Abstract
The Schizosaccharomyces pombe spo14-B221 mutant was originally isolated as a sporulation-deficient mutant. However, the spo14+ gene is essential for cell viability and growth. spo14+ is identical to the previously characterized stl1+ gene encoding a putative homologue of Saccharomyces cerevisiae Sec12, which is essential for protein transport from the endoplasmic reticulum (ER) to the Golgi apparatus. In the spo14 mutant cells, ER-like membranes were accumulated beneath the plasma membrane and the ER/Golgi shuttling protein Rer1 remained in the ER. Sec12 is a guanine nucleotide exchange factor for the Sar1 GTPase. Overproduction of psr1+ coding for an S. pombe Sar1 homologue suppressed both the sporulation defect of spo14-B221 and cold-sensitive growth of newly isolated spo14-6 and spo14-7 mutants. These results indicate that Spo14 is involved in early steps of the protein secretory pathway. The spo14-B221 allele carries a single nucleotide change in the branch point consensus of the fifth intron, which reduces the abundance of the spo14 mRNA. During meiosis II, the forespore membrane was initiated near spindle pole bodies; however, subsequent extension of the membrane was arrested before its closure into a sac. We conclude that Spo14 is responsible for the assembly of the forespore membrane by supplying membrane vesicles.
INTRODUCTION
Sporulation, gametogenesis in yeasts, involves two overlapping processes, meiosis and spore formation. Four haploid nuclei produced by meiosis are packaged into individual spores. Spore formation begins with the assembly of a double-layered intracellular membrane, termed the forespore membrane (Yoo et al., 1973). Spore wall material is deposited in the lumenal space of the forespore membrane, and its inner leaflet becomes the plasma membrane of spores. The forespore membrane formation provides a model system for the study of de novo synthesis of membranes within the cytoplasm.
Electron microscopic studies (Yoo et al., 1973; Hirata and Tanaka, 1982; Tanaka and Hirata, 1982; Kishida et al., 1990) and recent fluorescence microscopic observations (Asakawa et al., 2001; Nakamura et al., 2001; Nakase et al., 2001; Nakamura et al., 2002) with the fission yeast Schizosaccharomyces pombe have revealed fundamental aspects of forespore membrane formation. The formation of this membrane begins during meiosis II by the fusion of membrane vesicles. The spindle pole body (SPB) in yeast is an equivalent structure to the centrosome in animals and plays a crucial role in the formation of spindle microtubules. The SPB undergoes a morphological change into a multilayered structure before forespore membrane assembly during meiosis II. Using immunofluorescence microscopy, Hagan and Yanagida (1995) observed the alteration of the SPB structure from a dot to a crescent in the corresponding stage of meiosis. Because most of the sporulation-deficient mutants fail to modify the SPB, this SPB change might be indispensable for sporulation (Hirata and Shimoda, 1994). We have recently identified a novel coiled-coil protein, named Spo15, that is associated with the SPB and is essential for its modification (Ikemoto et al., 2000).
After the SPB modification, membrane vesicles are gathered and fuse to generate forespore membranes near the modified SPB (Hirata and Tanaka, 1982; Tanaka and Hirata, 1982). The sporulation-specific gene product Spo3 is a potential membrane protein and localizes to the forespore membrane (Nakamura et al., 2001). The assembly of this membrane is defective in the spo3 null mutant. One of the spo3 alleles, spo3-KC51, is dose-dependently suppressed by psy1+, which encodes a protein similar to mammalian syntaxin-1A, a component of the plasma membrane docking/fusion complex. In fact, Psy1 localizes to the plasma membrane during vegetative growth. The psy1+ gene is essential for vegetative growth, and its transcription is further enhanced during sporulation. Interestingly, Psy1 disappeared from the plasma membrane of mother cells immediately after the first meiotic division and relocalized to the nascent forespore membrane. These results support the idea that the forespore membrane is assembled by the fusion of membrane vesicles assisted by a syntaxin-like protein Psy1 (Nakamura et al., 2001).
The origin of these vesicles for the extension of the forespore membrane remains to be elucidated. One plausible mechanism is that the forespore membranes are assembled by the fusion of vesicles from the endoplasmic reticulum (ER) and/or the Golgi apparatus. Transport of membrane vesicles is carried out by a set of SEC gene products. The yeast secretory pathway was elucidated by analyzing a number of temperature-sensitive mutants that are defective in the regulatory or constitutive transport machinery (Novick et al., 1980). Some late-acting SEC genes have been reported to be necessary for sporulation in both Saccharomyces cerevisiae and S. pombe (Neiman, 1998; Nakase et al., 2001).
In this study, we have characterized the spo14+ gene product and analyzed phenotypes of spo14 mutants. Spo14 is identical to Stl1 (d'Enfert et al., 1992), which was supposed to be structurally and functionally related to the S. cerevisiae Sec12. Sec12 is a type II membrane protein, which regulates vesicle transport from the ER to the Golgi apparatus (Novick et al., 1980; Nakano et al., 1988; Kaiser and Schekman, 1990; d'Enfert et al., 1991, Rexach and Schekman, 1991). This protein is known as a GEF (GTP-GDP exchange factor) for a small GTPase, Sar1 (Barlowe and Schekman, 1991), which plays an essential part in the formation of transport vesicles at the ER (Nakano and Muramatsu, 1989; d'Enfert et al., 1991). Our analyses reported here support the notion that Spo14 is an S. pombe homologue of the budding yeast Sec12 that is essential for vesicle budding from ER. Furthermore, we examined defects in the forespore membrane assembly in spo14-B221 mutants in detail to elucidate the significance of the proteins in the secretion pathway in sporulation.
MATERIALS AND METHODS
Yeast Strains, Media, and Transformation
The S. pombe strains used in this study are listed in Table 1. The media used in this study have been previously described (Egel and Egel-Mitani, 1974; Gutz et al., 1974; Moreno et al., 1990). S. pombe cells were grown at 30°C and sporulated at 28°C unless otherwise stated. For examination of the sporulation defects in the spo14-B221 mutant, sporulation was induced at 23°C.
Table 1.
Strains
| Strain | Mating type | Genotype | Source |
|---|---|---|---|
| TN29 | h90 | leu1-32 ura4-D18 | Ikemoto et al. (2000) |
| TN8 | h90 | leu1-32 | Nakamura et al. (2001) |
| TN52 | h90 | ade6-M210 | This work |
| MKW5 | h90 | This work | |
| B221 | h90 | spo14-B221 ade6-M210 | Bresch et al. (1968) |
| MK14 | h90 | spo14-B221 | This work |
| MK14L | h90 | spo14-B221 leu1-32 | This work |
| MK05 | h90/h90 | ade6-M210/ade6-M216 | This work |
| MK06 | h90/h90 | spo14-B221/spo14-B221 ade6-M210/ade6-M216 | This work |
| MK07 | h90/h90 | ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 | This work |
| MKD14 | h90 | ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 spo14+/spo14∷ura4+ | This work |
| RM14 | h90 | spo14∷ura4+ ade6-M210 ura4-D18 leu1≪ spo14+ | This work |
| RM14-7 | h90 | spo14∷ura4+ ade6-M216 ura4-D18 leu1≪ spo14-7 | This work |
| MK14H | h90 | spo14∷ura4+ ade6-M210 leu1-32 ura4-D18 +pAL(spo14-HA) | This work |
| TN196 | h90 | spo14-B221 spo20-KC104 ade6-M210 | This work |
| TN226 | h90 | rer1-GFP≪ura4+ ura4-D18 | This work |
| TN230 | h90 | rer1-GFP≪ura4+ leu1-32 ura4-D18 | This work |
Cloning of spo14+
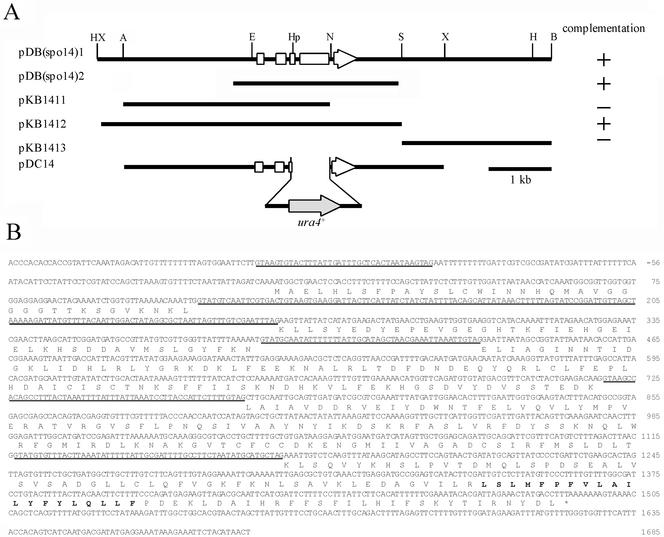
The spo14-B221 mutant (MK14L) was transformed with an S. pombe genomic library containing partial Sau3AI DNA fragments constructed in a multicopy plasmid, pDB248′ (Beach and Nurse, 1981). Approximately 105 independent Leu+ transformants were obtained. These transformants were allowed to sporulate on selective SSA plates, and they were then treated with 30% ethanol for 30 min to kill nonsporulating vegetative cells (Gutz et al., 1974). Cells were then spread again on SSA sporulation plates, which were then exposed to iodine vapor (Gutz et al., 1974). Iodine-positive (brown) colonies were removed and inspected for recovery of sporulation. Plasmid DNA was transferred from some of the Leu+ Spo+ colonies to an Escherichia coli strain (DH5α). Two isolated plasmids, designated pDB(spo14)1 and pDB(spo14)2, contained ∼8- and 3-kb DNA inserts, respectively. Purified plasmids completely complement the spo14-B221 mutant. Sequencing of pDB(spo14)2 revealed that the insert contained one open reading frame (ORF) that was identical to stl1+ (d'Enfert et al., 1992). Subcloning of pDB(spo14)1 confirmed that this ORF is responsible for the complementation of spo14-B221 (Figure 1A). This ORF represents the spo14+ gene itself, but not the multicopy suppressor, as described below.
