Abstract
Cyclical pamidronate infusions increase bone mass in children suffering from osteogenesis imperfecta. The histological basis for these effects remains unknown. Therefore, we compared parameters of iliac bone histomorphometry from 45 patients before and after 2.4 ± 0.6 years of pamidronate treatment (age at the time of the first biopsy, 1.4–17.5 years; 23 girls). Although biopsy size did not change significantly (P = 0.30), cortical width increased by 88%. Cancellous bone volume increased by 46%. This was due to a higher trabecular number, whereas trabecular thickness remained stable. Bone surface–based indicators of cancellous bone remodeling decreased by 26–75%. There was no evidence for a mineralization defect in any of the patients. These results suggest that, in the growing skeleton, pamidronate has a twofold effect. In remodeling, bone resorption and formation are coupled and consequently both processes are inhibited. However, osteoclasts and osteoblasts are active on different surfaces (and are thus uncoupled) during modeling of cortical bone. Therefore resorption is selectively targeted, and continuing bone formation can increase cortical width.
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder with increased bone fragility and low bone mass. The most commonly used classification distinguishes four clinical types (1). OI type I comprises patients with absence of bone deformities. Type II is lethal in the perinatal period. OI type III is the most severe form in children surviving the neonatal time. These patients have extremely short stature and limb and spine deformities secondary to multiple fractures. Patients with mild to moderate bone deformities and variable short stature are classified as OI type IV.
In the majority of patients with OI, the disease can be linked to mutations in one of the two genes coding for collagen type I α chains (COL1A1 and COL1A2) (2). However, in some patients no such mutations are detectable. The lack of a mutation affecting collagen type I therefore does not rule out a diagnosis of OI.
OI is characterized by a variety of bone tissue abnormalities (3). Iliac bone biopsy specimens of these patients are small and have thin cortices, suggesting a defect in cortical bone modeling. The number of trabeculae in the cancellous compartment is low and the activity of cancellous bone remodeling is elevated. The thickness of trabeculae increases less with age than in healthy children. Due to these abnormalities, the bone mass of OI patients increases at a slower rate than in healthy children.
We and others have shown that cyclical intravenous therapy with the bisphosphonate pamidronate has a beneficial effect in children and adolescents with severe OI (4–6). Lumbar spine areal bone mineral density (BMD) and metacarpal cortical width increase, fracture rate decreases, and mobility improves.
The effects of cyclical pamidronate therapy on the bone tissue of growing individuals are unknown at present. This is an important gap in the safety profile of this treatment form. Likewise, the histological basis for the marked bone mass effect of pamidronate therapy in children with OI has not been evaluated. We therefore obtained paired iliac bone biopsy samples in children and adolescents suffering from OI types I, III, and IV. The first biopsy was performed before the start of therapy, the second after an average treatment duration of 2.4 years. Here we present qualitative and quantitative analyses of paired biopsy samples from 45 patients.
Methods
Subjects.
This study comprised patients with a diagnosis of OI type I, III, or IV who received pamidronate therapy at the Shriners Hospital for Children in Montréal. Patients were eligible for pamidronate treatment if they had long-bone deformities or had suffered more than 3 fractures per year (including vertebrae) during the previous two years (4, 5). This applies to all patients with OI types III and IV, and generally to the more severe cases of OI type I.
According to the treatment protocol, iliac bone biopsies are to be performed before the start of therapy and after two years of treatment. Specimens were obtained from alternate locations to avoid interference of repair processes at the site of the previous biopsy. As the biopsy specimens were preferably obtained during elective orthopedic procedures, such as rodding of long bones, the actual timing of the biopsy could deviate from the treatment protocol. In patients who did not require orthopedic interventions, specimens were obtained under general anesthesia in a procedure performed exclusively for this purpose. Biopsies were not performed in patients with a body weight below 10 kg or who presented an elevated risk for anesthesia. Patients were included in the present evaluation if iliac bone samples of sufficient quality were available both from the start of therapy and after 1–4 years of treatment.
