Abstract
The degradation of extracellular matrix (ECM) by matrix metalloproteases is crucial in physiological and pathological cell invasion alike. Degradation occurs at specific sites where invasive cells make contact with the ECM via specialized plasma membrane protrusions termed invadopodia. Herein, we show that the dynamin 2 (Dyn2), a GTPase implicated in the control of actin-driven cytoskeletal remodeling events and membrane transport, is necessary for focalized matrix degradation at invadopodia. Dynamin was inhibited by using two approaches: 1) expression of dominant negative GTPase-impaired or proline-rich domain-deleted Dyn2 mutants; and 2) inhibition of the dynamin regulator calcineurin by cyclosporin A. In both cases, the number and extension of ECM degradation foci were drastically reduced. To understand the site and mechanism of dynamin action, the cellular structures devoted to ECM degradation were analyzed by correlative confocal light-electron microscopy. Invadopodia were found to be organized into a previously undescribed ECM-degradation structure consisting of a large invagination of the ventral plasma membrane surface in close spatial relationship with the Golgi complex. Dyn2 seemed to be concentrated at invadopodia.
INTRODUCTION
Degradation of the extracellular matrix (ECM) is a critical process during cell invasion in both physiological and pathological processes such as morphogenesis, differentiation, cell migration, apoptosis, and tumor invasion (reviewed in Basbaum and Werb, 1996). For example, metastatic tumor cells need to overcome the natural barriers impeding access to vascular or lymphatic pathways and to alter the extracellular environment to allow cancer growth in distant locations (reviewed in Foda and Zucker, 2001). This requires the direct participation of released and exposed proteases such as urokinase-type plasminogen activator, lysosomal proteases, and matrix metalloproteases (MMPs); MMPs in particular are thought to play a major role in the degradation of ECM. To reach the plasma membrane, proteases must be transported and processed by the secretory pathway. Although the mechanisms of release, intracellular trafficking and sorting of lysosomal proteases (reviewed in Dell'Angelica and Payne, 2001) and their regulation (Radons et al., 1994; Baldassarre et al., 2000), have been studied and partly elucidated, surprisingly, much less is known concerning the trafficking of the functionally more crucial MMPs, especially the membrane-bound forms (Hotary et al., 2000). Because the focalized delivery/exposure of MMPs is likely to be a crucial factor in physiological ECM remodeling events and cell invasive behavior (Basbaum and Werb, 1996), a key feature of the trafficking of MMPs is their targeting to specialized plasma membrane structures, where ECM degradation occurs (Chen, 1989; Mueller and Chen, 1991; Chen and Wang, 1999). At the ultrastructural level, these structures have been suggested to consist of 200-nm-wide and up to 3-μm-long membrane protrusions extending into the matrix (Mueller and Chen, 1991; Bowden et al., 2001), prominent in invasive cells. Because of these features, they have been termed invadopodia. The molecular composition of invadopodia at sites of ECM degradation is partially known. Invadopodial protrusions are enriched in integrins and associated tyrosine kinase signaling machinery, metalloproteases and, quite prominently, in actin and actin-associated proteins (Mueller et al., 1992; Monsky et al., 1994; Chen, 1996; Nakahara et al., 1998; Bowden et al., 1999; Deryugina et al., 2001). Herein, we report that the GTPase dynamin plays an essential role in the focal degradation of ECM at invadopodia.
The 100-kDa GTPase dynamin has been demonstrated to be required in endocytic membrane fission, caveolae internalization, and protein trafficking at the Golgi apparatus (Schmid et al., 1998; Hinshaw, 2000; McNiven et al., 2000a). The various dynamin isoforms are multidomain proteins featuring, in addition to a GTPase domain, a pleckstrin homology domain (PH) implicated in membrane binding, a GTPase effector domain shown to be essential for self-assembly and stimulated GTPase activity, and a C-terminal proline-rich domain (PRD), which contains several SH3-binding sites. Dynamin partners generally bind to the PRD and may either stimulate dynamin's GTPase activity or target dynamin to the plasma membrane (Schmid et al., 1998; Hinshaw, 2000). Of note, the binding of phosphoinositides to the well-characterized PH domain of dynamin affect both GTPase activity and self-assembly (Lee et al., 1999; Vallis et al., 1999; Muhlberg and Schmid, 2000) and the interactions between the dynamin PH domain and phosphoinositides are important for dynamin function in vivo; indeed, deletion of the PH domain impairs at least some of dynamin's functions (Achiriloaie et al., 1999; Vallis et al., 1999). Recent work points to the ubiquitous dynamin 2 form (Dyn2) as being capable of interacting with the actin cytoskeleton in regulating actin filament reorganization and subsequently cell shape via cortactin (McNiven et al., 2000b), actin comet formation (Lee and De Camilli, 2002; Orth et al., 2002), internalization of particles during phagocytosis (Gold et al., 1999), and the formation of podosomes (Ochoa et al., 2000), an actin-containing plasma membrane structure proposed to mediate cell adhesion and motility of phagocytic cells (Zambonin-Zallone et al., 1988; Nitsch et al., 1989). Because these findings propose Dyn2 as a key regulatory molecule in at least some types of actin-driven cytoskeletal machineries, and in linking the cytoskeleton to both membrane trafficking/remodeling and signaling events (Hinshaw, 2000), we examined whether Dyn2 plays a role in ECM invasion mediated by invadopodia.
We report that 1) Dyn2 activity is necessary for ECM degradation by invasive tumor cells; 2) invadopodia are organized in a novel ECM-degradation structure (EDS) consisting of a large invagination of the ventral plasma membrane surface in close spatial relationship with the Golgi complex; and 3) the effect of Dyn2 in focal matrix degradation is mediated by an essential role in the organization and function of these novel matrix degradation structures.
