Abstract
Plasma membranes in eukaryotic cells display asymmetric lipid distributions with aminophospholipids concentrated in the inner and sphingolipids in the outer leaflet. This asymmetry is maintained by ATP-driven lipid transporters whose identities are unknown. The yeast plasma membrane contains two P-type ATPases, Dnf1p and Dnf2p, with structural similarity to ATPase II, a candidate aminophospholipid translocase from bovine chromaffin granules. Loss of Dnf1p and Dnf2p virtually abolished ATP-dependent transport of NBD-labeled phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine from the outer to the inner plasma membrane leaflet, leaving transport of sphingolipid analogs unaffected. Labeling with trinitrobenzene sulfonic acid revealed that the amount of phosphatidylethanolamine exposed on the surface of Δdnf1Δdnf2 cells increased twofold relative to wild-type cells. Phosphatidylethanolamine exposure by Δdnf1Δdnf2 cells further increased upon removal of Drs2p, an ATPase II homolog in the yeast Golgi. These changes in lipid topology were accompanied by a cold-sensitive defect in the uptake of markers for bulk-phase and receptor-mediated endocytosis. Our findings demonstrate a requirement for Dnf1p and Dnf2p in lipid translocation across the yeast plasma membrane. Moreover, it appears that Dnf1p, Dnf2p and Drs2p each help regulate the transbilayer lipid arrangement in the plasma membrane, and that this regulation is critical for budding endocytic vesicles.
INTRODUCTION
Numerous cell types, from mammals down to yeast, exhibit a nonrandom distribution of phospholipids across their plasma membranes (Devaux, 1991; Cerbon and Calderon, 1995). In general, the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are sequestered in the inner leaflet, whereas sphingomyelin (SM) and glycosphingolipids are enriched in the outer leaflet. Regulated loss of this asymmetric lipid arrangement triggers a variety of physiological events. For example, cell surface exposure of PS promotes the reaction cascade of blood coagulation (Rosing et al., 1980) and acts as a signal for recognition and removal of apoptotic cells by macrophages (Fadok et al., 2000). However, a thorough understanding of the establishment of lipid asymmetry and its significance for the functioning of individual cells has yet to emerge.
It has been postulated that lipid asymmetry is generated and maintained by ATP-driven lipid transporters or translocases (Devaux, 1991). The use of short-chain lipid analogs has led to the discovery of an aminophopholipid translocase that catalyzes a fast, inwardly directed transport of PS and PE across the plasma membrane (Seigneuret and Devaux, 1984). This activity, first described in human erythrocytes and later demonstrated in many nucleated cell types, is generally held accountable for the selective accumulation of aminophospholipids in the inner leaflet of the plasma membrane. In addition, Saccharomyces cerevisiae (yeast) and certain mammalian epithelial cells also display a rapid, ATP-dependent internalization of phosphatidylcholine (PC) across their plasma membranes (Pomorski et al., 1999; Grant et al., 2001). It is unclear whether the latter cell types contain a PC-specific translocase next to an aminophospholipid translocase or a translocase of another kind that translocates both aminophospholipids and PC, because none of these activities have been unambiguously identified.
A prime candidate aminophospholipid translocase is ATPase II (Zachowski et al., 1989). This vanadate-sensitive Mg2+-ATPase of 115–120 kDa has been purified from erythrocytes, chromaffin granules, and clathrin-coated vesicles (reviewed in Daleke and Lyles, 2000). Cloning of the gene encoding ATPase II from bovine chromaffin granules revealed it to be a member of a previously unrecognized subfamily of P-type ATPases (Tang et al., 1996). Members of this subfamily differ from the cation-transporting P-type ATPases in that they lack negatively charged amino acids within transmembrane segments critically involved in cation transport. The abrogation of low temperature internalization of NBD-labeled PS in yeast cells in which the homologous gene, DRS2, was deleted has been interpreted as evidence for the biochemical function of the chromaffin granule P-type ATPase as aminophospholipid translocase (Tang et al., 1996; Gomès et al., 2000). However, the dependence of NBD-PS uptake on the expression of DRS2 could not be confirmed in two independently constructed null strains (Siegmund et al., 1998; Marx et al., 1999). Combined with the localization of Drs2p to the late Golgi (Chen et al., 1999), these findings have challenged the idea that Drs2p would function as a major aminophospholipid translocase in the yeast plasma membrane. Moreover, a genetic screen for yeast mutants hypersensitive to a cytolytic PE-binding peptide has recently led to the identification of another protein, named Ros3p, whose removal causes a marked reduction in the uptake of NBD-PE and -PC across the plasma membrane (Kato et al., 2002). Ros3p belongs to an evolutionary conserved protein family whose members are unrelated to P-type ATPases or other known transporters.
At present, a consensus as to the identity of the lipid translocase(s) responsible for establishing lipid asymmetry across the plasma membrane is lacking, and the biochemical function of ATPase II, Drs2p, and related P-type ATPases remains elusive. Genome sequencing projects revealed five members of this P-type ATPase subfamily in yeast, six in Caenorhabditis elegans, and more than a dozen in humans. A few of the human genes have been implicated in hereditary disorders. Mutations in FIC1, for example, cause familial intrahepatic cholestasis (Bull et al., 1998). The human ATP10C gene has been linked to the neurological disorders Angelman syndrome and autism (Meguro et al., 2002). Precisely what cellular process is affected in these diseases is unknown. However, recent progress in the functional analysis of the homologous genes in yeast has provided some important clues.
The DRS2 gene in yeast was originally identified in a genetic screen for mutants with a cold-sensitive defect in ribosome synthesis (Ripmaster et al., 1993). Yet, subsequent studies showed that deletion of DRS2 is synthetically lethal with mutations in the genes for ADP-ribosylation factor 1 (ARF1) and clathrin heavy chain (CHC1; Chen et al., 1999) and that Δdrs2 cells are defective in the generation of a specific class of clathrin-coated vesicles carrying invertase and acid phosphatase to the plasma membrane (Gall et al., 2002). Collectively, these findings suggest a primary role for Drs2p in budding of clathrin-coated vesicles from the late Golgi. The yeast genome contains four genes encoding P-type ATPases closely related to Drs2p, namely NEO1 (YIL048W), DNF1 (YER166W), DNF2 (YDR093W), and DNF3 (YMR162C; Catty et al., 1997; Hua et al., 2002). A recent analysis of strains carrying all possible viable combinations of null alleles for these genes revealed that Drs2p, Dnf1p, Dnf2p, and Dnf3p constitute an essential protein family with overlapping functions in membrane trafficking between the Golgi and endosomal/vacuolar system (Hua et al., 2002). However, whether lipid translocation is part of the mechanism by which these P-type ATPases contribute to membrane trafficking remains to be established.
The yeast plasma membrane contains only two Drs2p-related P-type ATPases, namely Dnf1p and Dnf2p. Here we show that Dnf1p and Dnf2p are essential for inward translocation of NBD-PE, -PS, and -PC across the plasma membrane. Loss of Dnf1p and Dnf2p leads to an increased cell surface exposure of endogenous PE, which is enhanced by additional removal of Drs2p. Concurrent with an altered phospholipid arrangement in the plasma membrane, Δdnf1Δdnf2Δdrs2 cells exhibit a defect in the uptake of endocytic tracer FM4-64 and in the ligand-induced internalization of α-factor receptor. These results point to a functional link between P-type ATPase-dependent lipid translocation and budding of endocytic vesicles from the plasma membrane.
MATERIALS AND METHODS
Yeast Strains
Yeast strains are listed in Table 1. For all experiments shown, strains were grown at 30°C to midlogarithmic phase (0.5–1.0 OD600) in standard synthetic dextrose (SD) medium, except US50–18C and AD13, which were grown in yeast extract-peptone-dextrose (YEPD) medium. Drs2p, Neo1p, Dnf1p, Dnf2p, and Dnf3p were tagged at their COOH termini with three copies of the hemagglutinin (HA) epitope using the PCR knock-in approach (Wach et al., 1997) and plasmid p3xHAt-HIS5 (S. Munro, MRC-LMB, Cambridge, UK). Expression of tagged ATPases was verified by Western blot analysis using rabbit anti-HA antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα ura3-52 his3-Δ200 leu2-3,112 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al. (1998) |
| SEY6211 | MATa ura3-52 his3-Δ200 leu2-3,112 trp1-Δ901 ade2-101 suc2-Δ9 | Robinson et al. (1998) |
| TPY051 | SEY6211 Δbar1∷URA3 | This work |
| TPY053 | TPY051 Δbar1∷URA3 Δdnf2∷loxP-HIS3-loxP | This work |
| TPY054 | TPY053 Δbar1∷URA3 Δdnf2∷loxP | This work |
| TPY055 | TPY051 Δbar1∷URA3 Δdnf1∷loxP-HIS3-loxP | This work |
| TPY058 | TPY054 Δbar1∷URA3 Δdnf2∷loxP Δdnf1∷loxP-HIS3-loxP | This work |
| TPY059 | TPY058 Δbar1∷URA3 Δdnf2∷loxP · Δdnf1∷loxP | This work |
| TPY061 | TPY051 Δbar1∷URA3 Δdrs2∷loxP-HIS3-loxP | This work |
| TPY063 | TPY055 Δbar1∷URA3 Δdnf1∷loxP | This work |
| TPY066 | TPY059 Δbar1∷URA3 Δdnf2∷loxP · Δdnf1∷loxP Δdrs2∷loxP-HIS3-loxP | This work |
| TPY091 | SEY6210 drs2∷DRS2 3xHAt HIS5Sp | This work |
| TPY093 | SEY6210 dnf3∷DNF3 3xHAt HIS5Sp | This work |
| TPY095 | SEY6210 dnf1∷DNF1 3xHAt HIS5Sp | This work |
| TPY097 | SEY6210 dnf2∷DNF2 3xHAt HIS5Sp | This work |
| TPY098 | SEY6210 neo1∷NEO1 3xHAt HIS5Sp | This work |
| TPY140 | TPY063 Δbar1∷URA3 Δdnf1∷loxP dnf2∷DNF2 3xHAt HIS5Sp | This work |
| TPY142 | TPY054 Δbar1∷URA3 Δdnf2∷loxP dnf1∷DNF1 3xHAt HIS5Sp | This work |
| US50-18C | MATα PDR1-3 ura3 his1 | Decottignies et al. (1998) |
| AD13 | US50-18C Δpdr5∷hisG Δyor1∷hisG | Decottignies et al. (1998) |
A bar1::URA3 deletion plasmid (pLH309; L. Hicke, Nortwestern University, Evanston, IL) was linearized with EcoRI and transformed into SEY6211 to produce 6211Δbar1 (TPY051). For the deletion of DRS2, NEO1, DNF1, DNF2, and DNF3 genes, 450–550-base pair fragments of the promotor and ORF 3′-end of each gene were amplified by PCR from yeast genomic DNA. The gene promotors and ORF ends were cloned into NotI/EcoRI and SpeI/MluI sites located on either site of a loxP-HIS3-loxP cassette that was ligated into the EcoRI/SpeI sites of a pBluescript KS− vector (Stratagene, La Jolla, CA; the loxP-HIS3-loxP plasmid was a gift of T. Levine, University College London, UK). Gene deletion constructs were linearized with NotI and MluI and transformed into TPY051. Multiple deletions were performed sequentially in TPY051 by repeated use of the loxP-HIS3-loxP cassette and subsequent removal of the HIS3 marker by excisive recombination using Cre recombinase (Sauer, 1987). In each case, the correct integration or excision event was confirmed by PCR.
