Abstract
Caveolin-1 is the principal structural component of caveolae microdomains, which represent a subcompartment of the plasma membrane. Several independent lines of evidence support the notion that caveolin-1 functions as a suppressor of cell transformation. For example, the human CAV-1 gene maps to a suspected tumor suppressor locus (D7S522/7q31.1) that is frequently deleted in a number of carcinomas, including breast cancers. In addition, up to 16% of human breast cancers harbor a dominant-negative mutation, P132L, in the CAV-1 gene. Despite these genetic associations, the tumor suppressor role of caveolin-1 still remains controversial. To directly assess the in vivo transformation suppressor activity of the caveolin-1 gene, we interbred Cav-1 (−/−) null mice with tumor-prone transgenic mice (MMTV-PyMT) that normally develop multifocal dysplastic lesions throughout the entire mammary tree. Herein, we show that loss of caveolin-1 gene expression dramatically accelerates the development of these multifocal dysplastic mammary lesions. At 3 wk of age, loss of caveolin-1 resulted in an approximately twofold increase in the number of lesions (foci per gland; 3.3 ± 1.0 vs. 7.0 ± 1.2) and an approximately five- to sixfold increase in the total area occupied by these lesions. Similar results were obtained at 4 wk of age. However, complete loss of caveolin-1 was required to accelerate the appearance of these dysplastic mammary lesions, because Cav-1 (+/−) heterozygous mice did not show any increases in foci development. We also show that loss of caveolin-1 increases the extent and the histological grade of these mammary lesions and facilitates the development of papillary projections in the mammary ducts. Finally, we demonstrate that cyclin D1 expression levels are dramatically elevated in Cav-1 (−/−) null mammary lesions, consistent with the accelerated appearance and growth of these dysplastic foci. This is the first in vivo demonstration that caveolin-1 can function as a transformation suppressor gene.
INTRODUCTION
Caveolae are 50–100-nm plasma membrane microdomains that function in vesicular trafficking and signal transduction (Galbiati et al., 2001). Approximately 10 years ago, the principal structural component of caveolae was identified as a 21- to 24-kDa integral membrane protein and termed caveolin (Glenney and Zokas, 1989; Rothberg et al., 1992) (now referred to as caveolin-1) (Scherer et al., 1996).
Caveolin-1 is expressed in numerous cell types, including fibroblasts, adipocytes, smooth muscle cells, endothelial cells, and epithelial cells (Razani et al., 2001). Interestingly, caveolin-1 was first described as a major substrate of the v-Src tyrosine kinase in Rous sarcoma virus-transformed fibroblasts, suggesting that caveolin-1 may be a target for modification or inactivation by activated oncogenes (Glenney, 1989). Subsequent cell culture experiments demonstrated that caveolin-1 mRNA and protein expression levels are dramatically down-regulated in NIH 3T3 cells transformed by a number of activated oncogenes, including v-Abl, Bcr-abl, H-RasG12V, and polyomavirus middle T antigen (PyMT); in addition, the ability of these transformed cells to grow in soft agar was inversely correlated with caveolin-1 expression levels (Koleske et al., 1995). These findings initially suggested the hypothesis that caveolin-1 may function as a tumor/transformation suppressor protein.
To stringently test this hypothesis, we and others reexpressed caveolin-1 in oncogenically transformed NIH 3T3 cells and other human tumor-derived cell lines. Interestingly, recombinant expression of caveolin-1 inhibited tumor cell proliferation and was found to dramatically attenuate anchorage-independent growth (Engelman et al., 1997; Lee et al., 1998; Razani et al., 2000; Zhang et al., 2000; Park et al., 2001; Fiucci et al., 2002). Conversely, antisense mediated ablation of caveolin-1 expression was sufficient to induce the transformation of NIH 3T3 cells. NIH 3T3 cells harboring the caveolin-1 antisense vector underwent anchorage-independent growth in soft agar, formed tumors in nude mice, and showed hyperactivation of the Ras-p42/44 mitogen-activated protein (MAP) kinase cascade (Galbiati et al., 1998). Importantly, this phenotype was reversible, because loss of the caveolin-1 antisense vector restored caveolin-1 to normal levels and reverted the transformed phenotype of these NIH 3T3 cell lines. Thus, in this cellular context, it seems that loss of caveolin-1 expression is sufficient to induce cellular transformation in cultured cells. Similarly, it has been reported that caveolin-1 levels are down-regulated in a number of human breast cancer cell lines and in tumors derived from transgenic mouse models of breast cancer (Sager et al., 1994; Engelman et al., 1998b; Lee et al., 1998; Suzuki et al., 1998; Razani et al., 2000; Zhang et al., 2000).
What is known about the human caveolin-1 gene? Interestingly, CAV-1 is localized to the D7S522 locus in the q31.1 region of human chromosome 7 (Engelman et al., 1998c,d,e, 1999). This chromosomal region (D7S522/7q31.1) is a suspected tumor suppressor locus and a common fragile site (known as FRA7G) that is frequently deleted in a number of human cancers (Zenklusen et al., 1994a,b, 1995; Kerr et al., 1996; Shridhar et al., 1997; Jenkins et al., 1998), including mammary tumors (Zenklusen et al., 1994a,b). Thus, we and others have proposed that caveolin-1 may be the as yet unidentified tumor suppressor gene located at the D7S522/7q31.1 locus (Engelman et al., 1998c,d,e). However, in vivo evidence of the tumor suppressor function of caveolin-1 has not yet been presented.
There is also evidence that caveolin-1 may play an important role in the onset or progression of human breast cancers. We and others have demonstrated that normal mammary epithelial cells express significant levels of caveolin-1, by using cultured human mammary epithelial cells and mouse mammary tissue sections (Engelman et al., 1998b,c, 1999; Lee et al., 1998; Sager et al., 1994). Sager and colleagues identified caveolin-1 as one of 26 genes down-regulated in human mammary adenocarcinoma-derived cells through differential display and subtractive hybridization techniques (Sager et al., 1994). Independently, Lee et al. (1998) demonstrated that a number of human breast cancer cell lines, including T47D cells, demonstrate a significant reduction in caveolin-1 expression levels, compared with normal mammary epithelial cells. Additionally, they showed that reintroduction of caveolin-1 in T47D cells resulted in a ∼50% reduction in cellular proliferation and an ∼15-fold reduction in the ability of these cells to form colonies in soft agar (Lee et al., 1998). Furthermore, adenoviral-mediated expression of caveolin-1 in a metastatic mammary epithelial cell line (MTLn3) dramatically inhibited EGF-stimulated lamellipod extension, cell migration, and anchorage-independent growth (Zhang et al., 2000). Similarly, Fiucci et al. (2002) recently showed that recombinant expression of caveolin-1 in another human breast cancer cell line, namely MCF-7 cells, decreases their ability to form colonies in soft agar and inhibits their capacity for matrix invasion.
Using genetic screening of primary human breast cancer samples, Hayashi et al. (2001) detected a novel sporadic mutation (P132L) in the CAV-1 gene in up to 16% of tumors examined. Importantly, recombinant expression of Cav-1 (P132L) in NIH 3T3 cells was sufficient to induce cellular transformation and hyper-activate the p42/44 MAP kinase cascade, thus, mimicking the phenotype previously observed by expression of antisense caveolin-1 (Galbiati et al., 1998). In support of these findings, we have recently shown that Cav-1 (P132L) behaves in a dominant-negative manner, causing the intracellular retention of wild-type caveolin-1 at the level of the Golgi complex (Lee et al., 2002).
We and others have now generated caveolin-1 (−/−) deficient mice by targeted gene disruption (Drab et al., 2001; Razani et al., 2001). This technological advance allows exploration of the in vivo role of caveolin-1 in the context of a whole-organismal model. Interestingly, the first reports on Cav-1 null mice describe the hyperproliferation of mouse embryo fibroblasts in culture and lung hypercellularity due to the presence of an increased number of endothelial cells (Drab et al., 2001; Razani et al., 2001), consistent with the idea that caveolin-1 normally functions as a negative regulator of cell growth (Galbiati et al., 2001). However, in the absence of other genetic alterations or carcinogenic treatments, Cav-1 null mice do not display an increased incidence of spontaneous tumors compared with wild-type mice, at up to 9 mo of age (Razani et al., 2001).
