Abstract
Prohormones are directed from the trans-Golgi network to secretory granules of the regulated secretory pathway. It has further been proposed that prohormone conversion by endoproteolysis may be necessary for subsequent retention of peptides in granules and to prevent their release by the so-called “constitutive-like” pathway. To address this directly, mutant human proinsulin (Arg/Gly32:Lys/Thr64), which cannot be cleaved by conversion endoproteases, was expressed in primary rat islet cells by recombinant adenovirus. The handling of the mutant proinsulin was compared with that of wild-type human proinsulin. Infected islet cells were pulse labeled and both basal and stimulated secretion of radiolabeled products followed during a chase. Labeled products were quantified by high-performance liquid chromatography. As expected, the mutant proinsulin was not converted at any time. Basal (constitutive and constitutive-like) secretion was higher for the mutant proinsulin than for wild-type proinsulin/insulin, but amounted to <1% even during a prolonged (6-h) period of basal chase. There was no difference in stimulated (regulated) secretion of mutant and wild-type proinsulin/insulin at any time. Thus, in primary islet cells, unprocessed (mutant) proinsulin is sorted to the regulated pathway and then retained in secretory granules as efficiently as fully processed insulin.
INTRODUCTION
The regulated secretory pathway allows for quantal release of appropriate amounts of secretory proteins in rapid response to extracellular stimuli. This is the hallmark of many exocrine and neuroendocrine cells and depends upon the efficient sorting of secretory products to secretory granules and storage in these organelles before stimulation of exocytosis. Although much progress has been made in the understanding of this highly specialized pathway since its original description by Palade (1975) and because the clear distinction was made between its kinetics and those of the constitutive secretory pathway that is active in all cells (Kelly, 1985), a number of key events remain to be understood. In particular, the precise mechanisms allowing for sorting of proteins to the regulated secretory pathway remain unclear. It is well established that an initial sorting event (Moore and Kelly, 1985, 1986) occurs within the trans-Golgi network (TGN) (Orci et al., 1987a; Halban and Irminger, 1994) allowing for delivery of proteins to nascent secretory granules; so-called “sorting for entry” (Arvan and Castle, 1992, 1998). The mechanism of this sorting event remains controversial (Thiele et al., 1997; Thiele and Huttner, 1998; Molinete et al., 2000). It has further been suggested that storage of proteins within granules is ensured by an additional (and possibly dominant) mechanism, so-called “sorting by retention” (Arvan and Castle, 1992, 1998). This latter sorting event has been suggested to depend upon the physicochemical properties of proteins in granules, and notably upon their ability to condense.
Insulin secretion from the pancreatic β-cell is a useful and well-studied model system for the study of regulated secretion, for two main reasons. First, sorting to the regulated pathway of this cell is particularly well regulated. We thus demonstrated many years ago that >99% of all newly synthesized proinsulin is directed to this pathway in primary rat β-cells (Rhodes and Halban, 1987). Second, and by the very nature of the proteins handled by the β-cell, it affords a particularly attractive model system for the study of sorting mechanisms. Proinsulin is converted by endoproteolysis to insulin and C-peptide. Whereas both proinsulin and C-peptide are believed to remain soluble within granules, insulin can form Zn-hexamers and in turn crystallizes in the majority of mammalian species (Emdin et al., 1980). Others have capitalized on such differences in physicochemical properties to study the fate of proinsulin and insulin respectively in the regulated pathway and they concluded that proinsulin is sorted normally to granules but that it is not retained well within this secretory compartment unless it can be converted to insulin (Kuliawat et al., 2000). It has further been proposed that proteins not retained within granules are removed in vesicles budding off from them and secreted by the so-called “constitutive-like” pathway, presumably after passage through the endosomal compartment (Turner and Arvan, 2000).
Previous studies on proinsulin sorting/retention have been performed in transformed β-cells or other transformed regulated secretory cell types (Kuliawat et al., 2000). Yet, it is clear that even the most well-differentiated transformed cells do not faithfully reproduce all features of the primary cell counterpart. This is certainly true of the β-cell (Nagamatsu and Steiner, 1992; Neerman-Arbez and Halban, 1993; Neerman-Arbez et al., 1993). The present study was designed to overcome this problem by following the fate of proinsulin and insulin in primary rat β-cells and with a view to determining definitively whether proinsulin endoproteolysis is important for efficient regulated secretion. To this end, either native human proinsulin or a mutant that is not subject to endoproteolytic cleavage (Docherty et al., 1989) was expressed in rat islet cells by recombinant adenovirus infection. The kinetics of secretion from pulse-labeled cells were followed under basal and stimulated conditions during 7 h of chase. The results show clearly that unprocessed proinsulin is sorted to granules, as expected, and retained within them as efficiently as fully processed insulin.
MATERIALS AND METHODS
Materials
All Materials were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Islet Isolation and Establishment of Islet Cells in Monolayer Culture
Islets of Langerhans were isolated from the pancreas of adult male Wistar rats (180–220 g) by using the collagenase digestion method and purification by centrifugation on a discontinuous density gradient as described in detail previously (Rouiller et al., 1990). To obtain a suspension of islet cells, islets were digested with trypsin (Rouiller et al., 1990). The dispersed islet cell population, consisting largely of single cells, was placed in culture overnight to allow for recovery from the isolation/dispersion procedure [2.8 × 105 cells/10 ml DMEM, 10% fetal calf serum, 11.2 mM glucose-(DMEM), in 10-cm-diameter plastic Petri dishes suitable for bacterial culture and to which mammalian cells do not attach]. The following day, cells were concentrated in the same culture medium and seeded in droplets (6 × 104 cells/70-μl droplet) on the surface of plastic Petri dishes suitable for mammalian cell culture and precoated with matrix secreted from 804G (rat bladder carcinoma) cells (a generous gift of Desmos, San Diego, CA) to enhance cell attachment and spreading (Bosco et al., 2000). After 18–24 h in culture, the cells had formed monolayers, shown previously to allow for optimal infection of the largest number of primary islet cells by recombinant adenovirus (Molinete et al., 2001).
