Abstract
The mechanisms by which mammalian cells remodel the actin cytoskeleton in response to motogenic stimuli are complex and a topic of intense study. Dynamin 2 (Dyn2) is a large GTPase that interacts directly with several actin binding proteins, including cortactin. In this study, we demonstrate that Dyn2 and cortactin function to mediate dynamic remodeling of the actin cytoskeleton in response to stimulation with the motogenic growth factor platelet-derived growth factor. On stimulation, Dyn2 and cortactin coassemble into large, circular structures on the dorsal cell surface. These “waves” promote an active reorganization of actin filaments in the anterior cytoplasm and function to disassemble actin stress fibers. Importantly, inhibition of Dyn2 and cortactin function potently blocked the formation of waves and subsequent actin reorganization. These findings demonstrate that cortactin and Dyn2 function together in a supramolecular complex that assembles in response to growth factor stimulation and mediates the remodeling of actin to facilitate lamellipodial protrusion at the leading edge of migrating cells.
INTRODUCTION
Growth factor stimulated cell migration is dependent upon the synergistic activation of multiple structural and enzymatic proteins. An initial step in this process is the regulated disassembly and reorganization of the actin cytoskeleton that normally provides strength and form to static cells. This rigid organization is replaced by a more pliable, dynamic actin meshwork seen largely in the leading ruffle or lamellipodia of the cell. The Ras superfamily of small GTPases, including Rac1, RhoA, and Cdc42, play important roles in this reorganization process (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995). These regulatory switches are believed to activate multiple kinases while recruiting actin-associated proteins such as the WAVE/Wiskott Aldrich syndrome protein (WASp) family (Miki et al., 1996), the actin-related proteins of the Arp2/3 complex (Welch, 1999; Mullins, 2000), and cortactin (Weed et al., 1998) to the plasma membrane.
Recently, we reported that the large GTPase dynamin (Dyn2) is present in the ruffles of platelet-derived growth factor (PDGF)-stimulated fibroblasts and directly interacts with the filamentous actin (F-actin) binding protein cortactin (McNiven et al., 2000b). Dynamin possesses mechanochemical properties (Sweitzer and Hinshaw, 1998) that function to model membrane and sever various endocytic and secretory vesicles from donor compartments (for reviews, see Hinshaw, 2000; McNiven et al., 2000a). Cortactin, an F-actin binding protein whose activity is modulated upon phosphorylation by Src (Wu et al., 1991; Wu and Parsons, 1993; Huang et al., 1997), localizes to regions of dynamic actin and binds to the proline-rich domain (PRD) of Dyn2 via its C-terminal Src homology (SH) 3 domain (McNiven et al., 2000b). Furthermore, cortactin is proposed to function during cell motility, likely through enhancing actin filament nucleation and branching (Weed et al., 2000; Olazabal and Machesky, 2001).
To test how Dyn2 and cortactin might interact to regulate cell motility, we initially followed the distribution of these proteins by using immunofluorescence microscopy of NIH/3T3 fibroblasts stimulated with the motogen PDGF. PDGF stimulates Src kinase signaling cascades, resulting in reorganization of the actin cytoskeleton and the induction of cell motility (Parsons and Parsons, 1997; Heldin et al., 1998). Surprisingly, we found that only minutes after PDGF stimulation, both Dyn2 and cortactin were concentrated in numerous punctate spots that became organized into large circular structures or “waves.” These waves formed along the cell surface, constricted over time, and disappeared. Multiple waves may form in a cell but occur only once after stimulation. Most importantly, there was a substantial disassembly of actin stress fibers within the waves. These waves are comprised of multiple proteins in addition to Dyn2 and cortactin, including actin, the Wiskott Aldrich syndrome protein (N-WASp), the actin-related proteins of the Arp2/3 complex, and gelsolin. Importantly, wave formation required functional Dyn2 and cortactin, because expression of dominant-negative mutants or microinjection of purified Dyn2 antibodies inhibited wave formation and the reorganization of actin filaments. Thus, the physical interaction between these proteins seems to be regulated by growth factor stimulation as confirmed by morphological and biochemical observations.
Similar alterations in plasma membrane architecture have been reported by others observing morphological changes in PDGF-stimulated cells. In separate studies Mellstrom (Mellstrom et al., 1983, 1988) and Schliwa (Schliwa et al., 1984) observed actin-rich “dorsal ruffles” on the surface of PDGF- or phorbol ester (12-0-tetradecanoylphorbol-13-acetate)-treated human glia or fibroblast cells. They suggested that these structures may mediate actin cytoskeletal reorganization or receptor recycling. Recently, others have also observed these structures in PDGF-stimulated cells and identified the presence of several cytoskeletal and signaling proteins. Scott and colleagues observed that the WASp family member WAVE-1, as well as Abl-tyrosine kinase and the protein kinase A RII regulatory subunit are in these structures (Westphal et al., 2000). Furthermore, waves also contain Arf-GAPs (ASAP1 and ACAP1 and 2) as well as the adhesion proteins vinculin and paxillin (Jackson et al., 2000; Randazzo et al., 2000). These studies suggest that these proteins are involved in a signal-dependent, yet undefined cytoskeletal rearrangement process. Because time-lapse video microscopy of waves was not performed in these studies, the dynamics and transient nature of these structures were unclear. In this current study, we demonstrate that first, Dyn2 and cortactin are integral components of waves; second, waves are extremely dynamic, transient structures that generally occur coincident with the leading edge and constrict inward; and third, waves result in a disassembly of actin stress fibers that precede lamellipodial protrusion.
The findings presented herein demonstrate a novel functional interaction for both Dyn2 and cortactin in a transient, multiprotein complex that assembles in response to PDGF stimulation and functions to rapidly reorganize the actin cytoskeleton. Thus, the interaction of a large GTPase with mechanochemical properties (Dyn2) and a Src substrate, F-actin–binding, and regulatory protein (cortactin), provides the cell with a valuable tool to regulate and support dynamic membrane and cytoskeletal remodeling at the onset of cell motility.