Figure 1.
Cloning, disruption, and the nucleotide sequence of the spo14+ gene. (A) Restriction map, subcloning, and disruption of the spo14+ gene. Plasmids pDB(spo14)1 and pDB(spo14)2 were independently isolated. The white arrow indicates the region and direction of the spo14+ ORF. Restriction enzyme sites: A, ApaI; B, BamHI; E, EcoRI; H, HindIII; Hp, HpaI; N, NsiI; S, SacI; X, XbaI. (B) Nucleotide sequence of spo14+ and its predicted amino acid sequence. Putative introns are underlined. A region indicated by bold letters is a hydrophobic stretch of 20 amino acid residues.
Disruption of the spo14+ Gene
The plasmids used for gene disruption were constructed as follows. A 6.0-kb of ApaI-XbaI fragment containing the spo14+ ORF was cloned into the corresponding site of pBluescript II-KS+ (Stratagene, La Jolla, CA). A 0.7-kb HpaI-NsiI fragment of the resulting plasmid was replaced by ura4+, yielding pDC14. (Figure 1A). The linear ApaI-XbaI fragment containing the interrupted spo14 allele was used to transform the strain MK07. Disruptions were confirmed by genomic Southern hybridization.
Plasmid Construction
Plasmids used in this work are listed in Table 2. Plasmid pKB282 was constructed as follows. The NdeI-NdeI region of pDblet (Brun et al., 1995) containing the ura4+ gene was eliminated, filled in, and then ligated with BglII linker, yielding pDblet-dash. pDblet-dash was digested with BglII and ligated with a 2.9-kb ade6+ fragment, yielding pKB282. To examine whether the overproduction of the spo14-B221 gene can resolve the sporulation defect of the spo14-B221, the spo14-B221 allele was cloned directly from the spo14-B221 genomic DNA by PCR using the primers 5′-CCCGGGCCC(ApaI)TTCATAATGGCTATAG-3′ and 5′-CCCGAGCTC(SacI)TTTCATTCAT AGTTATG-3′. The resulting fragment was digested with ApaI and SacI, and cloned into pAL-KS (Tanaka et al., 2000) to create pAL(spo14-B221). Plasmid pBR(leu1) was constructed by inserting leu1+ gene into the PvuII site of pBR322. Plasmid pGEX-KG(NotI) was constructed by inserting NotI linker into the HindIII site of pGEX-KG (Guan and Dixon, 1991). Plasmid pAL (spo14-HA) was constructed as follows. A 1.8-kb NotI-SacI fragment of pSLF372 (Forsburg and Sherman,1997), which contains the HA epitope and the nmt1 terminator (Maundrell, 1993), was ligated into the corresponding sites of pAL-KS to create pTN144. The 5-kb ApaI-XbaI fragment of pDB(spo14)1 was inserted at the corresponding sites in pTN144, yielding pAL(spo14I-HA). Two oligonucleotides were used to amplify the C terminal region of the spo14+ gene by PCR using 5′-CCCGTCGAC(SalI)AATGGCTGAACTCCAC-3′ and 5′-ATTTGCGGCCGC(NotI)AAAGGTCATAGTTTT-3′ as primers. The PCR product was digested with NsiI and NotI and then ligated into the corresponding site of pAL(spo14I-HA), yielding pAL(spo14-HA). Plasmid pREP81(13g6-GFP) was constructed as described by Brazer et al. (2000). Plasmid pKB282(rer1-GFP) was constructed through two steps. The rer1+ gene was amplified by PCR using 5′-CCCGTCGAC(SalI)AATGCTTGCAATCTTGTC-3′ and 5′-CCC-GCGGCCGC(NotI)AGTGTGAGCCAAATTTTTTCTTACCG-3′ as primers. The resulting PCR product was digested with SalI and NotI and ligated into the corresponding site of pTN143, yielding pAL(rer1-GFP). As the second step, pAL(rer1-GFP)was digested with ApaI and SacI and cloned into the corresponding site of pKB282 to create pKB282(rer1-GFP). Plasmid pIU(rer1-GFP) was constructed as follows. Two oligonucleotides were used to amplify the rer1+ gene by PCR using 5′-CCCGTCGAC(SalI)CATGGAATTCATTCAGCGTC-3′ and 5′-CCCGCGGCCGC(NotI)AGTGTGAGCCAA-ATTTTTTCTTACCG-3′ as primers. The resulting PCR product was digested with SalI and NotI and ligated into the XhoI and NotI sites of pIUGFP, yielding pIU(rer1-GFP). The pIU(rer1-GFP) plasmid was linearized by restricting it with SphI near the center of the rer1+ sequence and introduced into the strain (TN29).
Table 2.
Plasmids
| Plasmid | Characteristics | Source of reference |
|---|---|---|
| pDB248′ | 2μm origin, LEU2-based vector | Beach and Nurse (1981) |
| pAL-KS | ars1, LEU2-based vector | Tanaka et al. (2000) |
| pAU-SK | ars1, URA3-based vector | Tanaka et al. (2000) |
| pREP1 | ars1, LEU2-based vector carrying a thiamine-repressible nmt1 promoter | Maundrell (1993) |
| pREP41 | ars1, LEU2-based vector carrying a thiamine-repressible nmt41 promoter | Maundrell (1993) |
| pREP81 | ars1, LEU2-based vector carrying a thiamine-repressible nmt81 promoter | Maundrell (1993) |
| pTN143 | GFP and nmt1 terminator in pAL-KS | Ikemoto et al. (2000) |
| pSLF372 | 3XHA and nmt81 promoter | Forsburg and Sherman (1997) |
| pTN280 | 3XHA and nmt81 promoter | This work |
| pTN144 | 3XHA and nmt1 terminator in pAL-KS | This work |
| pTN253 | 3XHA and nmt1 terminator in pAU-SK | This work |
| pTN281 | GFP in pREP81 | This work |
| pBR(leu1) | leu1+in pBR322 | This work |
| pDblet | ars2004X2, ura4+-based vector | Brun et al. (1995) |
| pKB282 | ade6+in pDblet | This work |
| pIUGFP | GFP and nmt1 terminator in ura4+-based integration vector | This work |
| pDB(spo14)1 | Genomic spo14+ isolated from as S. pombe genomic library | This work |
| pDB(spo14)2 | Genomic spo14+ isolated from as S. pombe genomic library | This work |
| pAL(spo14) | spo14+ in pAL-KS | This work |
| pDC(spo14) | for spo14 disruption | This work |
| pREP41(spo14) | spo14+ in pREP41 | This work |
| pREP41(SEC12) | SEC12 in pREP41 | This work |
| pREP41(psr1) | psr1+ in pREP41 | This work |
| pAL(spo14-B221) | spo14-B221 in pAL-KS | This work |
| pAL(spo14-HA) | spo14+ in pTN144 | This work |
| pAU(spo14-HA) | spo14+ in pTN253 | This work |
| pREP81(13g6-GFP) | 13g6 in pTN281 | This work |
| pREP1(gma12-HA) | gma12-HA in pREP1 | T. Yoko-o |
| pREP81(GFP-psy1) | GFP-psy1 in pREP81 | Nakamura et al. (2001) |
| pAL(spo3-GFP) | spo3+ in pTN143 | Nakamura et al. (2001) |
| pKB282(rer1-GFP) | rer1-GFP in pKB282 | This work |
| pIU(rer1-GFP) | rer1+ in pIUGFP | This work |
Generation of Anti-Spo14 Antibody
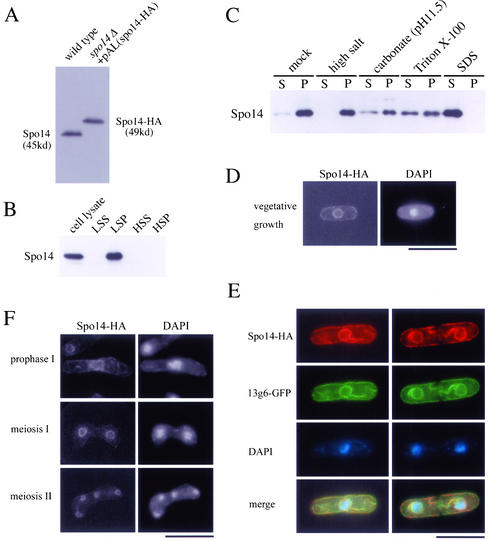
A GST-tagged protein of Spo14 produced in E. coli was used for the polyclonal antibodies. GST-Spo14 was obtained as follows. A 1.2-kb DNA fragment carrying the entire spo14 coding region was amplified by PCR with two oligonucleotides, 5′-CCCGTCGAC(SalI)AATGGCTGAACTCCAC-3′ and 5′-ATTTGCGGCCGC(NotI)AAAGGTCATAGTTT-3′. The amplified DNA was digested with SalI and NotI and then inserted into the same sites of the GST-tag expression vector pGEX-KG(Not) to make pGEX(spo14). The pGEX(spo14) was digested with SphI and NotI to eliminate the C-terminal region of Spo14, filled in, and self-ligated, yielding pGEX(spo14S), and the pGEX(spo14S) was transformed into E. coli BL21. The fusion protein was purified from SDS gels and used to immunize rabbit. Rabbit sera thus produced a 45-kDa protein in crude yeast extracts on an immunoblot (Figure 2A). For affinity purification, the sera were loaded over an AffiGel-15 (Bio-Rad, Hercules, CA) column containing the purified denatured GST-tagged Spo14 and eluted with 4.5 M MgCl2. Eluates were immediately dialyzed against PBS containing 30% glycerol and used for Western blot analysis.