One hundred twenty-five patients with a diagnosis of OI type I, III, or IV received pamidronate therapy for at least 1 year and thus were eligible for the present study. Pretreatment biopsies could not be obtained in 33 patients, in most cases because body weight was below 10 kg. Pretreatment samples from 11 patients were insufficiently preserved for quantitative analysis. Of the remaining 81 patients, 22 had not yet undergone a second bone biopsy at the time of the present evaluation, and in 14 patients the second biopsy was not adequate for quantitative analysis. Thus, 45 patients (23 girls, 22 boys) were included in the present study. The diagnostic distribution was as follows: OI type I, n = 11; OI type III, n = 10; OI type IV, n = 24. Age at the time of the pretreatment biopsy ranged from 1.4 to 17.5 years (mean ± SD, 8.4 ± 4.3 years). At the time of the second biopsy, these patients had received pamidronate therapy for 2.4 ± 0.6 years (range 1.0–4.0 years). One of the patients did not have anthropometric, densitometric, and biochemical evaluation at the time of the second biopsy.
Histomorphometric results in the study group were compared with those of two control groups, a cohort of subjects who were free from metabolic bone disorders and a group of OI patients who had not received bisphosphonate therapy prior to biopsy. The healthy control group comprised 58 subjects between 1.5 and 22.9 years of age, as described earlier (7). These individuals had undergone iliac bone biopsies during minor orthopedic procedures. The OI control group consisted of 123 children and adolescents (59 girls, 64 boys; age 1.4–21 years, median 8.4 years) with a diagnosis of OI type I (n = 47), OI type III (n = 22), or OI type IV (n = 54). Histomorphometric data from 72 of these patients have been published earlier (3).
The OI-IV groups in the study population and in the OI control cohort did not include patients who fulfilled the Sillence criteria for OI type IV, but who could be further classified as having OI type V, VI, or VII on the basis of our expanded classification (8–10).
The study was approved by the Shriners Hospital Institutional Review Board, and informed consent was obtained from legal guardians.
Treatment.
Pamidronate was administered intravenously on 3 consecutive days in all patients. The timing and dosage of these 3-day cycles varied with age. Children below 2 years of age received 0.25 mg/kg on the first day of the first cycle, 0.5 mg/kg on days 2 and 3 of the first cycle, and 0.5 mg/kg daily on all 3 days in subsequent cycles. Cycles were repeated every 2 months. Children from 2 to 3 years of age received 0.38 mg/kg on the first day of the first cycle, 0.75 mg/kg on days 2 and 3 of the first cycle, and 0.75 mg/kg daily on all 3 days of subsequent cycles. Cycles were repeated every 3 months. Above 3 years of age, the first 3-day cycle consisted of a dose of 0.5 mg/kg on the first day and 1 mg/kg on days 2 and 3. In subsequent cycles the dose was 1 mg/kg daily for 3 days. Cycles were repeated every 4 months. Thus, the yearly dose of the drug was the same at all ages. Each dose was diluted in 0.9% saline solution and administered slowly over 4 hours, as described (4, 5).
Calcium intake was maintained as adequate according to the recommended daily allowance in all patients. All patients underwent physiotherapy and occupational therapy evaluation and support, including exercises and design of special devices for transportation and sitting.
Bone biopsy and histomorphometry.
Whenever possible, labeling was performed prior to biopsy using demeclocycline (15–20 mg/kg per day taken orally during two 2-day periods separated by a 10-day free interval). Twenty-nine of the patients completed this labeling course before both biopsies. Transiliac bone samples were collected 4 or 5 days after the labeling. Biopsy preparation and histomorphometric analyses were performed with the standard procedures used at the Shriners Hospital, as described previously (7). Dynamic bone formation parameters on cortical surfaces were measured in those 16 patients who had a pair of samples with fully intact cortices that could be clearly separated from the cancellous compartment. Wall thickness was not measured in the present study, because reversal lines are difficult to visualize in severe OI. Consequently, activation frequency could not be determined. Osteoclasts were classified as large when their diameter exceeded 50 μm. Measurements were carried out using a digitizing table with OsteoMeasure software (Osteometrics Inc., Atlanta, Georgia, USA). Nomenclature and abbreviations follow the recommendations of the American Society for Bone and Mineral Research (11).