MATERIALS AND METHODS
Immunological Reagents
Anti-dynamin polyclonal antibodies MC63, Dyn2, and anti-cortactin monoclonal antibody were used as described previously (McNiven et al., 2000b). Anti-dynamin polyclonal antibody MC65 (Henley et al., 1998) was used in the immunoprecipitation experiments. Dyn2 polyclonal antibody against the PRD was used in the antibody microinjection experiments. Anti-p34 polyclonal antibody was from L. Machesky (University of Birmingham, Birmingham, United Kingdom), anti-N-WASp polyclonal antibody was from P. Aspenström (Ludwig Institute of Cancer Research, Uppsala, Sweden), Arf6 rabbit polyclonal antibody was from J. Donaldson (National Institutes of Health, Bethesda, MD), cofilin antibodies were from J.S. Condeelis (Albert Einstein College of Medicine, Bronx, NY), and gelsolin antibodies were from H.L. Yin (University of Texas Southwestern, Dallas, TX). Antibodies against Rac1 (Upstate Biotechnology, Lake Placid, NY; Santa Cruz Biotechnology, Santa Cruz, CA.; and BD Biosciences, San Jose, CA), RhoA and Cdc42 (Santa Cruz Biotechnology) were used according to the manufacturers' instructions. The anti-ERK2 (Santa Cruz Biotechnology) and anti-active extracellular signal-regulated kinase (ERK)1/2 (Promega, Madison, WI) antibodies were used as directed. Phospho-tyrosine polyclonal antibody was from Promega.
Plasmids and Transfections
Full-length Dyn2(aa) and Dyn2(aa)ΔPRD-green fluorescent protein (GFP) were as reported previously (Cao et al., 1998; McNiven et al., 2000b). Full-length cortactin and CortΔSH3 were as described previously (McNiven et al., 2000b). All constructs were transfected into cells by using either the LipofectAMINE Plus reagent kit (Invitrogen, Carlsbad, CA) or GeneJammer transfection reagent (Stratagene, La Jolla, CA). Transfection conditions were according to the manufacturers' instructions. Transfected cells were grown for 36 h before experimentation to allow for sufficient expression of protein.
Immunofluorescence Localization and PDGF Stimulation
NIH/3T3, primary human foreskin fibroblasts (HFs), PANC-1, or PC-3 cells were plated on glass coverslips in media with 10% serum. After 24 h, the culture medium was replaced with media supplemented with 0.2% serum and the cells were cultured an additional 24 h before stimulation with human recombinant PDGF-bb (Peprotec; Invitrogen). The low-serum media was removed by aspiration and replaced with prewarmed low-serum media containing 30 ng/ml PDGF. After incubating for 5 min at 37°C, cells were rinsed twice with Dulbecco's phosphate-buffered saline, fixed and prepared for immunocytochemistry as reported previously (McNiven et al., 2000b). Digital images were acquired using IPLab (Scanalytics, Fairfax, VA) software and an OrcaII camera (Hamamatsu Photonics, Hamamatsu City, Japan) attached to an Axiovert 35 microscope (Carl Zeiss, Jena, Germany) equipped with a 100-W mercury arc lamp.
Live-Time Phase Contrast and Fluorescence Video Microscopy
For live-time imaging, cells were grown on glass in modified 35-mm imaging dishes. The growth conditions were as described above except the media were buffered with 15 mM HEPES, pH 7.2. An Axiovert 35 microscope (Carl Zeiss) equipped with a 37°C heated stage was used to acquire the images. For phase contrast imaging, a 40× dry lens (Carl Zeiss) with a 0.75 numerical aperture (NA) was used. For live-time fluorescence imaging a 63× oil immersion lens (Carl Zeiss) with 1.40 NA was used. Images were captured every 10 s for 5 min before exchanging the media for low-serum media supplemented with 30 ng/ml PDGF. Images were captured every 10 s for up to 60 min poststimulation. The images were acquired and processed using IPLab.
Quantitation of Actin Fluorescence Intensity in Dorsal Waves
Cells were prepared for visualization of actin as described above. All cells imaged for fluorescence intensity quantitation were acquired at identical exposures, from one coverslip, during a single 4-h period. Cells with waves were identified visually. IPLab software was used to define a region of interest (ROI), and the total fluorescence intensity per unit area within the ROI was determined. Three independent measurements using identically sized ROIs were taken outside the cell (background), inside the wave (postwave), and outside the wave (prewave) for each wave scored. The background fluorescence intensity value was subtracted from the post- and prewave values, and the percentage of reduction in intensity was determined for each wave. To address any contribution of actin intensity differences due to cell thickness, we transfected pEGFP-N1 to express soluble GFP in the cells. The intensity of the GFP signal was quantitated as described for the actin, and the actin intensities were normalized to these values. A total of 125 waves was used in the quantitation, and average values of all measurements were used to determine the final average percentage of difference.
Dyn2 Immunoprecipitation and Western Blotting
NIH/3T3 cells were grown to ∼60% confluence in 150-mm plastic culture dishes. Cells to be stimulated were serum starved for 24 h before PDGF stimulation. Unstimulated and stimulated (30 ng/ml PDGF for 5 min) cells were rinsed twice with ice-cold Dulbecco's phosphate-buffered saline and harvested by scraping in ice-cold suspension buffer containing protease inhibitors (Complete; Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors. The cell suspension was centrifuged at 500 × g for 2 min to obtain a cell pellet; 1.0 ml of radioimmunoprecipitation buffer minus SDS was added to the pellet and tumbled for 10 min at 4°C. The cell lysate was centrifuged at 13,000 × g for 10 min at 4°C to remove cell debris. Equal masses of whole cell extract were immunoprecipitated using anti-dynamin MC65 polyclonal antibody. The immunoprecipitated protein complexes were subjected to SDS-PAGE in a 15% polyacrylamide gel and electrotransferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). Western blot analysis was performed using monoclonal antibodies against cortactin, RhoA, and Cdc42 and polyclonal antibodies against Dyn2, p34, N-WASp, and Rac1. For ERK2, phospho-ERK1/2 and phospho-tyrosine blotting, 15.0 μg of total cell lysates was subjected to SDS-PAGE in an 8.5% polyacrylamide gel and electrotransferred to polyvinylidene difluoride membrane. The ERK2, anti-active ERK1/2 antibodies and anti-phospho-tyrosine antibodies were used as directed by the manufacturers. The ECL reagent kit (Amersham Biosciences, Piscataway, NJ) was used for detection and the membranes were exposed to BioMax x-ray film (Eastman Kodak, Rochester, NY).
Inhibition of Wave Formation by Using αDyn 2 Antibody Microinjection
NIH/3T3 cells cultured in 0.2% serum media were microinjected with either buffer containing 400 μM fluorescein isothiocyanate (FITC)-dextran (3000 mol. wt.; Molecular Probes) or buffer containing αDyn2 polyclonal antibody at 8.0 mg/ml + FITC-dextran. The cells recovered for 2 h at 37°C before stimulation with PDGF for 5 min. For F-actin visualization, cells were fixed and stained using rhodamine-phalloidin (Sigma-Aldrich, St. Louis, MO). Injected cells were identified via FITC-dextran and the presence of a wave was determined by visualizing actin rearrangement. The percentage of wave formation was scored in noninjected, buffer only (no antibody)-injected and antibody-injected cells from the same coverslip. The average percentage of wave formation was determined (as described above) from 100 injected cells from two independent experiments.