Figure 2.
Localization of Spo14 to the ER membrane. (A) Western blot analysis of S. pombe cell extracts using anti-Spo14 antibody. MKW5 (wild-type) and MK14H (spo14Δ+ pAL(spo14-HA)) cells were grown in liquid complete medium (YE). Protein extracts were subjected to immunoblot analysis with the anti-Spo14 antibody. (B) Subcellular fractionation of membranes. MKW5 cells were spheroplasted and homogenated. The cell lysates was subjected to differential centrifugation to separate LSP, HSP, and HSS fractions as described in Elagoz et al. (1999). Each fraction was resolved on SDS-PAGE and subjected to immunoblot analysis using anti-Spo14 antibody. (C) The LSP fraction was extracted with lysis buffer (mock) or the same buffer containing either 0.5 M NaCl (high salt), 0.1 M sodium carbonate (pH 11.5), 1.5% Triton X-100, or 0.1% SDS. After extraction, mixtures were fractionated into the supernatant (S) and the pellet (P) by centrifugation (100,000 × g, 1 h). Each fraction was analyzed by Western blotting using anti-Spo14 antibody. (D) Localization of Spo14-HA in vegetative cells. Strain MK14H was cultured in MM medium and processed for immunofluorescence microscopy with anti-HA mAb (3F10) followed by Alexa 488-conjugated anti-rat IgG antibody. Bar, 10 μm. (E) Colocalization of Spo14-HA with an ER marker protein, 13g6. A wild-type strain TN29 carrying pAU(Spo14-HA) and pREP81(13g6-GFP) was grown to midlog phase in MM at 28°C. After fixation, cells were stained with anti-HA antibody (3F10) followed by Alexa 594-conjugated anti-rat IgG antibody and DAPI. Bar, 10 μm. (F) Localization of Spo14 during meiosis. Strain MK14H carrying pAL(spo14-HA) was cultured in SSL−N to induce meiosis. Spo14-HA was visualized as described in Figure 2D. Bar, 10 μm.
Immunofluorescence Microscopy
For cell fixation, we followed the method of Hagan and Hyams (1988) and used glutaraldehyde and paraformaldehyde. The Spo14-HA and Gma12-HA were visualized by indirect immunofluorescence microscopy using rat anti-HA antibody 3F10 (Boehringer Mannheim, Mannheim, Germany) and Alexa 488– or Alexa 594–conjugated goat anti-rat IgG (Molecular Probes, Eugene, OR). The SPB was visualized by indirect immunofluorescence microscopy using rabbit anti-Sad1 antibody and Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes). For microtubule staining, TAT-1 anti-α-tubulin antibody (Woods et al., 1989) and Cy3-conjugated secondary antibody (Sigma Chemical Co., St. Louis, MO) were used. To visualize the nuclear chromatin region, we stained the cells with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Stained cells were observed under a fluorescence microscope (model BX50; Olympus, Tokyo, Japan) equipped with a charge-coupled device (CCD) camera (Cool-SNAP; Roper Scientific, San Diego, CA).
RT-PCR
Total RNA was prepared from S. pombe cultures (Jensen et al., 1983). Reverse transcription (RT)-PCR was performed with a commercial kit (Amersham Pharmacia Biotech, Inc., Uppsala, Sweden), and the treatment of total RNA with DNase I before RT-PCR was carried out according to the supplier's instructions. The forward and reverse primers for PCR were 145s 5′-GCTGGAGCAGATTGCAGC-3′ and 145as 5′-ATTTGCGGCCGCAAAGGTCATAGTTTT-3′.
In Vitro Mutagenesis of spo14 by PCR
To screen for conditionally lethal alleles, the whole spo14+ ORF was randomly mutagenized by the error-prone PCR method (Leung et al., 1989). The forward and reverse primers for PCR were 5′-CCCAGTGGAATTC(EcoRI)TTGTAAGTGTAC-3′ and 5′-CCC-GAGCTC(SacI)TTTCATTCATAGTTATG-3′, respectively. The amplified DNA fragment contained the promoter and terminator regions, besides the coding region, of the spo14 gene. PCR was carried out in a reaction mixture composed of mutagenesis buffer (50 mM KCl, 10 mM Tris-HCl, pH 8.3, 1 mM MgCl2, 0.01% Triton X-100, and 0.1 mM MnCl2) and primers, recombinant Taq DNA polymerase (Toyobo, Osaka, Japan) and dNTPs (2 mM each). Plasmid pAL(spo14) was included in the reaction mixture as a template. The amplified fragment was digested with EcoRI and SacI and cloned into pBR (leu1) vector. The resulting library was digested at the NruI site within the leu1+ gene and then integrated at the leu1 locus of the spo14 disruptant (MKD14). The transformant colonies on SSA plates were treated with ethanol to kill nonsporulating vegetative cells and then spread on minimal medium. Candidates were replica-plated onto minimal plates containing phloxin B, and they were then incubated at either 20 or 37°C. Two novel spo14 mutants that showed cold-sensitive growth were further characterized.
Nucleotide Sequence Analysis of the spo14 Mutant Alleles
The entire spo14 ORF and the promoter region were amplified by PCR using genomic DNA from the spo14-B221, spo14-6, or spo14-7 as a template and then cloned into pAL-KS. The nucleotide sequences of six clones derived from each independent PCR amplification were determined in their entirety.
Nucleotide Sequence Accession Number
The sequence data for spo14+ are available from EMBL/GenBank/DDBJ under accession no. AB036755.
RESULTS
The Spo14-B221 Mutant Shows Defects in Ascospore Formation but Not in Vegetative Growth or Meiotic Nuclear Division
As we previously reported, spo14-B221 mutants exhibit cold sensitivity in ascospore formation (Kishida and Shimoda, 1986; Kishida et al., 1990; Hirata and Shimoda, 1992). To investigate meiosis and sporulation in the spo14-B221 mutant in more detail, we monitored meiotic nuclear divisions and ascus formation in homozygous diploid strains MK05 (spo14+/spo14+) and MK06 (spo14-B221/spo14-B221). At both 23 and 30°C, first and second meiotic divisions in the spo14-B221 mutant proceeded with kinetics similar to those in the isogenic wild-type strain. However, virtually no asci were produced in the spo14-B221 strain at 23°C. These results suggest that the spo14-B221 mutant is able to complete meiosis, but that it is defective in ascospore formation.
Next, we observed effects of the spo14-B221 mutation on properties of vegetative growth. The growth rate of the spo14-B221 mutant was not significantly different from those of wild-type cells. Cell size and morphology of the mutant were also normal. We thus concluded that the spo14-B221 mutant proceeds through normal mitotic and meiotic divisions but shows defects in ascospore formation.
The spo14+ Gene Encodes a Functional Homologue of Budding Yeast Sec12
The spo14+gene was cloned by means of its ability to complement the sporulation defect of the spo14-B221 mutant (MATERIALS AND METHODS). This gene is identical to the previously characterized stl1+ gene, which is a putative homologue of the S. cerevisiae SEC12 gene (d'Enfert et al., 1992). The comparison of the genomic sequence (this study) with the corresponding cDNA sequence (d'Enfert et al., 1992) shows that the predicted spo14+ gene is split by five introns (Figure 1B). Interestingly, the first intron is located in the 5′ untranslated region of the gene (Figure 1B). We refer to this gene as spo14+ hereafter in this article.