EXPERIMENTAL PROCEDURES
Constructs and Antibodies
All the Dyn2 expression constructs used have been described previously: 1) wild-type Dyn2 aa splice variant (Cao et al., 1998); 2) wild-type Dyn2(aa)-green fluorescent protein (GFP) chimera, Dyn2(aa)-GFP (Cao et al., 1998); 3) Dyn2-GFP chimera carrying an inactivating point mutation in the nucleotide binding site, Dyn2(aa)K44A-GFP (Ochoa et al., 2000); and 4) Dyn2-GFP chimera lacking the PRD Dyn2(aa)ΔPRD-GFP (McNiven et al., 2000b). The anti-dynamin peptide antibodies MC63 (to the conserved NH2-amino terminus of dynamin) and Dyn 2 (to the dynamin 2 proline-rich tail) have been described previously (Cook et al., 1994; Henley and McNiven, 1996). Anti-phosphotyrosine and anti-cortactin monoclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and Upstate Biotechnology (Lake Placid, NY), respectively. Polyclonal anti-giantin antibodies were a generous gift of Dr. M.A. De Matteis (Consorzio Mario Negri Sud, S. Maria Imbaro, Italy). Rab5 and Grb2 expression constructs were generous gifts of Drs. Cecilia Bucci (“Federico II” University, Naples, Italy) and Larry Samelson (National Institutes of Health, Bethesda, MD), respectively.
Transfection
Cells were plated at 50% confluence, and the next day they were incubated 1 h at 37°C with 0.45 μg/cm2 DNA of interest and 2 μl/μg DNA TransFast (Promega, Madison, WI). Complete medium was then added. Experiments involving overexpression of exogenous proteins were usually performed 24 h after transfection.
ECM Degradation Assay
Fluorescent matrix-coated coverslips were prepared and the assay carried out as described previously (Mueller and Chen, 1991; Mueller et al., 1992; Bowden et al., 2001). Briefly, thin layers of fluorescein-, rhodamine B- (Sigma-Aldrich, St. Louis, MO) or Alexa 546 (Molecular Probes, Eugene, OR)-conjugated gelatin (Sigma-Aldrich) were placed on coverslips, cross-linked with 0.5% glutaraldehyde for 15 min at 0°C, and incubated for 3 min at room temperature with 5 mg/ml NaBH4. Finally, after a wash and a short 10-min incubation in 70% ethanol, coverslips were quenched with complete DMEM/F-12 (1:1) (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum for 1 h a 37°C before cell plating. Cells were then cultured on ECM-coated coverslips over periods ranging from 2 to 15 h, depending on the experiment.
Immunofluorescence and Quantification of ECM Degradation
After treatments, cells were fixed in 4% paraformaldehyde for 15 min, permeabilized in phosphate-buffered saline (PBS) containing 0.02% saponin, 0.2% bovine serum albumin (BSA), and 50 mM NH4Cl, incubated with primary antibodies of interest or phalloidin-tetramethylrhodamine B isothiocyanate (TRITC) (Sigma-Aldrich) for 1 h and then incubated with fluorophore-conjugated secondary antibodies (Molecular Probes) for 45 min. Finally, coverslips were mounted in the antifade reagent SlowFade (Molecular Probes). All experiments were observed using an LSM 510 laser scanning confocal microscope (Carl Zeiss, Jena, Germany). Immunofluorescence images were acquired at high confocality (pinhole = 1 Airy unit) to achieve the thinnest possible optical slices at the substrate–cell interface. To determine the number of degrading cells for each experiment we considered 100 random fields (containing at least 50 transfected cells) at a 63× magnification. Values were then expressed as the percentage of transfected cells that presented at least one degradation patch (irrespective of extension) relative to the total number of cells analyzed. To determine, instead, areas of degradation we used the same criteria to select cells, and the area of each degradation patch was measured using the LSM 510 software together with an electronic spreadsheet. The total area for each condition was then normalized for cell number and expressed as a percentage of control. Experiments were repeated at least three times.
Correlative Light-Electron Microscopy (CLEM)
CLEM was carried out on invadopodia by using a recently described protocol (Polishchuk et al., 2000; Polishchuk and Mironov, 2001). Briefly, A375 cells transfected with wild-type or mutant Dyn2(aa)-GFP were grown on CELLocate coverslips (Eppendorf, Hamburg, Germany) coated with fluorescent gelatin, and, after appropriate times, were fixed with 0.05% glutaraldehyde plus 4% paraformaldehyde in 0.2 M HEPES (pH 7.4) for 5 min, and then with 4% paraformaldehyde in the same buffer for 30 min. Next, cells were incubated in 0.02% saponin-containing blocking solution (PBS supplemented with 0.2% BSA and 50 mM NH4Cl); after washing, samples were analyzed under the confocal microscope and the cell bearing the structure of interest was localized within the grid coordinates. Samples were then processed for conventional electron microscopy (EM) with 1% OsO4 plus 1.5% potassium ferrocyanide in 0.1 M cacodylate buffer (pH 7.3) for 2 h on ice (all incubations before this step were performed at room temperature) in the dark, and then dehydrated, embedded in epoxy resin, and polymerized for at least 24 h. CELLocate coverslips were then dissolved with 40% hydrofluoric acid, and samples were extensively washed with buffer. Finally, serial sections of the cell of interest were produced parallel to the substrate. One hundred-nanometer serial sections were collected on slot grids covered with formvar-carbon supporting film and examined at 80 kV in a 109 electron microscope (Carl Zeiss). The images collected by confocal microscopy and EM were aligned with Adobe Photoshop, and the structure of interest was identified on the basis of its position in space.