Quantitation of Wave Formation
Stimulated cells were processed for immunocytochemistry and costained with anti-dynamin MC63 or anti-cortactin together with rhodamine-phalloidin. Wave formation was determined by visual inspection and confirmed by its presence in both fluorescence channels. The average percentage of wave formation in cells expressing Dyn2(aa)ΔPRD or CortΔSH3 was determined from six independent experiments. At least 100 cells were counted in each experiment. The error bars reflect the SE.
Quantitation of Lamellipod Protrusion
Cells were grown for video microscopy as described above. For phase contrast imaging, a 10× dry lens with a 0.3 NA was used (Carl Zeiss). Images were captured every 5 s for 5 min. before exchanging the media with low-serum DMEM supplemented with 30 ng/ml PDGF. Images were captured every 5 s for an additional 40 min poststimulation. Cells were scored as positive or negative for waves as determined by visual inspection. Frames 1 and 400 (elapsed time 33.3 min) were compared, and outlines of the cell periphery were traced using Adobe Photoshop (Adobe Systems, Mountain View, CA). The area of lamellipod protrusion and the starting area of each cell were quantitated using NIH Image (values in square pixels). The ratio of the area of lamellipod protrusion to the initial area of the cell was calculated and expressed as a percentage; the higher the percentage, the greater the area of protrusion. Importantly, the total cell area before and after PDGF stimulation remained essentially unchanged, indicating that the change in area is due to lamella protrusion and not simply cell spreading. The average percentage of lamellipod protrusion values and SEs were calculated for each condition and graphed (cells with waves, n = 18; cells without waves, n = 17; unstimulated cells, n = 6).
RESULTS
Incorporation of Dyn2 and Cortactin into Dorsal “Waves” in PDGF-stimulated Cells
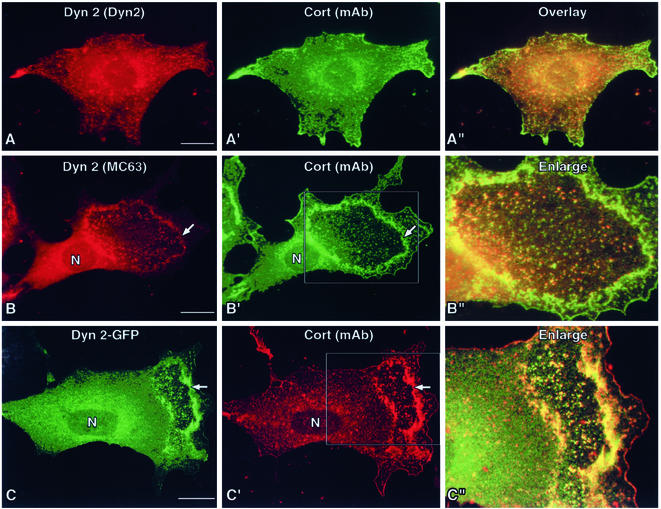
To test whether the organization of cortactin and Dyn2 changed upon stimulation with PDGF, NIH/3T3 fibroblasts were stimulated with 30 ng/ml PDGF. PDGF stimulation results in a robust motility response in cultured cells (Mellstrom et al., 1988; Parsons and Parsons, 1997; Heldin et al., 1998). After stimulation (1–5 min), cells were fixed and double stained with antibodies specific to Dyn2 and cortactin. Resting cells (no PDGF) possessed a normal cytoplasmic distribution of Dyn2 and cortactin along the cell cortex and internal membrane compartments (Figure 1A). Within minutes after stimulation (Figure 1, B–C"), substantial amounts of both proteins redistributed into numerous large puncta that assembled into circular waves. These Dyn2/cortactin spots were of a consistent size and congregated to form the edges of each wave. High-magnification images of waves demonstrated that the overlap between Dyn2 and cortactin in these structures was substantial. Furthermore, this colocalization was observed using multiple probes, including three different antibodies to each protein as well as GFP-tagged proteins (Figure 1; our unpublished data). Dorsal waves were not unique to fibroblasts, because they formed in multiple cell types, including NIH/3T3 and HFs, and two types of highly motile and aggressive neoplastic epithelial cell lines, human pancreatic ductular (PANC-1) and prostate (PC-3; our unpublished data).
Figure 1.
PDGF treatment induces the formation of Dyn2-cortactin–rich membrane waves. Immunofluorescence microscopy of NIH/3T3 cells, either resting or stimulated with 30 ng/ml PDGF-bb for 5 min and dual labeled with reagents to Dyn2 and cortactin. (A–A") In resting cells, both proteins associate modestly within the cell cortex and on internal membrane structures and cells show a flattened nonpolarized morphology. (B, B′ and C, C′) In contrast, cells exposed to PDGF show dramatic changes in shape and in the distribution of Dyn2 and cortactin proteins. Cell staining with multiple antibody reagents and GFP-tagged proteins show Dyn2 and cortactin seem to coassemble into punctate spots that coalesce into wave-like rings (arrows) marking the leading edge. (B" and C") Higher magnification images of the waves show substantial overlap of Dyn2 and cortactin in the puncta of the wave structures. Bars, 10 μm.
Optical sections through cells suggested that wave formation occurred along the dorsal membrane surface, compared with ventral Dyn2-cortactin structures such as podosomes (Ochoa et al., 2000). To confirm this, we performed scanning electron microscopy (SEM) on stimulated HF cells. In contrast to podosomes, SEM images of stimulated HF cells clearly resolved protrusions along the dorsal plasmalemma (our unpublished data). Higher magnification images revealed that the rims of the dorsal waves consisted of numerous bumps of uniform size.