To determine the consequences of complete loss of spo14+ function, we disrupted the spo14+ gene using a plasmid, pDC(spo14), constructed by deleting ∼50% of the ORF and replacing this with the ura4+ gene (Figure 1A). Tetrad analysis indicated that every ascus contained two viable and two inviable spores and that all viable spores were Ura−. Microscopic observation of nonviable progenies showed that spores germinated but ceased growth after a few divisions. This demonstrates that spo14+ is essential for vegetative growth and viability. A spo14-B221/spo14Δ diploid strain could not form spores, indicating that the cloned gene is spo14+ itself.
Generally, the S. pombe genes responsible for mating, meiosis and sporulation are transcribed under conditions of nutritional starvation (Yamamoto et al., 1997; Horie et al., 1998; Abe and Shimoda, 2000). Northern analysis revealed that spo14+ was transcribed during growth and was not further enhanced after the shift to a nitrogen-free medium (Figure 6C). In summary, Spo14 functions not only in sporulation but also in vegetative growth.
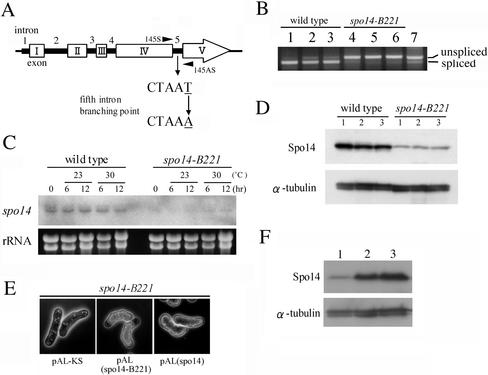
Figure 6.
Analysis of the spo14-B221 mutation. (A) Identification of the spo14-B221 mutation point. (B) RT-PCR analysis to assess splicing of the fifth intron. The PCR primers (145S and 145AS) are shown in Figure 6A. MKW5 (wild-type) and MK14 (spo14-B221) cells precultured in growth medium (MM+N) at 30°C were incubated in liquid sporulation medium (MM−N) at 23 or 30°C for 10 h. Strains: lanes 1–3, MKW5; lanes 4-6, MK14. Lanes 1 and 4, 0 h at 30°C; lanes 2 and 5, 10 h at 23°C, lanes 3 and 6, 10 h at 30°C. Lane 7, same as lane 1, but without DNase treatment. (C) Northern analysis of the spo14 mRNA in the spo14-B221 mutant. MKW5 and MK14 cells precultured in MM+N at 30°C were incubated in MM−N at 23 or 30°C. The equality of RNA loading was confirmed by staining gels with ethidium bromide (bottom). (D) Expression of Spo14 in the spo14-B221 mutant. MKW5 and MK14 cells precultured in MM+N at 30°C were incubated in MM−N at 23 or 30°C. Protein extracts were subjected to immunoblot analysis with the anti-Spo14 antibody, as well as anti–α-tubulin antibody as the loading control. Lane 1, 0 h at 30°C; lane 2, 10 h at 23°C; lane 3, 10h at 30°C. (E) Overexpression of spo14-B221 complemented the sporulation defect of the spo14-B221 mutant. Strain MK14L (h90 spo14-B221) carrying either pAL-KS, pAL(spo14-B221), or pAL(spo14) was incubated on SSA medium at 23°C for 5 d. (F) Expression of Spo14-B221 in the spo14-B221 mutant. MK14L cells transformed with either pAL-KS (lane 1) or pAL(spo14-B221) (lane 2) and MKW5 (lane 3) were precultured in MM+N at 30°C, and then cells were incubated in MM−N for 8 h. Immunoblot analysis was carried out as described in Figure 6D.
Identification of Spo14 Proteins
To identify and characterize the spo14+ gene product, we generated a rabbit polyclonal antiserum raised against a GST-fused Spo14 (see MATERIALS AND METHODS). This antiserum was used to detect Spo14 in crude S. pombe cell extracts by immunoblotting. The antibody recognized a single band with an apparent molecular mass of 45 kDa, which was in accord with that calculated from the nucleotide sequence (Figure 2A). In spo14Δ cells expressing HA-tagged Spo14, the band was shifted upward due to the tagging peptide (∼49 kDa; Figure 2A). Therefore, we concluded that the antibody specifically recognized the spo14+ gene product. S. cerevisiae Sec12 is known to be glycosylated (Nakano et al., 1988). The apparent molecular mass was not decreased after treatment with endo H glycosidase, suggesting that Spo14 is not strikingly glycosylated.
Spo14 Localizes to the ER
To elucidate the localization of Spo14, we first conducted subcellular fractionation experiments. Cells were converted to spheroplasts by lyticase treatment, homogenized, and subjected to differential centrifugation. In Western blotting using anti-Spo14 antibody (Figure 2B), Spo14 was almost exclusively present in a low-speed pellet (LSP) fraction that contained ER and vacuoles. To test whether Spo14 was soluble in a membrane-enclosed compartment or was an integral membrane protein, the LSP fraction was treated with high concentrations of salt solution, an alkaline solution, and detergents. Figure 2C shows that Spo14 remained sedimentable after treatment with a high concentration of salts and alkali but was partially solubilized by detergents. These facts suggested a firm association of Spo14 with the lipid bilayer.
To analyze further the localization of Spo14, we performed immunofluorescence microscopy. Because the polyclonal anti-Spo14 antibody mentioned above could not detect Spo14 protein by indirect immunofluorescence, we used the spo14Δ strain expressing Spo14-HA, which could be detected by anti-HA antibody. The spo14Δ cells containing either single or multiple copy Spo14-HA grew and sporulated normally. In vegetative cells, the Spo14-HA fluorescence gave a ring-like staining pattern surrounding the nucleus, and there was also staining in the cell periphery (Figure 2D). Essentially identical data were obtained with strains harboring a single copy of the spo14-HA fusion allele integrated chromosomally. Typical ER-resident proteins show similar staining images. The protein tentatively named 13g6 has been demonstrated to be localized to the ER (Brazer et al., 2000). Thus, Spo14-HA was coexpressed with 13g6-GFP. As shown in Figure 2E, both proteins were clearly colocalized, demonstrating that Spo14 is associated with the ER.
We also investigated Spo14 localization during meiosis and sporulation. The Spo14-HA was localized around nuclei during the sporulation process. Interestingly, signals at the cell periphery disappeared (Figure 2F). This observation suggests that intracellular membranes including the ER and Golgi apparatus possibly undergo dynamic alterations in this cell reforming process.
Isolation of Conditional Lethal Spo14 Mutants
S. cerevisiae Sec12 plays an essential part in protein secretion. To determine whether Spo14 is involved in this process, we attempted to isolate conditional lethal mutants by random PCR mutagenesis (see MATERIALS AND METHODS). We isolated two low temperature-sensitive mutants, spo14-6 and spo14-7, both of which grew normally at 28 and 37°C but poorly at 20°C. These mutant alleles were amplified by PCR and sequenced. The spo14-6 mutation caused a single nucleotide change (from T to C) at the 101st nucleotide from the initiation codon, resulting in valine to alanine replacement at the amino acid position 34. The spo14-7allele contained a single nucleotide change (from T to C) at the 1201st nucleotide that caused a change from leucine to histidine at amino acid position 299. Both mutation sites were located in the cytoplasmic region of Spo14 protein.
Because SAR1 suppresses the temperature sensitivity of sec12 mutants in S. cerevisiae (Nakano and Muramatsu, 1989), it is possible that the corresponding homologues of S. pombe may interact with each other. In fission yeast, an SAR1-like gene, once designated sar1+ (d'Enfert et al., 1992), has been isolated by the suppression of temperature-sensitive growth of the budding yeast sec12 mutant. Because the gene symbol sar1+ has already been used (Wang et al., 1991), we propose a new nomenclature, psr1+ (for pombe Sar1). To examine whether overproduction of Psr1 suppresses the spo14 mutation in S. pombe, we introduced a multicopy plasmid harboring psr1+ into spo14-6 and spo14-7 strains. Overexpression of psr1+ complemented the cold sensitivity of both the spo14-6 and the spo14-7 mutants.
Spo14 Functions in the Vesicle Transport from the ER to the Golgi Apparatus in Vegetative Growth
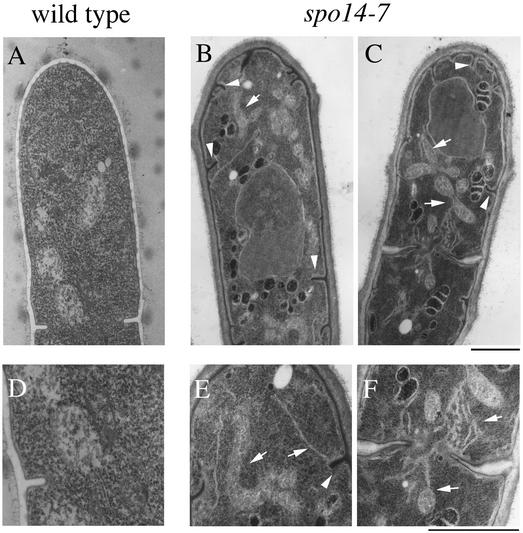
To determine whether protein transport was defective in the conditional lethal spo14-7 mutant, we determined the terminal phenotypes of the mutant after incubation at 20°C for 4 h. Prominent ER-like membrane structures were accumulated in the cytoplasm of spo14-7 mutant cells. Furthermore, invagination of plasma membranes and abnormal nuclear structures were often observed in the mutant cells (Figure 3, B, C, E, and F). Similar results were obtained when spo14-6 mutant was incubated at the restrictive temperature. In contrast, no abnormal membrane structure was accumulated in wild-type cells (Figure 3, A and D) or spo14-B221 cells.