Bone densitometry.
Bone densitometry was performed in the anteroposterior direction at the lumbar spine (lumbar vertebra 1 to 4) using a Hologic QDR 2000W or 4500A device (Hologic Inc., Waltham, Massachusetts, USA). Results for areal BMD (unit g/cm2; bone mineral content relative to projection area) were transformed to age-specific z scores using data provided by the densitometer manufacturer.
Areal BMD as determined in this study is a composite measure of three-dimensional mineral density and bone length in the anteroposterior direction (12). A size-independent measure of three-dimensional density was derived by calculating the ratio between bone mineral content and the extrapolated external volume of the measured bones (volumetric BMD). This was done as described by Carter et al. (12) using the formula: volumetric BMD = (bone mineral content)/(projection area)1.5.
Anthropometric and biochemical measurements.
Weight and height measurements were converted to age- and sex-specific z scores on the basis of reference data published by the National Center for Health Statistics (13).
Urine creatinine concentration was determined colorimetrically. Urinary cross-linked N-telopeptides of type I collagen (NTX) were quantified by ELISA (Osteomark; Ostex International Inc., Seattle, Washington, USA) using the second void sample of the morning. Results for urinary NTX/creatinine ratios in OI patients were expressed as a percentage of age-specific mean values using published reference data (14). Patients were fasting at the time of urine sampling.
Collagen type I mutation analysis.
Genomic DNA from peripheral blood leukocytes was analyzed in 42 patients, as described by Korkko et al. (15). All exons of the COL1A1 and COL1A2 genes and their respective exon-intron boundaries, with the exception of the six exons encoding the N-propeptides, were amplified by PCR. PCR products were screened for mutations by conformation-sensitive gel electrophoresis (16). Those products containing heteroduplexes were then sequenced using the ThermoSequenase kit (Amersham Biosciences, Cleveland, Ohio, USA).
Statistical analyses.
Variables were tested for normal distribution using the Kolmogorov-Smirnov test. Normally distributed data were expressed as mean and SD. Geometric means and geometric SD were calculated for non-normally distributed variables. These variables were log-transformed before performance of tests that require normal distribution. Nonparametric statistics were used for periosteal measures, because results of 0 occurred in several samples and geometric means therefore could not be calculated. The difference of results at base line and during therapy was tested for significance using Student’s paired t test or the Wilcoxon test, as appropriate. Differences between bone formation parameters on endocortical and cancellous surfaces were tested for significance using ANOVA. Post-hoc comparison between bone surfaces was performed using Bonferroni’s adjustment.
To compare histomorphometric measures in the study group with those of healthy subjects, results of each patient were expressed as a percentage of the published age-specific mean value (7). The difference to the mean result expected in healthy children (i.e., 100%) was tested for significance using the one-sample t test.
Histomorphometric measures in the study group were also compared with those from OI patients who had not received bisphosphonate therapy. The age-specific mean value in untreated patients was derived by linear regression of each histomorphometric parameter with age. This analysis was performed for the three OI types separately. For parameters that did not significantly vary with age in a given OI type, the mean value of the entire group was used. The results of each patient were expressed as a percentage of the age-specific mean value in untreated patients. The difference to the mean result expected for untreated OI patients (100%) was tested for significance using the one-sample t test.
Associations are given as Pearson correlation or Spearman rank correlation, as appropriate. All tests were two-tailed, and throughout the study P < 0.05 was considered significant. These calculations were performed using SPSS software, version 9.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
Results
Clinical data.
The participants of the present study were on average very short, with no significant change during the observation period (Table 1). Lumbar spine BMC increased by 130%, whereas projection area increased by only 36%. This translated into increases in areal and volumetric BMD of 74% and 48%, respectively. The urinary NTX/creatinine ratio decreased by 56% during therapy.
Table 1.
