Dgrips mediate these interactions.
The finding that all Dgrips interact with γ-tubulin directly is intriguing because it suggests that the Dgrips75, 79, 128, and 163 that were originally thought to be the cap subunits, directly contact γ-tubulin in the γTuRC. If this is the case, the current γTuRC model, which hypothesizes that the γTuRC ring consists exclusively of γTuSCs (Oegema et al., 1999; Moritz et al., 2000), would need to be revised. We speculate that each of the Dgrips75, 79, 128, and 163 could form dimers with γ-tubulin molecules. These dimers along with several γTuSCs may be required to form the ring of the γTuRC.
Structural studies revealed that WD repeats form a β-propeller fold that could mediate protein-protein interactions (Smith et al., 1999). We suggest that Dgp71WD could provide a scaffold via its WD-repeats to tether all of the Dgrips together in the γTuRC. Consistent with this idea, we found that four Dgrips (84, 91, 128, and 163) interact directly with Dgp71WD. Clearly, further structural and biochemical studies are necessary to determine whether some or all of the Dgrips75, 79, 128, and 163 participate in the formation of the γTuRC ring. Also, it is important to determine whether and how any of these Dgrips participate in the formation of the cap structure of the γTuRC.
We found that coexpressing any one of the grip motif–containing subunits, Dgrips84, 91, 128, and 163, with γ-tubulin was sufficient to promote γ-tubulin to bind to GTP. However, coexpressing γ-tubulin with Dgp71WD, which does not contain grip motifs, did not facilitate γ-tubulin binding to GTP. This finding showed that the interactions between γ-tubulin and Dgrips have a significantly different consequence from the interaction between γ-tubulin and Dgp71WD. Furthermore, it suggests that the grip motif–containing subunits play a role in regulating the GTP binding properties of γ-tubulin. Because GTP is important for α- and β-tubulin function, we suspect that Dgrips may be important for γ-tubulin function. Consistent with this idea, we found that γ-tubulin expressed alone could not incorporate into γTuRC in vitro; but coexpressing one of the Dgrips with γ-tubulin was sufficient for the incorporation (unpublished data).
Previously, human and Chlamydomonas γ-tubulins expressed alone using rabbit reticulocyte lysate and baculovirus expression systems, respectively, were used to study the microtubule binding and nucleating activities of γ-tubulin (Li and Joshi, 1995; Vassilev et al., 1995; Leguy et al., 2000). However, whether the γ-tubulins could bind to guanine nucleotides was not determined (Li and Joshi, 1995; Vassilev et al., 1995; Leguy et al., 2000). Because we found that γ-tubulin expressed alone did not bind GTP, caution should be used in analyzing the function of γ-tubulin in the absence of Dgrips.
The interaction we observed between Dgp71WD and γ-tubulin should also be interpreted cautiously. Because γ-tubulin found in γTuRC and γTuSC can be cross-linked to GTP, the inability of γ-tubulin to bind to GTP when coexpressed with Dgp71WD may indicate that γ-tubulin does not assume the same structural conformation as in the γTuRC. One possibility is that the γ-tubulin coexpressed with Dgp71WD was not folded properly. If so, the interaction between Dgp71WD and γ-tubulin that we observed may not reflect a true interaction in the γTuRC. Alternatively, it is possible that the γ-tubulin expressed with Dgp71WD was folded correctly, but assumed a slightly different conformation to disfavor GTP binding in our in vitro assays. If so, the interaction between Dgp71WD and γ-tubulin is likely to reflect a true interaction in the γTuRC. Structural studies of γTuRC will be important to resolve whether γ-tubulin directly contact Dgp71WD in the complex.
ACKNOWLEDGMENTS
We thank the members of the Zheng lab for helpful discussions during the course of this work. We also express our sincere appreciation to the editor and anonymous reviewers for helpful scientific and editorial suggestions. This work was supported by National Institutes of Health grant RO1-GM56312-01 and the Pew Scholar's Award to Y.Z.
Abbreviations used:
- γTuRC
γ-tubulin ring complex
- γTuSC
γ-tubulin small complex
- Dgrip
Drosophila gamma ring protein
- β-ME
β-mercaptoethanol
- MT
microtubule
- Dgp71WD
Drosophila gamma ring protein of 71 kDa with WD repeats
- NR
nonimmunized rabbit IgG
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0034. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0034.