Figure 3.
Fine structures of the conditional spo14-7 mutants at a restrictive temperature. RM14 and RM14-7 were grown to a midlog phase at 30°C and then shifted to the restrictive temperature (20°C). After incubation for 4 h, cells were fixed for electron microscopy. Arrowheads and arrows represent invagination of plasma membranes and the ER-like structures, respectively. A, RM14; B and C, RM14-7. D, E, and F, higher magnified images of A, B, and C, respectively. Bars, 1 μm.
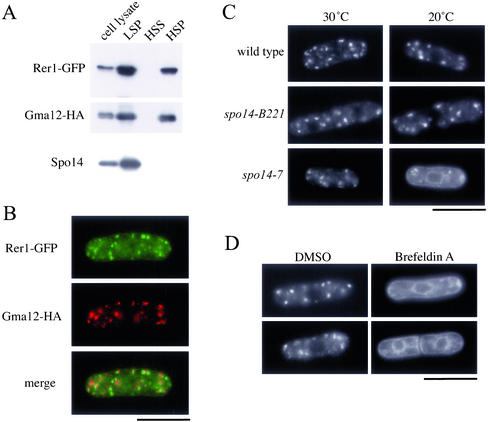
To confirm that Spo14 regulates the ER-Golgi protein transport, we observed the localization of S. pombe Rer1 protein in the spo14 mutant. S. cerevisiae Rer1 is a membrane protein present in the Golgi apparatus, and it is required for the retrieval of a variety of ER membrane proteins (Nishikawa and Nakano 1993; Boehm et al. 1994, 1997; Sato et al., 1995, 1997). Rer1 directly interacts with the transmembrane domain of Sec12, which contains a retrieval signal (Sato et al., 1996, 2001). The S. cerevisiae Rer1 protein fused to GFP rapidly shuttles between the Golgi apparatus and the ER. In the mutant, where membrane traffic from the ER to the Golgi apparatus is prevented, Rer1 localization is restricted to the ER (Sato et al., 2001). Rer1 is conserved evolutionarily among eukaryotes, and in fact, the S. pombe genome also contains a single copy of the homologous gene (Sato et al., 1999). We named this gene rer1+. To verify that Rer1 resides preferentially in the Golgi apparatus, we examined the subcellular localization of Rer1-GFP in the strain TN230 carrying a single chromosomal copy of the rer1+ gene tagged with GFP and driven by the authentic promoter. Previous studies have shown that ER and vacuolar membranes distribute mainly in the low-speed pellet (LSP) fraction, whereas Golgi membranes are recovered in both LSP and high-speed pellet (HSP) fractions (Nakano et al., 1988; Gaynor et al., 1994). Subcellular fractionation experiments revealed that Rer1 was present in both LSP and HSP fractions (Figure 4A). Rer1-GFP was visualized as numerous dots scattered in the cytoplasm. Localization of Rer1 in wild-type cells was compared with that of Gma12, Golgi-associated protein (Chappell et al., 1994). A fluorescent microphotograph indicates that Rer1-GFP and Gma12-HA are largely colocalized in the cytoplasm (Figure 4B). These observations strongly suggest that S. pombe Rer1 may also localize to the Golgi apparatus. We noticed, however, that fluorescent signals of Rer1-GFP and Gma12-HA were not completely overlapped. Perhaps, this is due to the differences in their localization within the Golgi apparatus. In fact, S. cerevisiae Rer1 is mostly present in early stages of Golgi apparatus formation, its localization is slightly different from that of Kex2, a trans-Golgi protein (Sato et al., 1995).
Figure 4.
Effect of the spo14-7 mutation on the ER/Golgi shuttling protein, Rer1. (A) Subcellular fractionation of Rer1-GFP. Strain TN230 transformed with pREP1(gma12-HA) was cultured in MM supplemented with thiamine (20 μM) at 28°C. Cells were converted to spheroplasts, homogenized, and subjected to differential centrifugation to fractionate into LSP, HSP, and HSS. Each fraction was resolved by SDS-PAGE and subjected to immunoblot analysis using either anti-GFP, anti-HA, or anti-Spo14 antibody to detect Rer1-GFP, Gma12-HA, and Spo14, respectively. (B) Double-immunofluorescence staining of Rer1 and Gma12. Strain TN230 transformed with pREP1(gma12-HA) was cultured in MM minimal medium supplemented with thiamine (20 μM) at 28°C. Cells were then stained with anti-HA antibody followed by Alexa 594–conjugated anti-rat IgG antibody and DAPI. Bar, 10 μm. (C) ER-to-Golgi transport was blocked in spo14-7. TN52 (wild-type), B221 (spo14-B221), and RM14-7 (spo14-7) cells were transformed with pKB282(rer1-GFP). These strains were grown in liquid medium (MM) at 20°C for 3 h. Localization of Rer1-GFP was observed under a fluorescence microscope. Bar, 10 μm. (D) Effect of Brefeldin A on the localization of Rer1-GFP. Strain TN226 (wild-type) was incubated in MM medium to log phase. Brefeldin A dissolved in dimethyl sulfoxide (DMSO) was added to the culture medium to a final concentration of 100 μg/ml. After incubation for 2 h, Rer1-GFP was observed under a fluorescence microscope. Bar, 10 μm.
Next, we observed the localization of Rer1-GFP in spo14 mutants. In wild-type and spo14-B221 cells, Rer1-GFP signals showed punctate distribution, thus suggesting a Golgi apparatus localization, at either 30 or 20°C. In the cold-sensitive spo14-7 mutant, however, the Rer1-GFP signal showed an ER-like pattern at a restrictive temperature (20°C; Figure 4C). Similar results were obtained with the spo14-6 cold-sensitive mutant. Brefeldin A is known to stimulate the membrane recycling from the Golgi apparatus to the ER in many organisms, including fission yeast (Pelham, 1991; Klausner et al., 1992; Turi et al., 1994; Brazer et al., 2000). The treatment with this drug altered the Rer1-GFP signal from the punctate (Golgi) pattern to the ER pattern as observed in spo14-6 and spo14-7 (Figure 4D). In conclusion, Spo14 is involved in the membrane traffic from the ER to the Golgi apparatus, like S. cerevisiae Sec12.
In budding yeast, secretion of the periplasmic proteins such as invertase and acid phosphatase is blocked in conditional sec mutants at restrictive temperatures (Novick et al., 1980, 1981; Ferro-Novick et al., 1984). However, the secretion of invertase and acid phosphatase is not blocked in S. pombe conditional spo14 mutants, although the glycosylation of the proteins was defective. Recently, we showed that the secretion of invertase and acid phosphatase was not affected in another secretion mutant, spo20-KC104, at the restrictive temperature, whereas the Golgi apparatus is accumulated (Nakase et al., 2001). The mechanism of the secretion in S. pombe might be partially different from that in S. cerevisiae.
Sporulation Deficiency of spo14-B221 Mutants is Due to Reduction in the Abundance of spo14 mRNA
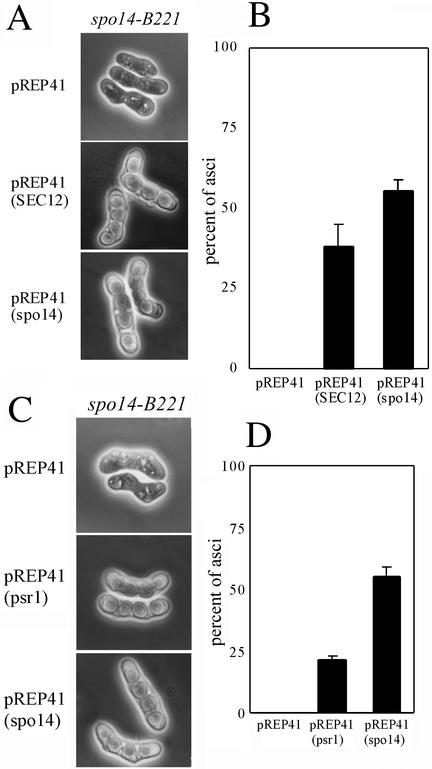
Next, we examined the role of Spo14 in sporulation. To determine whether S. cerevisiae SEC12 complements the sporulation defect of the spo14-B221 mutant, we introduced SEC12 driven by the nmt1 promoter into the S. pombe spo14-B221 mutant. This expression plasmid, pREP41(SEC12), rescued the spo− phenotype (Figure 5A). The degree of suppression of sporulation deficiency by the expression of SEC12 was comparable to that achieved by the expression of spo14+ (Figure 5B), and most spores were viable. Thus, we conclude that S. pombe Spo14 plays a similar role to that of S. cerevisiae Sec12 not only in vegetative growth but also in sporulation.