Replication-deficient Adenovirus Expressing Native or Mutant Human Proinsulin
cDNA encoding mutant (Arg/Gly32:Lys/Thr64) human preproinsulin was obtained from Dr. K. Docherty (University of Aberdeen, Aberdeen, Scotland). Preparation of replication-deficient recombinant adenovirus expressing wild-type human proinsulin (Adeno-proins.wt), mutant proinsulin (Adeno-proins.mut), or control without insert (“control” virus:Adeno-GFP) was as described previously (Molinete et al., 2001). Both the mutant proinsulin and the control virus expressed green fluorescent protein (GFP), allowing for direct assessment of infection in living cells. Monolayers were washed in DMEM and then infected with adenovirus for 90 min in DMEM. The infected cells were washed two times with phosphate-buffered saline and then cultured for 24 h to allow for expression of transduced protein. The multiplicity of infection was established empirically for each virus to obtain infection of >90% of the cells with no visible signs of toxicity (as evidenced by rounding up of cells and detachment from the surface of the dish) and as described previously (Molinete et al., 2001).
Pulse-Chase Experiments
One day after infection with recombinant adenovirus, the cells were washed three times with Krebs-Ringer bicarbonate buffer, 10 mM HEPES, pH 7.4, 0.25% bovine serum albumin (KRB-BSA), 16.7 mM glucose, preincubated 15 min at 37°C, and then pulse labeled (30 min) in 100 μl of this same buffer with 100 μCi of [3H]leucine (specific radioactivity 100–200 Ci/mmol; Anawa Trading, Wangen, Switzerland). The labeled cells were washed three times and then incubated in 2 ml of KRB-BSA, 2.8 mM glucose, 0.5 mM leucine for a basal chase period of 1 or 6 h. After each period, the basal chase medium was centrifuged (600 rpm; 10 min) to remove any floating cells and kept frozen before analysis. The cells were incubated for a further 1 h in KRB-BSA, 16.7 mM glucose with 0.1 mM isobutylmethyl xanthine (IBMX) and 5 μM forskolin to stimulate secretion. After this period of stimulation, the chase medium was centrifuged as described above and the cells were extracted in 1 M acetic acid, 0.1% BSA and both were kept frozen before analysis. Chase media were acidified by addition of 1 M HCl to a final concentration of 20 mM before chromatography. To study the kinetics of degradation of mutant proinsulin during the first hours of chase and to see whether lactacystin could inhibit such degradation, cells in monolayer were infected with Adeno-proins.mut or Adeno-GFP and 1 d later preincubated for 90 min in DMEM without serum with or without (controls) 20 μM lactacystin and then handled as described above for a 10-min labeling period followed by a 2-h chase under basal conditions with or without (controls) 20 μM lactacystin. Cells were extracted in acetic acid at set times and the extracts analyzed by high-performance liquid chromatography (HPLC).
Reverse-Phase HPLC
Radiolabeled proinsulin/insulin-related peptides were separated and quantified by reverse phase-HPLC as described in detail previously (Sizonenko and Halban, 1991; Sizonenko et al., 1993; Irminger et al., 1994). The system was calibrated for human peptides by using authentic unlabeled standards of human proinsulin, des-64,65- and des-31,32-split proinsulin, and insulin (generous gifts of Eli Lilly, Indianapolis, IN) and for rat peptides by using radiolabeled products secreted from pulse-chased INS-1 (rat insulinoma) cells (Sizonenko and Halban, 1991). To identity mutant (Arg/Gly32:Lys/Thr64) human proinsulin and calibrate the HPLC system, INS cells were infected with the mutant proinsulin adenovirus, pulse labeled, chased under basal conditions for 1 h, and then stimulated with a mixture of secretagogues for 1 h (Neerman-Arbez et al., 1993). Radiolabeled products in the stimulated medium were analyzed by HPLC and compared with those secreted from INS cells infected with control virus. The radioactive products were immunoprecipitated using guinea pig anti-insulin serum by using standard conditions known to allow for quantitative precipitation of both proinsulin and insulin (Halban et al., 1980). Nonimmune serum was used as control. Immunoprecipitation supernatants and pellets were analyzed by HPLC after removal of immunoglobulins by using a SepPak cartridge (Waters Division, Millipore, Milford, MA).
Statistics
Unless stated otherwise, data are presented as mean ± SE for three independent experiments. Statistical significance of differences between groups was assessed using Student's unpaired t test.