Recently, we have noted two novel phenotypes in the mammary glands of Cav-1 null mice in the C57Bl/6 background: 1) mild mammary epithelial cell hyperplasia in virgin mice (Lee et al., 2002), and 2) precocious lactation in pregnant mice (Park et al., 2002). During pregnancy, we have shown that caveolin-1 null mice demonstrate accelerated development of the lobulo-alveolar compartment, premature lactation, and hyperactivation of the Jak-2/STAT5a signaling cascade (Park et al., 2002). However, it remains unknown whether Cav-1 null mice are more susceptible to tumor formation induced by treatments with chemical agents or by breeding with tumor-prone animals, such as the mouse mammary tumor virus (MMTV)-PyMT mice.
Herein, we examine the role of caveolin-1 in the early steps of mammary tumor formation by interbreeding Cav-1 (−/−) null mice with MMTV-PyMT mice to generate MMTV-PyMT/Cav-1 (−/−) mice, all in the FVB/N background. We show that complete loss of caveolin-1 gene expression dramatically accelerates the appearance and growth of multifocal dysplastic lesions in a very early period of mammary gland development, defined as the ductal stage (2–5 wk) (Cardiff et al., 2000). These dysplastic changes are typically associated with the development of tumors (Maglione et al., 2001).
This report is the first demonstration that loss of caveolin-1 gene expression in vivo can dramatically accelerate dysplastic cellular growth/transformation in a given cell type.
MATERIALS AND METHODS
Materials
Mouse monoclonal antibodies that specifically recognize total STAT5a and activated STAT5a [phopho-STAT5a (pY694)] were purchased from Transduction Laboratories (Lexington, KY). Rabbit polyclonal antibodies directed against total extracellular signal-regulated kinase (ERK)-1/2 and activated phospho-ERK-1/2 were obtained from Cell Signaling Technology (a subsidiary of New England Biolabs, Beverly, MA). The cyclin D1 rabbit polyclonal antibody was purchased from NeoMarkers (Fremont, CA). An anti-pan-cytokeratin rabbit polyclonal antibody (BioGenex, San Ramon, CA) was used to equalize for epithelial cell content during immunoblot analysis.
Animal Studies
All animals were housed and maintained in a barrier facility at the Institute for Animal Studies (Albert Einstein College of Medicine, Bronx, NY). Cav-1 null mice were generated as described previously (Razani et al., 2001). However, all the Cav-1 null mice used in this study were in the FVB/N background. Transgenic FVB/N mice expressing the polyoma middle T antigen under the control of an MMTV long terminal repeat promoter (PyMT) were as described previously (Guy et al., 1992). All matings were performed with male mice heterozygous for the PyMT transgene. First, PyMT/Cav-1 (+/−) male mice were generated by crossing PyMT/Cav-1 (+/+) male mice with Cav-1 (−/−) female mice. Then, PyMT/Cav-1 (+/−) male mice were interbred with Cav-1 (+/−) female mice, yielding PyMT/Cav-1 (+/+), PyMT/Cav-1 (+/−), and PyMT/Cav-1 (−/−) mice. Offspring were genotyped by polymerase chain reaction of genomic DNA derived from tail clippings. Genotyping for Cav-1 was performed as described previously (Razani et al., 2001). Genotyping for the PyMT transgene was performed according to Jackson Laboratories (Bar Harbor, ME) protocols. All mice analyzed in this study were virgin females. In addition, all the PyMT transgenic mice studied were heterozygous for the PyMT transgene. Animal protocols used for this study were approved by the Albert Einstein College of Medicine Institute for Animal Studies.
Whole-Mount Analysis of Mammary Glands
Fourth (inguinal) mammary glands were excised, spread onto glass slides, fixed, and stained essentially as we described previously (Park et al., 2002). Briefly, glands were fixed in Carnoy's fixative (6 parts 100% ethyl alcohol:3 parts CHCl3:1 part glacial acetic acid) for 2–4 h at room temperature. The samples were then washed in 70% ethyl alcohol for 15 min and changed gradually to distilled water. Once hydrated, the mammary squashes were stained overnight in carmine alum (1 g of carmine, C1022; Sigma-Aldrich, St. Louis, MO) and 2.5 g of aluminum potassium sulfate (A7167; Sigma-Aldrich) in 500 ml of distilled water. The samples were then dehydrated using stepwise ethanol concentrations and left in xylenes to clear the fat. Mammary squashes were stored in methyl salicylate. Whole-mounts were digitally photographed with a ruler on a stereomicroscope, by using the same magnification and lighting conditions. Total area measurements for the dysplastic foci were quantified using NIH Image J 1.27 software. Whole-mount analysis at each time point was performed with female littermate mice.
Histological Analysis of Mammary Tissue
Fourth (inguinal) mammary glands were excised, formalin fixed for 24 h, and embedded in paraffin. Sections were cut at 5 μm, stained with hematoxylin and eosin, and evaluated by an experienced histopathologist. Analyses and descriptions were performed in accordance with the guidelines put forth by the mammary gland pathology consensus meeting in Annapolis (Cardiff et al., 2000).
Immunoblot Analysis
Mice were sacrificed and the mammary fat pads were isolated. Tissue samples were then homogenized in an appropriate volume of lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 60 mM octyl glucoside), containing protease inhibitors (Roche Diagnostics, Indianapolis, IN). Tissue lysates were then centrifuged at 12,000 × g for 10 min to remove insoluble debris. Protein concentrations were analyzed using the bicinchoninic acid reagent (Pierce Chemical, Rockford, IL) and the volume required for 20 μg of protein was determined. Samples were then separated by SDS-PAGE (10% acrylamide) and transferred to nitrocellulose. The nitrocellulose membranes were stained with Ponceau S (to visualize protein bands), followed by immunoblot analysis. All subsequent wash buffers contained 10 mM Tris pH 8.0, 150 mM NaCl, 0.05% Tween 20, which was supplemented with 1% bovine serum albumin (BSA) and 2% nonfat dry milk (Carnation, Solon, OH) for the blocking solution and 1% BSA for the antibody diluent. Primary antibodies were used at a 1:500 dilution. Horseradish peroxidase-conjugated secondary antibodies were used to visualize bound primary antibodies with the Supersignal chemiluminescence substrate (Pierce Chemical).
Immunohistochemisty
Paraffin-embedded mammary glands were sectioned at 5 μm. Sections were then deparaffinized first by treatment with xylene for 3 min (2×) and rehydrated by passage through a graded series of ethanol. Antigen retrieval was performed by microwaving the slides in 100 mM sodium citrate buffer for 15 min. Endogenous peroxide activity was quenched by incubating the slides for 10 min in 1% H2O2. Slides were then washed in phosphate-buffered saline (PBS) and blocked with a solution containing 10% horse serum, 1% BSA, 0.1% Triton X-100 in PBS for 1 h at room temperature. Samples were washed with PBS and incubated with the primary antibody in blocking solution for 12–16 h at 4°C. Slides were then washed with PBS (three washes; 5 min each) and incubated with a biotinylated secondary antibody in blocking solution for 30 min at room temperature. Slides were further washed in PBS (three washes; 5 min each) and incubated with the avidin/biotin-horseradish peroxidase reagent for 30 min at room temperature. Next, samples were washed in PBS and incubated with the diaminobenzidine reagent until color production developed. Finally, the slides were washed in distilled H2O to remove excess diaminobenzidine, counterstained with hematoxylin, dehydrated, and mounted with coverslips.
RESULTS
MMTV-PyMT Mice as a Model System for Spontaneous Breast Cancer
Polyomavirus middle T antigen transgenic mice (PyMT) provide an ideal model to study the effect of a given gene product on early tumorigenesis, because multifocal dysplastic foci develop in the mammary epithelium of PyMT mice as early as 3 wk of age and eventually progress to adenocarcinomas (Guy et al., 1992).