Figure 5.
Spo14 is a functional homologue of Sec12. (A) Overexpression of S. cerevisiae SEC12 suppressed sporulation deficiency of spo14-B221. Strain MK14L (spo14-B221) transformed with either pREP41, pREP41(SEC12), or pREP41(spo14) was incubated on SSA at 23°C for 5 d. (B) Sporulation frequency of the transformants. Mean of percent asci of three independent transformants is shown with SEs (vertical bars). (C) Overexpression of psr1+ suppressed sporulation deficiency of spo14-B221. Strain MK14L (spo14-B221) transformed with either pREP41, pREP41(psr1), or pREP41(spo14) was incubated on SSA medium at 23°C for 5 d. (D) Sporulation frequency of the transformants. Mean of percent asci of three independent transformants is shown with SEs (vertical bars).
As mentioned above, S. cerevisiae SAR1 suppresses the phenotype of temperature-sensitive sec12 mutants. In addition, overexpression of psr1+ suppresses the temperature sensitivity of budding yeast sec12 (d'Enfert et al., 1992). To examine whether overproduction of Psr1 suppresses the sporulation defect of the spo14-B221 mutation in S. pombe, we introduced a multicopy plasmid harboring psr1+ into spo14-B221 strains. The transformants recovered the sporulation-deficient phenotype (Figure 5, C and D), and the produced spores were viable. These results strongly suggest that Spo14 regulates Psr1 in a manner analogous to that of S. cerevisiae Sec12 and Sar1.
As described above, spo14+ is an essential gene, whereas the spo14-B221 mutant shows a sporulation-specific phenotype. Nucleotide sequence analysis demonstrated that spo14-B221 contained a single nucleotide change (from T to A) in the fifth intron (Figure 6A). The branch point consensus sequence CURAY was mutated to CURAA in spo14-B221. To examine whether splicing efficiency of the fifth intron was reduced in the mutant, we conducted RT-PCR analysis using primers 145S and 145AS encompassing this intron (Figure 6A). RNA preparations were obtained from wild-type and spo14-B221 mutant, which were incubated either in growth or sporulation media. As expected, splicing of the fifth intron in spo14-B221 cells was severely reduced either in vegetative growth or sporulation and at both permissive and restrictive temperatures (Figure 6B).
Next, the abundance of spo14 mRNA was compared between wild-type and spo14-B221 strains by Northern analysis. Interestingly, the spo14 mRNA was hardly detectable in spo14-B221 cells cultured in the medium with or without a nitrogen source (Figure 6C). Possibly, decreased efficiency of splicing may cause an instability of mRNA molecules rather than the accumulation of unspliced mRNA. In fact, Western analysis showed that Spo14 was remarkably decreased in spo14-B221 mutant (Figure 6D). As the apparent size of Spo14 was comparable to the wild-type protein, it might be produced from the barely spliced spo14 mRNA in the spo14-B221 mutant. Therefore, we suspect that overexpression of spo14-B221 should complement the sporulation defect. The transformant with a multicopy plasmid pAL(spo14-B221) sporulated well (Figure 6E) and contained approximately the wild-type level of Spo14 (Figure 6F). Because the spo14-B221 mutant grows normally, these data also suggest that the sporulation process requires higher amounts of Spo14 protein than does vegetative growth.
The spo14-B221 Mutant Is Defective in Forespore Membrane Formation
We examined how the spo14-B221 mutation impairs sporulation in more detail. During meiosis II, SPBs structurally change from a compact dot to a crescent (Hagan and Yanagida, 1995; Ikemoto et al., 2000). However, Immunostaining of the SPB showed that modified crescent-shaped SPBs were observed in spo14-B221 mutant cells (Figure 7B) at a frequency comparable to wild-type cells during the second meiotic division. Essentially the same results were obtained when the SPB was stained with the GFP-tagged Spo15, which is another SPB-associated protein (Ikemoto et al. 2000). Therefore, the sporulation defect of the spo14-B221 mutant is not due to the failure of the SPB modification.
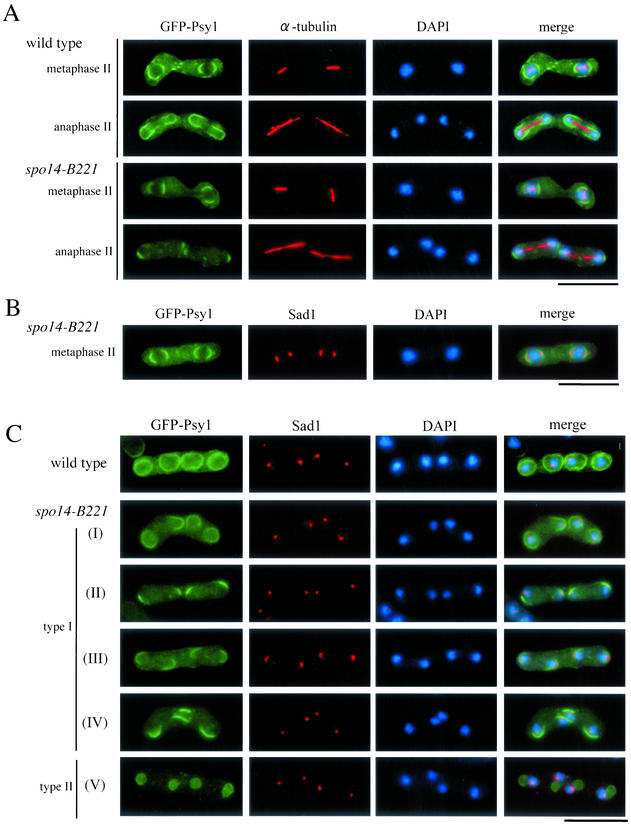
Figure 7.
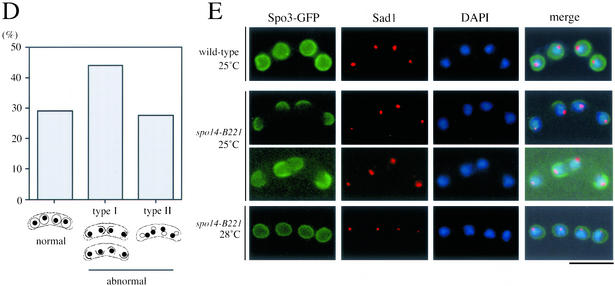
Aberrant assembly of forespore membranes in spo14-B221. (A and B) Assembly of the forespore membrane during metaphase II and anaphase II. TN8 (wild-type) and MK14L (spo14-B221) transformed with pREP81(GFP-psy1) were cultured in SSL−N to induce meiosis at a semipermissive temperature (25°C) for 8 h. Fixed cells were doubly stained with the anti–α-tubulin (A) or anti-Sad1 antibody (B) and DAPI. Bars, 10 μm. (C) Classification of terminal phenotypes of the forespore membrane in spo14-B221 zygotes. Strain, culture conditions, and staining procedures are the same as described in Figure 7B. Type I, zygotes in which forespore membranes were formed at the normal site but did not extend; Type II, zygotes in which four aggregates of GFP-Psy1 were formed close to nuclei. (D) Relative frequency of the cell types of Figure 7C. Stained cells were categorized into three classes, normal, abnormal type-I and abnormal type-II. The values depicted show one representative result (N = 400) of three independent experiments. (E) Observation of forespore membrane using another forespore membrane marker, Spo3-GFP. MK14L cells were transformed with pAL(spo3-GFP) to visualize the forespore membrane by Spo3-GFP. Cells were incubated in SSL−N at either a permissive (28°C) or a semipermissive temperature (25°C). Fixed cells were doubly stained with anti-Sad1 antibody and DAPI. Bar, 10 μm.
Next, we investigated the assembly of forespore membranes in the spo14-B221 mutant. We have recently succeeded in tracing the assembly process of the forespore membrane using GFP-tagged Psy1, a syntaxin-like protein (Nakamura et al., 2001). A spo14-B221 strain, MK14L, transformed by pREP81(GFP-psy1) was incubated in sporulation medium. Overexpression of GFP-Psy1 did not overcome the sporulation defect of spo14-B221. Progression of meiosis was monitored by observing the duplication of SPBs and the elongation of spindle microtubules. At the permissive temperature (30°C), the forespore membrane normally developed to engulf haploid nuclei in spo14-B221 cells. At a restrictive temperature (23°C), forespore membrane formation did not initiate in most of the mutant cells. At a semipermissive temperature (25°C), the membrane formation initiated near the SPBs (Figure 7, A and B), but further development of the membrane was impaired (Figures 7, A and C). In ∼70% of the spo14-B221 zygotes, abnormal forespore membranes were formed (Figures 7, C and D). In 44% of the zygotes, the forespore membrane formation was arrested (Figure 7, C, type I, and D). The rest of the zygotes contained four aggregates of GFP-Psy1 near nuclei (Figure 7C, type II, and D). Comparison of the forespore membrane assembly at semipermissive (25°C) and permissive (28°C) temperatures using another marker, Spo3-GFP, confirmed the results obtained with GFP-Psy1 (Figure 7E). These results indicate that forespore membrane formation initiates normally, but its subsequent development might be aberrant in spo14-B221 mutants. In conclusion, Spo14 appears to be responsible for the normal construction of the forespore membrane.