Anthropometric, densitometric, and biochemical results
Qualitative evaluation of bone samples.
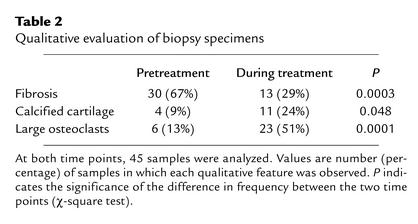
During pamidronate treatment, fewer samples showed fibrotic bone marrow, but more samples contained calcified cartilage or abnormally large osteoclasts (Table 2). Double labels were present in all bone specimens from patients who had received two courses of tetracycline prior to biopsy.
Table 2.
Qualitative evaluation of biopsy specimens
Histomorphometric measures of iliac bone structure.
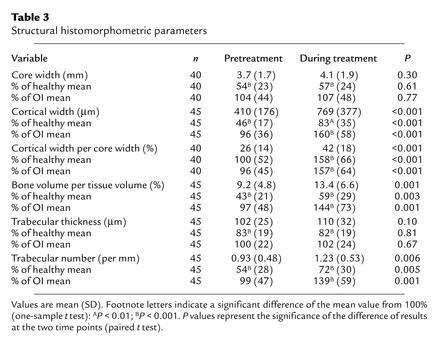
External bone size (core width) did not change significantly during pamidronate therapy, but average cortical width increased by 88% (Table 3). In 43 of the 45 patients, cortices were thicker in the second than in the first bone sample. Cortical width in the second sample was 60% higher (P < 0.001) than expected for age-matched untreated OI patients with the same type of the disease. Similar results were obtained when cortical width was expressed as a percentage of core width.
Table 3.
Structural histomorphometric parameters
Average cancellous bone volume increased by 46%. This was entirely due to an increase in trabecular number, whereas no significant changes occurred in trabecular thickness. The duration of pamidronate therapy was not associated with the changes in either cortical width or cancellous bone volume (P > 0.05 in both cases).
Histomorphometric measures of cancellous bone formation.
Both osteoid thickness and relative osteoid surface decreased during pamidronate therapy (Table 4). Osteoblast and mineralizing surfaces dropped to 25% and 32% of the pretreatment level, respectively. During therapy, the fraction of osteoid seam length showing mineralizing activity was significantly smaller than at base line. Therefore, mineralization lag time was prolonged during pamidronate treatment, even though mineral apposition rate did not change.
Table 4.
Histomorphometric parameters of cancellous bone formation
Bone formation rate per bone surface (BFR/BS) decreased by about 70%, from an elevated pretreatment level to a value that was significantly below that of healthy controls. The duration of pamidronate therapy was significantly associated with the decrease in BFR/BS (P = 0.008). The urinary NTX/creatinine ratio was significantly associated with BFR/BS at base line (r2 = 0.11; P = 0.04) but not during therapy (r2 = 0.04; P = 0.16). When both measures were expressed as a percentage of the age-specific mean for healthy children, there was no significant relationship at either time point (P > 0.3).
Histomorphometric measures of cancellous bone resorption.
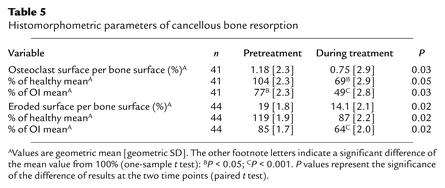
Pretreatment osteoclast surface was not significantly increased over the mean value for age-matched healthy subjects (Table 5). During pamidronate therapy, osteoclast and eroded surface decreased by 36% and 26%, respectively.
Table 5.
Histomorphometric parameters of cancellous bone resorption
Bone formation rate on periosteal and endocortical surfaces.
Bone formation activity was very variable on periosteal surfaces, ranging from 0 (i.e., no label uptake) to 462 μm3/μm2/yr (Table 6). No significant change in periosteal BFR/BS was noted during pamidronate therapy.
Table 6.