RESULTS
Identification of Mutant Proinsulin by HPLC and Quantification of Human and Rat Proinsulin/Insulin
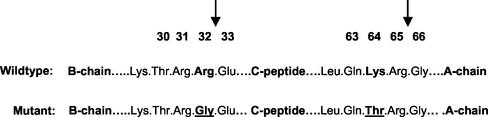
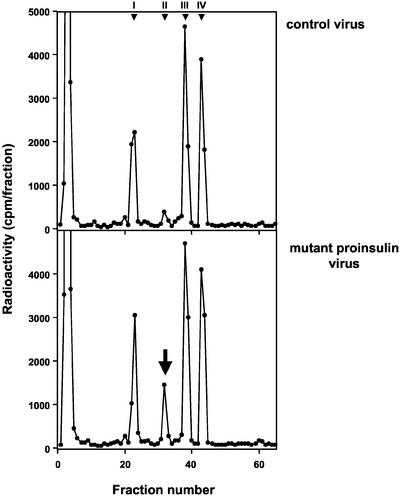
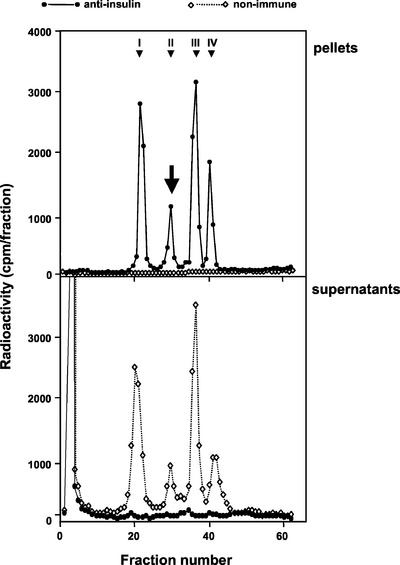
The mutant human proinsulin used in this study carries two mutations leading to the replacement of Arg32 by Gly and of Lys64 by Thr (Figure 1). The mutant proinsulin has been shown to be resistant to cleavage by both conversion endoproteases PC2 and PC3 and cannot therefore be processed (Docherty et al., 1989; Taylor and Docherty, 1992). Although there are well-established methods for analysis and quantification of proinsulin, and its conversion intermediates, insulin and C-peptide of both rat and human origin, it is not easy to predict where a mutant molecule will elute. It was thus first necessary to establish a method for the unequivocal identification and quantification of the mutant proinsulin in presence of normal rat islet cell products. The direct comparison of radiolabeled products secreted from islets infected with Adeno-GFP (control), Adeno-proins.wt, or Adeno-proins.mut revealed the presence of an as yet unidentified peak coeluting with rat des-31.32-split proinsulin at 30 min (Figure 2, bottom). To determine whether this peak corresponded to the mutant proinsulin, INS-1 cells were pulse labeled and secreted products subject to immunoprecipitation by using anti-insulin serum recognizing also proinsulin and its conversion intermediates. It was assumed that this broad cross-reactivity would allow for precipitation of the mutant proinsulin molecule despite its modified sequence. Such was the case (Figure 3). INS-1 cells infected with Adeno-proins.mut presented a peak of radioactivity in addition to the known peaks expected for rat proinsulin and its conversion products. This peak, eluting at ∼30 min was immunoprecipitated by anti-insulin but not by nonimmune serum, confirming that it is indeed the mutant proinsulin.
Figure 1.
Primary sequence of mutant (Arg/Gly32:Lys/Thr64) and wild-type human proinsulin. The two amino acids altered in the mutant are underlined. The sites of cleavage of proinsulin by the conversion endoproteases are indicated by the arrows.
Figure 2.
HPLC analysis of INS-1 cells expressing mutant (Arg/Gly32:Lys/Thr64) human proinsulin. INS cells were infected with control adenovirus expressing only GFP (top) or with adenovirus expressing the mutant human proinsulin (bottom). After a pulse chase, secretion was stimulated and labeled secretory products analyzed by HPLC. There was a new peak eluting at 30–32 min in cells infected with the mutant human proinsulin virus (bottom), as indicated by the arrow. Peak identity: I, rat insulins; II, rat des-31,32-split proinsulins and mutant (Arg/Gly32: Lys/Thr64) human proinsulin; III, rat des-64,65-split proinsulins; and IV, rat proinsulins.
Figure 3.
Immunoprecipitation and HPLC analysis of mutant (Arg/Gly32:Lys/Thr64) human proinsulin. INS-1 cells were infected with adenovirus expressing the mutant human proinsulin. After a pulse chase, secretion was stimulated and secretory products were immunoprecipitated by using anti-insulin or nonimmune serum and then analyzed by HPLC. Top, immunoprecipitation pellets. Bottom, immunoprecipitation supernatants. The mutant proinsulin (arrow) was precipitated quantitatively by anti-insulin serum. Peak identity: I, rat insulins; II, rat des-31,32-split proinsulins and mutant (Arg/Gly32:Lys/Thr64) human proinsulin; III, rat des-64,65-split proinsulins; and IV, rat proinsulins.
It was apparent from comparison of the elution profile of cells expressing only (endogenous) rat proinsulin, its conversion intermediates and insulin, with those expressing in addition either human proinsulin (and its conversion intermediates and insulin) or the mutant proinsulin, that there was coelution of products of immediate interest. In particular, human proinsulin coeluted with des-64,65-split rat proinsulin and the mutant human proinsulin coeluted with des-31,32-split proinsulin. Control experiments revealed no impact of the expression of either native or mutant human proinsulin on the kinetics of conversion of rat proinsulin (see below; Table 2). It was thus possible to calculate the contribution of the rat proinsulin conversion intermediates to the native or mutant human proinsulins, allowing for precise calculation of the radioactivity in all proinsulin-related products of both rat and human origin.
Table 2.
Conversion of human and rat proinsulin in pulse-chased rat islet cells
| Adenovirus | Proinsulin Conversion (%)
|
||
|---|---|---|---|
| Adeno-proins.wt | Adeno-proins.mut | ||
| Proinsulin species | Human wt | Rat | Rat |
| 1 h Basal medium | 55.4 ± 7.8 | 35.1 ± 2.4 | 46.8 ± 8.5 |
| + 1 h stimulated medium | 96.5 ± 1.2 | 96.7 ± 0.1 | 97.9 ± 0.2 |
| 1 + 1 h cell extracts | 91.3 ± 3.2 | 94.7 ± 0.2 | 93.2 ± 0.1 |
| 6 h Basal medium | 70.7 ± 8.3 | 64.3 ± 4.5 | 72.8 ± 2.7 |
| + 1 h stimulated medium | 100 | 99.2 ± 0.1 | 99.5 ± 0.1 |
| 6 + 1 h cell extracts | 96.7 ± 0.6 | 97.8 ± 0.5 | 97.7 ± 0.4 |
mut, Arg/Gly32:Lys/Thr64 human proinsulin; wt, wild-type human proinsulin.