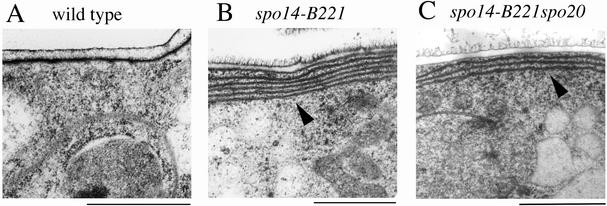
spo14-B221 Cells Accumulate ER-like Membranes during Sporulation
Because S. cerevisiae Sec12 is involved in the vesicle transport from the ER to the Golgi apparatus, the sporulation defect might be due to the blockage of vesicle transport from the ER to the Golgi apparatus in spo14-B221 mutant during sporulation. To confirm this possibility, we observed intracellular membranous organization by electron microscopy. The wild-type and spo14-B221 cells were sporulated on MEA medium and then processed for freeze substitution procedures. As shown in Figure 8B, membranous structures were markedly accumulated in the peripheral region of spo14-B221 cells, though such aberrant structures were not observed in vegetative cells. These aberrant membranes might result from a block of vesicle transport from the ER. Recently we reported that S. pombe Sec14 homologue (Spo20) is essential for post-Golgi protein transport (Nakase et al., 2001). Aberrant membrane structures shown in spo14 mutants have not been observed in a spo20 single mutant (Hirata and Shimoda, 1992). As shown in Figure 8C, the spo14-B221 spo20-KC104 double mutant also exhibited prominent peripheral membranes, indicating that the development of these membranes induced by spo14-B221 mutation is not suppressed by the spo20-KC104 mutation. These observations suggest that the observed peripheral membranes under sporulation conditions represent an abnormal accumulation of ER-related membranes.
Figure 8.
Accumulation of peripheral ER-like membranes during meiosis and sporulation in spo14-B221 mutants. Cells were sporulated on SSA medium at 23°C for 24 h. Strains used are as follows: TN52 (wild-type), B221 (spo14-B221), and TN196 (spo14-B221 spo20-KC104). Arrowheads in B and C indicate ER-like membranes. Bars, 1 μm.
DISCUSSION
The spo14+ Gene Encodes an S. cerevisiae Sec12-like Protein and Is Required for the Protein Transport from the ER to the Golgi Apparatus
The isolated spo14+ gene was identical to stl1+, which had been identified as a gene that complements the temperature sensitivity of the S. cerevisiae sec12 mutant (d'Enfert et al., 1992). Several lines of evidence obtained in this study showed that Spo14 played a function equivalent to S. cerevisiae Sec12 in the protein transport pathway, as follows: 1) Spo14 localizes to the ER membrane; 2) Spo14 and S. cerevisiae Sec12 mutually complemented the mutational defects; 3) abnormal ER-like membrane structures were accumulated in spo14-7 at the restrictive temperature; 4) an ER/Golgi shuttling protein Rer1 remained in the ER when conditional spo14-7 mutants were incubated at the restrictive temperature; 5) overexpression of psr1+, an S. pombe homologue of S. cerevisiae SAR1, suppressed the cold sensitivity of spo14-6 and spo14-7 mutations in the same way that SAR1 overexpression rescued sec12 temperature-sensitive alleles in S. cerevisiae. Recently, Matynia et al. (2002) reported that S. pombe Psr1 is also involved in the transport from the ER to the Golgi apparatus. From these results, we conclude that S. pombe Spo14 plays a crucial role in membrane traffic from the ER to the Golgi apparatus, probably by regulating the Psr1 activity.
Sporulation-specific Phenotype of the spo14-B221 Mutant
As described above, sporulation deficiency of spo14-B221 is caused by the reduction of the spo14 mRNA level. The apparent decrease in the spo14 mRNA could be explained by transcriptional repression of spo14 and/or increased instability of the nascent spo14 mRNA. Recently an idea has been proposed that transcription, splicing, capping, and addition of poly(A) tails are coupling in limited areas of the nucleus (McCracken et al., 1997). According to this RNA factory model, decreased splicing efficiency results in the reduction of transcriptional activity. Alternatively, insufficient splicing of the fifth intron may cause transcript instability, because splicing intermediates are thought to be unstable. In either case, the sporulation defect of the spo14-B221 mutant is likely to be due to the level of Spo14, implying that the sporulation process requires higher amounts of Spo14 than does the vegetative growth. This finding was further supported by the result that ER-like membrane structures are accumulated in spo14-B221 mutant during sporulation, although the mutant can grow normally. Transcription of a number of general secretory genes of S. cerevisiae including SEC12 is further stimulated during sporulation (Chu et al., 1998). We also recently demonstrated that psy1+ encoding a syntaxin 1A-like protein is essential for vegetative growth and that its transcription is further enhanced during meiosis (Nakamura et al., 2001). Requirement of sporulating cells for the general secretory machinery is explainable by the bulk de novo synthesis of the forespore membrane. The fact that reduced levels of Sec12 homologous proteins (Spo14) actually result in premature arrest of the forespore membrane supports this notion.
The Role of Spo14 in Forespore Membrane Formation
Our fluorescence microscopic observations reveal that, in both spo14-B221 and spo3Δ mutants, forespore membrane formation initiates normally near the SPB during meiosis II, but subsequent development into membrane compartments containing a nucleus, called prespores, cannot be completed (this study and Nakamura et al., 2001). However, the terminal phenotypes of these two mutants are different. The spo3Δ zygotes formed four amorphous aggregates of GFP-Psy1 near nuclei or extremely small nucleated prespores (Nakamura et al., 2001). In contrast, the development of prespores in spo14-B221 is blocked in the course of forespore membrane assembly. We speculate that Spo3 that localizes to the forespore membrane is responsible for assembly and/or integrity of the membrane, whereas Spo14 may be involved in the supply of membrane vesicles from the ER.
We reported that Spo14 is preferentially present around the nucleus and the cell periphery. Interestingly, the peripheral localization was lost during meiosis, though the localization around nuclei remained. We do not know whether this alteration is due to the Spo14-specific one or rearrangement of ER structure, because other ER markers, such as 13g6-GFP, disappeared during meiosis. An answer to this intriguing question awaits further molecular and cytological analysis.
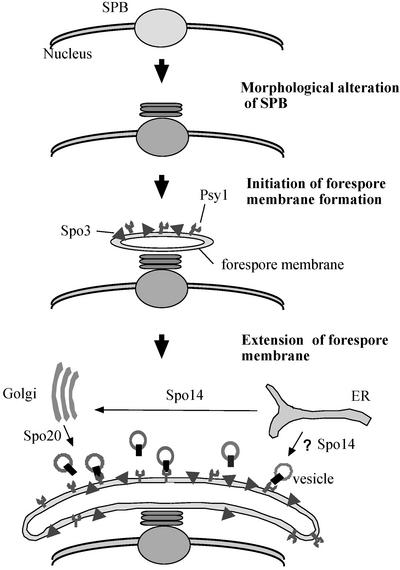
On the basis of the present study and our previous reports (Ikemoto et al., 2000; Nakase et al., 2001, Nakamura et al., 2001), we propose a model for construction of the forespore membrane (Figure 9). From metaphase II to anaphase II, the SPB undergoes morphological alteration to a multilayered form, depending on Spo15 (Ikemoto et al., 2000). The t-SNARE protein Psy1, probably as well as a SNAP-25 homologue, is recruited to the modified SPB and is localized to nascent forespore membranes. These t-SNARE proteins on the target membrane, the precursor structure of the forespore membrane, are implicated in the fusion with small vesicles. Another forespore membrane protein Spo3 may contribute to its assembly and integrity by promoting efficient membrane fusion or stabilizing the nascent architecture. Thus, the forespore membrane extends and eventually encapsulates each of the haploid nuclei. The secretory pathway components Spo14 and Spo20 serve to supply membrane vesicles for the forespore membrane.
Figure 9.
A model for assembly of the forespore membrane.
ACKNOWLEDGMENTS
We thank A. Nakano and coworkers of Riken and K. Takegawa of Kagawa University for invaluable discussions; K. Tanaka of the University of Tokyo, S. Forsburg of the Salk Institute; J. Huberman of Roswell Park Cancer Institute; T. Yoko-o of National Institute of Advanced Industrial Science and Technology and Y. Hiraoka of Kansai Advanced Research Center for plasmids; K. Gull of the University of Manchester for anti–α-tubulin antibody, TAT-1; S. Fujita of Mitsubishi Kagaku Institute of Life Sciences for anti-GFP antibody; and O. Niwa of Kazusa DNA Research Institute for affinity-purified antibodies against Sad1. We are grateful to Y. Nakase of Osaka City University for technical assistance. We also thank M. Yamamoto and Y. Watanabe of the University of Tokyo for S. pombe genomic library, plasmids, and strains. This study was supported by Grant-in-Aid for Scientific Research on Priority Areas (C) “Genome Biology” to C.S., and (A) “Cell Cycle Control” and “Life of Proteins” to T.N. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and Saneyoshi Scholarship Foundation to T.N.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0504. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0504.