Localization of P-type ATPases
Strains expressing tagged ATPases were grown in 500 ml SD medium, harvested, spheroplasted, and then lysed in a hypo-osmotic buffer as described (Holthuis et al., 1998). Subcellular membranes were collected at 100,000 × gav (60 min, 4°C) and loaded on top of a sucrose gradient prepared in gradient buffer (10 mM HEPES-KOH, pH 7.2, 1 mM EDTA, 0.8 M sorbitol) using the following steps: 0.5 ml 60%, 1 ml 40%, 1 ml 37%, 1.5 ml 34%, 2 ml 32%, 2 ml 29%, 1.5 ml 27%, and 1.5 ml 22% (wt/wt) sucrose. After centrifugation at 130,000 × gav in a Beckman SW40Ti rotor (18 h, 4°C), 20 × 0.6-ml fractions were collected from the top. Equal volumes per fraction were used to assay for Kex2p endoprotease activity (Cunningham and Wickner, 1989) and for Western blot analysis. HA-tagged ATPases were detected as described above. Other antibodies were directed against Tlg1p, Tlg2p, Vam3p, Pep12p (Holthuis et al., 1998), Dpm1p (Molecular Probes, Eugene, OR), Mir1p (R. Lill, University of Marburg, Germany), and Sso2p (S. Keränen, Biotechnology and Food Research, Espoo, Finland). Protein blots were probed with HRP-conjugated secondary antibodies (Bio-Rad, Hercules, CA), which were detected using ECL (Amersham, Little Chalfont, UK).
For fluorescence microscopy, cells were fixed, mounted and stained with 9F10 rat monoclonal anti-HA antibody (Boehringer, Mannheim, Germany) at a dilution of 1:200, as described previously (Holthuis et al., 1998). Texas Red goat anti-rat IgG (Molecular Probes) was used at 1:100 as secondary antibody. Image acquisition was as described below.
NBD-Lipid Uptake
Palmitoyl-(NBD-hexanoyl)-PS (NBD-PS), myristoyl-(NBD-hexanoyl)-PE (NBD-PE), myristoyl-(NBD-hexanoyl)-PC (NBD-PC), myristoyl-(NBD-hexanoyl)-phosphatidic acid (NBD-PA) and myristoyl-(NBD-hexanoyl)-phosphatidylglycerol (NBD-PG) were from Avanti Polar Lipids (Birmingham, AL). All NBD-lipid stocks (10 mM) were prepared in DMSO. NBD-lipid uptake experiments were performed essentially as described (Grant et al., 2001). Briefly, cells (10 OD600/ml) were incubated in SD medium at 2°C. NBD-lipids were added to a final concentration of 100 μM under vortexing. After incubation for 60 min with periodic mixing, cells were collected by centrifugation, resuspended in ice-cold SD medium without glucose but containing 2% sorbitol and 20 mM NaN3 (SSA medium), and transferred to a new tube. Cells were washed twice in ice-cold SSA medium containing 4% (wt/vol) BSA and once in SSA medium before analysis by fluorescence microscopy or flow cytometry. To compare the uptake of NBD-lipids with that of the endocytic tracer FM4-64 (Molecular Probes), an aliquot of resuspended cells was incubated for 60 min at 2°C with 40 μM FM4-64 (from a 32 mM DMSO stock). For labeling mitochondrial and nuclear DNA, cells were preincubated with 1 μg/ml DAPI (Sigma, St. Louis, MO) at 30°C for 30 min before harvesting. For ATP depletion, cells were preincubated in SSA medium for 45 min at 30°C.
Fluorescence Microscopy
Fluorescence microscopy and image acquisition were carried out using a Leica DMRA microscope (Leitz, Wetzlar, Germany) equipped with a cooled CCD camera (KX85, Apogee Instruments Inc., Tucson, AZ) driven by Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). All images were acquired using a Plan-APO 100×/1.4 NA oil objective with the following filter sets: BP 460–500, FT 505, BP 512–542 (NBD); BP 515–560, FT 580, LP 590 (FM4-64/Texas Red); BP 340–380, FT 400, LP 425 (DAPI).
Flow Cytometry
Flow cytometry of NBD-labeled cells was performed on a Becton Dickinson FACS (San Jose, CA) equipped with an argon laser using Cell Quest software, as described (Grant et al., 2001). One microliter of 1 mg/ml propidium iodide in H2O was added to 5 × 107 cells in 500 μl SSA medium just before dilution (40-fold in SSA medium) and FACS analysis. Twenty thousand cells were analyzed without gating during the acquisition. A histogram of the red fluorescence (propidium iodide; SP 610, LP 630) was used to set the gate that excluded dead cells from the analysis. Green fluorescence (NBD; SP 560, BP 515–545) of living cells was plotted on a histogram and the mean fluorescence intensity calculated.
TNBS Labeling
Cells grown overnight in 200 ml SD medium containing 250 μCi [32P]KH2PO4 (Amersham) were washed twice in labeling buffer (40 mM NaCl, 120 mM NaHCO3, pH 8.4), resuspended to 10 OD600/ml, and incubated at 2°C. Two milliliters of cell suspension was mixed with 2 ml of ice-cold labeling buffer containing 20 mM trinitrobenzene sulfonic acid (TNBS; Sigma), pH 8.5, and incubated for 60 min at 2°C with periodic shaking. To terminate labeling, 10 ml of stop buffer (50 mM Tris-HCl, 200 mM glycylglycine, pH 6.8) was added. After 5 min on ice, cells were washed twice in stop buffer. For membrane fractionation, 50 OD600 of TNBS-labeled cells were broken by vortexing with glass beads in 1 ml of stop buffer containing 0.6 M sorbitol and protease inhibitors. A postnuclear supernatant was loaded on top of a sucrose gradient consisting of the following steps: 0.5 ml 60%, 1 ml 40%, 1 ml 37%, 1 ml 35%, and 1 ml 30% (wt/wt) sucrose in gradient buffer. After centrifugation at 130,000 × gav in a Beckman TLS-55 rotor (16 h, at 4°C), 10 × 0.5-ml fractions were collected from the top. Equal volumes per fraction were used for Western blot analysis and lipid extraction. Lipids were extracted as described (Bligh and Dyer, 1959), using 20 mM acetic acid in the aqueous phase, and separated by two-dimensional TLC (I: chloroform/methanol/25% aqueous ammonium hydroxide, 65:25:4; II: chloroform/aceton/methanol/acetic acid/water, 50:20:10:10:5). Radiolabeled lipids were quantified using a STORM 860 phosphorimager and ImageQuant v1.2 software (Molecular Dynamics, Sunnyvale, CA). Lipid spots stained by iodine were scraped and quantitated by phosphate determination (Rouser et al., 1970). Phospholipids were identified by comparison with commercial standards (Sigma).
Drug Sensitivity Assays
Sensitivity assays with PE-binding peptide Ro09-0198 (kindly provided by Masato Umeda, The Tokyo Metropolitan Institute of Medical Science, Japan) and amphotericin B (Sigma) were performed as described previously (Kato et al., 2002).
Endocytosis Assays
For bulk phase endocytosis assays, cells (2 OD600/ml) were incubated at 2°C in SD medium containing 40 μM FM4-64. Cells were then either mounted on poly-l-lysine–coated glass slides for immediate inspection or incubated with shaking at 15°C for 1 or 4 h before mounting. Images were collected as described under Fluorescence Microscopy. 35S-labeled α-factor uptake experiments were performed at 24°C on cells preincubated at 24°C for 20 min, using the continuous presence protocol (Dulic et al., 1991).
RESULTS
Drs2p-related P-type ATPases Dnf1p and Dnf2p Localize to the Plasma Membrane
The S. cerevisiae genome contains four genes encoding P-type ATPases closely related to Drs2p, namely NEO1, DNF1, DNF2, and DNF3 (Catty et al., 1997; Hua et al., 2002). The four share 29–31% amino acid sequence identity (45–48% similarity) with Drs2p. Within this P-type ATPase cluster, Dnf1p and Dnf2p show the highest degree of structural similarity, sharing 69% sequence identity (83% similarity). Of the 11 other P-type ATPases found in yeast, the closest related to the Drs2p family are the Ca2+ transporters (21% identity between Drs2p and Pmc1p).