Correlative Immunoelectron Microscopy
A375 cells transfected with wild-type or mutant Dyn2(aa)-GFP fusion proteins were grown on CELLocate coverslips, and, after appropriate times, were fixed for electron microscopy as described above. Samples were analyzed under the confocal microscope and the cell bearing the structure of interest was localized within the grid coordinates. Next, cells were incubated in 0.02% saponin-containing blocking solution (PBS supplemented with 0.2% BSA and 50 mM NH4Cl) and then 2 h with anti-GFP antibody (Abcam, Cambridge, United Kingdom), extensively washed, and revealed with Goldenhance-EM (Nanoprobes, Stony Brook, NY) according to manufacturer's instructions. Samples were then processed for conventional EM as described above. The images collected by confocal microscopy and EM were aligned and analyzed as described above.
RESULTS
To study the morphogenesis of invadopodia, we chose to use an invasive and highly metastatic clone of an established human melanoma cell line, A375MM (Ayala et al., 1999). Invadopodia were visualized as described previously by culturing cells on a cross-linked fluorophore-conjugated gelatin matrix (Mueller and Chen, 1991; Mueller et al., 1992; Bowden et al., 2001). This ECM layer is very stable, insoluble, and resistant to bland proteolysis such that only aggressively invasive protease-secreting cells are able to attack it, causing the formation of dark (i.e., nonfluorescent) areas of degradation that can be counted and quantified. In a typical experiment, up to 60% of A375MM melanoma cells efficiently degrade this matrix and display a characteristic pattern of degradation that consists, depending on the time of incubation, of clusters of 2-3-15-16 degradation patches (Figure 1), generally immediately close to the Golgi complex and often (25% of cases) directly below it (Figure 1). With increasing incubation times, the degradation patches tend to become larger, coalescing into larger ones as degradation progresses. The metalloprotease-specific broad-spectrum inhibitor BB-94 (ineffective on other proteases; Davies et al., 1993) completely inhibited the appearance of degradation patches (our unpublished data), indicating that ECM degradation by A375MM cells was totally metalloprotease dependent. Although in A375MM cells we could detect the first degradation events as early as 2 h after plating, most of the experiments that follow refer to 15-h incubation.
Figure 1.
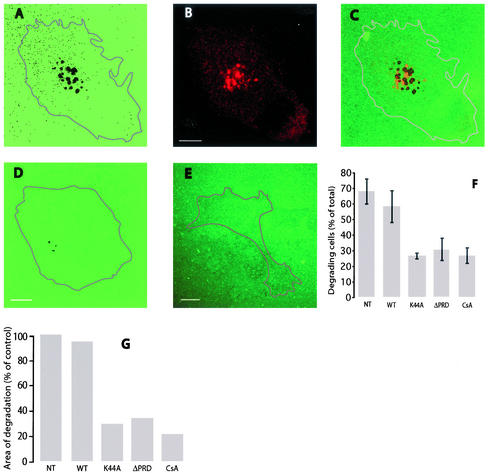
ECM degradation by A375MM cells and effects of dominant negative dynamin 2 mutants and cyclosporin A on ECM degradation. A375MM melanoma cells were plated on cross-linked fluorophore-conjugated matrix for 15 h, fixed, stained with antigiantin antibodies to label the Golgi complex, and analyzed at the confocal microscope. A substrate level optical section shows the dark areas of degradation (A), the Golgi complex (B), and their relative positions in the merge (C). A375MM cells transiently transfected with wtDyn2aa-GFP, Dyn2(aa)K44A-GFP (D), or Dyn2(aa)ΔPRD-GFP (E) were cultured as described above and fixed. The percentage of degrading cells (F) and the areas of degradation (G) in nontransfected (NT), wild-type-, and mutant-transfected samples were quantified as described in Experimental Procedures Cell contours have been digitally rendered in the micrographs. Bar, 10 μm.
Inhibition of Dynamin 2 Suppresses Focal ECM Degradation
To determine whether Dyn2 plays a functional role in the degradative process we followed two independent strategies. The first was to directly exploit two different mutations of Dyn2 previously shown to act in a dominant negative manner: 1) the K44A point mutation in the nucleotide binding site, which impairs guanine nucleotide binding capability (Herskovits et al., 1993); and 2) the PRD deletion (McNiven et al., 2000b), unable to bind SH3-containing proteins. Strikingly, transient overexpression of the dominant negative Dyn2 mutants, Dyn2(aa)K44A-GFP and Dyn2(aa)ΔPRD-GFP, substantially decreased both the number of degrading cells and the total area of degradation (Figure 1). As a control, when the GFP chimera of the wild-type Dyn2 aa splice variant (wtDyn2aa; GenBank sequence B53165) was transiently overexpressed, both ECM degradation activity, expressed in terms of number of degrading cells and area of degradation, and the general appearance of cells were similar to untransfected cells (Figure 1). The second approach was a pharmacological one based on the fact that the neuron-specific dynamin 1, binds to (Lai et al., 1999) and is a substrate of, the calcium-dependent protein phosphatase calcineurin. Dephosphorylation of dynamin 1 by calcineurin decreases its GTPase activity (Liu et al., 1994) and enhances interaction with its partners (Slepnev and De Camilli, 2000). When we tested the calcineurin inhibitor cyclosporin A (CsA) in A375MM cells, we found it to dramatically decrease both the number of degrading cells and the total area of degradation (Figure 1).