Islet cells were infected as indicated with adenovirus expressing wild-type (Adeno-proins.wt) or mutant (Arg/Gly32:Lys/Thr64) proinsulin (Adeno-proins.mut) and pulse labeled for 30 min. The cells were then chased under basal conditions for 1 or 6 h followed in each instance by a 1-h period of stimulation with secretagogues. Radioactive products were analyzed by HPLC. Proinsulin conversion is presented as (insulin/insulin + conversion intermediates + proinsulin) × 100. Data are for the conversion of human wild-type proinsulin or endogenous rat proinsulin in cells infected with one or the other recombinant adenovirus. There was no detectable conversion of the mutant proinsulin at any time (our unpublished data).
Proinsulin Biosynthesis and Conversion
Islet cells were infected with Adeno-proins.wt or Adeno-proins.mut and 24 h later, they were pulse labeled for 30 min. After a brief 15-min chase period under basal conditions (shown previously to be needed to ensure maximal labeling of proinsulin and during which time there was no detectable secretion of labeled products), the cells were extracted and analyzed by HPLC to quantify the radiolabeling of proinsulin as well as the small amounts of conversion products present at this time (Table 1). The mutant human proinsulin was expressed at 39% that of the wild type that in turn was expressed at 17% of rat proinsulin. There was no significant difference in the labeling of rat proinsulin for cells infected with Adeno-proins.wt or Adeno-proins.mut.
Table 1.
Incorporation of radioactivity into human and rat proinsulin
| Virus | Total Proinsulin-related Radioactivity (dpm × 10−3)
|
|
|---|---|---|
| Human | Rat | |
| Adeno-proins.wt | 88 ± 15 | 507 ± 99 |
| Adeno-proins.mut | 34 ± 1 | 451 ± 55 |
Rat islet cells were infected with adenovirus expressing wild-type (Adeno-proins.wt) or mutant (Arg/Gly32:Lys/Thr64) proinsulin (Adeno-proins.mut) and pulse labeled for 30 min. Cell extracts were analysed by HPLC to quantify radioactivity in human and rat proinsulin.
As expected, the mutant proinsulin was not converted at any time point (Docherty et al., 1989; Taylor and Docherty, 1992). The extent of proinsulin conversion for the rat or the wild-type human molecule increased as expected with time of chase (Table 2). The differences in the conversion of human and rat proinsulin are related to differences in the primary sequence of human proinsulin and the two nonallelic rat proinsulins as shown previously (Sizonenko and Halban, 1991; Sizonenko et al., 1993). Note that no effort was made to separate the products of the two nonallelic rat preproinsulin genes; this was not considered relevant to the present study because the rat peptides served only as internal control. No significant differences in conversion of rat proinsulin were seen between islet cells infected with adenovirus expressing wild-type or mutant proinsulin, and neither was there any difference between infected and noninfected cells (our unpublished data). Furthermore, no differences in conversion were seen in cells over a 2-h period under (basal) conditions in which essentially all labeled proinsulin and insulin was retained in the cells (our unpublished data). This is taken to indicate that there was no untoward effect of viral infection per se as can occur if the multiplicity of infection is too high.
On the basis of these results, it became apparent that the model system behaved as expected and that the fate of converted (wild-type) or of nonconverted (mutant) human proinsulin could be compared directly in the same setting (infected rat islet cells), with the rat proinsulin-related peptides serving as internal standard and to confirm that adenoviral infection or expression of human proinsulin did not affect islet cell function.
Fate of Newly Synthesized (Radiolabeled) Proinsulin/Insulin
To determine the impact of blocking proinsulin endoproteolysis on its fate within the cell, and most notably its handling by the regulated pathway, adeno-infected, pulse-labeled islet cells were chased under basal conditions (2.8 mM glucose) for 1 or 6 h followed in each instance by a 1-h period of stimulation (16.7 mM glucose, 0.1 mM IBMX, 5 μM forskolin). Radiolabeled products were quantified in the basal and stimulated chase buffers and in cell extracts at the end of the stimulation. To follow the fate of rat proinsulin and the wild-type human molecule, proinsulin, conversion intermediates and insulin were summed [and indicated as “(pro)insulin”]. Given that the mutant proinsulin was not subject to proteolytic cleavage, only the intact molecule was taken into consideration.
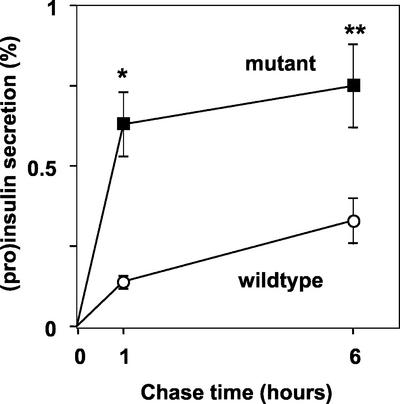
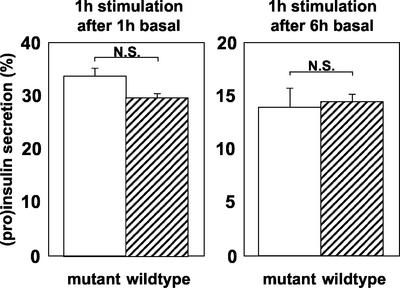
Basal secretion was significantly higher for mutant (unconverted) proinsulin than for human (pro)insulin at both 1 and 6 h of chase (Figure 4). Significantly, however, <1% of mutant proinsulin had been secreted even after 6 h of basal chase. There was no difference in the percentage of labeled mutant and wild-type (pro)insulin released during the 1-h periods of stimulation after either 1 or 6 h of basal chase (Figure 5).