To facilitate the localization of each Drs2p family member, the corresponding endogenous gene was tagged by inserting three copies of the HA epitope at the COOH terminus of the ORF. Tagging did not interfere with P-type ATPase function. This can be inferred from the fact that NEO1 is an essential gene and that cells in which the only copy of this gene is tagged were viable. In addition, cells expressing only a tagged version of Drs2p, Dnf1p, and Dnf2p lacked the mutant phenotype observed upon disruption of the corresponding gene.
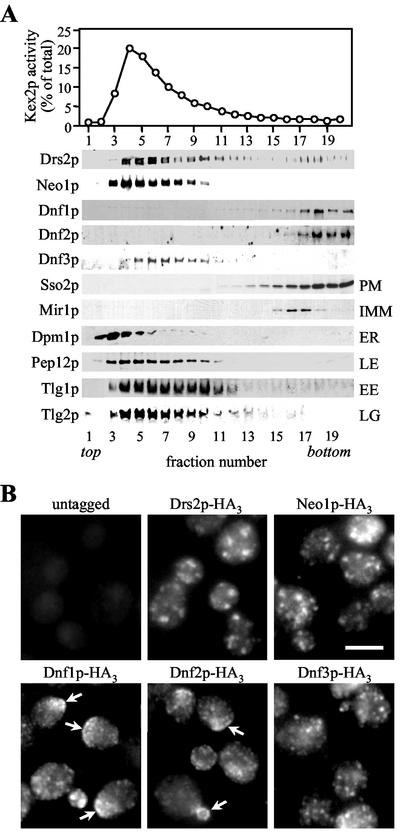
For the identification of plasma membrane-resident family members, tagged strains were subjected to subcellular fractionation on sucrose gradients to separate plasma membranes from intracellular organelles. Figure 1A shows that the fractionation profiles of HA-tagged Dnf1p and Dnf2p coincide with that of plasma membrane marker Sso2p, but are distinct from fractionation profiles of markers for mitochondria (Mir1p), ER (Dpm1p), late Golgi (Kex2p, Tlg2p), early endosomes (Tlg1p), late endosomes (Pep12p), and vacuoles (Vam3p, unpublished data). In contrast, the bulk of membranes containing HA-tagged Drs2p, Neo1p, or Dnf3p segregated from the plasma membrane. The fractionation profile of Drs2p matched that of Kex2p and Tlg2p, consistent with the previous localization of Drs2p to the late Golgi (Chen et al., 1999). Neo1p displayed a fractionation profile similar to that of Pep12p, suggesting its association with late endosomes. Dnf3p peaked in fractions with a slightly higher sucrose density that contained the bulk of late Golgi/early endosomal membranes.
Figure 1.
P-type ATPases Dnf1p and Dnf2p reside in the plasma membrane. (A) Yeast strains expressing HA-tagged Drs2p, Neo1p, Dnf1p, Dnf2p, or Dnf3p were lysed, and cellular membranes were fractionated on a sucrose step gradient. Gradient fractions were assayed by enzyme activity for Kex2p or immunoblotted using antibodies against the HA-epitope or several organellar markers. Fractionation profiles of markers shown are from the gradient containing membranes of cells expressing HA-tagged Dnf1p, but were determined for each gradient individually, giving similar results. PM, plasma membrane; IMM, inner mitochondrial membrane; ER, endoplasmic reticulum; LE, late endosomes; EE, early endosomes; LG, late Golgi. (B) Fluorescence microscopy of cells stained with a rat monoclonal anti-HA antibody. Arrows indicate regions of heavily labeled plasma membrane. Bar, 10 μm.
We next examined the localization of the tagged P-type ATPases by immunofluorescence microscopy. Staining against Drs2p, Neo1p, and Dnf3p in each case revealed punctate structures that were evenly distributed throughout the cell (Figure 1B). In contrast, Dnf1p and Dnf2p generally localized to smaller punctate structures that were partially internal, but often found on, or directly underneath the plasma membrane. Both proteins were also found concentrated in regions of continuous plasma membrane, in particular those marking small buds or emerging bud sites (Figure 1B, arrows). The different localization patterns observed for the individual Drs2p family members are very similar to those described in a recent study (Hua et al., 2002).
Collectively, our data show that a significant portion of Dnf1p and Dnf2p is localized to the plasma membrane. The fine internal membrane structures labeled for these proteins could be endocytic and/or exocytic vesicles and may suggest that Dnf1p and Dnf2p cycle between the plasma membrane and an intracellular compartment, as proposed previously (Hua et al., 2002). Dnf3p, Drs2p, and Neo1p, on the other hand, are primarily associated with intracellular organelles, most likely the late Golgi and early/late endosomes.
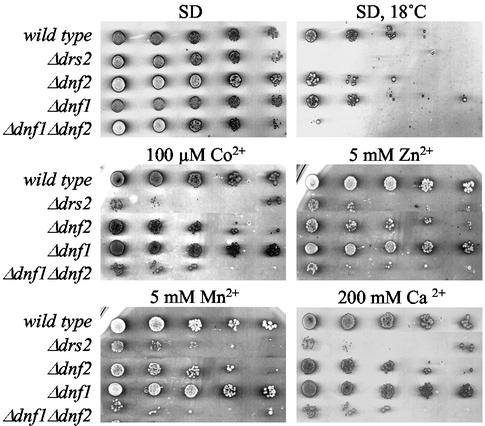
Δdnf1Δdnf2 and Δdrs2 Mutants Display Similar Growth Phenotypes
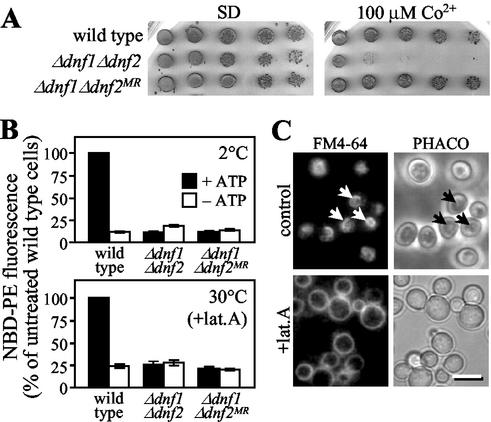
To study the functions of Dnf1p and Dnf2p, we created strains lacking the corresponding ORFs. Neither gene proved essential. Strains lacking both genes were also viable. However, in contrast to Δdnf1 and Δdnf2 cells, growth of the Δdnf1Δdnf2 double mutant was blocked at temperatures below 20°C (Figure 2). Moreover, Δdnf1Δdnf2 cells were sensitive to low concentrations of Co2+ (100 μM), Ni2+ (250 μM), Zn2+ (5 mM), and Mn2+ (5 mM) or high concentrations of Ca2+ (200 mM) or Mg2+ (500 mM; Figure 2 and unpublished data). These phenotypes are also displayed by cells lacking Drs2p (Ripmaster et al., 1993; Siegmund et al., 1998). This suggests that members of the Drs2p family, although associated with different organelles, serve similar biochemical functions.
Figure 2.
Sensitivity of Δdnf1Δdnf2 and Δdrs2 mutant strains to heavy metals and low temperature. Serial fivefold dilutions of wild-type and mutant cells were spotted onto SD medium or SD medium containing the indicated amounts of divalent cations added as chlorides. Plates were scanned after 3 d of incubation at 30°C or after 6 d at 18°C, as indicated.
The Δdnf1Δdnf2 Mutant Is Defective in the Inward Translocation of NBD-PE, -PS, and -PC Across the Plasma Membrane
The sensitivity of Δdnf1Δdnf2 and Δdrs2 cells toward heavy metals may reflect a role for Drs2p family members as transporters of heavy metals, but could also be a consequence of the previously proposed function of these proteins in lipid translocation (Tang et al., 1996). The availability of a strain lacking the plasma membrane-associated members of this subclass of P-type ATPases provided a new opportunity to evaluate their role in lipid transport.