Bone formation rate on endocortical and periosteal surfaces
All samples showed label uptake on endocortical surfaces. Base-line BFR/BS was approximately 150% (P = 0.004) and 100% (P = 0.09) higher on external and internal endocortical surfaces, respectively, than on cancellous surfaces. During pamidronate therapy, BFR/BS decreased on the endocortical surface of the external cortex but did not change significantly on the internal cortex. Compared with the cancellous surface, BFR/BS was now three times (P = 0.07) and seven times (P < 0.001) higher on external and internal endocortical surfaces, respectively.
Relationship between treatment effects and collagen type I mutation.
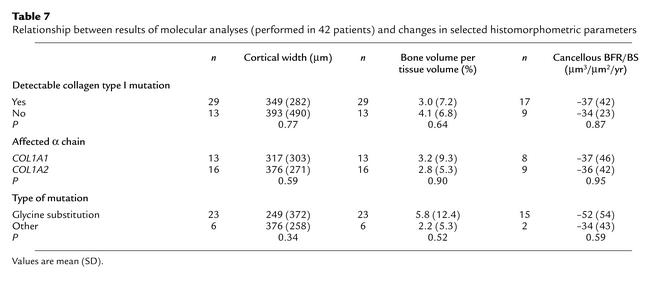
Patients who had a detectable mutation in one of the two α chains of collagen type I had a histomorphometric response to pamidronate therapy that was similar to that of patients without a mutation (Table 7). In 23 patients, mutations were predicted to lead to substitutions of glycine by another amino acid. Three mutations affected splice sites, two were frameshift mutations, and one was a nonsense mutation. The treatment effect did not appear to depend on the type of mutation or on which collagen type I alpha chain was affected. Among glycine substitutions there was no relationship between the distance of the affected glycine residue from the N-terminus and changes in cortical thickness, cancellous bone volume, or trabecular BFR/BS (P > 0.05 in each case; Spearman rank correlation).
Table 7.
Relationship between results of molecular analyses (performed in 42 patients) and changes in selected histomorphometric parameters
Discussion
Safety issues
In this study on children and adolescents with OI, pamidronate therapy led to a marked reduction in remodeling activity of trabecular bone. The relative magnitude of these changes was similar to that reported for adults who received bisphosphonates (17–19). Lower bone turnover probably accounts for the observation that marrow fibrosis decreased in our patients during pamidronate therapy.
The slowdown in remodeling activity may contribute to making bones stronger, because each remodeling event creates a transient structural weakness in the tissue (20). On the other hand, a decrease in remodeling activity is not necessarily beneficial in the long term, as microdamage might accumulate in the bone tissue (21). Slow bone turnover may also explain why more samples contained calcified cartilage during pamidronate treatment. Growth plate cartilage material probably is not completely removed during the conversion of primary to secondary spongiosa. There is no indication at present that the decreased remodeling activity and the increased amount of calcified cartilage cause clinical problems. Nevertheless, this possibility must be closely monitored, especially in patients who receive pamidronate for a long time. Indeed, bone turnover was more suppressed in patients who had been on treatment for a longer period.
No patient of the present study had signs of a mineralization defect. Mineralization disorders were relatively frequent with the first-generation bisphosphonate etidronate but are rarely observed with pamidronate (22). Nevertheless, there is one well-documented case of an adolescent who developed osteomalacia and rickets while he was receiving intravenous pamidronate treatment for fibrous dysplasia, a condition where hypophosphatemia frequently compounds the picture (23). In our patients, mineralization lag time was prolonged during therapy, but there was no accumulation of osteoid. Quite to the contrary, osteoid thickness and surface decreased considerably. The prolonged mineralization lag time therefore is a sign of sluggish remodeling activity rather than of a mineralization defect.
Therapeutic effects of cyclical pamidronate therapy
Effect on bone mass parameters.