REFERENCES
- Abe H, Shimoda C. Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics. 2000;154:1497–1508. doi: 10.1093/genetics/154.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa H, Kitamura K, Shimoda C. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol Genet Genom. 2001;265:424–435. doi: 10.1007/s004380000429. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- Beach D, Nurse P. High-frequency transformation of the fission yeast Schizosaccharomyces pombe. Nature. 1981;292:140–142. doi: 10.1038/290140a0. [DOI] [PubMed] [Google Scholar]
- Boehm J, Ulrich HD, Ossig R, Schmitt HD. Kex2-dependent invertase secretion as a tool to study the targeting of transmembrane proteins which are involved in ER→Golgi transport in yeast. EMBO J. 1994;13:3696–3710. doi: 10.1002/j.1460-2075.1994.tb06679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Letourneur F, Ballensiefen W, Ossipov D, Demolliere C, Schimitt HD. Sec12p requires Rer1p for sorting to coatomer (COPI)-coated vesicles and retrieval to the ER. J Cell Sci. 1997;110:991–1003. doi: 10.1242/jcs.110.8.991. [DOI] [PubMed] [Google Scholar]
- Brazer SC, Williams HP, Chappell TG, Cande WZ. A fission yeast kinesin affects Golgi membrane recycling. Yeast. 2000;16:149–166. doi: 10.1002/(SICI)1097-0061(20000130)16:2<149::AID-YEA514>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bresch C, Muller G, Egel R. Genes involved in meiosis and sporulation of a yeast. Mol Gen Genet. 1968;102:301–306. doi: 10.1007/BF00433721. [DOI] [PubMed] [Google Scholar]
- Brun C, Dubey DD, Huberman JA. pDblet, a stable autonomously replicating shuttle vector for Schizosaccharomyces pombe. Gene. 1995;164:173–177. doi: 10.1016/0378-1119(95)00497-t. [DOI] [PubMed] [Google Scholar]
- Chappell TG, Hajibagheri MAN, Ayscough K, Pierce K, Warren G. Localization of a d-1,2 galactosyltransfetase activity to the Golgi apparatus of Schizosaccharomyces pombe. Mol Biol Cell. 1994;5:519–528. doi: 10.1091/mbc.5.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- d'Enfert C, Wuestehube LJ, Lila T, Schekman R. Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J Cell Biol. 1991;114:663–670. doi: 10.1083/jcb.114.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C, Gensse M, Gaillardin C. Fission yeast and a plant have functional homologues of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. EMBO J. 1992;11:4205–4211. doi: 10.1002/j.1460-2075.1992.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Elagoz A, Callejo M, Armstrong J, Rokeach LA. Although calnexin is essential in S. pombe, its highly conserved central domain is dispensable for viability. J Cell Sci. 1999;112:4449–4460. doi: 10.1242/jcs.112.23.4449. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S, Novick P, Field C, Schekman R. Yeast secretory mutants that block the formation of active cell surface enzymes. J Cell Biol. 1984;98:35–43. doi: 10.1083/jcb.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL, Sherman DA. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, te Heesen S, Graham TR, Aebi M, Emr SD. Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J Cell Biol. 1994;127:653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King RC, editor. Handbook of Genetics. Vol. 1. New York: Plenum Press; 1974. pp. 395–446. [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Tanaka K. Nuclear behavior during conjugation and meiosis in the fission yeast Schizosaccharomyces pombe. J Gen Appl Microbiol. 1982;28:263–274. [Google Scholar]
- Hirata A, Shimoda C. Electron microscopic examination of sporulation-deficient mutants of the fission yeast Schizosaccharomyces pombe. Arch Microbiol. 1992;158:249–255. doi: 10.1007/BF00245240. [DOI] [PubMed] [Google Scholar]
- Hirata A, Shimoda C. Structural modification of spindle pole bodies during meiosis II is essential for the normal formation of ascospores in Schizosaccharomyces pombe: ultrastructural analysis of spo mutants. Yeast. 1994;10:173–183. doi: 10.1002/yea.320100205. [DOI] [PubMed] [Google Scholar]
- Horie S, Watanabe Y, Tanaka K, Nishiwaki S, Fujioka H, Abe H, Yamamoto M, Shimoda C. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol Cell Biol. 1998;18:2118–2129. doi: 10.1128/mcb.18.4.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Nakamura T, Kubo M, Shimoda C. S. pombe sporulation-specific coiled-coil protein Spo15 is localized to the spindle pole body and essential for its modification. J Cell Sci. 2000;113:545–554. doi: 10.1242/jcs.113.3.545. [DOI] [PubMed] [Google Scholar]
- Jensen R, Sprague GF, Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci USA. 1983;80:3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kishida M, Shimoda C. Genetic mapping of eleven spo genes essential for ascospore formation in the fission yeast Schizosaccharomyces pombe. Curr Genet. 1986;10:443–447. doi: 10.1007/BF00419871. [DOI] [PubMed] [Google Scholar]
- Kishida M, Hirata A, Shimoda C. A cold- sensitive spo14 mutation affecting ascospore formation in the fission yeast Schizosaccharomyces pombe. Plant Cell Physiol. 1990;31:433–437. [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Chen E, Goeddel DV. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction: technique. J Cell Mol Biol. 1989;1:11–15. [Google Scholar]
- Matynia A, Salus SS, Sazer S. Three proteins required for early steps in the protein secretory pathway also affect nuclear envelope structure and cell cycle progression in fission yeast. J Cell Sci. 2002;115:421–431. doi: 10.1242/jcs.115.2.421. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, Bentley DL. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klarl A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1990;194:793–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nakamura-Kubo M, Hirata A, Shimoda C. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+ encoding syntaxin-like protein. Mol Biol Cell. 2001;12:3955–3972. doi: 10.1091/mbc.12.12.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nakamura-Kubo M, Nakamura T, Shimoda C. A novel fission yeast Cdc7-Dbf4-like kinase complex required for the initiation and progression of meiotic second division. Mol Cell Biol. 2002;22:309–320. doi: 10.1128/MCB.22.1.309-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R. A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol. 1988;107:851–863. doi: 10.1083/jcb.107.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–2691. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y, Nakamura T, Hirata A, Routt SM, Skinner HB, Bankaitis VA, Shimoda C. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell. 2001;12:901–917. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman AM. Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J Cell Biol. 1998;140:29–37. doi: 10.1083/jcb.140.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Nakano A. Identification of a gene required for membrane protein retention in the early secretory pathway. Proc Natl Acad Sci USA. 1993;90:8179–8183. doi: 10.1073/pnas.90.17.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Multiple targets for brefeldin A. Cell. 1991;67:449–551. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Rexach MF, Schekman R. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991;14:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Nishikawa S, Nakano A. Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER), characterization of the RER1 gene products as a component involved in ER localization of Sec12p. Mol Biol Cell. 1995;6:1459–1477. doi: 10.1091/mbc.6.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p as common machinery for the endoplasmic reticulum localization of membrane proteins. Proc Natl Acad Sci USA. 1997;94:9693–9698. doi: 10.1073/pnas.94.18.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ueda T, Nakano A. The Arabidopsis thaliana RER1 gene family: its potential role in the endoplasmic reticulum localization of membrane proteins. Plant Mol Biol. 1999;41:815–824. doi: 10.1023/a:1006329828395. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J Cell Biol. 2001;152:935–944. doi: 10.1083/jcb.152.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol. 1996;134:279–293. doi: 10.1083/jcb.134.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirata A. Ascospore development in the fission yeasts. Schizosaccharomyces pombe and S. japonicus. J Cell Sci. 1982;56:263–279. doi: 10.1242/jcs.56.1.263. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yonekawa T, Kawasaki Y, Kai M, Furuya K, Iwasaki M, Murakami H, Yanagida M, Okayama H. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol Cell Biol. 2000;20:3459–3469. doi: 10.1128/mcb.20.10.3459-3469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turi TG, Webster P, Rose JK. Brefeldin A sensitivity and resistance in Schizosaccharomyces pombe. Isolation of multiple genes conferring resistance. J Biol Chem. 1994;269:24229–24236. [PubMed] [Google Scholar]
- Wang Y, Boguski M, Riggs M, Rodgers L, Wigler M. sar1, a gene from Schizosaccharomyces pombe encoding a protein that regulates ras1. Cell Regul. 1991;2:453–465. doi: 10.1091/mbc.2.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Imai Y, Watanabe Y. Molecular and Cellular Biology of the Yeast Saccharomyces, ed. J.R. Pringle, J.B. Broach, and E.W. Jones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. Mating and sporulation in Schizosaccharomyces pombe; pp. 1037–1106. [Google Scholar]
- Yoo BY, Calleja GB, Johnson BF. Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch Mikrobiol. 1973;91:1–10. doi: 10.1007/BF00409533. [DOI] [PubMed] [Google Scholar]