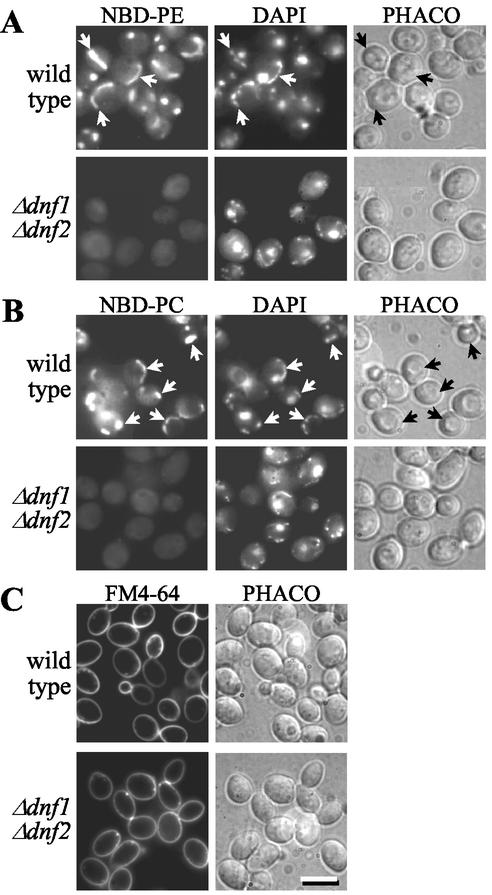
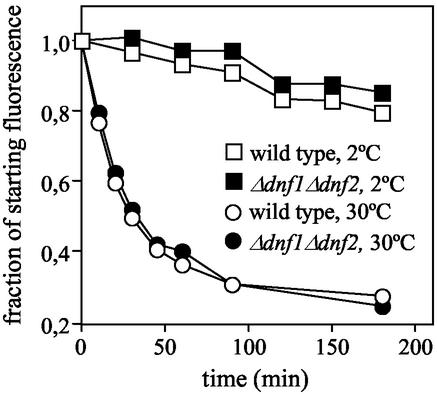
As a first approach, we analyzed Δdnf1Δdnf2 cells for defects in the internalization of NBD-lipids. Consistent with a previous study (Grant et al., 2001), wild-type cells incubated at 2°C internalize and distribute NBD-PE, -PS, and -PC primarily to mitochondria, as indicated by colocalization of NBD and DAPI fluorescence (Figure 3, A and B; and unpublished data). Under these conditions, the dye FM4-64, an endocytic tracer, which allowed visualization of membrane traffic from the plasma membrane to the vacuole, was not internalized and remained associated with the plasma membrane (Figure 3C). This suggests that internalization of NBD lipids occurs independently of endocytosis and requires a translocation step across the plasma membrane. Strikingly, the ability to accumulate NBD-PE, -PC, and -PS was greatly reduced in Δdnf1Δdnf2 cells (Figure 3, A and B, and unpublished data). TLC analysis of lipid extracts prepared from cells and incubation media revealed that this decrease in NBD-lipid labeling cannot be ascribed to an enhanced hydrolysis in the mutant (unpublished data). Moreover, NBD-PE efflux rates measured in Δdnf1Δdnf2 cells were indistinguishable from those in wild-type cells (Figure 4), indicating that loss of Dnf1p and Dnf2p unlikely affects NBD-lipid accumulation by an enhanced outward movement of lipids across the plasma membrane. Therefore, the simplest explanation for the inability of Δdnf1Δdnf2 cells to accumulate appreciable amounts of NBD-PE, -PS, or -PC is a defect in the inward transport of these lipids across the plasma membrane.
Figure 3.
Δdnf1Δdnf2 cells are defective in the nonendocytic uptake of NBD-PE and -PC. Wild-type and Δdnf1Δdnf2 cells, prestained with 1 μg/ml DAPI for 30 min at 30°C, were incubated for 60 min at 2°C with 100 μM NBD-lipid (A and B) or 40 μM FM4-64 (C) before imaging by fluorescence (NBD, DAPI, FM4-64) or phase contrast (PHACO) microscopy. Note that wild-type cells accumulate NBD lipids primarily in mitochondria, as indicated by colocalization with DAPI fluorescence (arrows). For each NBD lipid examined, TLC analysis revealed that >80% of cell-associated NBD fluorescence corresponded to intact lipid. Bar, 10 μm.
Figure 4.
Loss of Dnf1p and Dnf2p does not affect NBD-lipid efflux rates across the plasma membrane. Wild-type and Δdnf1Δdnf2 cells were allowed to accumulate similar amounts of NBD-PE at 30°C, washed in ice-cold SD medium, and then incubated at 2 or 30°C in SD medium containing 4% (wt/vol) BSA to extract NBD lipids appearing on the cell surface. Aliquots of cells were removed at various time points after addition of BSA, washed in ice-cold 4% (wt/vol) BSA-containing SSA medium, and then analyzed by flow cytometry. Relative NBD-PE fluorescence intensities at the start of the experiment were 148 a.u. for wild-type, and 104 a.u. for Δdnf1Δdnf2 cells. Note that both cell types accumulated NBD-PE primarily in mitochondria.
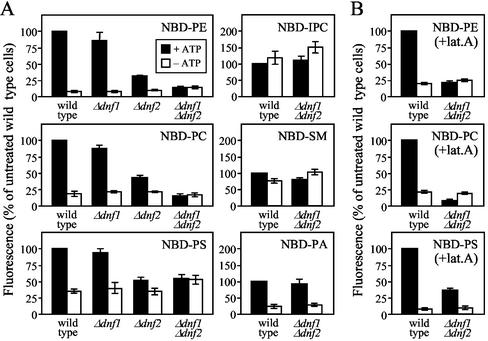
For a quantitative assessment of NBD-lipid internalization, the NBD fluorescence associated with mutant and wild-type cells was measured by flow cytometry. As shown in Figure 5A, ATP depletion of wild-type cells caused a 70–90% reduction in the internalization of NBD-PE, -PS, and -PC. On removal of Dnf1p, uptake of both choline- and aminophospholipid analogs was slightly reduced (10–15%). Loss of Dnf2p led to a more pronounced decline in NBD-lipid uptake (50–68%), again affecting all three lipid analogs to a similar extent. In cells lacking both Dnf1p and Dnf2p, the internalization of NBD-PE, -PS, and -PC was further reduced, but now to a level similar to that in ATP-depleted wild-type cells. Moreover, ATP depletion of the Δdnf1Δdnf2 mutant did not result in any further reduction of cell-associated NBD fluorescence levels. Similar results were obtained when lipid internalization experiments were performed at 30°C in the presence of latrunculin A (Figure 5B), a drug blocking the endocytic uptake of FM4-64 (Figure 6C; Morton et al., 2000). The only difference was that under these conditions NBD-PS uptake in the Δdnf1Δdnf2 mutant, although greatly affected (62% reduction), was no longer reduced to the same level as that in ATP-depleted wild-type cells (90% reduction). Based on these findings, we conclude that Dnf1p and Dnf2p are essential to sustain an energy-dependent influx of NBD-PE, -PS, and -PC across the plasma membrane.
Figure 5.
Loss of Dnf1p and Dnf2p abolishes ATP-dependent influx of NBD-labeled PE, PS, and PC without affecting uptake of PA and sphingolipid analogs. Cells, preincubated in SD medium (+ATP) or SSA medium (−ATP) for 45 min at 30°C, were labeled with 100 μM NBD-lipid for 60 min at 2°C (A) or 30 min at 30°C (B) and then washed and analyzed by flow cytometry. For labeling at 30°C, preincubation was with 20 μM latrunculin A (+lat. A) to block endocytosis. Accumulation of NBD lipids was expressed as the percentage fluorescence intensity relative to control wild-type cells (+ATP); 100% in A corresponds to 81 ± 16 a.u. (NBD-PE), 25 ± 6 a.u. (NBD-PS), 36 ± 8 a.u. (NBD-PC), 14 ± 2 a.u. (NBD-SM), 6 ± 1 a.u. (NBD-IPC), and 19 ± 3 a.u. (NBD-PA); 100% in B corresponds to 636 ± 58 a.u. (NBD-PE), 451 ± 45 a.u. (NBD-PS), and 483 ± 44 a.u. (NBD-PC). Results represent the means ± SEM of three independent experiments.
Figure 6.
Metal sensitivity of Δdnf1Δdnf2 cells is secondary to the defect in NBD-lipid uptake. (A) Serial fivefold dilutions of wild-type, Δdnf1Δdnf2 and metal-resistant Δdnf1Δdnf2 cells (Δdnf1Δdnf2 MR) were spotted onto SD plates or SD plates containing 100 μM CoCl2. Plates were scanned after 3 d of incubation at 30°C. (B) Cells, preincubated in SD medium (+ATP) or SSA medium (−ATP), were labeled with 100 μM NBD-PE at 2 or 30°C and then analyzed by flow cytometry as in Figure 5. For labeling at 30°C, preincubation was with 20 μM latrunculin A (+lat. A). NBD-PE uptake was expressed as percentage fluorescence intensity relative to control wild-type cells (+ATP). Results represent the means ± SEM of three independent experiments. (C) Wild-type cells, preincubated in SD medium (control) or SD medium containing 20 μM latrunculin A (+lat. A) for 45 min at 30°C, were labeled with 40 μM FM4-64 for 30 min at 30°C and then imaged as in Figure 3. Arrows mark labeled vacuolar membranes. Bar, 10 μm.
By plating out Δdnf1Δdnf2 cells on heavy metal-containing medium, we obtained numerous revertants in which the metal sensitive phenotype was lost (Figures 2 and 6A). In all cases tested, these revertants were still blocked in energy-dependent lipid uptake (Figure 6B), suggesting that metal sensitivity of Δdnf1Δdnf2 cells is a secondary phenotype of the defect in lipid translocation.
The Lipid Translocation Defect in Δdnf1Δdnf2 Cells Is Specific
We next determined whether the Dnf1p/Dnf2p-dependent translocation machinery is capable of discriminating phospholipids with a glycerol backbone from those containing a sphingoid base. To this end, wild-type and Δdnf1Δdnf2 cells were incubated with NBD-labeled sphingomyelin (NBD-SM) and inositol phosphorylceramide (NBD-IPC). On internalization, both lipids distributed primarily to mitochondria (unpublished data). However, internalization of NBD-SM and -IPC was less efficient than that of NBD-glycerolipids and independent of ATP, Dnf1p, and Dnf2p (Figure 5A), suggesting that it occurs by passive diffusion. These results indicate that the Dnf1p/Dnf2p-dependent transport machinery distinguishes sphingolipids from glycerolipids and recognizes only the latter as substrates for translocation across the plasma membrane.
We also analyzed wild-type and Δdnf1Δdnf2 cells for their ability to internalize NBD-labeled phosphatidic acid (NBD-PA) and phosphatidylglycerol (NBD-PG). Uptake experiments were performed at 2°C. At this temperature, >80% of the internalized lipid could be recovered in the intact form (unpublished data). NBD-PA uptake in yeast has previously been reported to occur via a nonendocytic pathway and independent of ATP (Trotter, 2000). However, we observed that ATP-depletion of wild-type cells caused a major reduction in the uptake of both NBD-PA (82%) and NBD-PG (85%; Figure 5A and unpublished data). The reason for this discrepancy is unclear. Nevertheless, internalization of NBD-PA and -PG was independent of Dnf1p and Dnf2p (Figure 5A and unpublished data). These results suggest that the defect in NBD-PE, -PS, and -PC uptake in Δdnf1Δdnf2 cells is specific and unlikely due to a general malfunctioning of ATP-dependent transport processes across the yeast plasma membrane. This notion is further supported by our finding that NBD-PE efflux rates, which are sensitive to ATP-depletion (Hanson and Nichols, 2001) and depend on expression of the yeast ABC transporters Pdr5p and Yor1p (Decottignies et al., 1998), are not affected by removal of Dnf1p and Dnf2p (Figure 4).