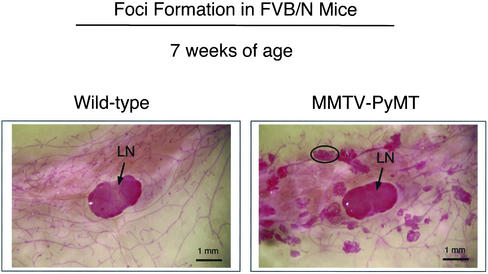
Whole-mount preparations are a well-established and recommended method to identify early premalignant lesions of the mammary epithelium (Cardiff et al., 2000). These lesions have also been termed mammary intraepithelial neoplasia (MIN) or hyperplastic atypias and are thought to be the lesions which develop into tumors (Maglione et al., 2001). These dysplastic lesions are present in the majority of mammary glands by 3 wk of age in PyMT mice and spread throughout the entire mammary fat pad by 7 wk of age (Figure 1).
Figure 1.
Whole-mount analysis of the mammary glands of wild-type and PyMT mice in the FVB/N background. At 7 wk of age, the right fourth (inguinal) mammary glands were excised from virgin female mice, spread onto glass slides, fixed, and stained with carmine alum. Note the widespread appearance of multifocal dysplastic foci in PyMT FVB/N mice. A single dysplastic lesion is encircled. Arrows point at the subiliac lymph node (LN) present in the center of the images. All images were taken at the same magnification. Bar, 1 mm.
Complete Loss of Caveolin-1 Expression Accelerates the Development of Multifocal Dysplastic Lesions in the Mammary Gland
To directly assess the in vivo transformation suppressor activity of the caveolin-1 gene, we interbred Cav-1 (−/−) null mice with tumor-prone PyMT mice. PyMT/Cav-1 (+/−) male mice were first generated by crossing PyMT/Cav-1 (+/+) male mice with Cav-1 (−/−) female mice. Then, PyMT/Cav-1 (+/−) male mice were interbred with Cav-1 (+/−) female mice, yielding PyMT/Cav-1 (+/+), PyMT/Cav-1 (+/−), and PyMT/Cav-1 (−/−) mice. Offspring were genotyped by polymerase chain reaction of genomic DNA derived from tail clippings. All mice analyzed in this study were FVB/N virgin females. In addition, all the PyMT transgenic mice studied were heterozygous for the PyMT transgene.
To determine whether complete loss of caveolin-1 (Cav-1) affected the development of multifocal dysplastic lesions, whole-mount mammary gland analysis was performed on female littermate mice. Mammary glands (four) were harvested from PyMT/Cav-1 (+/+) and PyMT/Cav-1 (−/−) mice at exactly 3 wk of age, fixed in ethanol/acetic acid for 2–4 h, and stained overnight with carmine dye. Virtually identical experiments were also carried out using 4-wk-old virgin female mice.
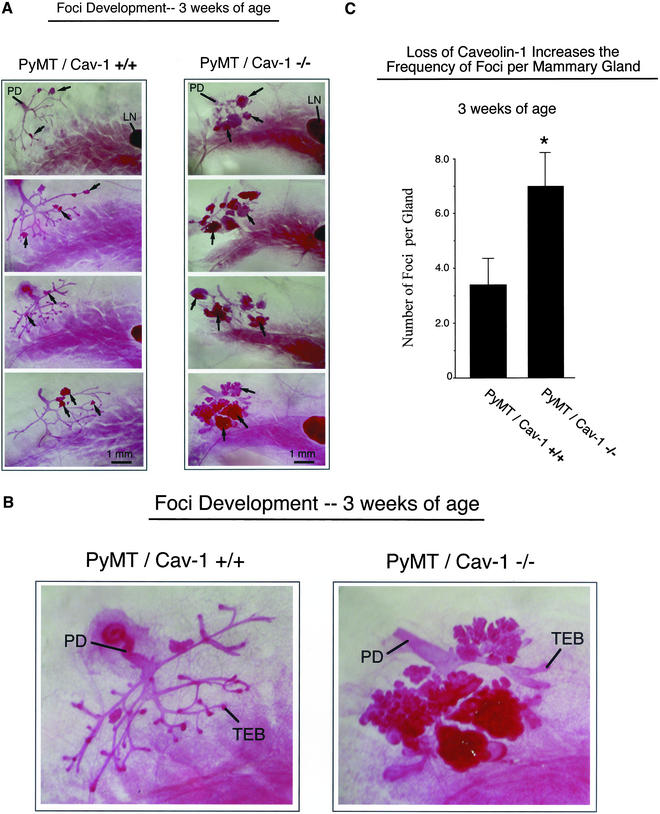
At 3 wk of age, PyMT/Cav-1 (+/+) mice demonstrate small hyperplastic focal atypias in the older portions of the ductal tree originating from the nipple. In contrast, PyMT/Cav-1 (−/−) mice show a striking increase in the frequency and size of these multifocal dysplastic lesions (Figure 2, A and B). Interestingly, at 3 wk of age not all PyMT/Cav-1 (+/+) mammary glands showed the presence of foci, whereas in PyMT/Cav-1 (−/−) mammary glands the incidence of foci was 100%. Quantitation of the number of lesions per gland revealed that loss of caveolin-1 resulted in an approximately twofold increase in the number of lesions; PyMT/Cav-1 (+/+) mice had 3.3 ± 1.0 lesions (n = 6), whereas PyMT/Cav-1 (−/−) mice had 7.0 ± 1.2 lesions (n = 6) (Figure 2C).
Figure 2.
Loss of caveolin-1 gene expression accelerates the development of multifocal dysplastic lesions in the mammary gland (3 wk of age). (A) Mammary glands (inguinal) were harvested from virgin female PyMT/Cav-1 (+/+) (left) and PyMT/Cav-1 (−/−) (right) mice at exactly 3 wk of age, fixed in ethanol/acetic acid for 2–4 h, and stained overnight with carmine dye (original magnification, 5×). Four representative images are shown for each genotype. The primary duct (PD), which originates from the nipple area, is visible in the top left corner of all the images. Arrows point at a few examples of dysplastic foci. Note the increased size and number of the dysplastic foci in the mammary glands of PyMT/Cav-1 (−/−) mice, compared with littermate PyMT/Cav-1 (+/+) mice. The subiliac lymph node (LN) is apparent to the right in many of the images. All images were taken at the same magnification. Bar, 1 mm. (B) Higher magnification (12.5×) views of the early mammary epithelial tree at 3 wk of age, including the primary duct from PyMT/Cav-1 (+/+) (left) and PyMT/Cav-1 (−/−) (right) mice. Terminal end buds (TEB) are present in both genotypes. Both images were taken at the same magnification. (C) Quantitation of the number of lesions per gland revealed that loss of caveolin-1 resulted in an approximately twofold increase in the number of lesions (see asterisk); PyMT/Cav-1 (+/+) mice had 3.3 ± 1.0 lesions (n = 6), whereas PyMT/Cav-1 (−/−) mice had 7.0 ± 1.2 lesions (n = 6).
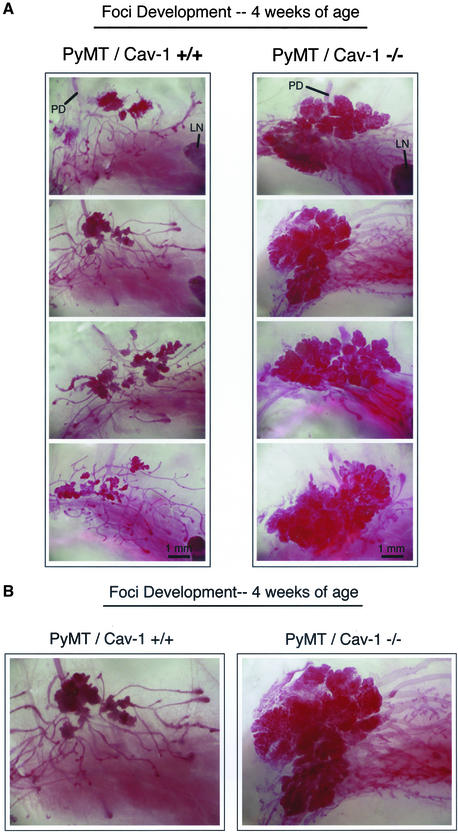
To follow the progression of these early tumorigenic events in the mammary gland, 4-wk-old mice were also examined by whole-mount analysis (Figure 3, A and B). Again, complete loss of caveolin-1 gene expression results in a dramatic increase in the total mass of the mammary lesions [PyMT/Cav-1 (+/+), n = 10; PyMT/Cav-1 (−/−), n = 6]. However, at this time point the number of dysplastic foci per gland cannot be accurately counted because the foci have overgrown, especially in PyMT/Cav-1 (−/−) mice.