Localization of Wild-Type and Mutant Dyn2
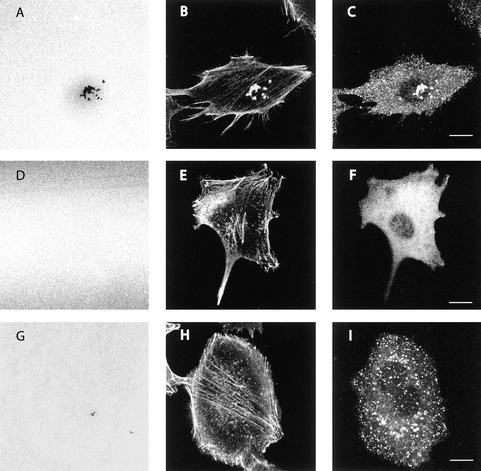
We proceeded to determine whether Dyn2 was localized to invadopodia at areas of degradation by using two different peptide antibodies against dynamin (MC63-ab to the conserved NH2-amino terminus and Dyn2-ab to the dynamin 2 proline-rich tail). A typical array of proteins localize to these areas, among which are cortactin, tyrosine-phosphorylated proteins, and actin (Figure 2). Hence, for practical purposes, the operational definition of invadopodia at the light microscopy level is the colocalization of a series of proteins (e.g.,. actin, cortactin, and tyrosine-phosphorylated proteins) at sites of degradation (Bowden et al., 2001). When the localization of Dyn2 was examined, we found a marked concentration of Dyn2 in colocalization with cortactin, actin, and tyrosine-phosphorylated proteins at areas of degradation in A375MM cells (Figure 2). Thus, dynamin clearly localizes to the invadopodia. The same was observed in wild-type Dyn2-GFP–transfected cells (Figure 3). In contrast, the Dyn2 mutants exhibited a very altered distribution. Dyn2ΔPRD-GFP displayed a completely diffuse staining pattern (Figure 3), as expected (McNiven et al., 2000b). Strikingly, in the few Dyn2ΔPRD-GFP–expressing cells with recognizable invadopodia-like staining, endogenous Dyn2 was found to be localized at the rare remaining sites of degradation; this was determined through the use of the Dyn2-ab antibody directed toward the proline-rich domain of dynamin (thus unable to recognize transfected Dyn2ΔPRD-GFP; our unpublished data). Thus, ECM degradation clearly correlated with the presence of endogenous (functional) dynamin. Dyn2K44A-GFP, instead, appeared in numerous discrete puncta of fluorescence (Figure 3) much smaller than those observed in the wt-transfected cells and uniformly distributed throughout the entire surface of the plasma membrane. This was clearly at variance with control cells, where Dyn2-positive structures were only present at the ventral surface and usually clustered in groups at sites of ECM degradation (Figure 3). To determine, however, whether in Dyn2K44A-GFP–transfected cells invadopodial structures were formed but unable to degrade the ECM, we studied the distribution of other markers in combination with Dyn2K44A. As a result, a very limited number of the numerous Dyn2K44A-positive puncta seemed to colocalize with either actin (Figure 3), cortactin, or tyrosine-phosphorylated proteins (our unpublished data), suggesting that invadopodial structures were not formed.
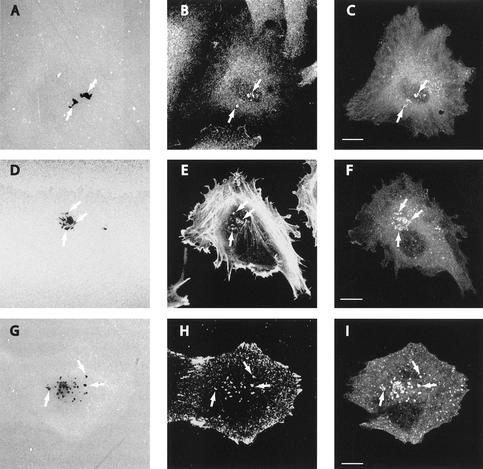
Figure 2.
Localization of dynamin 2 and invadopodia markers to EDS. A375MM melanoma cells were plated on cross-linked fluorophore-conjugated matrix for 15 h, and then fixed, stained with anti-dynamin (Dyn2-ab), anti-cortactin, or anti-phosphotyrosine antibodies or phalloidin-TRITC and analyzed at the confocal microscope. ECM degradation patches (A, D, and G, arrows) from substrate level optical sections clearly coincided with either cortactin (B), actin (E), or phosphotyrosine (H) and a reinforcement of the Dyn2 signal (C, F, and I). Bar, 10 μm.
Figure 3.
Effects of dominant negative dynamin 2 mutants on EDS. A375MM cells transiently transfected with wtDyn2aa-GFP (A–C), Dyn2(aa)ΔPRD-GFP (D–F), or Dyn2(aa)K44A-GFP (G–I) were cultured as described above, fixed, stained with anti-phalloidin-TRITC, and analyzed at the confocal microscope. Substrate level optical sections show the fluorescent matrix (A, D, and G), actin staining (B, E, and H), and the GFP signal (C, F, and I). Bar, 10 μm.