Loss of Dnf1p and Dnf2p Causes an Increased Cell Surface Exposure of Endogenous Aminophospholipids
Although loss of Dnf1p and Dnf2p blocks inward transport of NBD-PE across the plasma membrane (Figures 3 and 5), removal of the ABC transporters Pdr5p and Yor1p from a yeast PDR1–3 gain-of-function mutant has been shown to enhance the net uptake of NBD-PE (Decottignies et al., 1998). To investigate whether transport of NBD-lipids is indicative for that of their natural counterparts, the amount of endogenous aminophospholipids exposed on the surface of wild-type, Δdnf1Δdnf2, PDR1–3, and PDR1–3Δpdr5Δyor1 cells was measured by TNBS labeling.
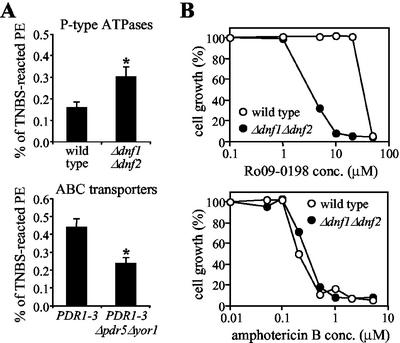
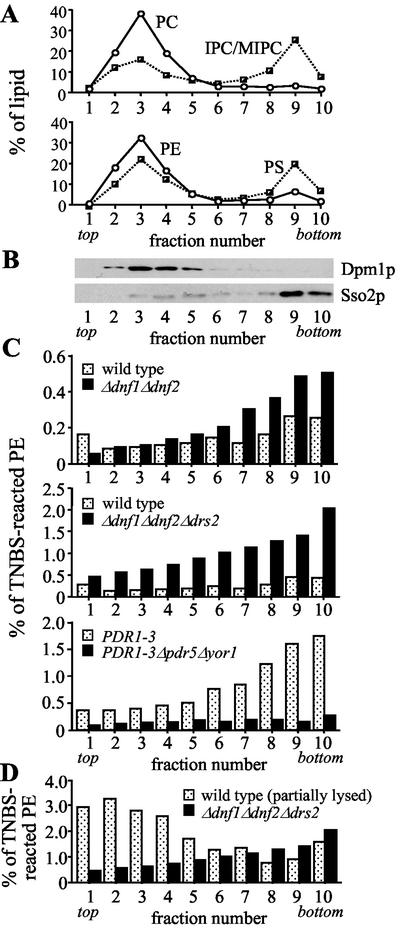
In intact wild-type cells, a small fraction of total cellular PE reacted with TNBS (0.16 ± 0.02%; Figure 7A). TNBS labeling of PS was virtually undetectable (<0.02% of total). If cells were first permeabilized, TNBS labeled up to 55% of all cellular aminophospholipids (PE and PS) and >90% of them in the presence of detergent. Given that ∼15% of the total cellular PE is localized in the plasma membrane (Figure 8A), the above results would suggest that in wild-type cells at most 1% of plasma membrane-associated PE is available for external labeling with TNBS. Whether this TNBS-labeled fraction reflects the total pool of PE in the outer leaflet of the plasma membrane remains to be established. Nevertheless, removal of Dnf1p and Dnf2p caused a twofold increase in the percentage of TNBS-labeled PE (from 0.16 ± 0.02 to 0.30 ± 0.05%; Figure 7A). This doubling is unlikely due to changes in cell viability or permeability (see legend of Figure 7). Indeed, when lysates prepared from TNBS-treated wild-type and Δdnf1Δdnf2 cells were fractionated on sucrose gradients to separate plasma membranes from intracellular organelles, the differences in percentage of TNBS-labeled PE between the two cell types occurred primarily in fractions enriched for plasma membrane (Figure 8, B and C). Thus, in line with a reduced net uptake of NBD-PE (Figures 2 and 3), loss of Dnf1p and Dnf2p increased the amount of endogenous PE in the outer leaflet of the plasma membrane. In contrast, removal of the ABC transporters Pdr5p and Yor1p from PDR1–3 cells reduced the PE level in the outer plasma membrane leaflet (Figures 7A and 8C), conform to an increased net uptake of NBD-PE (Decottignies et al., 1998).
Figure 7.
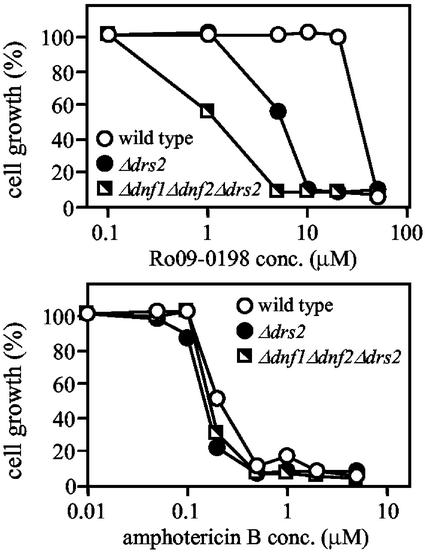
Loss of Dnf1p and Dnf2p causes increased cell surface exposure of endogenous PE. (A) 32P-labeled wild-type and mutant cells were reacted with 10 mM TNBS for 60 min at 2°C, washed, and subjected to lipid analysis as described in MATERIALS AND METHODS. Percentages of TNBS-reacted PE relative to total cellular PE are shown as the means ± SEM from three independent experiments performed in triplicate. Asterisks denote significant differences in labeling between deletion mutants and their parental wild-type strains (p < 0.01). The percentage of viable cells, as determined by propidium iodide exclusion, was 97.8 ± 1.0 (wild-type), 97.3 ± 1.0 (Δdnf1Δdnf2), 97.2 ± 0.6 (PDR1–3), and 95.9 ± 1.3 (PDR1–3Δpdr5Δyor1). (B) Sensitivity of wild-type and Δdnf1Δdnf2 cells to PE-binding peptide Ro09-0198 and amphotericin B. Cells were diluted to 1 × 106 cells/ml in SD medium containing Ro09-0198 or amphotericin B at the indicated concentrations. After 24-h incubation at 30°C, OD600 values were measured and the values of cultures incubated without drugs was normalized to 100% growth.
Figure 8.
Removal of Drs2p from Δdnf1Δdnf2 cells enhances cell surface exposure of endogenous PE. Wild-type and mutant cells were labeled with 10 mM TNBS as in Figure 7, lysed, and fractionated on sucrose step gradients. (A) Fractionation profiles of the different phospholipid species. (B) Fractionation profiles of plasma membrane (Sso2p) and ER (Dpm1p). (C and D) Distribution of TNBS-reacted PE, expressed as percentage of the total PE per gradient fraction. Lipid and protein profiles shown in A and B are from fractionated wild-type cells, but are representative for the profiles obtained with fractionated mutant cells. Results shown are from one experiment that was representative of two. The percentage of viable cells was 99.2 (wild-type), 89.9 (wild-type, partially lysed before TNBS labeling), 98.7 (Δdnf1Δdnf2), 93.8 (Δdnf1Δdnf2Δdrs2), 99.2 (PDR1–3), and 99.3 (PDR1–3Δpdr5Δyor1).
As a complementary approach, we analyzed wild-type and Δdnf1Δdnf2 cells for their sensitivity to Ro09-0198, a tetracyclic peptide that binds specifically to cell surface exposed PE and subsequently induces cytolysis (Aoki et al., 1994; Kato et al., 2002). As shown in Figure 7B, Δdnf1Δdnf2 cells displayed an increased sensitivity to peptide-induced cytolysis, with a LD50 of Ro09-0198 that was approximately 10-fold lower than that for wild-type cells. There was no significant difference between Δdnf1Δdnf2 and wild-type cells with respect to their sensitivity to amphotericin B, a polyene macrolide antibiotic that binds to membrane ergosterol and induces cellular leakage (Brajtburg et al., 1990; Figure 7B). Moreover, PE levels in Δdnf1Δdnf2 and wild-type cells were very similar, whether determined on total cellular membranes (Table 2) or on fractions enriched for plasma membrane (average values for fraction numbers 8–10 of gradients shown in Figure 8C were 37 and 36 mol % PE, respectively). These results suggest that loss of Dnf1p and Dnf2p affects the distribution of PE across the plasma membrane, rather than PE synthesis or the overall organization of the plasma membrane. Taken together, our findings support a role for Dnf1p and Dnf2p in the inward translocation of natural PE across the yeast plasma membrane.
Table 2.