Lumbar spine BMC more than doubled during the observation period. A proportion of this increase can be explained by the concomitant increase in vertebral size. After size changes are taken into account, the estimated volumetric BMD still increased by an average rate of 19% per year. Adults who receive bisphosphonate therapy typically experience an increase in lumbar spine areal BMD of 2–5% per year (24, 25). Most of this increase in adults is probably due to a higher mineralization density of bone tissue (26), and this effect may also have contributed to the increase in BMD in the present study. However, the markedly better treatment response in children with OI can be explained by the finding that both cancellous bone volume and cortical width increased. Neither of these effects is usually found in bisphosphonate-treated adults (17–19). Increasing cortical width appears to be a generalized phenomenon during pamidronate therapy of children with OI, as we had found a similar increase (averaging 27% per year) in metacarpal bones (4).
In growing individuals, the amount of bone present in an iliac cross section increases through three mechanisms (27): remodeling of cancellous bone with a positive balance, production of new trabeculae by endochondral ossification, and cortical bone modeling. These mechanisms appear to be differentially affected by cyclical pamidronate therapy.
Effect on cancellous bone remodeling.
It is somewhat surprising that antiresorptive treatment with pamidronate led to a larger relative decrease in bone formation parameters than in bone resorption measures. Yet, histomorphometric resorption parameters do not quantify osteoclast function but only reflect the amount of bone surface that has an eroded appearance or is occupied by osteoclasts. Our results are therefore in accordance with the hypothesis that pamidronate may render osteoclasts dysfunctional without causing apoptosis (28). This is also supported by the observation that many osteoclasts had a clearly abnormal aspect.
A decrease in remodeling activity will not by itself lead to a major increase in the amount of bone, unless there is a positive remodeling balance. This should lead to an increase in trabecular thickness (27), which, however, was not observed in this study. Thus, pamidronate did not have a detectable effect on the remodeling balance. Similarly, studies in adults did not reveal any changes in cancellous bone volume during bisphosphonate therapy (17–19).
Effect on production of trabeculae.
The increase in cancellous bone volume was entirely due to an increase in trabecular number. During growth, new primary trabeculae arise at the growth plate–metaphyseal bone interface. About 80% of these primary trabeculae are quickly removed during the ensuing conversion to secondary spongiosa (29). It is likely that, during antiresorptive therapy, a larger proportion of primary trabeculae “survive” and become secondary trabeculae. Indeed, pamidronate increased the number of trabeculae in the tibial metaphysis of growing rats, without an effect on trabecular thickness (30). These results of these animal studies exactly match the observations of the present study.
Effect on cortical bone modeling.
Cortical width during growth is determined by modeling of the endocortical and periosteal surfaces (27). In modeling, formation is not linked to prior resorption, which is the major difference from the much better known remodeling process (27, 31). Thus, antiresorptive therapy should not interfere with bone formation activity that is dedicated to modeling. Indeed, pamidronate had a smaller effect (or no effect) on BFR/BS on periosteal and endocortical (modeling) surfaces than on cancellous (remodeling) surfaces. Thus, modeling-directed bone formation activity appeared to continue, allowing for the rapid increase in cortical width.
Effect on whole-body bone metabolism versus iliac bone metabolic activity.
It would be useful for clinical management if a noninvasive marker of bone metabolism such as NTX indicated to what degree bone turnover is suppressed in individual patients. However, the urinary NTX/creatinine ratio during therapy was not associated with histomorphometric measures of the activity of cancellous bone metabolism. Thus, it is unclear whether the urinary NTX/creatinine ratio is useful for monitoring pamidronate therapy in children and adolescents with OI.
Relationship between treatment effect and collagen type I mutations.
Histomorphometric changes during therapy did not depend on whether a mutation affecting collagen type I was detectable or not and had no obvious relationship with the location or type of mutation. Thus children with OI appear to benefit from pamidronate therapy regardless of the results of collagen type I mutation analysis.
In conclusion, bone mass increases in children and adolescents with OI who are treated with cyclical intravenous pamidronate, because cortical width and trabecular number increase. These effects may reflect the influence of the treatment on cortical modeling and endochondral ossification, respectively. Potential adverse effects of this therapy are the marked decrease in cancellous bone remodeling and an increase in the amount of residual calcified cartilage within secondary spongiosa. It is unclear at present whether this structural abnormality has detrimental effects on bone stability in the long run. It is therefore prudent to reserve pamidronate treatment for patients in whom the obvious clinical benefits outweigh the potential long-term risks.