Figure 4.
Proinsulin and insulin secretion from primary rat islet cells under basal conditions. Islet cells were infected with adenovirus expressing mutant (Arg/Gly32:Lys/Thr64) or wild-type human proinsulin and then pulse labeled. Secretion of labeled products was followed during a 6-h chase under basal conditions. Labeled products were quantified by HPLC. For the wild type, data are for the sum of proinsulin, conversion intermediates, and insulin. There was no conversion of the mutant proinsulin. Secretion is expressed as a percentage of the total proinsulin/insulin in chase medium plus cell extracts. There was significantly more mutant proinsulin secreted at both 1 h (*p < 0.01) and 6 h (**p < 0.05) of chase. Note, however, that <1% of mutant proinsulin had been secreted during the entire 6-h basal chase period.
Figure 5.
Stimulated secretion of mutant (Arg/Gly32:Lys/Thr64) or wild-type proinsulin/insulin from primary rat islet cells. Islet cells were infected with adenovirus expressing mutant (Arg/Gly32:Lys/Thr64) or wild-type human proinsulin and then pulse labeled. Cells were chased under basal conditions for 1 h or for 6 h followed in each instance by a 1-h period of stimulation (glucose, IBMX, and forskolin). Labeled products in the stimulated medium were analyzed and data presented as described in the legend to Figure 4.
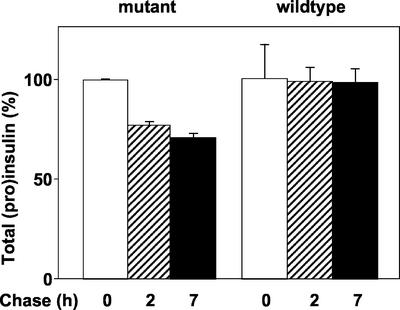
The total amount of radioactive (pro)insulin was estimated by summing that in the basal and stimulated media and in cell extracts after a total of 2 h (1 h basal + 1 h stimulated) or 7 h (6 h basal + 1 h stimulated) of chase. There was no significant loss of the wild-type molecules during the entire 7-h period (Figure 6, right). By contrast, 24% of the mutant proinsulin was lost from the system after 2 h of chase (Figure 6, left). There was no further loss between 2 and 7 h of chase.
Figure 6.
Degradation of human mutant proinsulin in primary rat islet cells. Islet cells were infected with adenovirus expressing mutant (Arg/Gly32:Lys/Thr64) or wild-type human proinsulin, pulse labeled, and chased for 1 or 6 h under basal conditions followed in each instance by 1 h of stimulation, for total chase times of 2 and 7 h, respectively. The radiolabeled proinsulin/insulin was measured in basal and stimulated chase media and in cell extracts and summed. There was loss of 23% mutant proinsulin due to degradation during the first 2 h of chase but not thereafter. There was no significant loss of wild-type human proinsulin.
Kinetics of Degradation of Mutant Proinsulin and Its Inhibition by Lactacystin
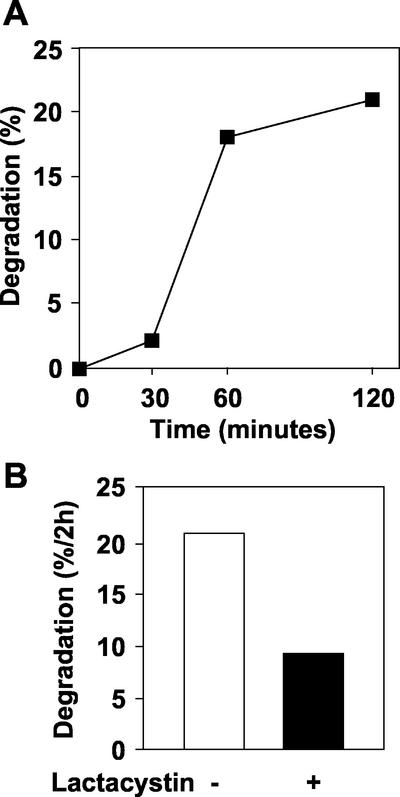
To monitor the kinetics of degradation of the mutant proinsulin in greater detail during the first 2 h of chase, cells were labeled for a shorter period of time (10 min) and the extent of degradation monitored at 30, 60, and 120 min of chase. The results indicated only 2% degradation by 30 min but with a major increase to 18% by 60 min and with only a modest further increase to 21% by 120 min (Figure 7). These kinetics are reminiscent of proteosomal degradation of proteins retained in the ER. This was substantiated by the observation that the extent of degradation over the full 2-h period was decreased to just 9% by the proteosomal inhibitor lactacystin (Figure 7). There was no apparent effect of lactacystin on the conversion of endogenous rat proinsulin and neither was there any effect on basal secretion (our unpublished data).
Figure 7.
Kinetics of degradation of newly synthesized mutant proinsulin and inhibition by lactacystin. To monitor the kinetics of degradation of newly synthesized mutant (Arg/Gly32:Lys/Thr64) proinsulin, islet cells infected with adenovirus expressing the mutant proinsulin were pulse labeled (10 min) and chased under basal conditions for 30, 60, or 120 min in presence or absence of the proteosome inhibitor lactacystin (20 μM). (A) Degradation of mutant proinsulin with time of chase. (B) Effect of lactacystin on degradation of mutant proinsulin during a 2-h chase. Data are means of duplicate observations from one of two independent experiments.