Phospholipid composition of wild type and P-type ATPase mutant strainsa
| Strain | Lipid
|
|||||
|---|---|---|---|---|---|---|
| PE | PS | PC | PI | Othersb | ||
| Phosphate distribution | wild type | 33.0 ± 1.6 | 8.3 ± 1.3 | 29.0 ± 1.8 | 17.8 ± 1.3 | 11.8 ± 1.4 |
| Δdnf1 Δdnf2 | 30.2 ± 2.3 | 8.4 ± 1.4 | 30.8 ± 1.9 | 18.5 ± 3.2 | 12.2 ± 1.8 | |
| Δdrs2 | 29.9 ± 4.3 | 8.6 ± 2.4 | 34.8 ± 2.6** | 15.1 ± 4.0 | 11.6 ± 0.8 | |
| Δdnf1 Δdnf2 Δdrs2 | 22.4 ± 1.8** | 5.9 ± 1.1* | 39.3 ± 1.6** | 22.1 ± 1.9** | 10.4 ± 1.9 | |
| 32P label distribution | wild type | 32.4 ± 1.5 | 8.2 ± 1.4 | 30.1 ± 2.2 | 18.1 ± 1.5 | 11.2 ± 0.7 |
| Δdnf1 Δdnf2 | 29.6 ± 2.5 | 8.7 ± 1.0 | 30.6 ± 1.1 | 19.8 ± 2.9 | 11.3 ± 0.8 | |
| Δdrs2 | 30.4 ± 3.2 | 7.9 ± 2.5 | 36.2 ± 3.6* | 14.8 ± 4.1 | 10.6 ± 1.4 | |
| Δdnf1 Δdnf2 Δdrs2 | 22.4 ± 2.3** | 6.4 ± 0.8 | 39.5 ± 1.7** | 21.9 ± 2.2* | 9.8 ± 1.6 | |
Cells were labeled overnight with [32P]KH2PO4 and the phospholipids extracted and analysed as described in Materials and Methods.
No significant differences were observed between membranes of wild type and mutant strains with respect to their phosphatidic acid, phosphatidyl glycerol, phosphosphingolipid and cardiolipin content. Results are expressed as percentage of total phospholipids and represent the mean ± SD of two independent experiments performed in duplicate. Significant differences compared to wild type were determined by t test (
, p < 0.05;
, p < 0.01).
Loss of Drs2p in Δdnf1Δdnf2 Cells Enhances Cell Surface Exposure of Aminophospholipids and Decreases the Aminophospholipid Content of Cellular Membranes
Siegmund et al. (1998) found that loss of the Golgi-associated P-type ATPase Drs2p had no significant effect on the amount of cell surface-exposed PE. However, sucrose density fractionation of TNBS-treated wild-type and Δdrs2 cells showed that plasma membrane-enriched fractions contained on average 0.19 and 0.38% TNBS-reacted PE, respectively. Likewise, removal of Drs2p from Δdnf1Δdnf2 cells increased the plasma membrane pool of TNBS-reacted PE from 0.47 to 1.60% (average values of fraction numbers 8–10, Figure 8C). In addition, plasma membrane fractions of the triple mutant now also contained detectable levels of TNBS-reacted PS (0.16 vs. <0.02% in wild-type and Δdnf1Δdnf2 cells).
The enhanced reactivity of aminophospholipids toward TNBS cannot be ascribed solely to changes in cell viability and/or permeability. First, plasma membrane fractions of Δdrs2 and Δdnf1Δdnf2Δdrs2 cells contained three- to fivefold higher percentages of TNBS-reacted aminophospholipids than those containing the bulk of intracellular membranes (fraction numbers 2–4; Figure 8, A and C, and unpublished data). Second, fractionation of wild-type cells that were partially lysed by glass bead vortexing before TNBS labeling showed that aminophospholipids in intracellular membranes labeled more efficiently than those in the plasma membrane (Figure 8D). Third, wild-type and mutant cells displayed very similar kinetics of TNBS labeling. Finally, removal of Drs2p from wild-type or Δdnf1Δdnf2 cells caused a four- to fivefold decrease in the LD50 of PE-binding peptide Ro09-0198 (Figure 9).
Figure 9.
Loss of Drs2p causes hypersensitivity toward PE-binding peptide Ro09-0198. Sensitivity of wild-type, Δdrs2 and Δdnf1Δdnf2Δdrs2 cells toward PE-binding peptide Ro09-0198 or amphotericin B was determined as described in legend of Figure 7.
The enhanced TNBS-labeling and hypersensitivity toward PE-binding peptide of cells lacking Drs2p is not due to an increased cellular aminophospholipid content (Table 2). On the contrary, we found that removing Drs2p from Δdnf1Δdnf2 cells caused a significant drop in both PE (from 30 to 22 mol %) and PS levels (from 8 to 6 mol %). This reduction in aminophopholipid content was compensated by increased levels of PC (from 31 to 39 mol %) and phosphatidylinositol (PI; from 19 to 22 mol %; see Table 2). Comparative analysis of plasma membrane-enriched fractions from Δdnf1Δdnf2 and Δdnf1Δdnf2Δdrs2 cells revealed a similar drop in aminophospholipid content (fraction numbers 8–10 of gradients shown in Figure 8C contained on average 37 and 26 mol % PE, and 22 and 11 mol % PS, respectively).
Instead, our data suggest that Drs2p contributes significantly to the sequestration of aminophospholipids in the cytoplasmic leaflet of the plasma membrane.
Δdnf1Δdnf2Δdrs2 Cells Are Defective in the Internalization Step of Endocytosis
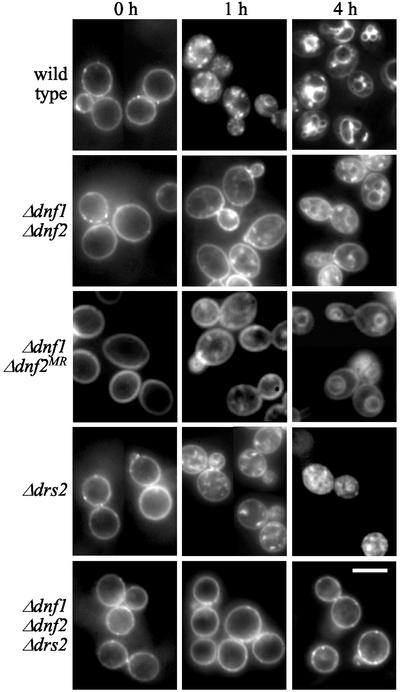
It has been postulated that inward lipid translocases play a fundamental role in endocytosis by facilitating plasma membrane invagination during budding of endocytic vesicles (Devaux, 1991; Farge et al., 1999). Being defective in inward lipid translocation across the plasma membrane, the Δdnf1Δdnf2 mutant offered an attractive model to experimentally address the above concept. When incubated at 30°C, Δdnf1Δdnf2 cells internalized and delivered the bulk phase endocytic marker FM4-64 to the vacuole at a rate similar to that in wild-type cells (unpublished data). However, when cells were stained with FM4-64 on ice and then shifted to 15°C to initiate endocytosis, the Δdnf1Δdnf2 mutant displayed a significant delay in the internalization of the dye (Figure 9). After 4 h at 15°C, when essentially all FM4-64 had reached the vacuolar membrane in wild-type cells, a considerable portion of the dye was still associated with the plasma membrane of Δdnf1Δdnf2 cells and only a minor fraction delivered to the vacuole. Interestingly, this cold-sensitive defect in FM4-64 uptake was retained in Δdnf1Δdnf2MR cells that had lost the metal-sensitive phenotype. Δdrs2 cells incubated at 15°C for 4 h accumulated FM4-64, primarily in endosomal intermediates, consistent with a defect in endosome-to-vacuole transport (Chen et al., 1999). FM4-64 uptake in the Δdrs2 mutant was delayed, but to a lesser extent than that in Δdnf1Δdnf2 cells. When Δdnf1Δdnf2 and Δdrs2 cells were warmed up to 24°C, all FM4-64 was delivered to the vacuole within 30 min, indicating that the transport defects were fully reversible (unpublished data). Strikingly, FM4-64 uptake in Δdnf1Δdnf2Δdrs2 cells was essentially abolished (Figure 9). Even after 4 h of incubation at 15°C, endosomal intermediates and vacuolar membranes were devoid of staining and the bulk of FM4-64 remained associated with the plasma membrane. Raising the temperature to 24°C only partially restored FM4-64 internalization in the triple mutant, because at 30 min after the shift a major portion of the dye was still associated with the plasma membrane (unpublished data).
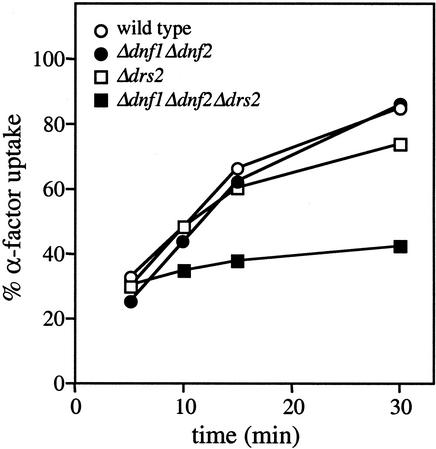
We next screened P-type ATPase mutants for defects in receptor-mediated endocytosis. In yeast, the first step of receptor-mediated endocytosis can be monitored by using 35S-labeled α-factor that, upon binding to its G-protein coupled receptor, is rapidly internalized from the cell surface (Dulic et al., 1991). The observation that binding of α-factor to its receptor was impaired below 20°C prevented us from screening P-type ATPase mutants for cold-sensitive defects in receptor-mediated endocytosis. When incubated at 24°C, Δdrs2 and Δdnf1Δdnf2 cells essentially showed wild-type kinetics of α-factor uptake (Figure 10). In contrast, the α-factor internalization rate in Δdnf1Δdnf2Δdrs2 cells was greatly reduced. Collectively, these results indicate that loss of Dnf1p, Dnf2p, and Drs2p causes a general defect in the internalization step of endocytosis.
Figure 10.
Analysis of bulk phase endocytosis in P-type ATPase mutant cells. Cells were stained with FM4-64 on ice and endocytic uptake of the dye initiated by shifting cells to 15°C. Images were captured at 0, 1, and 4 h after shift to 15°C. Bar, 10 μm.