Figure 3.
Loss of caveolin-1 gene expression accelerates the development of multifocal dysplastic lesions in the mammary gland (4 wk of age). (A) Mammary glands (inguinal) were harvested from virgin female PyMT/Cav-1 (+/+) (left) and PyMT/Cav-1 (−/−) (right) mice at exactly 4 wk of age, fixed in ethanol/acetic acid for 2–4 h, and stained overnight with carmine dye (original magnification, 5×). Four representative images are shown for each genotype. The primary duct (PD), which originates from the nipple area, is visible in the top region of all the images. The subiliac lymph node (LN) is apparent to the right in several of the images. The mammary epithelial tree has extended to the region of the lymph node in both genotypes. Note the dramatically increased size of the dysplastic areas in the mammary glands of PyMT/Cav-1 (−/−) mice, which seem to have coalesced to form larger singular masses. All images were taken at the same magnification. Bar, 1 mm. (B) Higher magnification (12.5×) views of the early mammary epithelial tree at 4 wk of age, including the primary duct from PyMT/Cav-1 (+/+) (left) and PyMT/Cav-1 (−/−) (right) mice. Both images were taken at the same magnification.
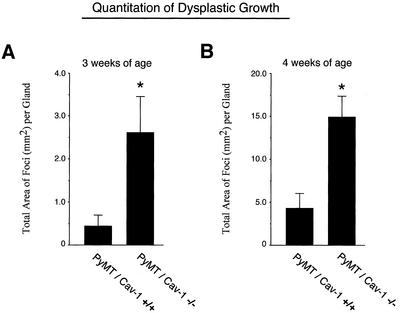
To quantify the growth of these lesions, digital images were acquired and the total area occupied by dysplastic lesions was measured for each mammary gland examined using NIH Image J software. At 3 wk of age, loss of caveolin-1 results in an approximately five- to sixfold increase in the total area of these lesions (Figure 4A). Similarly, at 4 wk of age loss of caveolin-1 leads to an approximately three- to fourfold increase (Figure 4B). Thus, loss of caveolin-1 dramatically stimulates the growth and development of these multifocal dysplastic mammary lesions.
Figure 4.
Quantitation of the growth of dysplastic lesions in PyMT/Cav-1 (+/+) and PyMT/Cav-1 (−/−) mice at 3 and 4 wk of age. To quantify the growth of these lesions, digital images were acquired and the total area occupied by dysplastic lesions was measured for each mammary gland examined using NIH Image software. (A) Mean total area of dysplastic foci per mammary gland at 3 wk. Note that at 3 wk of age, loss of caveolin-1 results in an approximately five- to sixfold increase in the total area of these lesions (see asterisk) [PyMT/Cav-1 (+/+), n = 6; PyMT/Cav-1 (−/−), n = 6]. (B) Mean total area of dysplastic foci per mammary gland at 4 wk. Note that at 4 wk of age, loss of caveolin-1 results in an approximately three- to fourfold increase in the total area of these lesions (see asterisk). [PyMT/Cav-1 (+/+), n = 10; PyMT/Cav-1 (−/−), n = 6]. Thus, loss of caveolin-1 dramatically stimulates the growth and development of these multifocal dysplastic mammary lesions.
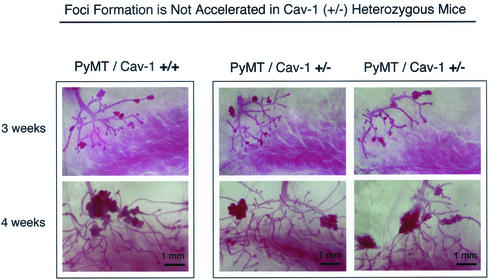
A Partial Caveolin-1 Deficiency Does Not Accentuate the Development of Dysplastic Mammary Lesions
In some mice heterozygous (+/−) for a tumor suppressor gene, such as p53 or INK4a, loss of one allele is sufficient to cause an increased incidence of tumors, either from down-regulated protein expression and/or function or through loss of heterozygosity achieved through somatic mutations (Harvey et al., 1993; Serrano et al., 1996). Thus, we next examined whether loss of a single caveolin-1 allele is sufficient to affect the development of these dysplastic mammary lesions. We have previously demonstrated that Cav-1 (+/−) heterozygote mice show an ∼50% reduction in caveolin-1 protein levels, compared with wild-type Cav-1 (+/+) mice (Razani et al., 2001).
For this purpose, we performed whole-mount analysis on the mammary glands of virgin female PyMT/Cav-1 (+/+) and PyMT/Cav-1 (+/−) mice at 3 and 4 wk of age. Interestingly, PyMT/Cav-1 (+/−) mice do not show any increases in the growth of these dysplastic lesions, compared with PyMT/Cav-1 (+/+) mice (Figure 5). Virtually identical results were obtained at 3 wk of age (Figure 5, top) and 4 wk of age (Figure 5, bottom). Also, total area measurements of the dysplastic foci seen in PyMT/Cav-1 (+/−) mice are exactly within the range measured for PyMT/Cav-1 (+/+) mice (our unpublished data). Thus, loss of both caveolin-1 alleles is required to accelerate the development of these mammary lesions.
Figure 5.
Partial caveolin-1 deficiency does not accentuate the development of dysplastic mammary lesions. Cav-1 (+/−) heterozygote mice show an ∼50% reduction in caveolin-1 protein levels, compared with wild-type Cav-1 (+/+) mice (Razani et al., 2001). Thus, we next examined whether loss of a single caveolin-1 allele is sufficient to affect the development of dysplastic mammary lesions. Mammary glands (inguinal) were harvested from virgin female PyMT/Cav-1 (+/+) (left) and PyMT/Cav-1 (+/−) (right) mice at exactly 3 and 4 wk of age, fixed in ethanol/acetic acid 2–4 h, and stained overnight with carmine dye (original magnification, 5×). Two representative PyMT/Cav-1 (+/−) heterozygote images are shown for each time point, along with one PyMT/Cav-1 (+/+) image for comparison. See Figures 2 and 3 for additional PyMT/Cav-1 (+/+) images. Interestingly, PyMT/Cav-1 (+/−) mice (right) do not show any changes in the size, number, and appearance of dysplastic foci, compared with PyMT/Cav-1 (+/+) mice (left), at either 3 or 4 wk of age. Thus, loss of both caveolin-1 alleles is required to accelerate the development of these mammary lesions. All images were taken at the same magnification. Top, 3 weeks of age; bottom, 4 weeks of age. Bar, 1 mm.
Loss of Caveolin-1 Increases the Extent and the Histological Grade of Mammary Lesions
In addition to following the onset and growth of dysplastic foci, we also determined whether loss of caveolin-1 affects the histological grade of these lesions. Fourth (inguinal) mammary glands were excised, formalin fixed for 24 h, and embedded in paraffin. Sections were cut at 5 μm, stained with hematoxylin and eosin, and evaluated by an experienced histopathologist. Analyses and descriptions were performed in accordance with the guidelines put forth by the mammary gland pathology consensus meeting in Annapolis (Cardiff et al., 2000). For example, herein we use the term MIN to histologically describe these lesions.