Acknowledgments
We are indebted to Liljana Lalic and Peter Roughley for helpful comments on mutation analysis. We thank Guy Charette and Josée Dépot for technical assistance with biopsy sample processing. This study was supported by the Shriners of North America.
Footnotes
See the related Commentary beginning on page 1239.
Horacio Plotkin’s present address is: Inherited Metabolic Diseases Section, Children’s Hospital and Department of Pediatrics, University of Nebraska, Omaha, Nebraska, USA.
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: osteogenesis imperfecta (OI); bone mineral density (BMD); bone formation rate per bone surface (BFR/BS).
References
- 1.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe, D.W., and Shapiro, J.R. 1998. Osteogenesis imperfecta. In Metabolic bone disease and clinically related disorders. L.V. Avioli and S.M. Krane, editors. Academic Press Inc. San Diego, California, USA. 651–695.
- 3.Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26:581–589. doi: 10.1016/s8756-3282(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 4.Glorieux FH, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin H, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 6.Astrom A, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child. 2002;86:356–364. doi: 10.1136/adc.86.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glorieux FH, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 8.Glorieux FH, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15:1650–1658. doi: 10.1359/jbmr.2000.15.9.1650. [DOI] [PubMed] [Google Scholar]
- 9.Glorieux FH, et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17:30–38. doi: 10.1359/jbmr.2002.17.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Ward LM, et al. Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone. 2002;31:12–18. doi: 10.1016/s8756-3282(02)00790-1. [DOI] [PubMed] [Google Scholar]
- 11.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 12.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 13.Hamill PV, et al. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–629. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 14.Bollen AM, Eyre DR. Bone resorption rates in children monitored by the urinary assay of collagen type I cross-linked peptides. Bone. 1994;15:31–34. doi: 10.1016/8756-3282(94)90888-5. [DOI] [PubMed] [Google Scholar]
- 15.Korkko J, et al. Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet. 1998;62:98–110. doi: 10.1086/301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguly A, Rock MJ, Prockop DJ. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA. 1993;90:10325–10329. doi: 10.1073/pnas.90.21.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravenboer N, et al. Bone histomorphometric evaluation of pamidronate treatment in clinically manifest osteoporosis. Osteoporos Int. 1999;9:489–493. doi: 10.1007/s001980050175. [DOI] [PubMed] [Google Scholar]
- 18.Chavassieux PM, et al. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavassieux PM, et al. Effects of alendronate on bone quality and remodeling in glucocorticoid-induced osteoporosis: a histomorphometric analysis of transiliac biopsies. J Bone Miner Res. 2000;15:754–762. doi: 10.1359/jbmr.2000.15.4.754. [DOI] [PubMed] [Google Scholar]
- 20.Burr DB, et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 21.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- 22.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 23.Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12:1746–1752. doi: 10.1359/jbmr.1997.12.10.1746. [DOI] [PubMed] [Google Scholar]
- 24.Orwoll E, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 25.Reid IR, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 26.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 27.Parfitt AM, Travers R, Rauch F, Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27:487–494. doi: 10.1016/s8756-3282(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 28.Halasy-Nagy JM, Rodan GA, Reszka AA. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone. 2001;29:553–559. doi: 10.1016/s8756-3282(01)00615-9. [DOI] [PubMed] [Google Scholar]
- 29.Fazzalari NL, Moore AJ, Byers S, Byard RW. Quantitative analysis of trabecular morphogenesis in the human costochondral junction during the postnatal period in normal subjects. Anat Rec. 1997;248:1–12. doi: 10.1002/(SICI)1097-0185(199705)248:1<1::AID-AR1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Pataki A, et al. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec. 1997;249:458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Frost HM. Skeletal structural adaptations to mechanical usage (SATMU). I. Redefining Wolff’s law: the bone modeling problem. Anat Rec. 1990;226:403–413. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]