DISCUSSION
Herein, we present evidence strongly implicating the Drs2p-related P-type ATPases Dnf1p and Dnf2p in the inward translocation of phospholipids across the yeast plasma membrane. Moreover, it appears that both plasma membrane- and Golgi-associated members of the Drs2p family contribute to regulation of the transbilayer phospholipid distribution in the plasma membrane and that this regulation is critical for budding of endocytic vesicles.
Evidence for a Functional Assignment of Dnf1p and Dnf2p as Inward Plasma Membrane Phospholipid Translocases
The Drs2p family in yeast consists of two plasma membrane members, namely Dnf1p and Dnf2p, and three other members that are associated with late Golgi and endosomal compartments. Removing both plasma membrane ATPases proved sufficient to abolish the ATP-dependent internalization of NBD-PE and -PC, and severely affected NBD-PS uptake. Because uptake of these lipids occurs under conditions that block endocytosis, it must involve a translocation step across the plasma membrane. Consequently, it appears that Dnf1p and Dnf2p are required for energy-coupled transport of PE, PS, and PC from the outer to the inner leaflet of the plasma membrane. In support of this notion, loss of Dnf1p and Dnf2p led to an increased cell surface exposure of endogenous PE, as evidenced by enhanced TNBS labeling and hypersensitivity to PE-binding peptide.
A screen for yeast mutants hypersensitive to PE-binding peptide recently led to the identification of Ros3p, an evolutionary conserved transmembrane protein whose removal causes a major reduction in the nonendocytic uptake of NBD-PE and -PC (Kato et al., 2002). In contrast to Dnf1p and Dnf2p, Ros3p is unrelated to any known ATPase. Hence, the possibility that Ros3p functions as an independent lipid translocase at the yeast plasma membrane seems not very likely. Instead, Ros3p may represent an essential component of the Dnf1p/Dnf2p-dependent translocation machinery or, alternatively, be required for the proper functioning or localization of Dnf1p and Dnf2p.
Apart from being defective in lipid transport, Δdnf1Δdnf2 cells displayed a striking sensitivity toward heavy metals. An important question is whether this metal sensitivity is a consequence of the involvement of Dnf1p and Dnf2p in lipid translocation or whether it reflects a role for these P-type ATPases as metal transporters. Arguing against a primary function in metal transport is the fact that Dnf1p and Dnf2p lack structural features that are diagnostic for metal-transporting P-type ATPases (Catty et al., 1997). Moreover, we observed that Δdnf1Δdnf2 cells could readily lose their metal sensitive phenotype. Our finding that such revertants are still blocked in energy-dependent lipid uptake suggests that the metal sensitive phenotype is secondary to the defect in lipid translocation.
Could a defect in lipid translocation across the plasma membrane explain an increased sensitivity toward heavy metals? Conceivably, an altered lipid transbilayer organization may compromise the activity of cation transporters embedded in the plasma membrane. For example, H+-linked transport systems in the yeast plasma membrane are sensitive to variations in surface potential that are due to changes in the transbilayer orientation of anionic phospholipids PS and PI (Cerbon and Calderon, 1995). Alternatively, a change in lipid composition of the outer leaflet might result in altered cation binding properties of the plasma membrane and thereby affect its barrier function. In line with such notion, in vitro studies have shown that metals like Ca2+ and Zn2+ can induce changes in the permeability of phospholipid vesicles that originate from specific ion–lipid interactions (Smaal et al., 1986).
Collectively, our data support a functional assignment of Dnf1p and Dnf2p as major, inward phospholipid-translocating ATPases of the yeast plasma membrane. However, final proof for a direct role in lipid transport requires reconstitution of purified Dnf1p and Dnf2p in chemically defined liposomes.
Significance of Drs2p-related P-type ATPases in Lipid Asymmetry
In addition to the fast, energy-coupled influx of aminophospholipids commonly observed in cells, yeast and certain mammalian epithelial cell types display an equally rapid, ATP-dependent internalization of PC across their plasma membranes (Pomorski et al., 1999; Grant et al., 2001). This would imply that some eukaryotic cells contain either a PC-specific translocase next to an aminophospholipid translocase or a translocase of another kind that translocates both aminophospholipids and PC. Our finding that loss of Dnf1p or Dnf2p affects transport of NBD-PS, -PE, and -PC to a similar extent suggests that the latter scenario is correct, at least in the case of yeast. The Drs2p family presently contains dozens of uncharacterized mammalian P-type ATPases and may well include aminophospholipid-specific translocases. However, the apparent inability of Dnf1p and Dnf2p to discriminate between choline and amino head groups is inconsistent with the idea that members of this family would serve exclusively as aminophospholipid transporters (Tang et al., 1996; Gomès et al., 2000). Removal of Dnf1p and Dnf2p severely affected, but did not eliminate ATP-dependent transport of NBD-PS, and had no effect on the ATP-dependent internalization of NBD-PA and -PG. This implies that the yeast plasma membrane is equipped with a Drs2p family-independent transport mechanism for the uptake of PS, PA, and PG. The molecular basis of this transport remains to be established.
Dnf1p/Dnf2p-dependent transport of NBD-PC was abolished when the glycerol backbone was replaced by a sphingoid base. Hence, the Dnf1p/Dnf2p-dependent transport machinery distinguishes sphingolipids from glycerolipids and recognizes only the latter as substrates for inward translocation. The yeast plasma membrane also contains outward lipid translocases that pump both glyceride- and ceramide-based lipids (Decottignies et al., 1998; our unpublished data). Candidates for these outward lipid pumps are members of the ABC transporter family (Decottignies et al., 1998; Raggers et al., 2000; this study). Conceivably, a selective P-type ATPase-dependent transport of PE, PS, and PC to the inner leaflet, concurrent with a less specific outward movement of both sphingolipids and glycerolipids by ABC transporters, could lead to a steady state segregation of aminophospholipids and sphingolipids across the plasma membrane. Consistent with this scenario, loss of Dnf1p and Dnf2p caused an increased cell surface exposure of endogenous PE, whereas loss of the ABC transporters Pdr5p and Yor1p produced the opposite effect.
In plasma membranes of wild-type cells, a small fraction of PE (<1%) was available for external labeling with TNBS. Loss of Dnf1p and Dnf2p abolished the energy-dependent NBD-PE influx, yet produced only a twofold increase in the amount of TNBS-reacted PE. Removal of the Golgi P-type ATPase Drs2p also increased the amount of cell surface-exposed PE, regardless of whether Dnf1p and Dnf2p were present. This increase in PE exposure likely reflects an altered aminophospholipid distribution across the bilayer, because it could not be ascribed to an elevated PE content of the plasma membrane. From these findings, it can be inferred that 1) Drs2p is functionally homologous to Dnf1p and Dnf2p; 2) Drs2p contributes significantly to the sequestration of aminophospholipids in the inner leaflet of the plasma membrane; and 3) lipid asymmetry is not a unique feature of the plasma membrane, but may be established already in the Golgi.
Hence, our data suggest that members of the Drs2p family serve a general function as regulators of membrane lipid topology. Loss of Dnf1p and Dnf2p may have a limited impact on the steady state lipid distribution across the plasma membrane because membrane cycling between the plasma membrane and intracellular organelles harboring functional homologues (Golgi and endosomes) would help preserve an asymmetric lipid arrangement. Likewise, removing Drs2p from the Golgi may affect the lipid distribution across the plasma membrane because the incoming flow of secretory vesicles with an aberrant lipid orientation would exceed the capacity of Dnf1p and Dnf2p to retain a tight lipid asymmetry.
Role for Drs2p-related P-type ATPases in Vesicle Budding
Concurrent with an altered lipid movement and distribution across the plasma membrane, Δdnf1Δdnf2 cells displayed a defect in the uptake of endocytic tracer FM4-64 at low temperature. Even though Δdnf1Δdnf2 cells readily lost their metal-sensitive phenotype, such revertants were still blocked in inward lipid translocation and retained their cold-sensitive defect in FM4-64 uptake. Removal of Drs2p both enhanced aminophospholipid exposure at the cell surface and aggravated the FM4-64 internalization defect in Δdnf1Δdnf2 cells. Moreover, the Δdnf1Δdnf2Δdrs2 triple mutant was now also defective in ligand-induced internalization of α-factor receptor. Collectively, these findings point to a functional link between P-type ATPase-dependent lipid transport and the formation of endocytic vesicles at the plasma membrane. This raises the question how lipid translocation would participate in vesicle budding.
One possibility is that lipid translocation is required to help deform the membrane during vesicle budding. A prerequisite for membrane bending is the generation of a lipid imbalance across the bilayer. Consistent with the bilayer couple hypothesis (Sheetz and Singer, 1974), net transfer of a small fraction of phospholipids between the two leaflets of a bilayer (<1%) suffices to convert discoid giant liposomes into tubular and connected vesicular structures (Farge and Devaux, 1992). A characteristic of the ER, and possibly also of early Golgi membranes (Buton et al., 2002), is that phospholipids can freely cross the bilayer in both directions. In these flexible membranes, assembly of COP coats may be sufficient to drive budding. The situation is different in the plasma membrane, the late Golgi and endosomes where the free “flip-flop” of phospholipids across the bilayer is constrained. These membranes lack the energy-independent lipid flippase described for the ER (Menon, 1995) and contain high levels of sterols and sphingolipids that reduce lipid diffusion rates. As a result, the transbilayer lipid imbalance required for vesicle budding at the plasma membrane and late Golgi might be hard to accomplish without assistance of inward-directed, ATP-driven lipid transporters. Members of the Drs2p family are prime candidates to serve such a role. Indeed, loss of Drs2p perturbs secretory vesicle formation at the late Golgi (Chen et al., 1999; Gall et al. 2002), whereas removal of Dnf1p and Dnf2p causes a cold-sensitive defect in endocytic vesicle budding at the plasma membrane (this study). However, unlike the block in energy-coupled lipid influx, the endocytic defect in Δdnf1Δdnf2 cells was reversible by raising the temperature. It is feasible that Dnf1p and Dnf2p actively participate in vesicle budding at the plasma membrane, but become indispensable for this process only when a drop in temperature passes a threshold where a decreased fluidity of the membrane prevents coat assembly from driving this process alone. Moreover, fusion of secretory vesicles provides an alternative mechanism for generating a lipid imbalance across the plasma membrane that could be exploited during budding of endocytic vesicles. By decreasing the flow of secretory vesicles, loss of Drs2p may render endocytosis more dependent on the activity of inward lipid translocases at the plasma membrane. This may explain why loss of Drs2p exacerbates the endocytic defect in Δdnf1Δdnf2 cells.