Ultrastructure of Dynamin-containing EDS
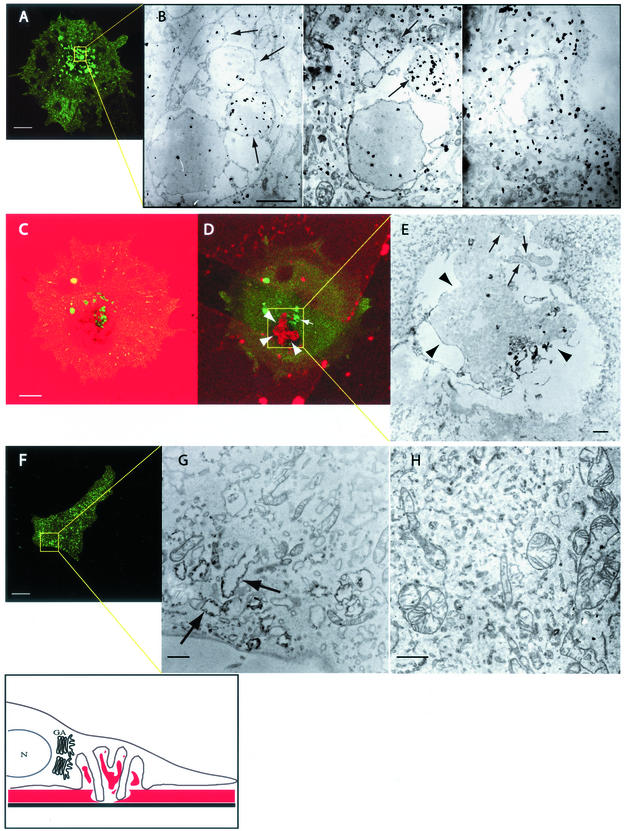
A detailed ultrastructural analysis of the cell structures associated to the areas of degradation (tentatively called ECM-degradation structures; EDSs) was necessary to define the mode of action of dynamin and to map its location within them. To this end, we used a correlative confocal light electron microscopy (CLEM) approach developed in our laboratory (Polishchuk et al., 2000) and by which individual objects can be analyzed both at the optical microscopic level and at the electron microscope. We transfected A375MM cells with wtDyn2aa-GFP and analyzed specific fluorescent EDS at the EM (Figure 4 and associated EM serial section series; serial.mov). Serial ultrathin sections were then produced, revealing the EDS to be a profound invagination of the ventral surface of the plasma membrane. Within the area delimited by the large invagination, large fragments of gelatin could often be seen (Figure 4 and associated movie of the confocal Z-stack series; stack.mov). In general, such invaginations averaged 8 μm in width and 2 μm in depth. A common, prominent feature was the many surface protrusions with diameters, ranging from hundreds of nanometers to a few micrometers, and averaging 500 nm in length, originating from various sites and that sometimes penetrate into the matrix (Figure 4). These protrusions are consistent with the reported “invading” structures originally described for invadopodia (Chen, 1989), but seemed to be part of a more complex superstructure. We next defined the localization of Dyn2 within these structures by correlative immuno-EM of wtDyn2-GFP–expressing cells using anti-GFP antibodies (Figure 4). Dynamin labeling was clearly concentrated in most invadopodial protrusions and within these, apparently not on the plasma membrane, suggesting association to cytosolic structures. In summary, the EDS are profound invaginations of the ventral surface of the plasma membrane filled with fragments of partially degraded ECM and from which a number of Dyn2-associated protrusions (invadopodia) originate contacting and locally degrading the ECM as exemplified in the drawing in Figure 4. In agreement with the results obtained through the use of confocal microscopy, the Golgi complex consistently occurred to be in close spatial relationship with the EDS (our unpublished data). To define the effects of mutant Dyn2 expression, we extended our ultrastructural analysis to the mutant Dyn2-expressing cells. As demonstrated above, Dyn2ΔPRD-GFP displayed a completely diffuse staining pattern (Figure 3). When examined at the EM level, the Dyn2ΔPRD-GFP–expressing cells did not feature structures recognizable as EDS, consistent with the diffuse staining of Dyn2 at the optical microscope level and the lack of detectable ECM degradation (Figure 4). Dyn2K44A-GFP–expressing cells, instead, displayed a number of Dyn2K44A-positive puncta. However (see above), when we examined their ultrastructure we found them to be simple membrane pits (0.5–1 μm in width and 0.2–0.3 μm in depth) with no evidence of either gelatin fragments or of invadopodial protrusions (Figure 4).
Figure 4.
Correlative light-electron microscopy of EDS. Transfected A375MM melanoma cells were plated on CELLocate coverslips coated with cross-linked fluorophore-conjugated matrix for 15 h, fixed, analyzed at the confocal microscope, and then processed for immunoelectron microscopy. The shown electron microscopy fields are boxed in yellow in the corresponding confocal images, arrows indicate invadopodial protrusions. The boxed area of a wtDyn2aa-GFP–transfected cell (A) was serially sectioned and observed at the EM. The movie serial.mov shows the complete serial section series. Three different serial sections (bottom, middle, and top) (B) show Dyn2-GFP labeling as identified with anti-GFP antibodies. The movie stack.mov shows the complete Z-stack rendering of the confocal image series, whereas C and D show two different confocal sections of a same cell (substrate level and middle section, respectively); fragments of partially degraded gelatin are clearly visible both in the confocal (D) and electron microscopy images (E, arrowheads). The boxed area of Dyn2K44A-GFP–transfected cells (F) was observed at the electron microscope; arrows point to structures vaguely resembling EDS but devoid of invadopodia and gelatin fragments. H shows an electron micrograph of a Dyn2ΔPRD-GFP–transfected cell. The drawing depicts a schematic reconstruction of EDS in relationship to the nucleus (N) and the Golgi apparatus (GA), ECM is indicated in red. Bar, 10 μm in confocal images, and 1 μm in EM images.
Dynamics of Invadopodia
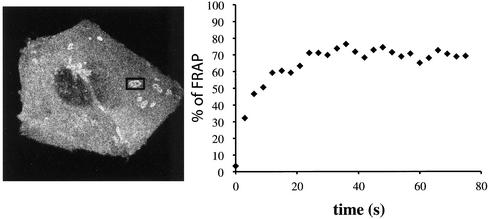
We next determined the dynamics of invadopodia organized into EDS and actively engaged in ECM degradation (as detectable by the presence of dark patches on the fluorescent substrate) by imaging Dyn2-GFP–transfected cells with time-lapse confocal microscopy to determine the appearance and positioning of EDS (relative to the area of degradation) in time. The results clearly indicated that the Dyn2-labeled EDS was stable both in appearance and positioning over a long period (up to 50 min; our unpublished data). Next, to assess the molecular dynamics of Dyn2 at sites of degradation, we determined the turnover of Dyn2-GFP at EDS, by measuring fluorescence recovery after photobleaching (FRAP) and found that fluorescence recovered very rapidly with a t1/2 of only 10 s after bleaching (Figure 5).