DISCUSSION
It is well established that unprocessed proinsulin is sorted within the TGN to immature, clathrin-coated granules (Orci, 1982, 1985; Orci et al., 1987a) and that conversion to insulin and C-peptide occurs in maturing granules (Orci, 1985, 1986; Orci et al., 1985, 1986, 1987b). The mutant proinsulin used in the present study contains two alterations, Agr/Gly32 and Lys/Thr64, resulting in the loss of two charged, basic residues and most significantly, that of paired basic residues at the two cleavage sites for the conversion endoproteases. The sorting of this mutant proinsulin to the regulated pathway seems to be as efficient as for the native protein, suggesting that, unlike for other proteins (Feliciangeli et al., 2001; Feliciangeli and Kitabgi, 2002), pairs of basic residues are not implicated in sorting of proinsulin within the TGN.
Having established normal sorting to the regulated pathway, the major question to be addressed in this study was whether unprocessed proinsulin was retained within granules (so-called sorting by retention; Arvan and Castle, 1998) as effectively as fully processed insulin. In previous studies, it was suggested that removal of soluble proteins from granules arises over several hours (Kuliawat et al., 2000). This is in itself surprising, given that maturation of granules in the β-cell is very rapid, occurring in parallel with progressive granule acidification and proinsulin conversion (Orci et al., 1987b, 1994) and largely complete within a couple of hours in primary rat β-cells. Notwithstanding, we elected to follow the fate of the mutant proinsulin over a total chase time of 6 h under basal conditions. Secretion under basal conditions is considered to be the sum of constitutive secretion (the bona fide constitutive pathway, with discharge by exocytosis of vesicles formed at the TGN), constitutive-like secretion (the so-called postgranular pathway, involving secretion from vesicles formed from granules), and true basal secretion from the regulated pathway (exocytosis from large dense-core secretory granules). Although there is no direct means of distinguishing between these three pathways, the total amount of material secreted under basal conditions from primary β-cells is in any event extremely low. Thus, <1% newly synthesized proinsulin or insulin had been released during a 6-h basal chase period. There was significantly more mutant proinsulin than wild-type proinsulin plus insulin released during this time. One could argue that this difference reflects secretion of mutant proinsulin not retained in granules and released via the constitutive-like pathway just as described by Kuliawat et al. (2000). If so, we estimate that only 0.6% newly synthesized mutant proinsulin was released in this manner. We do not consider this of physiological significance and neither can this trivial amount be considered as relevant to our understanding of the cell biology of the regulated secretory pathway. It is thus important to stress that primary cells were studied herein, whereas transformed cells with their known peculiarities (and perhaps a more active constitutive-like pathway) were used by others (Kuliawat et al., 2000). Another difference is that in the present studies the nonconverted (mutant) proinsulin was expressed in cells producing large amounts of native, endogenous (rat) proinsulin. The mutant proinsulin may thus have been retained in granules due to association with the endogenous insulin. If this does occur it would be another natural consequence of using primary insulin-producing cells. We cannot, however, exclude the possibility that the mutant proinsulin is retained in granules by virtue of aberrant physiochemical properties intrinsic to this unnatural molecule and leading to its condensation in granules. Regardless, the results do show unequivocally that conversion of proinsulin is not a prerequisite for retention in granules.
There was a considerable amount of mutant proinsulin lost from the system during the first 2 h of chase in the face of no loss of wild-type proinsulin plus insulin. This relatively rapid loss of ∼20% of mutant proinsulin is attributed to intracellular degradation. Its time course is not indicative of crinophagy (fusion of granules with lysosomes) that typically takes days rather than hours (Halban and Wollheim, 1980; Halban and Renold, 1983). Had degradation been due to transfer from granules to the endosomal system and from there to lysosomes (Turner and Arvan, 2000), again the timing would have been very different, with relative stability during the first few hours of chase followed by degradation thereafter. Rather, we suggest that this is consequent to (partial) misfolding and/or unusually slow transit through of the mutant molecule with recognition by the quality control process of the rough endoplasmic reticulum and proteolysis by 26S proteosomes (for reviews, see Jarosch et al., 2002; Kaufman et al., 2002). The inhibition of this degradation by the proteosome inhibitor lactacystin supports this hypothesis. Molecules that escape such degradation soon after synthesis and having been delivered from the rough endoplasmic reticulum to the Golgi complex, would remain very stable throughout the remainder of the experimental period, in keeping with our results. It is not clear from the present study whether mutant proinsulin retained in granules is correctly folded. However, the elution time of this molecule from HPLC is as predicted for a correctly folded proinsulin molecule lacking basic residues and the mutated residues are in any event predicted to be on the surface of the molecule (and accessible to the conversion endoproteases) (Blundell et al., 1972; Emdin et al., 1980). The mutations may thus slow rather than prevent normal folding.