Dorsal Waves Are Highly Dynamic and Incorporate Dyn2 As They Form and Progress
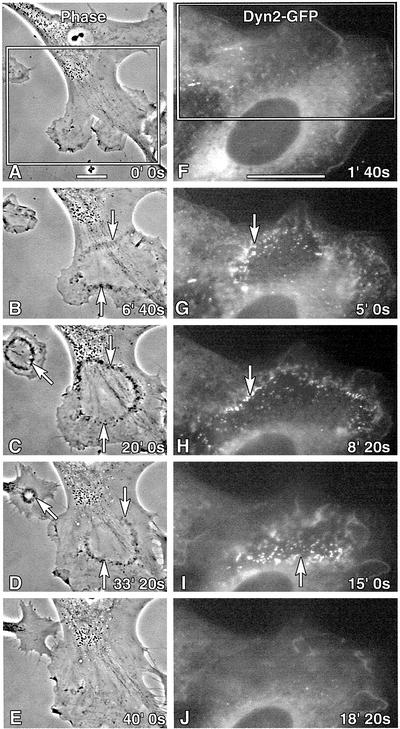
To observe wave formation in living cells, we performed time-lapse imaging of cultured fibroblasts. Both phase and fluorescence (Dyn2-GFP) microscopies were used in an attempt to follow membrane dynamics and Dyn2 distribution. As observed in the phase contrast image series (Figure 2, A–E), nonmotile, HF cells displayed multiple distinct, small protrusions at the moment of stimulation. These protrusions underwent modest ruffling, but the cells had not yet established a clear polarized phenotype (Figure 2A). Within 5 min after the addition of PDGF, phase dense particles were formed near the leading edge and coalesced into circular waves along the cell cortex (Figure 2B and video 1). Over the next 30 min, the waves constricted to a reduced diameter and disappeared (Figure 2, C–E). Concomitantly, the peripheral protrusions became more smooth and polarized, forming a single large lamellipodia with active ruffles. Fluorescent images of an NIH/3T3 fibroblast expressing Dyn2-GFP (Figure 2, F–J, and video 2) showed the fluorescent protein in the cortical cell ruffles at early times after stimulation. By 5 min, the Dyn2-GFP assembled into punctate foci that gathered at the edge of the circular wave (Figure 2G). As noted in the images of fixed cells, the Dyn2-GFP puncta were of uniform size and generally organized into linear chains that formed larger aggregates (Figure 2, G–I, and video 2). From this live imaging, we have made several significant conclusions. First, waves formed and disappeared within 30–40 min after PDGF stimulation. Second, waves formed predominantly at the anterior leading end of a cell. Third, waves formed only once while cortical membrane ruffling was continuous. And fourth, Dyn2-GFP is actively incorporated into waves.
Figure 2.
Waves are dynamic and actively incorporate Dyn2-GFP in living cells. Time-lapse images of living human foreskin (phase contrast, A–E, and video 1) or NIH/3T3 (Dyn2-GFP, F–J, and video 2) fibroblasts stimulated with PDGF. (A) HFs display multiple small lamellae at the cell periphery just after stimulation. (B) Within 6 min after PDGF treatment, phase-dense puncta coalesced into concentric waves at the anterior end of the cell (arrows). (C–E) Within 40 min after stimulation the circular waves have constricted to a tight annulus and disappeared. (E) Concomitantly, the individual lamellipodia “fused” to form one large, rounded leading edge. (F–J) NIH/3T3 cells expressing Dyn2-GFP and stimulated with PDGF. (G and H, arrows) Numerous Dyn2 puncta actively organized into wave structures within 5–10 min poststimulation. (H and I) Note the consistent size of the Dyn2 foci that seem to organize into linear chains that fuse together into larger spots or tubules. (J) Within 20 min, this wave has entirely disappeared. Bar, 10 μm.
Waves Function to Remodel the Actin cytoskeleton in Anterior Cytoplasm of Stimulated Cells
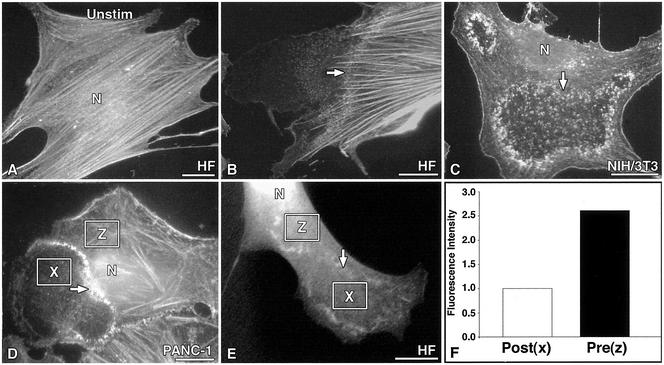
Because we had previously observed that Dyn2–cortactin interactions seemed to affect cell shape and the polymerization of actin filaments (McNiven et al., 2000b), we tested whether the dynamic dorsal waves altered actin distribution in stimulated cells. To this end, both fibroblasts and epithelial cells were examined. Cells were fixed either before or after PDGF stimulation and stained with the F-actin probe phalloidin (Figure 3). Static cells displayed numerous large actin stress fibers oriented uniformly along the long axis of the cell (Figure 3A). In contrast, 5 min after stimulation, cells displayed a dramatic remodeling of polymerized actin (Figure 3, B–D) and dorsal waves were easily resolved. Phalloidin staining in the waves was consistently punctate, characteristic of the distribution of cortactin and Dyn2 (Figures 1 and 2). Most evident was a marked decrease in the actin fluorescence of the cytoplasm distal to or “inside” the wave (Figure 3, B–D). Also, stress fibers seldom extended beyond or into the wave area. Persisting fibers were of a lower density (stress fibers/unit area), often looked thinner, and extended only a short distance into the waves (Figure 3, B–D). These observations suggested that a marked disassembly and reorganization of F-actin both in the fine subcortical meshwork and the large stress fibers was occurring.
Figure 3.
Loss of F-actin staining in stimulated cells corresponds with the sites of Dyn2-cortactin wave progression. Rhodamine-phalloidin staining of F-actin in resting vs. PDGF-stimulated cells. Three cell types are shown. (A) Resting cells display numerous, thick, brightly labeled stress fibers that traverse the long axis of the cell. (B–D) In contrast, stimulated cells display a dramatic loss of cortical actin and stress fibers that coincides with the advancing edge of the dorsal wave (arrows). Note the punctate organization of the actin within each wave structure and the marked difference of staining intensity on either side of the wave. (D) Actin fluorescence intensity was measured in the pre- (z) and postwave (x). (E) Fluorescence intensity values were normalized to cell thickness by expressing GFP alone and measuring the differences in intensity in the pre- (z) and postwave (x). (F) Quantitation of the average fluorescence intensity of actin for 125 waves (normalized for cell thickness; see MATERIALS AND METHODS). There is a 63% reduction of actin fluorescence in the anterior cytoplasm after wave migration. Bars, 10 μm.
To quantitate changes in levels of cytoplasmic F-actin proximal and distal to the waves, stimulated cells were stained with phalloidin, and the average pixel intensity was measured within defined boxes inside and outside the wave (Figure 3, D and E). A substantial 63.0% reduction in the average fluorescence intensity of actin staining in the postwave cytoplasm was measured (Figure 3F). To correct for the possibility of changes in fluorescence intensity due to cell thickness, GFP was expressed in these cells, and the actin intensity was normalized to these values (Figure 3E). The distribution of GFP throughout the cytoplasm was uniform, with only a small enrichment in large membrane distortions (our unpublished data). This control indicated that the areas inside and outside of the waves are of similar thickness. These findings confirmed that the transient Dyn2-containing waves mediated an ordered disassembly and reorganization of F-actin in the cell periphery.