Figure 5.
Dynamics of invadopodia. A375MM melanoma cells, transiently transfected with wtDyn2aa-GFP, were plated on cross-linked fluorophore-conjugated matrix for 15 h and then analyzed at the confocal microscope at 37°C. (A) Digital capture of the cell imaged in the experiment shown: a single EDS (boxed area) was bleached for 30 s and the fluorescence allowed to recover for 80 s at 0.5 frames/s. FRAP is plotted as percentage of FRAP in time (B).
DISCUSSION
We report herein that both the enzymic activity and the proper localization of the large GTPase dynamin 2 are necessary for focalized ECM degradation by invasive tumor cells. The ECM-degrading plasma membrane protrusions, invadopodia, were found to originate, and be organized into, a novel EDS consisting of a large invagination of the ventral plasma membrane surface in close spatial relationship with the Golgi complex. Dyn2 seemed to be markedly enriched within the surface protrusions and apparently not associated to the plasma membrane. Hence, Dyn2 seems to regulate focal matrix degradation coordinating the organization and function of these novel matrix degradation structures.
Our findings indicate that both the GTPase activity and proper localization of Dyn2 are necessary for the organization of EDS-associated structures and ultimately for ECM degradation. It is well established that the proline-rich domain of dynamin binds a variety of SH3 domain-containing proteins proposed to mediate and/or regulate dynamin function. Thus, although the dominant negative behavior of Dyn2ΔPRD-GFP suggests that the proline-rich domain of Dyn2 is necessary for proper localization, i.e., via cortactin or other actin-binding protein(s), it also implies that some other Dyn2 domain(s) mediates interaction with another partner protein(s) essential for invadopodia function. Because the dynamin mutants used in this study also inhibit endocytosis (McNiven et al., 2000b), we wondered whether their effect on ECM degradation at EDS might be due to a block of endocytosis. A number of direct and indirect indications rule out this possibility: 1) Typical endocytosis markers (e.g., transferrin receptor, internalized transferrin, and fluorophore-conjugated dextran) seemed to be excluded from the invadopodial area (our unpublished data). 2) The small G-protein Rab 5 has been shown to be an essential regulator of early endocytic events and expression of dominant negative (i.e., GDP-bound) and positive (GTP-bound) mutants interfere with both early endosome formation and fusion (Bucci et al., 1992). Transient transfection of dominant inactive and active Rab 5 mutants in A375MM cells produced the expected aberrant phenotypes but had no effect on ECM degradation at sites of invasion (our unpublished data). 3) Grb2, an adaptor protein known to couple receptors to intracellular signaling pathways, has been proposed to be required for a clathrin-independent endocytic pathway mediating epidermal growth factor receptor internalization (Yamazaki et al., 2002). Indeed, Grb 2 constructs containing inhibitory mutations in either of the two SH3 domains have been shown to prevent internalization of the epidermal growth factor receptor (Yamazaki et al., 2002). In our experiments, transient transfection of the same mutants did not affect ECM degradation at sites of invasion (our unpublished data). 4) Our correlative immunoelectron microscopy analysis clearly localized Dyn2 to the cytoplasmic area within the invadopodial protrusions, inconsistent with the localization and proposed mechanism of action of Dyn2 in endocytosis. 5) The phosphoinositide 3-kinase inhibitors LY294002 and wortmannin, known to perturb various steps of endocytic trafficking (reviewed in Backer, 2000) have no effect on ECM degradation at invadopodia (our unpublished data). and 6) Cyclosporin A does not significantly perturb endocytosis in nonneuronal cells (Yasutomi et al., 1992; Artalejo et al., 1996). Taken together, these observations suggest that the role of Dyn2 in ECM degradation is not related to its function in endocytosis. Finally, another theoretical possibility is that inhibition of dynamin function affects the intracellular transport and secretion of MMPs, because Dyn2 has also been shown to play a role in export from the Golgi complex (Cao et al., 2000). Although this explanation cannot be formally ruled out at present, it is difficult to reconcile, however, with the dramatic effects of mutant dynamin on the structure and composition of EDS.
On the basis of the above-mentioned indications, we favor the hypothesis that dynamin plays a direct role in controlling and maintaining the structure/function of the large actin-based structure where ECM degradation takes place. This is in line with a previous report suggesting a role for cortactin in ECM degradation at invadopodia (see below; Bowden et al., 1999) and with the recent finding that the actin nucleation regulator Neural Wiskott-Aldrich syndrome protein is localized to, and is necessary for, invadopodia function (Mizutani et al., 2002). Possibly, this occurs via one or more of the actin-regulating partners of dynamin 2. In fact, as introduced above, the dynamins are multidomain proteins featuring, among others, a C-terminal PRD, which binds a variety of SH3-containing proteins. Notably, among these are the actin-binding/-regulating proteins mABP1, syndapin, cortactin, and profilin (Witke et al., 1998; Qualmann et al., 1999; McNiven et al., 2000b; Kessels et al., 2001). For example, a candidate in mediating Dyn2 function at the EDS might be cortactin, a tyrosine-phosphorylated cortical actin-binding protein proposed to play a role in tumor cell invasion (reviewed in Weed and Parsons, 2001), and also known to 1) mediate the regulatory function of dynamin on actin in membrane ruffling (McNiven et al., 2000b), and 2) be localized to, and required for, ECM degradation at invadopodia (Bowden et al., 1999). Interestingly, dynamin is also the target of multiple regulatory inputs such as protein kinase C (Powell et al., 2000), phosphoinositides (Lee et al., 1999; Vallis et al., 1999; Muhlberg and Schmid, 2000), and calcineurin (Liu et al., 1994; Slepnev and De Camilli, 2000). Thus, dynamin and its interacting partners are possible molecular targets for the development of antiinvasive agents.