The present finding of highly efficient sorting of unprocessed proinsulin to secretory granules followed by retention and storage in this compartment contrasts with the findings of others. Arvan and colleagues have thus demonstrated in mammalian cells (Kuliawat et al., 2000) and in yeast (Zhang et al., 2001) that proinsulin processing is necessary for normal retention in the regulated secretory compartment. We do not challenge these previous findings. The experimental approaches and settings are so different as to prevent direct comparison. We intentionally restricted our study to primary islet cells. We consider this to be the gold-standard cellular setting for the study of the regulated secretory pathway for insulin and its biosynthetic precursors (Rhodes and Halban, 1987). The present study is the first to examine directly the fate of unprocessed proinsulin in β-cells over long periods. This is usually impossible given the rapid kinetics of conversion of this prohormone. The expression of human proinsulin in rat islet cells did not alter the handling of endogenous rat proinsulin. It was felt important to limit the level of expression of the human proinsulin so as not to overload the regulated secretory pathway and to ensure that there was no anomalous condensation of the mutant proinsulin simply by virtue of an unphysiologically high local concentration in the secretory pathway. With the provisos mentioned above (and notably the possibly aberrant behavior of the mutant proinsulin), we therefore conclude that the model system is valid for the purposes of this study. We do however agree with Arvan and coworkers that both sorting for entry (from the TGN to immature granules) and sorting by retention (in granules) are operational in secretory cells but that the relative contribution of each mechanism will vary from one cell setting to another and from one secretory protein to another (Arvan et al., 2002).
Our results are in fact in keeping with several other experiments in primary cells, both in vitro and in vivo. In human, type 2 (noninsulin-dependent) diabetes is associated with an increased ratio of proinsulin:insulin in the circulation. This is not believed to be due to an intrinsic defect in conversion. In this situation, proinsulin is secreted via the regulated pathway (Kahn and Halban, 1997). In mice, the homozygous Cpefat/Cpefat mutation in carboxypeptidase E leads to a profound inhibition of proinsulin conversion. Although it has been suggested that carboxypeptidase is a sorting receptor in the TGN (Cool et al., 1997), this hypothesis has been questioned (Thiele et al., 1997) and unconverted proinsulin in β-cells from these mice does seem to be sorted efficiently to the regulated pathway and stored within granules (Irminger et al., 1997; Varlamov et al., 1997). This is confirmed by the presence of granules with a pale homogenous content by electron microscopy (Naggert et al., 1995) typical of proinsulin-rich granules (Orci et al., 1994). In knockout mice lacking the conversion enzyme PC2, there is again apparently normal storage of unconverted proinsulin (along with conversion intermediates) in granules (Furuta et al., 1998). Even though proinsulin is thus clearly stored in granules in β-cells from both these mice, it could be reasoned that there is nonetheless some leakiness with time and this has not been tested experimentally. Regardless, the amounts of unprocessed proinsulin cleared from granules must be modest. Finally, we have shown previously that proinsulin that cannot be converted due to incorporation of analogs of lysine and arginine (thialysine and canavanine) is sorted to immature granules and secreted in response to secretagogues (Halban et al., 1984; Orci et al., 1984).
In conclusion, mutant proinsulin that cannot be processed by endoproteases is handled by the regulated pathway of β-cells as efficiently as the native prohormone and its conversion product insulin. Processing is thus not important per se for sorting to granules or retention in this storage compartment.
ACKNOWLEDGMENTS
We thank Dr. Kevin Docherty (University of Aberdeen, Aberdeen, Scotland) for the generous gift of the mutant preproinsulin plasmid and Stéphane Dupuis for expert technical assistance. This work was supported by the Swiss National Science Fund grant 3200-061776.00.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0299. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0299.
REFERENCES
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Zhang B, Feng L, Liu M, Kuliawat R. Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr Opin Cell Biol. 2002;14:448–453. doi: 10.1016/s0955-0674(02)00344-7. [DOI] [PubMed] [Google Scholar]
- Blundell T, Dodson G, Hogkin D, Mercola D. Insulin: the structure in the crystal and its reflection in biochemistry and biology. Adv Protein Chem. 1972;26:279–401. [Google Scholar]
- Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet β-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Docherty K, Rhodes CJ, Taylor NA, Shennan KI, Hutton JC. Proinsulin endopeptidase substrate specificities defined by site-directed mutagenesis of proinsulin. J Biol Chem. 1989;264:18335–18339. [PubMed] [Google Scholar]
- Emdin SO, Dodson GG, Cutfield JM, Cutfield SM. Role of zinc in insulin biosynthesis. Diabetologia. 1980;19:174–182. doi: 10.1007/BF00275265. [DOI] [PubMed] [Google Scholar]
- Feliciangeli S, Kitabgi P. Insertion of dibasic residues directs a constitutive protein to the regulated secretory pathway. Biochem Biophys Res Commun. 2002;290:191–196. doi: 10.1006/bbrc.2001.6137. [DOI] [PubMed] [Google Scholar]
- Feliciangeli S, Kitabgi P, Bidard JN. The role of dibasic residues in prohormone sorting to the regulated secretory pathway. A study with proneurotensin. J Biol Chem. 2001;276:6140–6150. doi: 10.1074/jbc.M009613200. [DOI] [PubMed] [Google Scholar]
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- Halban PA, Amherdt M, Orci L, Renold AE. Proinsulin modified by analogues of arginine and lysine is degraded rapidly in pancreatic B-cells. Biochem J. 1984;219:91–97. doi: 10.1042/bj2190091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Irminger JC. Sorting and processing of secretory proteins. Biochem J. 1994;299:1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Renold AE. Influence of glucose on insulin handling by rat islets in culture. Diabetes. 1983;32:254–261. doi: 10.2337/diab.32.3.254. [DOI] [PubMed] [Google Scholar]