Actin Remodeling Proteins Are Selectively Incorporated into Dyn2–Cortactin Wave Complex
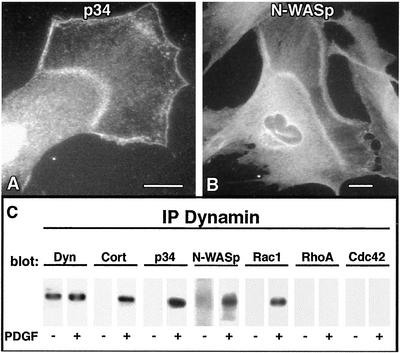
The evidence for a Dyn2–cortactin complex acting to regulate actin dynamics in cells is expanding (McNiven et al., 2000b; Ochoa et al., 2000; Lee and De Camilli, 2002; Orth et al., 2002). Because Dyn2, cortactin, and actin localized to the punctate foci in waves, we tested whether additional actin-binding proteins that regulate actin polymerization might also be a part of this dynamic remodeling complex. Because cortactin has been shown to associate with the Arp2/3 complex (Weed et al., 2000), we tested whether this actin-related protein complex, and the associated scaffolding protein N-WASp, were incorporated into the waves. PDGF-stimulated cells were stained with antibodies to a component of the Arp2/3 complex (p34) and N-WASp. Both of these antibodies stained the dorsal waves prominently, with an intensity and localization largely overlapping the Dyn2 and cortactin stain (Figure 4, A and B).
Figure 4.
Actin nucleation and branching proteins p34 (Arp2/3) and N-WASp are prominent components of dorsal waves. PDGF stimulation induces formation of a multicomponent protein complex. (A and B) Immunocytochemical staining of stimulated cells with antibodies to the p34 subunit of the Arp2/3 complex and N-WASp in stimulated NIH/3T3 fibroblasts. (C) Dyn2 immunoprecipitation from resting vs. PDGF-stimulated NIH/3T3 cells. Under resting conditions, dynamin antibodies (MC65) pull down Dyn2, modest levels of N-WASp, and no detectable cortactin or small GTPases. In contrast, in stimulated cells substantial amounts of cortactin, N-WASp, p34, and Rac1 are coimmunoprecipitated from the extract. Note that RhoA and Cdc42 are not immunoprecipitated. All proteins migrated at their expected molecular mass and were aligned to facilitate comparison. Representative results are shown. −, no stimulation; +, stimulation with 30 ng/ml PDGF. Bars, 10 μm.
To further test that Dyn2, cortactin, Arp2/3, and N-WASp comprised a dynamic complex that was assembled during stimulation, NIH/3T3 cells were treated for 5 min with PDGF or carrier alone and harvested for coimmunoprecipitation experiments. Parallel samples of stimulated and unstimulated cells were solubilized under nondenaturing conditions and Dyn2 was precipitated using a pan-dynamin antibody (MC65) to the N-terminal domain. The immunoprecipitated Dyn2 samples were blotted for multiple putative binding partners and actin regulatory proteins as had also been tested using immunocytochemistry. Figure 4C shows a representative blot of multiple experiments comparing proteins associated with Dyn2 from resting (−) and stimulated (+) cells. Although only modest or undetectable amounts of cortactin coprecipitated with Dyn2 in resting cells, this was increased considerably in the samples from PDGF-stimulated cells, as were p34 (Arp2/3), N-WASp, and Rac1; RhoA and Cdc42 were not observed to associate with the Dyn2 complex under either condition. These biochemical findings are consistent with the immunofluorescence observations described above, and support the concept that a complex containing Dyn2, cortactin, N-WASp, Arp2/3, F-actin, and other proteins is assembled within dorsal waves in response to motogenic stimulation.
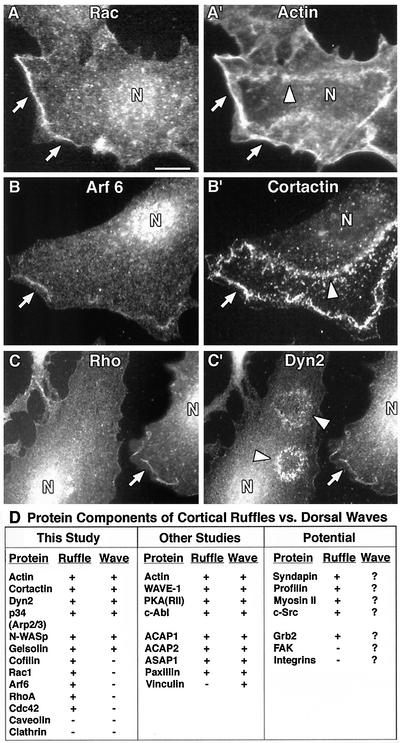
Because several small GTPases, including Rac1, Arf6, and RhoA, function as upstream regulators of the actin cytoskeleton in response to growth factors, and because Rac1 was immunoprecipitated in the Dyn2 complex, we tested whether these proteins were components of dorsal waves by using immunofluorescence. Surprisingly, as revealed by costaining with actin, cortactin, or Dyn2, all of the three Rac1 antibodies used stained cortical ruffles (CRs) but did not label the waves (Figure 5, A and A′). Similar to Rac1, Arf6, and RhoA prominently labeled cortical ruffles but did not label the waves proper (Figure 5, B, B′ and C, C′). The table in Figure 5D compares the components of cortical ruffles with those of dorsal waves and shows similar but distinct proteins between the structures. It is important to mention that even though the small GTPases we tested did not localize to waves, it does not eliminate their potential function as upstream “switches” that govern actin-dependent dorsal wave and cortical ruffle formation.
Figure 5.
Dorsal waves and cortical ruffles contain similar but distinct protein components. NIH/3T3 cells were stimulated with PDGF, fixed, and stained for numerous actin regulatory proteins. (A and A′) Rac1 staining with multiple reagents demonstrated significant labeling and colocalization with actin (and Dyn2 and cortactin; our unpublished data) in cortical ruffles, but not in the wave proper (arrows vs. arrowheads). (B, B′ and C, C′) Similarly, Arf6 and RhoA labeled cortical ruffles (arrows), but not waves (arrowheads), as revealed by cortactin (B′), Dyn2 (C′), and actin (our unpublished data) colabeling. These data suggest that these small GTPases are not sequestered within the Dyn2-positive dorsal waves. (D) Table summarizing cortical ruffle vs. dorsal wave components identified in this study and others. All components were identified using fluorescence microscopy. For references of proteins identified in other studies please see the text. +, positive immunofluorescence staining; −, negative staining; ?, currently unknown.