An alternative possibility is that lipid translocation is needed to create a membrane environment permissive for vesicle budding. Dnf1p, Dnf2p, and the Golgi-localized Drs2p protein all seem to contribute to the sequestration of aminophospholipids in the inner leaflet of the plasma membrane. Conceivably, a high concentration of aminophospholipids in the cytoplasmic leaflet is necessary for the recruitment or activity of peripherally associated proteins with a critical function in vesicle budding. For example, clathrin can be recruited on chemically defined liposomes, yet requires a high concentration of PE (40% of total lipid) to generate well-defined, clathrin-coated buds on the membranes (Takei et al., 1998). However, we found that removing multiple Drs2p family members led to a marked decrease in aminophospholipid content of cellular membranes. This result is difficult to reconcile with the idea that maintenance of high concentrations of aminophospholipids in the cytoplasmic leaflet accounts for the requirement of P-type ATPases in vesicle budding, because downregulation of aminophospholipid levels would be counterproductive. It is tempting to speculate that vesicle budding in P-type ATPase mutants is defective because aminophospholipid levels in the exoplasmic leaflet exceed a certain threshold and that downregulation of aminophospholipid levels serves as a compensatory measure to limit the defect. Formation of a vesicle requires the bilayer to adopt a positive curvature at the site of the emerging bud as well as an extreme negative curvature at the site of membrane fission. Lipid polymorphism has been recognized as a critical factor in generating these curvatures (Schmidt et al., 1999; Weigert et al., 1999). The cone shape of PE, for example, makes it ideally suited for fitting in areas of high constriction. During vesicle budding, these areas arise primarily in the inner leaflet at the level of the bud neck. Loss of lipid asymmetry and equilibration of PE between the two leaflets of the plasma membrane may put physical constraints on the ability of the bilayer to adopt the type of curvatures needed to form an endocytic vesicle.
Figure 11.
Analysis of the internalization step of receptor-mediated endocytosis in P-type ATPase mutant cells. Cells were assayed for internalization of 35S-labeled α-factor at 24°C. Percentage of α-factor uptake was determined by dividing internalized counts by the total cell-associated counts for each time point. Data shown are the average of two independent experiments.
ACKNOWLEDGMENTS
We gratefully acknowledge Sigrún Hrafnsdóttir, Nannette Kälin, and Maarten Egmond for stimulating discussions and critical reading of the manuscript; Carel van Oven for help with flow cytometric analysis; and André Goffeau, Linda Hicke, Sirkka Keränen, Roland Lill, Timothy Levine, Sean Munro, and Masato Umeda for generously providing strains, plasmids, antibodies, or drugs. This work was supported by the Dutch Foundation “De Drie Lichten,” a long-term EMBO fellowship (to T.P.) and grants from the Royal Netherlands Academy of Arts and Sciences (to J.H.), the Meelmeijer Foundation (to J.H. and G.v.M.) and the Swiss National Science Foundation (to H.R.). A research training grant from the EC, HPRN-CT-2000–00077, is acknowledged (to G.v.M., H.R., and P.F.D.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0501. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0501.
REFERENCES
- Aoki Y, Uenaka T, Aoki J, Umeda M, Inoue K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J Biochem. 1994;116:291–297. doi: 10.1093/oxfordjournals.jbchem.a124522. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull LN, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- Buton X, et al. Transbilayer movement of monohexylsphingolipids in endoplasmic reticulum and Golgi membranes. Biochemistry. 2002;41:13106–13115. doi: 10.1021/bi020385t. [DOI] [PubMed] [Google Scholar]
- Catty P, de Kerchove d'Exaerde A, Goffeau A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 1997;409:325–332. doi: 10.1016/s0014-5793(97)00446-8. [DOI] [PubMed] [Google Scholar]
- Cerbon J, Calderon V. Generation, modulation and maintenance of the plasma membrane asymmetric phospholipid composition in yeast cells during growth: their relation to surface potential and membrane protein activity. Biochim Biophys Acta. 1995;1235:100–106. doi: 10.1016/0005-2736(94)00311-c. [DOI] [PubMed] [Google Scholar]
- Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase, and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Wickner WT. Yeast KEX2 protease and mannosyltransferase I are localized to distinct compartments of the secretory pathway. Yeast. 1989;5:25–33. doi: 10.1002/yea.320050105. [DOI] [PubMed] [Google Scholar]
- Daleke DL, Lyles JV. Identification and purification of aminophospholipid flippases. Biochim Biophys Acta. 2000;1486:108–127. doi: 10.1016/s1388-1981(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Decottignies A, Grant AM, Nichols JW, de Wet H, McIntosh DB, Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Farge E, Devaux PF. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys J. 1992;61:347–357. doi: 10.1016/S0006-3495(92)81841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E, Ojcius DM, Subtil A, Dautry-Varsat A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am J Physiol. 1999;276:C725–C733. doi: 10.1152/ajpcell.1999.276.3.C725. [DOI] [PubMed] [Google Scholar]
- Gall WE, Geething NC, Hua Z, Ingram MF, Liu K, Chen S, Graham TR. Drs2p-dependent budding of clathrin-coated vesicles in vivo. Curr Biol. 2002;12:1623–1627. doi: 10.1016/s0960-9822(02)01148-x. [DOI] [PubMed] [Google Scholar]
- Gomès E, Jakobsen MK, Axelsen KB, Geisler M, Palmgren MG. Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell. 2000;12:2441–2454. [PMC free article] [PubMed] [Google Scholar]
- Grant AM, Hanson PK, Malone L, Nichols JW. NBD-labeled phosphatidylcholine and phosphatidylethanolamine are internalized by transbilayer transport across the yeast plasma membrane. Traffic. 2001;2:37–50. doi: 10.1034/j.1600-0854.2001.020106.x. [DOI] [PubMed] [Google Scholar]
- Hanson PK, Nichols JW. Energy-dependent flip of fluorescence-labeled phospholipids is regulated by nutrient starvation and transcription factors, PDR1 and PDR3. J Biol Chem. 2001;276:9861–9867. doi: 10.1074/jbc.M009065200. [DOI] [PubMed] [Google Scholar]
- Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologs in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato U, Emoto K, Fredriksson C, Nakamura H, Ohta A, Kobayashi T, Murakami-Murofushi K, Kobayashi T, Umeda M. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J Biol Chem. 2002;277:37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- Marx U, Polakowski T, Pomorski T, Lang C, Nelson H, Nelson N, Herrmann A. Rapid transbilayer movement of fluorescent phospholipid analogs in the plasma membrane of endocytosis-deficient yeast cells does not require the Drs2 protein. Eur J Biochem. 1999;263:254–263. doi: 10.1046/j.1432-1327.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Meguro M, Kashiwagi A, Mitsuya K, Nakao M, Kondo I, Saitoh S, Oshimura M. A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat Genet. 2002;28:19–20. doi: 10.1038/ng0501-19. [DOI] [PubMed] [Google Scholar]
- Menon AK. Flippases. Trends Cell Biol. 1995;5:355–360. doi: 10.1016/s0962-8924(00)89069-8. [DOI] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Pomorski T, Herrmann A, Muller P, van Meer G, Burger K. Protein-mediated inward translocation of phospholipids occurs in both the apical and basolateral plasma membrane domains of epithelial cells. Biochemistry. 1999;38:142–150. doi: 10.1021/bi981244n. [DOI] [PubMed] [Google Scholar]
- Raggers RJ, Pomorski T, Holthuis JCM, Kalin N, van Meer G. Lipid traffic: the ABC of transbilayer movement. Traffic. 2000;1:226–234. doi: 10.1034/j.1600-0854.2000.010305.x. [DOI] [PubMed] [Google Scholar]
- Ripmaster TL, Vaughn GP, Woolford JL., Jr DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7901–7912. doi: 10.1128/mcb.13.12.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Bata LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980;255:274–283. [PubMed] [Google Scholar]
- Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Wolde M, Thiele C, Fest W, Kratzin W, Podtelejnikov AV, Witke W, Huttner WB, Soling HD. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, Grant A, Angeletti C, Malone L, Nichols JW, Rudolph H K. Loss of Drs2p does not abolish transfer of fluorescence-labeled phospholipids across the plasma membrane of Saccharomyces cerevisiae. J Biol Chem. 1998;273:34399–34405. doi: 10.1074/jbc.273.51.34399. [DOI] [PubMed] [Google Scholar]
- Smaal EB, Mandersloot JG, de Kruijiff B, de Gier J. Consequences of the interaction of calcium with dioleoylphosphatidate-containing model membranes: changes in membrane permeability. Biochim Biophys Acta. 1986;860:99–108. doi: 10.1016/0005-2736(86)90503-1. [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- Trotter PJ. A novel pathway for transport and metabolism of a fluorescent phosphatidic acid analog in yeast. Traffic. 2000;1:425–434. doi: 10.1034/j.1600-0854.2000.010507.x. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Weigert R, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–33. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- Zachowski A, Henry JP, Devaux PF. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature. 1989;340:75–76. doi: 10.1038/340075a0. [DOI] [PubMed] [Google Scholar]