- Halban PA, Wollheim CB. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. J Biol Chem. 1980;255:6003–6006. [PubMed] [Google Scholar]
- Halban PA, Wollheim CB, Blondel B, Renold AE. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the β cell. Biochem Pharmacol. 1980;29:2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Irminger JC, Verchere CB, Meyer K, Halban PA. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J Biol Chem. 1997;272:27532–27534. doi: 10.1074/jbc.272.44.27532. [DOI] [PubMed] [Google Scholar]
- Irminger JC, Vollenweider FM, Neerman-Arbez M, Halban PA. Human proinsulin conversion in the regulated and the constitutive pathway of transfected AtT20 cells. J Biol Chem. 1994;269:1756–1762. [PubMed] [Google Scholar]
- Jarosch E, Geiss-Friedlander R, Meusser B, Walter J, Sommer T. Protein dislocation from the endoplasmic reticulum - pulling out the suspect. Traffic. 2002;3:530–536. doi: 10.1034/j.1600-0854.2002.30803.x. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes. 1997;46:1725–1732. doi: 10.2337/diab.46.11.1725. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Prabakaran D, Arvan P. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol Biol Cell. 2000;11:1959–1972. doi: 10.1091/mbc.11.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete M, Irminger JC, Tooze SA, Halban PA. Trafficking/sorting and granule biogenesis in the β-cell. Semin Cell Dev Biol. 2000;11:243–251. doi: 10.1006/scdb.2000.0173. [DOI] [PubMed] [Google Scholar]
- Molinete M, Dupuis S, Brodsky FM, Halban PA. Role of clathrin in the regulated secretory pathway of pancreatic β-cells. J Cell Sci. 2001;114:3059–3066. doi: 10.1242/jcs.114.16.3059. [DOI] [PubMed] [Google Scholar]
- Moore HPH, Kelly RB. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985;101:1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HPH, Kelly RB. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986;321:443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Steiner DF. Altered glucose regulation of insulin biosynthesis in insulinoma cells: mouse β TC3 cells secrete insulin-related peptides predominantly via a constitutive pathway. Endocrinology. 1992;130:748–754. doi: 10.1210/endo.130.2.1733723. [DOI] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouillé Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinemia in obese fat/fat mice is associated with a point mutation in the carboxypeptidase E gene and reduced carboxypeptidase activity in pancreatic islets. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Neerman-Arbez M, Halban PA. Novel, non-crinophagic, degradation of connecting peptide in transformed pancreatic β cells. J Biol Chem. 1993;268:16248–16252. [PubMed] [Google Scholar]
- Neerman-Arbez M, Sizonenko SV, Halban PA. Slow cleavage at the proinsulin B-chain/connecting peptide junction associated with low levels of endoprotease PC1/3 in transformed β cells. J Biol Chem. 1993;268:16098–16100. [PubMed] [Google Scholar]
- Orci L. Macro and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L. The insulin factory: a tour of the plant surroundings and a visit to the assembly line. Diabetologia. 1985;28:528–546. doi: 10.1007/BF00281987. [DOI] [PubMed] [Google Scholar]
- Orci L. The insulin cell: its cellular environment and how it processes (pro)insulin. Diabetes Metab Rev. 1986;2:71–106. doi: 10.1002/dmr.5610020106. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli JD, Anderson RGW. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Halban P, Amherdt M, Ravazzola M, Vassalli JD, Perrelet A. Nonconverted, amino acid analog-modified proinsulin stays in a Golgi-derived clathrin-coated membrane compartment. J Cell Biol. 1984;99:2187–2192. doi: 10.1083/jcb.99.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Halban P, Perrelet A, Amherdt M, Ravazzola M, Anderson RGW. pH-independent and -dependent cleavage of proinsulin in the same secretory vesicle. J Cell Biol. 1994;126:1149–1156. doi: 10.1083/jcb.126.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Vassalli JD, Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985;42:671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell SK, Quinn DL, Moore HPH. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987a;51:1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RGW, Vassalli JD, Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987b;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller DG, Cirulli V, Halban PA. Differences in aggregation properties and levels of the neural cell adhesion molecule (NCAM) between islet cell types. Exp Cell Res. 1990;191:305–312. doi: 10.1016/0014-4827(90)90019-7. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Halban PA. Differential rates of conversion of rat proinsulins I and II. Evidence for slow cleavage at the B-chain/C-peptide junction of proinsulin II. Biochem J. 1991;278:621–625. doi: 10.1042/bj2780621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko S, Irminger JC, Buhler L, Deng S, Morel P, Halban PA. Kinetics of proinsulin conversion in human islets. Diabetes. 1993;42:933–935. doi: 10.2337/diab.42.6.933. [DOI] [PubMed] [Google Scholar]
- Taylor NA, Docherty K. Sequence requirements for processing of proinsulin in transfected mouse pituitary AtT20 cells. Biochem J. 1992;286:619–622. doi: 10.1042/bj2860619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Gerdes HH, Huttner WB. Protein secretion: puzzling receptors. Curr Biol. 1997;7:R496–R500. doi: 10.1016/s0960-9822(06)00247-8. [DOI] [PubMed] [Google Scholar]
- Thiele C, Huttner WB. Protein and lipid sorting from the trans-Golgi network to secretory granules-recent developments. Semin Cell Dev Biol. 1998;9:511–516. doi: 10.1006/scdb.1998.0259. [DOI] [PubMed] [Google Scholar]
- Turner MD, Arvan P. Protein traffic from the secretory pathway to the endosomal system in pancreatic β-cells. J Biol Chem. 2000;275:14025–14030. doi: 10.1074/jbc.275.19.14025. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Fricker LD, Furukawa H, Steiner DF, Langley SH, Leiter EH. β-Cell lines derived from transgenic Cpe(fat)/Cpe(fat) mice are defective in carboxypeptidase E and proinsulin processing. Endocrinology. 1997;138:4883–4892. doi: 10.1210/endo.138.11.5506. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chang A, Kjeldsen TB, Arvan P. Intracellular retention of newly synthesized insulin in yeast is caused by endoproteolytic processing in the Golgi complex. J Cell Biol. 2001;153:1187–1198. doi: 10.1083/jcb.153.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]