Inhibition of Dyn2 and Cortactin Interaction Prevents Wave Formation
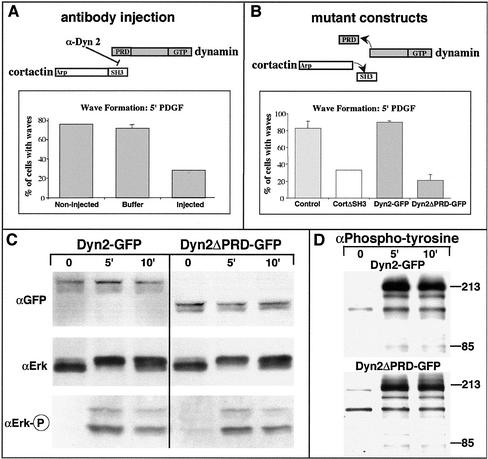
The data mentioned above demonstrated that Dyn2 and cortactin are significant components of circular dorsal waves (Figure 1). We next tested whether these proteins were required for wave formation. Previously, we showed that removal of the PRD and SH3 domains of Dyn2 and cortactin, respectively, prevented a Dyn2 and cortactin interaction (McNiven et al., 2000b). This was achieved in vivo through the expression of truncated dominant-negative proteins lacking the interaction domains and in vitro through the action of a Dyn2 PRD-specific antibody. These mutant proteins and inhibitory antibodies were used to test whether wave formation could be delayed or prevented by perturbing Dyn2–cortactin interaction and/or function. NIH/3T3 cells were either microinjected with affinity-purified Dyn2 PRD-specific antibodies or transfected with Dyn2ΔPRD-GFP or CortΔSH3 plasmids. After a 2-h (for antibody injection) or 36-h (plasmid transfection) recovery period, cells were stimulated, fixed, and stained for waves by using rhodamine-phalloidin. The number of nascent dorsal waves were counted and compared with cells injected with control buffers or transfected with wild-type constructs. Approximately 80% of control cells and cells expressing wild-type cortactin or Dyn2 formed dorsal waves. However, only 20–30% of cells injected with the Dyn2 PRD-specific antibody or expressing truncated mutant proteins formed waves (Figure 6, A and B). To demonstrate that the Dyn2ΔPRD protein was structurally inhibiting wave formation, and not inhibiting upstream signaling events through a block in receptor endocytosis, we expressed the truncated protein in NIH/3T3 cells and assayed for the integrity of mitogen-activated protein kinase signaling (Figure 6C). ERK1/2 activation was measured in cells expressing wild-type and truncated Dyn2 by immunoblotting for ERK2 and phosphorylated ERK1/2 in serum-starved cells and in cells stimulated for 5 and 10 min (Figure 6C). On PDGF stimulation, the characteristic up-shift of the ERK2 band was equally as robust in wild-type and mutant Dyn2-expressing cells (Figure 6C). This result was confirmed by blotting the cellular extracts for phosphorylated (active) ERK1/2. No active ERK1/2 was detected in serum-starved cells (0 min), and upon stimulation with PDGF, ERK1/2 was as efficiently phosphorylated in cells expressing Dyn2ΔPRD as in cells expressing wild-type Dyn2 (Figure 6C). Densitometric quantitation of the phospho-ERK1/2 bands confirmed that phosphorylation levels were within 95% of those obtained from mock transfected cells (our unpublished data). We also assayed for effects on global tyrosine phosphorylation in cells expressing the truncated Dyn2 (Figure 6D). The tyrosine phosphorylation pattern seemed identical in mutant and wild-type–expressing cells (Figure 6D). These experiments indicate that a functional/structural interaction between Dyn2 and cortactin is required for wave formation and that inhibition of wave formation was not due to impaired PDGF-receptor signaling.
Figure 6.
Wave formation is attenuated by inhibiting the Dyn2 and cortactin interaction, whereas downstream signaling is unaffected. (A) Quantitation of wave formation in PDGF-stimulated NIH/3T3 cells after injection with an anti-Dyn2 PRD-specific antibody. Cells injected with affinity-purified antibodies showed a 62.4% reduction in the formation of waves. Note that buffer-injected cells showed no reduction in wave formation. More than 50 cells were quantitated for each injectate. (B) Quantitation of reduced wave formation in NIH/3T3 fibroblasts expressing either truncated Dyn2 (Dyn2ΔPRD) lacking the PRD or truncated cortactin protein lacking its C-terminal SH3 domain (CortΔSH3). More than 80% of cells expressing wild-type constructs formed waves. In contrast, cells expressing mutant truncated proteins showed a 74.3% (Dyn2ΔPRD) and 60.2% (CortΔSH3) reduction in wave formation. More than 100 cells were quantitated for each construct expressed. (C) Immunoblot analysis of extracts from cells expressing wt Dyn2 or Dyn2ΔPRD-GFP by using antibodies to the downstream signaling protein ERK1/2. Total cell lysates from unstimulated (0) and stimulated (5 and 10 min) NIH/3T3 cells expressing Dyn2-GFP or Dyn2ΔPRD-GFP were immunoblotted for GFP, to show the full-length or truncated expressed forms, ERK2, and activated (phosphorylated) ERK1/2. On stimulation, a characteristic up-shift of ERK2 (phosphorylation) was seen (compare 0 min to 5 and 10 min) in cells expressing either wt or truncated Dyn2. The same blot probed with antibodies to phospho-ERK1/2 showed that the expression of Dyn2ΔPRD did not inhibit the signaling pathway. (D) Cell lysates were also probed with antibodies to phospho-tyrosine. Again, both control and mutant cells showed identical phosphorylation patterns. The level of Dyn2 expression in the cells was consistent in all conditions. Transfection efficiency of the cells was estimated to be 70% (GFP fluorescence).
Wave Formation Is Required for Protrusion of Lamellipodia
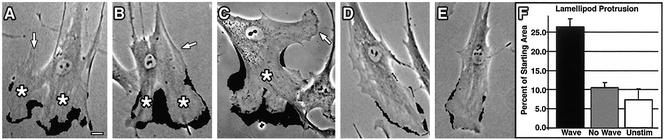
Consistent with the concept that Dyn2-cortactin waves mediate cytoskeletal remodeling in the front end of growth factor-stimulated cells, we observed two significant characteristics of wave formation. First, waves almost always formed in proximity to the leading edge of a cell (Figure 7, A–C, asterisks) and second, significant lamellipodial protrusion occurred only from areas at which waves formed (Figure 7, A–C, shaded areas). Peripheral areas of cells with waves that were not associated with waves protruded only modestly (Figure 7, A–C, arrows). Furthermore, the leading edge of stimulated cells that did not assemble waves did not undergo significant lamellipodial protrusion, compared with those that formed waves (Figure 7, D and E vs. A–C, shaded areas; and F). To quantitate this protrusion, HFs were either stimulated with PDGF or maintained in low-serum conditions and then imaged using phase microscopy. The average percentage of cell area that was protruded by a leading lamellipodia >33 min was determined (Figure 7F). Cells that formed waves (Figure 7, A–C, asterisks) underwent a rapid and dynamic lamellipod protrusion of ∼25% of the original starting area, on average >1000 μm2 (Figure 7F and video 1). Furthermore, robust lamellae protrusion was confined to where the wave formed and did not occur at different regions of the cell periphery (Figure 7, A–C, asterisks vs. arrows). In contrast, cells on the same coverslip that were stimulated but that did not form a wave did undergo membrane ruffling but protruded only 11% of the starting cell area, on average only 374 μm2 (Figure 7F). Control cells that were maintained in low-serum conditions (0.2% serum) protruded only 7.5% of the starting cell area (Figure 7F). These observations suggest that wave formation and progression promote lamellipodial protrusion.