The neuron-specific dynamin 1 binds to (Lai et al., 1999) and is a substrate of the calcium-dependent protein phosphatase calcineurin. Dephosphorylation of dynamin 1 by calcineurin decreases its GTPase activity (Liu et al., 1994) and enhances interaction with its partners (Slepnev and De Camilli, 2000). The immunosuppressants CsA and Tacrolimus (FK506) are calcineurin inhibitors reported to inhibit synaptic vesicle recycling, a dynamin 1-controlled event (Marks and McMahon, 1998) and to interfere with podosome organization (Ochoa et al., 2000). We found CsA to dramatically decrease both the number of degrading cells and the total area of degradation. Interestingly, CsA and Tacrolimus have been reported to decrease bone resorption in vitro by rat osteoclasts (Dempster et al., 1987), via inhibition of calcineurin (Awumey et al., 1999). The function of osteoclasts in bone resorption is mediated by focal matrix degradation. Hence, these findings together with our observations can be interpreted as suggesting an inhibitory effect of CsA on ECM degradation. An interesting clinical correlate of these findings arises from the observation that renal transplantation recipients treated with CsA alone show an increase in bone mineral density after 18 mo (Ponticelli and Aroldi, 2001).
Other actin-driven cellular structures/events shown to be regulated by Dyn2 are phagocytosis (Gold et al., 1999), membrane remodeling (McNiven et al., 2000b), actin comet movement (Lee and De Camilli, 2002; Orth et al., 2002), and the formation of the above-mentioned podosomes (Ochoa et al., 2000), dynamic plasma membrane protrusions characterized in nontumoral phagocytic cells or virus-transformed cells, and thought to be involved in mediating transient attachment during locomotion. The similarity in molecular composition between podosomes and invadopodia is remarkable and, in this regard, it is interesting to note that invadopodia have been initially characterized in Src-transformed cells cultured on a degradable ECM substrate and termed “invading podosomes” (Chen, 1989). There are, however, a number of fundamental differences between podosomes and invadopodia at both the morphological and functional levels that need to be considered. First, although podosomes have been described to be tubular invaginations of the plasma membrane associated with columnar arrays of actin (Nitsch et al., 1989; Ochoa et al., 2000), our CLEM ultrastructural analysis reveals that invadopodial protrusions are organized into profound invaginations of the plasma membrane (EDS) without evidence of inward tubulations of the plasma membrane. Moreover, we observe that at EDS, Dyn2 is clearly localized to the cytoplasmic area within the invadopodial protrusions whereas in podosomes, Dyn2 has been proposed to be a component of the sheath surrounding the tubular invaginations of podosomes (Ochoa et al., 2000), although the lack of an ultrastructural localization of Dyn2 at podosomes renders a comparative analysis with our immunoelectron microscopy observations difficult (Ochoa et al., 2000). At the functional level, podosomes have been consistently shown to be highly dynamic protrusive structures constantly forming and reforming within minutes. Although, similarly to podosomes (Ochoa et al., 2000), Dyn2 rapidly turns over with a half-life of ∼10 s at EDS, we observe instead, that at EDS, invadopodia engaged in active ECM degradation (as suggested by the underlying degradation patches) are immobile over a long period. Finally, expression of Dyn2K44A-GFP was not observed to affect the organization of podosomes (as observed at the optical level; Ochoa et al., 2000), but did disrupt invadopodia and block ECM degradation in our experiments. Finally, the highly invasive A375 human melanoma cells used in this study, form EDS and associated invadopodia only when grown on extracellular matrix proteins (our unpublished data) and do not form the podosome-like structures on glass or serum-coated surfaces. This is not surprising since the induction of functional invadopodia (i.e., associated to ECM degradation) is related to the activation of tightly regulated integrin-mediated signaling events (Coopman et al., 1996; Nakahara et al., 1998). Considering all the above-mentioned information and that they have been detected only in cells with at least the potential ability to degrade ECM, podosomes might represent vestigial or initial structures that in the appropriate conditions differentiate into the ECM degradation-competent, stable structures described herein. These aspects need to be investigated further because a wealth of information on podosomes is available that could be related to the biogenesis and function of invadopodia. As a final remark, invadopodia seem to be a powerful experimental paradigm to study the dynamic interrelationships between membrane transport, cytoskeleton regulation, and cell invasion through the ECM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Alexander Mironov for advice and discussion concerning the morphological aspects of this work. This study was supported by the Italian Association for Cancer Research (Milano, Italy), the Italian Foundation for Cancer Research (Milano, Italy), the Italian National Research Council (Rome, Italy) Progetto Finalizzato “Biotecnologie” (01.00035.PF49), and grant DK56647-02 (to M.A.M.).
Footnotes
Online version of this article contains video material for some figures. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0308. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0308.