REFERENCES
- Fava F, et al. Human 76p: a new member of the γ-tubulin-associated protein family. J Cell Biol. 1999;147:857–868. doi: 10.1083/jcb.147.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler S, Pereira G, Spang A, Knop M, Soues S, Kilmartin J, Schiebel E. The spindle pole body component Spc98p interacts with the gamma-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J. 1996;15:3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Gunawardane NR, Martin OC, Cao K, Zhang L, Dej K, Akihiro I, Zheng Y. Characterization and reconstitution of Drosophila gamma tubulin ring complex subunits. J Cell Biol. 2000;151:1513–1523. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane RN, Zheng Y, Oegema K, Wiese C. Purification, and reconstitution of Drosophila gamma tubulin complexes. Methods Cell Biol. 2001;67:1–25. doi: 10.1016/s0091-679x(01)67002-x. [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, Deng XW. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 2001;20:118–127. doi: 10.1093/emboj/20.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Keating D, Borisy G. Immunostructural evidence for the template mechanism of microtubule nucleation. Nat Cell Biol. 2000;2:352–357. doi: 10.1038/35014045. [DOI] [PubMed] [Google Scholar]
- Knop M, Schiebel E. Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 1997;16:6985–6995. doi: 10.1093/emboj/16.23.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;30:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- Leguy R, Melki R, Pantaloni D, Carlier M. Monomeric γ-tubulin nucleates microtubules. J Biol Chem. 2000;275:21975–21980. doi: 10.1074/jbc.M000688200. [DOI] [PubMed] [Google Scholar]
- Li Q, Joshi H. Gamma-tubulin is a minus end-specific microtubule binding protein. J Cell Biol. 1995;131:207–214. doi: 10.1083/jcb.131.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O, Gunawardane R, Iwamatsu A, Zheng Y. Xgrip109: a γ-tubulin-associated protein with an essential role in γ-tubulin ring complex (γTuRC) assembly and centrosome function. J Cell Biol. 1998;141:675–687. doi: 10.1083/jcb.141.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld M, Guenebaut V, Heuser J, Agard D. Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat Cell Biol. 2000;2:365–370. doi: 10.1038/35014058. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld M, Sedat J, Alberts B, Agard D. γ-Tubulin-containing rings in the centrosome. Nature. 1995a;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Fung JC, Sedat JW, Alberts BM, Agard DA. 3D structural characterization of centrosomes from early Drosophila embryos. J Cell Biol. 1995b;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Urbani L, Stearns T. The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, Preble AM, Patel UK, O'Connell KL, Dias DP, Moritz M, Agard D, Stults JT, Stearns T. GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol Biol Cell. 2001;12:3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BR. Gamma-tubulin. Curr Topics Dev Biol. 2000;49:27–54. doi: 10.1016/s0070-2153(99)49003-9. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Oakley CE, Yoon Y, Jung MK. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oegema K, Wiese C, Martin O, Milligan R, Iwamatsu A, Mitchison T, Zheng Y. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh JL, Nogales E, Oakely BR, McDonald K, Pidoux AL, Cande WZ. A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol Biol Cell. 2000;11:1225–1239. doi: 10.1091/mbc.11.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina N, Walker R, Oakley C, Oakley B. Gamma-tubulin and the C-terminal motor domain kinesin-like protein, KLPA, function in the establishment of spindle bipolarity in Aspergillus nidulans. Mol Biol Cell. 2001;12:3161–3174. doi: 10.1091/mbc.12.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg B, Khodjakov A, Rieder C, Palazzo R. The disassembly and reassembly of functional centrosomes in vitro. Proc Natl Acad Sci USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Sobel S, Synder M. A highly divergent gamma-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol. 1995;131:1775–1788. doi: 10.1083/jcb.131.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol Biol Cell. 2001;12:1751–1764. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel C, Gomes R, Sampaio P, Perdigao J, Gonzalez C. Gamma-tubulin is required for the structure and function of the microtubule organizing center in Drosophila neuroblasts. EMBO J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE. Behavior of structurally divergent alpha tubulin isotypes during Drosophila embryogenesis: evidence for post-translational regulation of isotype abundance. Dev Biol. 1992;154:205–217. doi: 10.1016/0012-1606(92)90060-t. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kimble M, Silflow C, LaVoie M, Kuriyama R. Identification of intrinsic dimer and overexpressed monomeric forms of gamma-tubulin in Sf9 cells infected with baculovirus containing the Chlamydomonas gamma-tubulin sequence. J Cell Sci. 1995;108:1083–1092. doi: 10.1242/jcs.108.3.1083. [DOI] [PubMed] [Google Scholar]
- Vinh DB, Kern JW, Hancock WO, Howard J, Davis TN. Reconstitution, and characterization of budding yeast gamma-tubulin. Complex Mol Biol Cell. 2002;13:1144–1157. doi: 10.1091/mbc.02-01-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. Gamma-Tubulin complexes and their interaction with microtubule-organizing centers. Curr Opin Struct Biol. 1999;9:250–259. doi: 10.1016/S0959-440X(99)80035-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Keating TJ, Wilde A, Borisy GG, Zheng Y. The role of Xgrip210 in gamma tubulin ring complex assembly and centrosome recruitment. J Cell Biol. 2000;151:1525–1535. doi: 10.1083/jcb.151.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wong M, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]