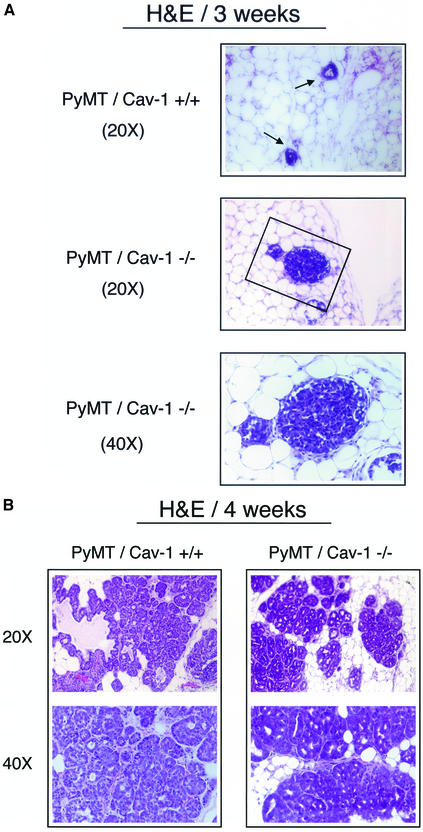
Foci Morphology at 3 wk of Age
PyMT/Cav-1 (+/+) mammary glands show a relatively small number of MIN foci involving the ducts and the terminal ductal lobular units (TDLUs) (Figure 6A, top). These lesions are low grade and are characterized by layers of atypical hyperchromatic epithelial cells with scant cytoplasm.
Figure 6.
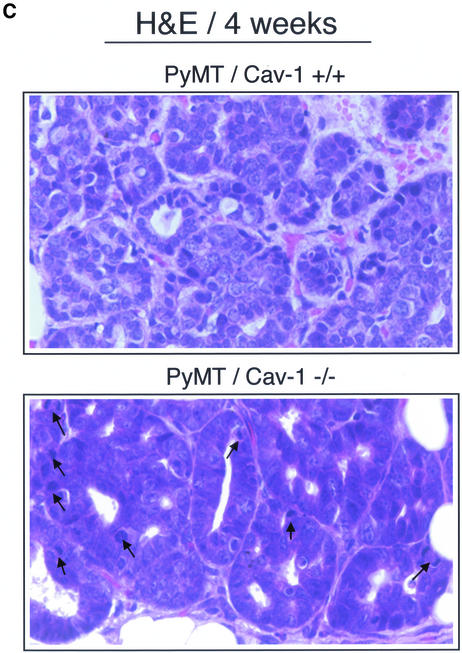
(cont. from facing page). Histological analysis of mammary gland lesions in PyMT/Cav-1 (+/+) and PyMT/Cav-1 (−/−) mice at 3 and 4 wk of age. Fourth mammary glands were excised, formalin fixed, paraffin embedded, sectioned at 5 μm, and counterstained with hematoxylin and eosin (H&E). The images shown are representative for each genotype. (A) Foci morphology at 3 wk of age. PyMT/Cav-1 (+/+) glands display a few small MIN foci involving the ducts (arrows). Note that the lesions are low grade and characterized by only two to three layers of hyperchromatic epithelial cells with scant cytoplasm (top). In contrast, PyMT/Cav-1 (−/−) glands demonstrate the presence of similar MIN lesions, but they are more advanced (middle). The boxed area is shown at higher magnification (bottom). Note that the epithelial lumen is completely obliterated by atypical epithelial cells. (B) Foci morphology at 4 wk of age. PyMT/Cav-1 (+/+) mammary glands contain medium grade MIN lesions with atypia (left). Nuclei are anaplastic with increased mitotic figures (1 and 2) per high-power field. In contrast, PyMT/Cav-1 (−/−) MIN foci seem more advanced than in PyMT/Cav-1 (+/+) mice at the same age (right). The lesions are high grade with marked atypia and show many mitotic figures (up to 20 per high-power field). Low- and medium-power images are shown for each genotype. (C) Mitotic figures. A higher power view of MIN foci in 4-wk-old PyMT/Cav-1 (+/+) and PyMT/Cav-1 (−/−) mice is shown. Arrows point at mitotic figures that are readily apparent in PyMT/Cav-1 (−/−) lesions. Thus, loss of caveolin-1 increases the extent and the histological grade of these mammary lesions.
Interestingly, PyMT/Cav-1 (−/−) mammary glands show similar MIN foci, but these lesions are more extensive than in PyMT/Cav-1 (+/+) mammary glands, with greater involvement of the ducts and TDLUs. For example, in many cases, the PyMT/Cav-1 (−/−) MIN-involved ducts are filled with cells, such that the lumen is no longer apparent (Figure 6A, middle and bottom).
Foci Morphology at 4 wk of Age
There are many more MIN foci present in the PyMT/Cav-1 (+/+) mammary glands than at 3 wk of age. These lesions involve both the ducts and TDLUs and are of medium grade with greater atypia than is present at 3 wk of age (Figure 6B, left). The number of layers of cells is increased and the nuclei seem more anaplastic. There is also an increased mitotic rate, with approximately one to two mitotic figures present per high power field (Figure 6C, top). However, in the ducts, no papillary projections were detected in PyMT/Cav-1 (+/+) mammary glands.
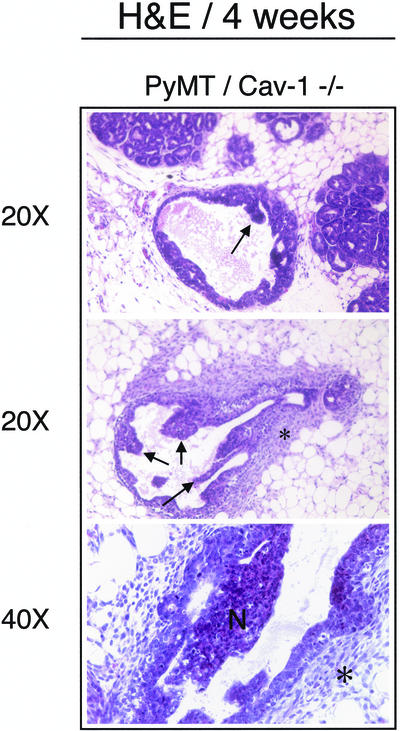
In contrast, the PyMT/Cav-1 (−/−) foci seem more advanced than in PyMT/Cav-1 (+/+) mice at the same age. First, the PyMT/Cav-1 (−/−) lesions are much more widespread. Additionally, these lesions are high grade with marked atypia (Figure 6B, right) and show many mitotic figures (up to 20 per high-power field) (Figure 6C, bottom). There is multifocal involvement of the ducts and the TDLUs with MIN. Furthermore, many papillary projections from the epithelial duct lining are present, with some containing areas of necrosis (Figure 7, top and middle). Moreover, there is increased fibrosis and the presence of inflammatory cells in the form of neutrophils surrounding the involved ducts (Figure 7, bottom). Finally, in PyMT/Cav-1 (−/−) mammary glands, the fatty stromal tissue between the ducts shows more fibrosis than in PyMT/Cav-1 (+/+) mice.
Figure 7.
Additional findings in PyMT/Cav-1 (−/−) mice at 4 wk of age. Note the presence of papillary lesions projecting into the lumen of the hyperplastic epithelial duct lining (top and middle; see arrows). In addition, there is marked fibrosis surrounding the involved ducts, with infiltration by neutrophils (middle and bottom; see asterisks). Finally, some of the papillary projections show areas of necrosis (bottom; indicated by the letter N).
Loss of Caveolin-1 Dramatically Up-Regulates Cyclin D1 Expression in Dysplastic Mammary Lesions
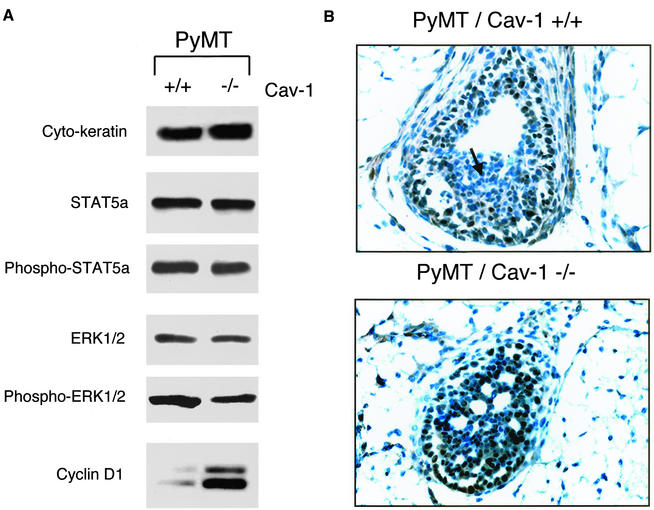
To identify a potential mechanism by which loss of caveolin-1 accelerates the development of dysplastic mammary lesions, we next performed immunoblot analysis on mammary gland tissue samples derived from 4-wk-old PyMT/Cav-1 (−/−) and PyMT/Cav-1 (+/+) mice. Tissue lysates were first normalized using a pan-cytokeratin antibody to ensure that the epithelial content between samples was equivalent.