REFERENCES
- Achiriloaie M, Barylko B, Albanesi JP. Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol Cell Biol. 1999;19:1410–1415. doi: 10.1128/mcb.19.2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Awumey EM, Moonga BS, Sodam BR, Koval AP, Adebanjo OA, Kumegawa M, Zaidi M, Epstein S. Molecular and functional evidence for calcineurin-A α and β isoforms in the osteoclast: novel insights into cyclosporin A action on bone resorption. Biochem Biophys Res Commun. 1999;254:248–252. doi: 10.1006/bbrc.1998.9785. [DOI] [PubMed] [Google Scholar]
- Ayala I, Babia T, Baldassarre M, Pompeo A, Fabra A, Kok JW, Luini A, Buccione R, Egea G. Morphological and biochemical analysis of the secretory pathway in melanoma cells with distinct metastatic potential. FEBS Lett. 1999;451:315–320. doi: 10.1016/s0014-5793(99)00620-1. [DOI] [PubMed] [Google Scholar]
- Backer JM. Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol Cell Biol Res Commun. 2000;3:193–204. doi: 10.1006/mcbr.2000.0202. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Dragonetti A, Marra P, Luini A, Isidoro C, Buccione R. Regulation of protein sorting at the TGN by plasma membrane receptor activation. J Cell Sci. 2000;113:741–748. doi: 10.1242/jcs.113.4.741. [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCμ associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 2001;63:613–627. doi: 10.1016/s0091-679x(01)63033-4. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Thompson HM, Krueger EW, McNiven MA. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- Chen WT. Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein. 1996;49:59–71. doi: 10.1159/000468616. [DOI] [PubMed] [Google Scholar]
- Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann NY Acad Sci. 1999;878:361–371. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- Cook TA, Urrutia R, McNiven MA. Identification of dynamin 2, an isoform ubiquitously expressed in rat tissues. Proc Natl Acad Sci USA. 1994;91:644–648. doi: 10.1073/pnas.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopman PJ, Thomas DM, Gehlsen KR, Mueller SC. Integrin alpha 3 beta 1 participates in the phagocytosis of extracellular matrix molecules by human breast cancer cells. Mol Biol Cell. 1996;7:1789–1804. doi: 10.1091/mbc.7.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993;53:2087–2091. [PubMed] [Google Scholar]
- Dell'Angelica EC, Payne GS. Intracellular cycling of lysosomal enzyme receptors: cytoplasmic tails' tales. Cell. 2001;106:395–398. doi: 10.1016/s0092-8674(01)00470-6. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Murrills RJ, Horbert WR, Arnett TR. Biological activity of chicken calcitonin: effects on neonatal rat and embryonic chick osteoclasts. J Bone Miner Res. 1987;2:443–448. doi: 10.1002/jbmr.5650020512. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–482. doi: 10.1016/s1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin, and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels MM, Engqvist-Goldstein AE, Drubin DG, Qualmann B. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J Biol Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- Lee A, Frank DW, Marks MS, Lemmon MA. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr Biol. 1999;9:261–264. doi: 10.1016/s0960-9822(99)80115-8. [DOI] [PubMed] [Google Scholar]
- Lee E, De Camilli P. From the cover: dynamin at actin tails. Proc Natl Acad Sci USA. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem Sci. 2000a;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape [in process citation] J Cell Biol. 2000b;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of β1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99:213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Yeh Y, Chen WT. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992;119:1309–1325. doi: 10.1083/jcb.119.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlberg AB, Schmid SL. Domain structure and function of dynamin probed by limited proteolysis. Methods. 2000;20:475–483. doi: 10.1006/meth.2000.0960. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nitsch L, Gionti E, Cancedda R, Marchisio PC. The podosomes of Rous sarcoma virus transformed chondrocytes show a peculiar ultrastructural organization. Cell Biol Int Rep. 1989;13:919–926. doi: 10.1016/0309-1651(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, De Camilli P. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Cao H, McNiven MA. From the cover: the large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci USA. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk RS, Mironov AA. Correlative video/light electron microscopy. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current Protocols in Cell Biology. New York: John Wiley & Sons; 2001. pp. 4.8.1–4.8.9. [Google Scholar]
- Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C, Aroldi A. Osteoporosis after organ transplantation. Lancet. 2001;357:1623. doi: 10.1016/S0140-6736(00)04765-6. [DOI] [PubMed] [Google Scholar]
- Powell KA, Valova VA, Malladi CS, Jensen ON, Larsen MR, Robinson PJ. Phosphorylation of dynamin I on Ser-795 by protein kinase C blocks its association with phospholipids. J Biol Chem. 2000;275:11610–11617. doi: 10.1074/jbc.275.16.11610. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol Biol Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radons J, Biewusch U, Grassel S, Geuze HJ, Hasilik A. Distinctive inhibition of the lysosomal targeting of lysozyme and cathepsin D by drugs affecting pH gradients and protein kinase C. Biochem J. 1994;302:581–586. doi: 10.1042/bj3020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, McNiven MA, De Camilli P. Dynamin and its partners: a progress report. Curr Opin Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Vallis Y, Wigge P, Marks B, Evans PR, McMahon HT. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr Biol. 1999;9:257–260. doi: 10.1016/s0960-9822(99)80114-6. [DOI] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- Witke W, Podtelejnikov AV, Di Nardo A, Sutherland JD, Gurniak CB, Dotti C, Mann M. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 1998;17:967–976. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Zaal K, Hailey D, Presley J, Lippincott-Schwartz J, Samelson LE. Role of Grb2 in EGF-stimulated EGFR internalization. J Cell Sci. 2002;115:1791–1802. doi: 10.1242/jcs.115.9.1791. [DOI] [PubMed] [Google Scholar]
- Yasutomi D, Odaka C, Saito S, Niizeki H, Kizaki H, Tadakuma T. Inhibition of programmed cell death by cyclosporin A; preferential blocking of cell death induced by signals via TCR/CD3 complex and its mode of action. Immunology. 1992;77:68–74. [PMC free article] [PubMed] [Google Scholar]
- Zambonin-Zallone A, Teti A, Carano A, Marchisio PC. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988;3:517–523. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.