Figure 7.
Wave formation is required for active lamellipod protrusion. Phase microscopy of PDGF-stimulated HFs showing the position of waves (*) in relationship to newly formed lamellipod protrusions. (A–C) Cells that formed waves actively protruded membrane (shaded areas). Arrows indicate regions of the periphery distal to waves that subsequently did not protrude membrane. (D and E) Cells stimulated with PDGF that did not form waves exhibited only very modest protrusion (shaded areas). (F) Quantitation of the percentage of total cell area protruded in stimulated cells that formed waves (black bar) vs. stimulated cells that did not form waves (gray bar) compared with unstimulated cells (white bar). Note that wave formation led to more than a 2.5-fold increase in the lamellipod area protruded and that this occurred only along membrane adjacent to waves. Bar, 10 μm.
DISCUSSION
We observed that immediately after treatment with PDGF, both Dyn2 and cortactin localized rapidly into large circular structures on the dorsal surface, near the cells' leading edges (Figure 1). It seems that Dyn2 and cortactin interaction is required to support wave formation and reorganization of polymerized actin, because waves do not occur upon expression of truncated dominant-negative forms of these proteins. Because both dynamin and cortactin interact with a variety of proteins via their PRD and SH3 domains, respectively, truncation of these proteins may have a variety of affects. Our conclusions are supported by additional observations such as injection of a Dyn2 PRD-specific antibody (Figure 6, A and B), and most importantly, the marked physical and temporal colocalization of dynamin and cortactin spots that coassemble in the wave structure. Furthermore, we found that waves contain only certain cytoskeletal and signaling proteins, including the Arp2/3 complex and N-WASp (Figures 4 and 5). Most importantly, there was a dramatic 63% reduction of polymerized actin within the postwave, suggesting that these structures remodel the actin cytoskeleton of the stimulated cells in response to motogenic stimulation (Figure 3). Studies by other groups have examined tyrosine receptor kinase signaling in the context of dynamin mutants and have suggested that some downstream signaling cascades are left unchanged despite an inhibition of receptor internalization (Vieira et al., 1996; Kao et al., 1998). Significantly, tyrosine kinase signaling was not inhibited by the expression of truncated Dyn2 (Figure 6, C and D). Together, these findings are consistent with the concept that disrupting Dyn2–cortactin interactions by removing the respective binding domains directly reduces wave formation as opposed to simply attenuating cell signaling.
Dorsal Waves Are Novel Multiprotein Complexes Distinct from Cortical Ruffles or Lamellipodia
Our current findings suggest that dorsal waves are distinct from the CRs and lamellipodia described in the INTRODUCTION. This is based on several criteria. First, CRs, although dynamic, occur as constant features of a migrating cell that may form and disappear continuously. In contrast, dorsal waves are ephemeral structures that form rapidly and occur only once after growth factor stimulation. Second, the morphologies of CRs, represented by large sheets of folded membrane, differ dramatically from the collection of membrane bumps and blisters that comprise dorsal waves. The wave structures described herein result in a marked deformation of the dorsal plasma membrane that can be resolved with phase microscopy or SEM (Figure 2; our unpublished data). Although we cannot exclude that these structures traverse and propagate through the dorsal-ventral axis of the cell, clearly the most obvious structural event occurs at the dorsal cell surface. Third, as expanded upon below, unlike CRs, dorsal waves seem to restructure the actin cytoskeleton and facilitate the protrusion of lamellipodia. Fourth, the protein components of these two dynamic structures are similar but distinct. As depicted in Figure 5, immunofluorescence staining by us and others shows a substantial amount of the small GTPases Rac1, Arf6, RhoA, and Cdc42 in the CRs but not in dorsal waves. Although the Dyn2 immunoprecipitation did pull down Rac1 from stimulated cells, we were unable to definitively localize this protein to the wave proper (Figure 5, A and A′), suggesting that a stimulated interaction at other cellular locations, for example, cortical membrane ruffles, may contribute to the band in the immunoprecipitation, or that Rac1 is of very low abundance in the wave. In support of this, Rac1, but not Cdc42 or RhoA, is known to recruit cortactin to cortical ruffles (Weed et al., 1998). Thus, it is possible that Dyn2 is associated with cortactin and Rac1 in cortical ruffles and not waves, as the immunoprecipitation and morphological data suggest. Furthermore, although RhoA and Cdc42 stain cortical membrane ruffles this does not necessitate that they are associated with Dyn2 or that they are in a Dyn2 complex within the ruffle.
Dyn2- and Cortactin-dependent Dorsal Waves Participate in Actin Remodeling in Response to PDGF Stimulation
Past studies suggested, but did not demonstrate, that dorsal waves might function as cytoskeletal remodelers (Mellstrom et al., 1983; Schliwa et al., 1984; Mellstrom et al., 1988). Herein, insights into this process were provided using microscopy of stimulated cells stained for actin, Dyn2, and cortactin (Figures 1–3). Our findings are consistent with the concept that the primary function of dorsal waves is to solate the cytoplasm proximal to the leading edge of a stimulated cell, thereby preparing a cell for motility. This prediction is supported by the observations that, first, there was a substantial disassembly (63%) of bundled actin filaments within the wave area (Figure 3); and second, there was an enrichment of Arp2/3, cortactin, and N-WASp in the waves (Figures 1 and 4); these proteins are required for lamellipodia formation and motility. Taken together, these findings suggest that dorsal waves mediate the disassembly of a strong and rigid actin cytoskeleton, allowing for the formation of a pliable and dynamic leading edge needed to support Arp2/3-N-WASp–dependent membrane protrusion and cell motility. Indeed, this prediction is supported by the observations described in Figure 7 that show a substantial statistical probability of lamellipodia formation and protrusion occurring in proximity with and just subsequent to wave formation. Thus, we currently view dorsal waves as a related but distinct precursor to a nascent lamellipod.