As caveolin-1 has been previously implicated as a negative regulator of Jak-2/STAT5a signaling (Park et al., 2002) and as a tonic inhibitor of the p42/44 MAP kinase cascade (Engelman et al., 1998a), we first evaluated the activation state of these pathways by using a panel of phospho-specific antibody probes. Figure 8A shows that the levels of total STAT5a and phospho-STAT5a remain unchanged in PyMT/Cav-1 (−/−) samples. Similarly, the levels of total ERK-1/2 and phospho-ERK-1/2 were not elevated in PyMT/Cav-1 (−/−) samples. Thus, hyperactivation of these signaling cascades does not seem to play a role in the accelerated development of dysplastic mammary lesions in Cav-1 (−/−) null mice.
Figure 8.
Loss of caveolin-1 dramatically up-regulates cyclin D1 expression in dysplastic mammary lesions. (A) Immunoblot analysis. Mammary glands were harvested from 4-wk-old PyMT/Cav-1 (−/−) and PyMT/Cav-1 (+/+) mice and homogenized in lysis buffer. Tissue lysates were prepared, separated by SDS-PAGE, and transferred to nitrocellulose membranes. Normalization of epithelial cell content was performed using a pan-cytokeratin antibody. Relative levels of phospho-STAT5a were determined by immunoblotting with a phospho-specific antibody probe that selectively recognizes activated STAT5a at its Jak-2 phosphorylation site (pY694). Similarly, the levels of phospho-ERK-1/2 were determined by immunoblotting with a phospho-specific antibody probe that selectively recognizes activated ERK-1/2 (pT202/pY204). Phospho-independent anti-ERK-1/2 IgG and anti-STAT5a IgG were used as controls for equal loading. Cyclin D1 expression levels were monitored using a specific rabbit polyclonal antibody. Note that cyclin D1 protein expression is dramatically elevated in PyMT/Cav-1 (−/−) samples, compared with matched-samples derived from PyMT/Cav-1 (+/+) mice. However, the levels of total STAT5a and phospho-STAT5a remain unchanged in PyMT/Cav-1 (−/−) samples. Similarly, the levels of total ERK-1/2 and phospho-ERK-1/2 were not elevated in PyMT/Cav-1 (−/−) samples. (B) Immunohistochemistry. To visualize the cellular distribution of cyclin D1, we performed immunohistochemical analysis on dysplastic mammary lesions derived from 4-wk-old PyMT/Cav-1 (−/−) and PyMT/Cav-1 (+/+) mice. Paraffin-embedded mammary glands were sectioned at 5 μm and immunostained with a rabbit polyclonal antibody to cyclin D1. Lesions of approximately the same size from each genotype were chosen to allow for a better comparison. Representative examples are shown. Note that the intensity of cyclin D1 immunostaining (brown color) is clearly increased in the PyMT/Cav-1 (−/−) dysplastic mammary lesions and the nuclei of these dysplastic mammary epithelial cells are more densely stained. The cyclin D1 staining pattern was also noticeably altered in PyMT/Cav-1 (−/−) samples. In PyMT/Cav-1 (+/+) mammary lesions, cyclin D1 immunostaining was confined to the outermost layers of mammary epithelial cells; little or no staining was observed in the center of the lesion (see arrow). In contrast, in PyMT/Cav-1 (−/−) mammary lesions, cyclin D1 immunostaining was present in virtually all the epithelial cells and extended to the center of the lesion. No changes in cyclin D1 immunostaining were observed in the surrounding stromal cells.
Another possible mechanism is the up-regulation of cyclin D1 expression levels. Cyclin D1 is an important cell cycle regulator and has been shown to be overexpressed in a variety of human neoplasms, including breast cancers (Yu et al., 2001). Interestingly, cyclin D1 expression is normally required for mammary epithelial cell transformation and mammary tumorigenesis induced by activated c-Neu/ErbB2 (Lee et al., 2000; Yu et al., 2001). Caveolin-1 is a known transcriptional repressor of cyclin D1 expression (Hulit et al., 2000), suggesting that loss of caveolin-1 gene expression may cause the up-regulation of cyclin D1 expression levels. In accordance with this hypothesis, Figure 8A shows that cyclin D1 protein expression levels are indeed dramatically elevated by approximately three- to fourfold in PyMT/Cav-1 (−/−) samples, compared with matched samples derived from PyMT/Cav-1 (+/+) mice.
To visualize the cellular distribution of cyclin D1, we next performed immunohistochemical analysis on dysplastic mammary lesions derived from 4-wk-old PyMT/Cav-1 (−/−) and PyMT/Cav-1 (+/+) mice. Lesions of approximately the same size from each genotype were chosen to allow for a better comparison. Figure 8B shows that the intensity of cyclin D1 immunostaining (brown color) is clearly increased in the PyMT/Cav-1 (−/−) dysplastic mammary lesions. In accordance with our results from Western blot analysis, the nuclei of the dysplastic mammary epithelial cells are more densely stained in PyMT/Cav-1 (−/−) samples. However, no changes in cyclin D1 immunostaining were observed in the surrounding stromal cell population.
Furthermore, the cyclin D1 staining pattern was noticeably altered in PyMT/Cav-1 (−/−) samples. In PyMT/Cav-1 (+/+) mammary lesions, cyclin D1 immunostaining was confined to the outermost layers of mammary epithelial cells; little or no staining was observed in the center of the lesion (see arrow). In contrast, in PyMT/Cav-1 (−/−) mammary lesions, cyclin D1 immunostaining was present in virtually all the epithelial cells and extended to the center of the lesion.
DISCUSSION
Carcinogenesis is a multistep process involving genetic alterations in vivo that allow a cell to acquire “transformed” properties, such as unregulated cell proliferation, the ability to bypass apoptotic pathways, increased invasiveness, the capability to undergo metastasis, and the ability to evade immunodetection (Cotran et al., 1999). These genetic alterations can result from inherited germline transmission or from acquired somatic changes during a cell's lifetime. Some may involve up-regulated expression or mutational activation of proto-oncogenes that control cell growth and proliferation, such as Neu (erbB2) and Ras. Likewise, loss of cell cycle checkpoints through the down-regulated expression or mutational inactivation of tumor suppressor genes, such as p53 or pRb, enables cells to grow and divide uncontrollably. Additionally, there are a whole host of genes implicated in the processes of tissue invasion and metastasis, including cadherins, matrix metalloproteinases, and growth factor receptors. Our current understanding of tumor progression is that it results from an accumulation of genetic events and that no single genetic alteration in vivo is sufficient for the development of a tumor.
The advent of mouse autochthonous tumor models has greatly facilitated the study of how a gene of interest affects spontaneous tumor progression. The utility of MMTV-PyMT transgenic mice as a spontaneous breast cancer model is now well-established (Guy et al., 1992, 1994; Webster et al., 1998; Maglione et al., 2001). These transgenic mice express the PyMT under the transcriptional control of the MMTV long terminal repeat, which directs expression to the mammary epithelium (Choi et al., 1987). MMTV-PyMT mice rapidly develop widespread multifocal adenocarcinomas involving the entire mammary epithelium, with dysplastic foci occurring as early as 3 wk of age (Guy et al., 1992). PyMT interacts with a number of signaling molecules, including the c-Src tyrosine kinase and phosphatidylinositol-3 kinase (PI-3 kinase), both of which have been shown to be essential for PyMT-mediated mammary tumorigenesis (Guy et al., 1994; Webster et al., 1998).
Numerous studies have now demonstrated that tumor progression in MMTV-PyMT mice is altered when they are interbred with other genetically modified mice, such as CSF-1, keratin-8, Mgat5, tenascin-C, and plasminogen gene knockout mice (Baribault et al., 1997; Bugge et al., 1998; Talts et al., 1999; Granovsky et al., 2000; Lin et al., 2001). MMTV-PyMT mice have also been used to demonstrate the selective up-regulation of certain gene products (MUC1, autocrine growth factors [amphiregulin and cripto], and matrix metalloproteinase 9) at different stages of mammary tumor development (Niemeyer et al., 1999; Kupferman et al., 2000; Graham et al., 2001).