How might Dyn2, cortactin, and the other wave protein components mediate these phenomena? We have been unable to identify any traditional endocytic processes occurring at the site of wave assembly. Staining of stimulated cells with antibodies to several endocytic markers, including clathrin, AP2, caveolin 1, or caveolin 2 did not label waves structures (Figure 5D). Instead, Dyn2, cortactin, and additional proteins, including the Arp2/3 complex, N-WASp, and gelsolin, may actively participate in the disassembly and remodeling of actin in the waves. This model is supported by the observations in this study and our previous findings that show an inhibition of Dyn2-cortactin binding induced a marked accumulation of F-actin in cells (McNiven et al., 2000b; Orth et al., 2002). Additional support comes from in vitro data from Schafer et al. (2002) demonstrating that Dyn2′s binding of cortactin regulates cortactin's ability to stimulate Arp2/3-dependent actin nucleation and that Dyn2 regulates F-actin organization (Schafer et al., 2002). Thus, a functional interaction between Dyn2 and cortactin seems to be important for regulating actin polymerization and organization in a variety of cell structures as discussed below.
Participation of Dyn2 and Cortactin in Other Dynamic, Actin-mediated Plasma Membrane Remodeling Events
The observations reported in this study are consistent with several past and current studies involving Dyn2, cortactin, and plasma membrane dynamics. Studies by Banker et al. identified actin- and cortactin-rich wave structures in cultured hippocampal neurons (Ruthel and Banker, 1998, 1999). These waves progressed to the growth cones where they mediated rapid and extensive protrusion, resulting in net-outgrowth of the peripheral membrane. The mechanism of growth cone protrusion was not defined in the Banker studies, but it was suggested that the wave might function to recruit protrusion machinery to the site of function. Whether dynamin functions during growth cone extension is unknown. Podosomes also look structurally similar to the dorsal waves reported herein. Unlike dorsal waves, podosomes are found on the ventral cell surface of migrating macrophages (DeFife et al., 1999; Linder et al., 1999), osteoclasts (Soriano et al., 1991; Tanaka et al., 1996), and cultured cells expressing constitutively active Src (Tarone et al., 1985; Nitsch et al., 1989). Podosomes mediate the deformation of the ventral plasma membrane during motility and/or bone reabsorbtion during osteogenesis. DeCamilli and colleagues (Ochoa et al., 2000) have demonstrated that podosomes contain Dyn2, cortactin, and vinculin, all of which have been found in dorsal waves (Figure 1; Randazzo et al., 2000). We believe that the dorsal waves studied herein are distinct from podosomes for multiple reasons. First, and most importantly, unlike podosomes, dorsal waves are transient, occurring only once in response to growth factor stimulation. Second, as stated above, podosomes are ventral, whereas waves are dorsal. Third, dorsal waves mediate stress fiber disassembly, which has not been shown for podosomes. Finally, other ventral membrane structures that are similar to podosomes, called “invadopodia” (Bowden et al., 1999), have been described in highly invasive, metastatic tumor cells. These structures contain the Dyn2 binding partner cortactin, resemble podosomes morphologically, and have been proposed to function as a site for exocytic release of specific metalloproteases that act to degrade extracellular matrix and facilitate invasion. Buccione and colleagues (Baldassarre et al., 2003) have recently observed that Dyn2 is a prominent component of invadopodia and is required to mediate matrix degradation. Whether other Dyn2 binding partners are components of invadopodia is currently under study. Thus, Dyn2 and its associated proteins seem to work together to mediate deformation of the plasma membrane and reorganization of the actin cytoskeleton to support related but distinct dynamic cellular processes. Identifying additional proteins or lipid-modifying components in these structures, while further defining how these transient structures function, represents a novel and important future direction for the field of cell motility and metastasis.
Supplementary Material
ACKNOWLEDGMENTS
We thank S.G. Weller, J. Chen, B.J. Oswald, and T.E. Peterson for technical assistance; P. Aspenström, J.S. Condeelis, J. Donaldson, L.M. Machesky, and H.L. Yin for antibodies and plasmids; and M.A. Accola, N.W. Gray, H.M. Thompson, S.G. Weller, and Y. Yoon for advice on this manuscript. This study was supported by funding from the Mayo Clinic and National Institutes of Health (grant DK44650 to M.A.M.).
Footnotes
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0466. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0466.
REFERENCES
- Baldassarre M, Pompeo A, Castaldi C, Cortelino S, Beznoussenko G, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;13:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCm associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven M. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFife KM, Jenney CR, Colton E, Anderson JM. Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J Histol Cytol. 1999;47:65–74. doi: 10.1177/002215549904700107. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochem Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Yansong N, Wang T, Gao Y, Haudenschild CC, Zhan X. Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:13911–13915. doi: 10.1074/jbc.272.21.13911. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are Arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol. 2000;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, Ceresa BP, Santeler SR, Pessin JE. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J Biol Chem. 1998;273:25450–25457. doi: 10.1074/jbc.273.39.25450. [DOI] [PubMed] [Google Scholar]
- Lee E, De Camilli P. Dynamin at actin tails. Proc Natl Acad Sci USA. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci USA. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA, Cao H, Pitts KR, Yoon Y. Pinching in new places: multiple functions for the dynamin family. Trends Biochem Sci. 2000a;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Interactions between dynamin and the actin binding protein cortactin modulate cell shape. J Cell Biol. 2000b;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellstrom K, Heldin CH, Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988;177:347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- Mellstrom K, Hoglund AS, Nister M, Heldin CH, Westermark B, Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983;4:589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- Mullins RD. How WASP-family proteins, and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr Opin Cell Biol. 2000;12:91–96. doi: 10.1016/s0955-0674(99)00061-7. [DOI] [PubMed] [Google Scholar]
- Nitsch L, Gionti E, Cancedda R, Marchisio PC. The podosomes of Rous sarcoma virus transformed chondrocytes show a peculiar ultrastructural organization. Cell Biol Int Rep. 1989;13:919–926. doi: 10.1016/0309-1651(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ochoa G-C, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Cao H, McNiven M, Baron R, De Camilli P. A functional link between dynamin and the actin cytoskeleton at podosome. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal IM, Machesky LM. Abp1p and cortactin, new “hand-holds” for actin. J Cell Biol. 2001;154:679–682. doi: 10.1083/jcb.200105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci USA. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Peterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil Cytoskeleton. 1998;40:160–173. doi: 10.1002/(SICI)1097-0169(1998)40:2<160::AID-CM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Role of moving growth cone-like “wave”structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol. 1999;39:97–106. doi: 10.1002/(sici)1097-4695(199904)39:1<97::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin 2 and cortactin regulate actin assembly and filament organization. Curr Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Nakamura T, Porter KR, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy JB, Baron R. c-Cbl is downstream of c-Src in a signaling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Weed S, Du Y, Parsons J. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111:2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999;9:423–427. doi: 10.1016/s0962-8924(99)01651-7. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.