Another hallmark of malignant tumors is anaplasia, which refers to the morphological description of a tumor cell as appearing undifferentiated. Central to our understanding of neoplasia is that poorly differentiated cells tend to be behave more malignantly than well-differentiated cells. Interestingly, caveolin-1 is most highly expressed in terminally differentiated cells, such as adipocytes and endothelial cells, suggesting that high levels of this protein may contribute to their well differentiated/non-proliferating state (Engelman et al., 1998d).
The aim of our current study was to test the hypothesis that caveolin-1 can function as a tumor/transformation suppressor in an in vivo setting. This hypothesis is supported by a wealth of genetic, cellular, and clinical evidence. Despite this evidence, the tumor/transformation suppressor role of caveolin-1 still remains controversial. Herein, we examined whether loss of caveolin-1 gene expression affects the early steps of tumor progression, including tumor initiation and growth. For this purpose, we interbred Cav-1 (−/−) null mice with tumor-prone transgenic mice (MMTV-PyMT) that normally develop multifocal dysplastic lesions throughout the entire mammary tree. Interestingly, at 3 wk of age not all PyMT/Cav-1 (+/+) mammary glands showed the presence of foci, whereas in PyMT/Cav-1 (−/−) mammary glands the incidence of foci was 100%. Most importantly, loss of caveolin-1 resulted in an approximately twofold increase in the number of lesions (foci per gland; 3.3 ± 1.0 vs. 7.0 ± 1.2) and an approximately five- to sixfold increase in the total area occupied by these lesions. Similar results were obtained at 4 wk of age. We also demonstrated that loss of caveolin-1 increased the extent and the histological grade of these mammary lesions, especially at 4 wk of age. Finally, we showed that cyclin D1 expression levels are dramatically elevated in Cav-1 (−/−) null mammary lesions, consistent with the accelerated appearance of these dysplastic foci. However, we did not observe any changes in the activation state of the Jak-2/STAT5a pathway or the p42/44 MAP kinase cascade.
The role of caveolin-1 as a negative regulator of cellullar proliferation is now well-established from in vitro studies on cultured cells. In addition to extensive evidence that caveolin-1 expression decreases cellular proliferation and anchorage-independent growth in transformed cells, Cav-1 (−/−) null primary embryonic fibroblasts proliferate significantly faster than their wild-type counterparts and demonstrate increased rates of DNA synthesis and increased S-phase fractions (Razani et al., 2001). The hypercellular lung phenotype observed in Cav-1 null mice also suggests that one or more cell types are experiencing a disruption in cell cycle regulation. Mechanistic insight into the relationship between caveolin-1 and cellular proliferation has been provided by experiments that show that caveolin-1 and the Ras-p42/44 MAP kinase cascade undergo a form of reciprocal regulation: 1) down-regulation of caveolin-1 expression by using an antisense approach leads to constitutive ERK activation (Galbiati et al., 1998); 2) up-regulation of caveolin-1 down-regulates p42/44 MAP kinase activity, as measured using an Elk-luciferase reporter system, as well as by using in vitro reconstitution experiments (Engelman et al., 1998a); and 3) down-regulation of p42/44 MAPK activity by treatment of Ras-transformed cells with a mitogen-activated protein kinase kinase inhibitor (PD98059) up-regulates caveolin-1 protein expression (Engelman et al., 1997). These findings establish a clear relationship between caveolin-1 and proliferative signaling pathways, such as the Ras-p42/44 MAPK cascade. Similar reciprocal regulation exists between caveolin-1 and the activated c-Neu-proto-oncogene (Engelman et al., 1998b).
In addition to the reciprocal relationships described above, there seems to be a direct connection between caveolin-1 and the Myc proto-oncogene. c-Myc encodes a nuclear phospho-protein that plays an active role in cellular functions, such as proliferation, differentiation, and apoptosis (Schmidt, 1999). Overexpression of c-Myc leads to shortening of the G1 phase of the cell cycle and inhibition of differentiation (Karn et al., 1989). We have shown that Myc activation leads to repression of caveolin-1 at the transcriptional level, suggesting that this may be another mechanism by which cells attain a transformed phenotype (Park et al., 2001).
Aside from repression of caveolin-1 by both Neu and Myc, caveolin-1 also demonstrates some repressor function. We have shown that overexpression of caveolin-1 causes transcriptional repression of cyclin D1, whereas expression of antisense caveolin-1 increases cyclin D1 levels, lending further support to the idea that caveolin-1 performs a growth regulatory function (Hulit et al., 2000). The cyclin D1 protein is the regulatory component of the holoenzyme that inactivates the retinoblastoma pRB protein, implying roles for cyclin D1 in cellular proliferation and transformation (Hulit et al., 2000). Interestingly, cyclin D1 expression is required for 1) mammary epithelial cell transformation induced by activated c-Neu/ErbB2 (Lee et al., 2000), and 2) in vivo mammary tumorigenesis in MMTV-Neu/ErbB2 transgenic mice (Yu et al., 2001).
How is the absence of caveolin-1 related to the accelerated phenotype we observed in these MMTV-PyMT transgenic mice? We suggest a mechanism involving the hyperactivation of cellular proliferation mediated through cyclin D1. D-Cyclins are involved in controlling cell cycle progression by activating their associated kinases cdk4 and cdk6. These cyclin-dependent kinases phosphorylate the retinoblastoma pRB protein, leading to transition through the G1 phase of the cell cycle (Sherr and Roberts, 1999). Clinically, the cyclin D1 gene is amplified in up to 20% of human breast cancers and the cyclin D1 protein is overexpressed in >50% of human mammary carcinomas (Bartkova et al., 1994; Gillett et al., 1994; McIntosh et al., 1995). Interestingly, transgenic mice overexpressing cyclin D1 in the mammary epithelium (MMTV-cyclin D1) develop significant mammary hyperplasia and mammary carcinomas, demonstrating that cyclin D1 overexpression can deregulate cell proliferation in the mammary epithelium and induce dysplastic or tumorigenic changes (Wang et al., 1994). We have previously demonstrated that caveolin-1 transcriptionally represses the cyclin D1 gene by using cultured cell systems (Hulit et al., 2000). Therefore, it is expected that loss of caveolin-1 expression would cause the transcriptional up-regulation of cyclin D1 levels, as we observed experimentally (Figure 8, A and B); as such, these increases in cyclin D1 protein expression could account for the accelerated appearance and growth of dysplastic mammary foci in PyMT/Cav-1 (−/−) mice.
Alternatively, the polyomavirus middle T antigen mediates cellular transformation through the activation of a number of signaling pathways, including the c-Src tyrosine kinase and PI-3 kinase (Guy et al., 1994; Webster et al., 1998). The relationship between Src and caveolin-1 was first established >10 years ago when caveolin-1 was first identified as a major v-Src substrate in Rous sarcoma virus-transformed fibroblasts (Glenney, 1989). Since then, additional experiments have elucidated that c-Src, a lipid-modified nonreceptor tyrosine kinase, is localized to caveolae membranes, causes a decline in caveolin-1 expression, and reduces the number of invaginated caveolae, suggesting caveolin-1 levels need to be down-regulated before a cell becomes transformed (Ko et al., 1998). Interestingly, caveolin-1 binding seems to inhibit the tyrosine kinase activity of Src family members, as mediated via the caveolin-scaffolding domain (residues 82–101; CSD), demonstrating yet another example of reciprocal regulation between caveolin-1 and a signaling molecule (Couet et al., 1997). Likewise, the PI-3 kinase pathway has both growth stimulatory and antiapoptotic/survival functions. Zundel et al. (2000) have demonstrated that ceramide induces apoptosis by inhibiting PI-3 kinase activity. However, this inhibition is not mediated through changes in PI-3 kinase expression or subunit association, but rather by recruitment to caveolin-1-enriched membrane microdomains. Additionally, overexpression of caveolin-1 is sufficient to inhibit PI-3 kinase activity and sensitizes cells to ceramide-induced cell death, whereas antisense caveolin-1 activates PI-3 kinase and reduces ceramide-induced cell death. These findings suggest other possible mechanisms by which loss of caveolin-1 gene expression could accelerate tumorigenic changes in the mammary epithelium of MMTV-PyMT transgenic mice, potentially through synergistic hyperactivation of the c-Src kinase and PI-3 kinase signaling cascades, and the cyclin D1 proliferation pathway.
In summary, our results indicate that caveolin-1 plays an important in vivo role in suppressing early tumor development and that its absence facilitates the appearance and growth of MIN lesions. These findings directly support a wealth of clinical, genetic, and cellular evidence implicating caveolin-1 as a tumor/transformation suppressor gene.
ACKNOWLEDGMENTS
We thank Dr. M. Cammer for help with image analysis, Dr. R. Mahmood for technical expertise with tissue processing and sectioning of the mammary glands, and Dr. D. Neufeld for histological image acquisition. This work was supported by grants from the National Institutes of Health, the Breast Cancer Alliance, the Muscular Dystrophy Association, and the American Heart Association, as well as a Hirschl/Weil-Caulier Career Scientist Award (all to M.P.L.). T.M.W., B.R., and A.W.C. were supported by a National Institutes of Health Medical Scientist Training Grant (T32-GM07288). D.S.P. was supported by a National Institutes of Health Graduate Training Program Grant (TG-CA09475). R.G.P. was supported by grants from the National Institutes of Health (R01-CA70897, R01-CA86072, and R01-CA75503), the Komen Breast Cancer Foundation, the Breast Cancer Alliance, Inc., and the Department of Defense. R.G.P. is the recipient of a Hirschl/Weil-Caulier Career Scientist Award.
Footnotes
DOI: 10.1091/mbc.E02–08–0503.
REFERENCES
- Baribault H, Wilson-Heiner M, Muller W, Penner J, Bakhiet N. Functional analysis of mouse keratin 8 in polyoma middle T-induced mammary gland tumors. Transgenic Res. 1997;6:359–367. doi: 10.1023/a:1018427215923. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Lund LR, Kombrinck KK, Nielsen BS, Holmback K, Drew AF, Flick MJ, Witte DP, Dano K, Degen JL. Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene. 1998;16:3097–3104. doi: 10.1038/sj.onc.1201869. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Choi YW, Henrard D, Lee I, Ross SR. The mouse mammary tumor virus long terminal repeat directs expression in epithelial and lymphoid cells of different tissues in transgenic mice. J Virol. 1987;61:3013–3019. doi: 10.1128/jvi.61.10.3013-3019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: WB Saunders Company; 1999. [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Drab M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998a;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J Biol Chem. 1998b;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31) FEBS Lett. 1998c;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer's disease, and muscular dystrophy. Am J Hum Genet. 1998d;63:1578–1587. doi: 10.1086/302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998e;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonté D, Engelman JA, Watanabe G, Burk R, Pestell R, Lisanti MP. Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–21066. [PubMed] [Google Scholar]
- Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RA, Morris JR, Cohen EP, Taylor-Papadimitriou J. Up-regulation of MUC1 in mammary tumors generated in a double-transgenic mouse expressing human MUC1 cDNA, under the control of 1.4-kb 5′ MUC1 promoter sequence and the middle T oncogene, expressed from the MMTV promoter. Int J Cancer. 2001;92:382–387. doi: 10.1002/ijc.1192. [DOI] [PubMed] [Google Scholar]
- Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- Harvey M, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Hulit J, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–9. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- Jenkins RB, et al. A molecular cytogenetic analysis of 7q31 in prostate cancer. Cancer Res. 1998;58:759–766. [PubMed] [Google Scholar]
- Karn J, Watson JV, Lowe AD, Green SM, Vedeckis W. Regulation of cell cycle duration by c-myc levels. Oncogene. 1989;4:773–787. [PubMed] [Google Scholar]
- Kerr J, Leary JA, Hurst T, Shih YC, Antalis TM, Friedlander M, Crawford E, Khoo SK, Ward B, Chenevix-Trench G. Allelic loss on chromosome 7q in ovarian adenocarcinomas: two critical regions and a rearrangement of the PLANH1 locus. Oncogene. 1996;13:1815–1818. [PubMed] [Google Scholar]
- Ko YG, Liu P, Pathak RK, Craig LC, Anderson RG. Early effects of pp60(v-src) kinase activation on caveolae. J Cell Biochem. 1998;71:524–535. [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferman ME, Fini ME, Muller WJ, Weber R, Cheng Y, Muschel RJ. Matrix metalloproteinase 9 promoter activity is induced coincident with invasion during tumor progression. Am J Pathol. 2000;157:1777–1783. doi: 10.1016/S0002-9440(10)64815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: Cav-1 (P132L) behaves in a dominant-negative fashion and Cav-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161:1357–1369. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, Nicholson B, Cardiff RD, MacLeod CL. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW, Horne CH. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995;11:885–891. [PubMed] [Google Scholar]
- Niemeyer CC, Spencer-Dene B, Wu JX, Adamson ED. Preneoplastic mammary tumor markers: cripto and amphiregulin are overexpressed in hyperplastic stages of tumor progression in transgenic mice. Int J Cancer. 1999;81:588–591. doi: 10.1002/(sici)1097-0215(19990517)81:4<588::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Park DS, Lee H, Frank PG, Razani B, Nguyen AV, Parlow AF, Russell RG, Hulit J, Pestell RG, Lisanti MP. Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyper-activation of the Jak-2/STAT5a signaling cascade. Mol Biol Cell. 2002;13:3416–3430. doi: 10.1091/mbc.02-05-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Razani B, Lasorella A, Schreiber-Agus N, Pestell RG, Iavarone A, Lisanti MP. Evidence that Myc isoforms transcriptionally repress caveolin-1 gene expression via an INR-dependent mechanism. Biochemistry. 2001;40:3354–3362. doi: 10.1021/bi002787b. [DOI] [PubMed] [Google Scholar]
- Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry. 2000;39:13916–13924. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- Razani B, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sager R, et al. RNA genetics of breast cancer: maspin as a paradigm. Cold Spring Harbor Symp Quant Biol. 1994;59:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988–2996. doi: 10.1038/sj.onc.1202751. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shridhar V, Sun QC, Miller OJ, Kalemkerian GP, Petros J, Smith DI. Loss of heterozygosity on the long arm of human chromosome 7 in sporadic renal cell carcinomas. Oncogene. 1997;15:2727–2733. doi: 10.1038/sj.onc.1201448. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Suzuki Y, Hanada K, Hashimoto A, Redpath JL, Stanbridge EJ, Nishijima M, Kitagawa T. Reduction of caveolin-1 expression in tumorigenic human cell hybrids. J Biochem. 1998;124:383–388. doi: 10.1093/oxfordjournals.jbchem.a022123. [DOI] [PubMed] [Google Scholar]
- Talts JF, Wirl G, Dictor M, Muller WJ, Fassler R. Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J Cell Sci. 1999;122:1855–1864. doi: 10.1242/jcs.112.12.1855. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG, Cardiff RD, Graham FL, Hassell JA, Muller WJ. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- Zenklusen JC, Bieche I, Lidereau R, Conti CJ. (C-A)n microsatellite repeat D7S522 is the most commonly deleted region in human primary breast cancer. Proc Natl Acad Sci USA. 1994a;91:12155–12158. doi: 10.1073/pnas.91.25.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen JC, Thompson JC, Klein-Szanto AJ, Conti CJ. Frequent loss of heterozygosity in human primary squamous cell and colon carcinomas at 7q31.1: evidence for a broad range tumor suppressor gene. Cancer Res. 1995;55:1347–1350. [PubMed] [Google Scholar]
- Zenklusen JC, Thompson JC, Troncoso P, Kagan J, Conti CJ. Loss of heterozygosity in human primary prostate carcinomas: a possible tumor suppressor gene at 7q31.1. Cancer Res. 1994b;54:6370–6373. [PubMed] [Google Scholar]
- Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, Lisanti MP. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem. 2000;275:20717–20725. doi: 10.1074/jbc.M909895199. [DOI] [PubMed] [Google Scholar]
- Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol. 2000;20:1507–1514. doi: 10.1128/mcb.20.5.1507-1514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]